Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most highly aggressive cancer worldwide with an extremely poor prognosis. Evidence has revealed that microRNA-587 (miR-587) is abnormally expressed in a series of cancers. However, its expressions and functions in HCC have not been clearly acknowledged.
Methods
We detected the expression level of miR-587 both in the Gene Expression Omnibus (GEO) database and 86 paired clinical HCC tissues together with paired adjacent normal tissues by quantitative real-time PCR (qRT-PCR). Afterwards, the transfected HCC cell line SMMC-7721 cells were collected for the cell proliferation assay, cell-cycle arrest, cell migration, and invasion assays to explore the roles of miR-587 in regulating cellular function. In addition, bioinformatics analysis, combined with qRT-PCR and dual-luciferase reporter assays, were performed to confirm whether ribosomal protein SA (RPSA) mRNA was the direct target gene of miR-587. Moreover, the Cancer Genome Atlas (TCGA) and GEO databases as well as 86 paired clinical HCC tissues were used to verify the negative regulation between miR-587 and RPSA.
Results
In the present study, both the GEO database (GSE36915 and GSE74618) analysis and qRT-PCR analysis of 86 paired clinical tissues showed that miR-587 was significantly downregulated in HCC tissues. The overexpression of miR-587 inhibited proliferation, cell cycle, migration, and invasion in SMMC-7721 cells. In addition, miR-587 directly interacted with the 3′-untranslated region (UTR) of RPSA. Moreover, miR-587 overexpression directly suppressed RPSA expression, and the two genes were inversely expressed in HCC based on the analyses in TCGA and GEO (GSE36376) databases and qPCR analysis of 86 paired clinical tissues.
Conclusion
Our results demonstrate that miR-587 is downexpressed in HCC and regulates the cellular function by targeting RPSA.
1. Introduction
Primary liver cancer is the fourth leading cause of cancer-related death worldwide, and the incidence rates continue to rise faster than that of any other cancer in both men and women [1]. HCC accounts for 85–90% of all cases of primary liver cancer, which poses a significant threat to health and life in China [2]. MicroRNAs (miRNAs) are a set of small, noncoding RNAs of 20-25 nucleotides which play a pivotal role in regulating gene expressions by directly targeting the 3′-UTR of mRNAs and negatively regulating its transcription and translation [3]. Growing evidences reveal that miRNAs exert critical functions in the progression of multiple human cancers [4, 5]. As to HCC, numerous studies have reported that up- and downregulations of miRNAs are closely related to the occurrence and prognosis of HCC [6, 7]. For instance, Ji et al. [8] report that the upregulation of hsa-miR-210 can predict the poor outcome of HCC. Xiang et al. [9] show that downregulated miR-520d-3p is correlated with the poor survival of HCC patients, which may attribute to its promotion on cell proliferation, migration, and invasion in HCC cells. Wei et al. [10] conclude that miR-137 is correlated with the poor prognosis of HCC patients based on the microarray data of GSE31384. Related studies show that miR-587 can be abnormally expressed in colorectal cancer [11], melanoma [12], and glioma [13]. However, its expressions and cellular functions in HCC have not been fully elucidated. In this study, we aim to systematically investigate the expression levels and biofunctions of miR-587 in HCC.
RPSA, a laminin receptor 1, is a member of the nonintegrin family. The amino acid sequence of RPSA is highly conserved during evolution and has important biological functions by interactions with the extracellular matrix glycoprotein laminin [14, 15]. Munien et al. [16] report that RPSA transcript levels are higher in malignant melanoma cells than normal cells. However, the expression and biofunction of RPSA in HCC are not fully explored.
In this study, we analyzed the relative expressions of miR-587 in HCC tissues and adjacent normal tissues and explored its cellular functions in SMMC-7721 cells. Furthermore, whether RPSA was a target mRNA of miR-587 was predicted and verified, and their regulatory relationship in HCC was analyzed.
2. Materials and Methods
2.1. Tissues and Patients
Eighty-six cancerous tissues and adjacent normal tissues from patients pathologically diagnosed as HCC from April 2013 to September 2016 at the Affiliated Tumor Hospital of Guangxi Medical University, China, were sampled. The tissues were stored at -80°C in RNAlater (Qiagen, Valencia, CA, USA). All patients enrolled met the criteria that were defined as follows: (a) patients undergoing surgical resection initially, (b) who were diagnosed according to histopathological criteria, and (c) without any other malignant tumors. Clinical characteristics consisted of age, sex, smoking status, alcohol use, tumor size, HBV infection, number of involved lesions, serum alpha-fetoprotein (AFP) level, liver cirrhosis, ascites, the Barcelona Clinic Liver Cancer (BCLC) stage, TNM stage, tumor thrombus, distant metastasis, microvascular thrombi (MVI), and early postoperative recurrence (≤12months). All data were obtained from medical records and pathological reports of the patients. The ethical approval for our research on human HCC tissues after surgical resection was obtained from the Ethics Review Committee of the Affiliated Tumor Hospital of Guangxi Medical University.
2.2. Cell Lines and Culture Conditions
The liver cancer cell line SMMC-7721 cells were preserved in our laboratory and identified by short tandem repeat (STR) analysis (GENEWIZ Inc., South Plainfield, NJ, USA). Cells were cultured in Dulbecco's modified Eagle medium (DMEM, Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, USA) and 1% antibiotics (penicillin, 100 μ/ml; streptomycin sulfates, 100 mg/ml) in a humidified atmosphere with 5% CO2 at 37°C.
2.3. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from the enrolled tissues and cultured cells. The concentration of total RNA was determined using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Sepharose was used to bind the integrality of RNA. Then total RNAs in tissues and cells were reverse transcribed into cDNA using the miRcute miRNA cDNA kit (Tiangen Biotechnology, Beijing, China) and PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Dalian, China). Additionally, a qRT-PCR platform (Roche Lightcycler 480 system, Roche Diagnostics, Basel, Switzerland) was employed to determine expression levels of miR-587 and RPSA using the miRcute miRNA qPCR detection kit (Tiangen Biotechnology, Beijing, China) and FastStart Universal SYBR Green Master (ROX) kit (Roche, Germany). Relative expression levels of miR-587 and RPSA were normalized to their endogenous controls U6 and Actin beta using the 2−ΔΔCt method.
2.4. Cell Transfection
Synthesized RNA duplexes of miR-587 mimics, miR-587 inhibitors, and conjugated negative controls (NC) were purchased from Tiangen Biochemical Technology (Beijing, China). According to the manufacturer's instructions, cells (1 × 105 per well) were seeded into 12-well plates. miR-587 mimic and mimic NC (both at the concentration of 50 nM) as well as miR-587 inhibitor and inhibitor NC (100 nM) were added to cells at the logarithmic phase of growth. Lipo6000 (Invitrogen, USA; 5 μl per well) was used for transfection per well as well. The cells were harvested after 48 h of transfection to determine the relative expressions and analyze altered functions of miR-587.
2.5. Cell Proliferation Assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to detect the proliferation of SMMC-7721 cells. SMMC-7721 cells (approximately 5000 per well) transfected by mimics or inhibitors were collected and seeded into 96-well plates, and their viability at 24, 48, 72, and 96 h was measured. The cells were incubated with 100 μl culture medium mixed with 10 μl MTT (Sigma-Aldrich, St. Louis, USA) solution for 4 h. And then, the mixed medium was replaced by dimethylsulfoxide (DMSO, Sigma-Aldrich; Shanghai, China), 150 μl per well. Subsequently, the plate was agitated on the shaking table (TSB-108, Qilinbeier, Jiangsu, China) for about 15 min in the dark. The optical density (OD) value at 490 nm was quantitated for each sample to obtain the viability of SMMC-7721 cells.
2.6. Cell Wound Healing Assay
SMCC-7721 cells (1 × 105 per well) were seeded into 6-well plates. After adherence, the cells were transfected with miR-587 mimic and inhibitor as well as the corresponding NC for 48 h, respectively. A wound (a clear straight line) was vertically scraped across each well using a sterile 200 μl pipette tip. Phosphate buffered saline (PBS; Gibco) was used to clean the floating cells. Subsequently, the 6-well plates were cultured with serum-free DMEM. The cells were photographed after 0 and 24 h of incubation using a digital camera system (Olympus Corporation, Tokyo, Japan).
2.7. Transwell Invasion Assay
Transwell assay was carried out to detect the cell invasion in 24-well plates (BD Bioscience, USA) with Transwell filters of 8 μm pore size (Costar, USA). Transfected cells (1 × 105 per well) cultured with serum-free DMEM were added to the upper chamber. The lower chamber was filled with DMEM containing 10% serum. The cells were allowed to invade for 24 h, and those on the lower side of Matrigel filter were fixed with paraformaldehyde, stained with crystal violet, and photographed and counted using a microscope.
2.8. Measurement of Cell Cycle
After 48 h of transfection, about 1 × 106 cells were collected and fixed in 70% ethanol overnight at -20°C and then washed three times by PBS. The cells were added with 0.25 mg/ml RNase A for incubation at 37°C for 30 min. And 5 μl propidium iodide (PI, KeyGen, Nanjing, China) was further added for 30 min incubation at room temperature in the dark. Cell suspension was collected for analyzing cell cycle using the FACSCalibur Flow Cytometer (BD Bioscience, USA).
2.9. miR-587 Target Prediction
The TargetScan Release 7.1 prediction algorithm was used to predict the potential target genes of miR-587 (http://www.targetscan.org/vert_71/). The cumulative weighted context++ score was utilized to screen the putative miR-587 targets. Genes with a cutoff value of less than -0.4 were considered as the potential targets. Meanwhile, the significantly upregulated genes in HCC were collected in TCGA databases. The final targets were confirmed based on the interaction between the predicted miR-587 targets and the upregulated genes in HCC.
2.10. Dual-Luciferase Reporter Assay
A miR-RPSA-3′-UTR plasmid containing the potential miR-587 binding sites was prepared by using the Dharma FECT Duo Transfection Reagent for the human RPSA-3′-UTR luciferase reporter assay. 293T cells were cotransfected with the luciferase plasmid (100 ng per well) and miR-587 mimics (100 nM) in 96-well plates. After the cells were transfected for 48 h at 37°C, the luciferase activity was detected using the dual-luciferase reporter assay system (Promega, Madison, USA).
3. Statistical Analyses
All statistical analyses in this study were performed using the SPSS 22.0 software (Chicago, IL, USA). Graphs were generated using the GraphPad Prism 5 software (La Jolla, CA, USA). The differential gene analysis of multiplatform transcriptional expression data in GEO and TCGA was performed using the “limma” package in R language. Gene expression profiling datasets of miR-587 and RPSA were examined based on the two databases using Student's t-test. Additionally, a paired t-test was performed to analyze the relative expressions of miR-587 and RPSA in clinical HCC tissues compared with adjacent normal tissues. Qualitative variables were compared using chi-square tests. Mean ± standard error of the mean (SEM) was presented to describe continuous variables. Pearson's correlation analysis was used to analyze the correlation between miR-587 and RPSA expressions. A two-sided p value of less than 0.05 was set as the threshold for statistical significance.
4. Results
4.1. miR-587 Was Downregulated in HCC Tissues
Two GEO (GSE36915 and GSE74618) databases were included for the validation of miR-587 expressions. Consistently, the GSE36915 study containing 68 HCC tissues and 21 noncancerous tissues validated that miR-587 was significantly downregulated in HCC tissues compared with noncancerous tissues (p < 0.001, Figure 1(a)). And GSE74618 containing 218 HCC tissues and 20 noncancerous tissues revealed the same result that HCC tissues had lower miR-587 expression levels than noncancerous tissues (p = 0.01, Figure 1(b)). To confirm the results according to bioinformatics analysis from the two databases, 86 paired HCC and adjacent normal tissues were collected in this study. The qRT-PCR analysis showed the same result that expression levels of miR-587 were significantly reduced in HCC tissues compared with adjacent normal tissues (p = 0.02, Figure 1(c)). The relationship between miR-587 expression and clinicopathologic features of HCC patients is displayed in Table 1. The results showed that miR-587 was associated with AFP (p = 0.002) and MVI (p = 0.041).
Figure 1.
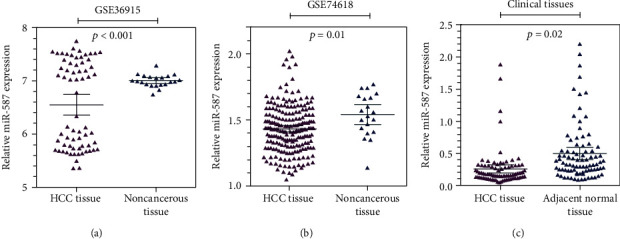
Downexpression levels of miR-587 in HCC. The gene expression analysis based on (a) GSE36915, (b) GSE74618 datasets from GEO database, and (c) data from 86 paired clinic HCC tissues shows that miR-587 was downexpressed in HCC tissues compared with noncancerous tissues (p < 0.001, p = 0.01, and p = 0.02, respectively). miR: microRNA.
Table 1.
Relationship between miR-587/RPSA expressions and clinicopathologic features of HCC patients.
| Clinicopathological | miR-587 | Patients | RPSA | Patients | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p value | Low | High | p value | |||
| Features | (n = 43) | (n = 43) | (n = 86) | (n = 43) | (n = 43) | (n = 86) | ||
| Gender | 0.534 | 0.132 | ||||||
| Female | 7 | 5 | 12 | 9 | 4 | 13 | ||
| Male | 36 | 38 | 74 | 34 | 39 | 73 | ||
| Age(years) | 0.245 | 0.357 | ||||||
| ≤55 | 32 | 27 | 59 | 27 | 31 | 58 | ||
| >55 | 11 | 16 | 27 | 16 | 12 | 28 | ||
| Smoking status | 0.808 | 0.194 | ||||||
| Negative | 31 | 32 | 64 | 36 | 31 | 67 | ||
| Positive | 12 | 11 | 22 | 7 | 12 | 19 | ||
| Alcoholism | 0.795 | 0.047 | ||||||
| Negative | 34 | 33 | 67 | 39 | 32 | 71 | ||
| Positive | 9 | 10 | 19 | 4 | 11 | 15 | ||
| Size (cm) | 0.223 | 0.194 | ||||||
| ≤5 | 14 | 9 | 23 | 12 | 7 | 19 | ||
| >5 | 29 | 34 | 63 | 31 | 36 | 67 | ||
| Number | 0.645 | 0.516 | ||||||
| ≤2 | 28 | 30 | 58 | 22 | 25 | 47 | ||
| >2 | 15 | 13 | 28 | 21 | 18 | 39 | ||
| HBV | 0.802 | 0.336 | ||||||
| Negative | 11 | 10 | 21 | 10 | 14 | 24 | ||
| Positive | 32 | 33 | 65 | 33 | 29 | 62 | ||
| AFP (ng/ml) | 0.002 | 0.272 | ||||||
| ≤400 | 25 | 11 | 35 | 20 | 15 | 35 | ||
| >400 | 18 | 32 | 51 | 23 | 28 | 51 | ||
| Liver cirrhosis | 0.058 | 0.534 | ||||||
| No | 5 | 12 | 17 | 7 | 5 | 12 | ||
| Yes | 38 | 31 | 69 | 36 | 38 | 74 | ||
| Ascites | 0.725 | 0.501 | ||||||
| No | 39 | 38 | 77 | 37 | 39 | 76 | ||
| Yes | 4 | 5 | 9 | 6 | 4 | 10 | ||
| BCLC stage | 0.828 | 0.387 | ||||||
| 0 + A | 24 | 25 | 48 | 22 | 18 | 40 | ||
| B + C | 19 | 18 | 38 | 21 | 25 | 46 | ||
| TNM stage | 0.996 | 0.308 | ||||||
| I-II | 21 | 20 | 41 | 22 | 16 | 38 | ||
| III-IV | 22 | 21 | 43 | 21 | 24 | 45 | ||
| Distant metastasis | 0.062 | 0.501 | ||||||
| Absent | 34 | 40 | 74 | 39 | 37 | 76 | ||
| Present | 9 | 13 | 22 | 4 | 6 | 10 | ||
| Tumor thrombus | 0.826 | 0.110 | ||||||
| No | 25 | 26 | 51 | 32 | 25 | 57 | ||
| Yes | 18 | 17 | 35 | 11 | 18 | 29 | ||
| Microvascular cancer thrombus | 0.041 | 0.190 | ||||||
| No | 29 | 37 | 66 | 15 | 21 | 36 | ||
| Yes | 14 | 6 | 20 | 28 | 22 | 50 | ||
| Early recurrence (≤12 months) | 0.235 | 0.021 | ||||||
| No | 28 | 33 | 61 | 34 | 24 | 68 | ||
| Yes | 15 | 10 | 25 | 9 | 19 | 18 | ||
miR: microRNA; AFP: alpha-fetoprotein; BCLC stage: Barcelona Clinic Liver Cancer stage.
4.2. miR-587 Inhibited Cell Proliferation in HCC Cells
QRT-PCR assay was used to detect the transfection efficiency of SMMC-7721 cells, and results showed that the relative expression level of miR-587 in cells transfected with the miR-587 mimic was 237.94 times higher than that in cells transfected with mimic NC (p = 0.001), and miR-587 expressions in cells transfected with the miR-587 inhibitor decreased by 18.77% compared with those in cells transfected with inhibitor NC (p = 0.037) (Figure 2(a)). Then, the MTT assay was conducted to detect the viability of SMMC-7721 cells. The results revealed that the upregulation of miR-587 suppressed cell proliferation at 72 (p = 0.008) and 96 h (p = 0.019) (Figure 2(b)). Conversely, the downregulation of miR-587 fostered cell proliferation at 72 (p = 0.037) and 96 h (p = 0.001) (Figure 2(c)). The results of the MTT assay revealed that the overexpression of miR-587 evidently suppressed the proliferation of SMMC-7721 cells.
Figure 2.
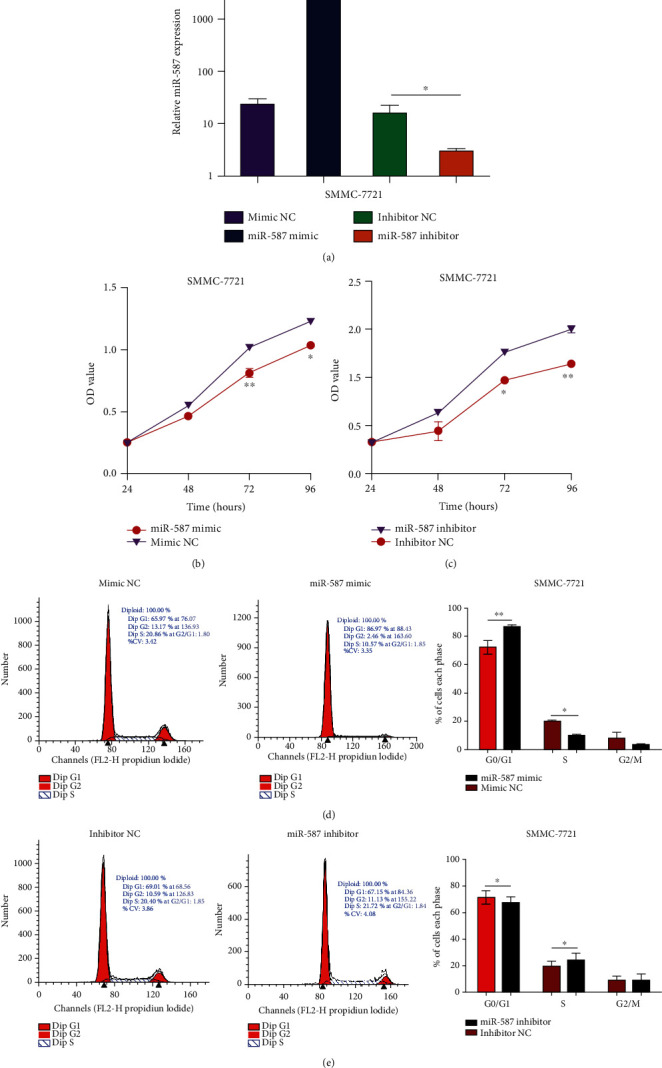
miR-587 suppressed HCC cell proliferation. (a) The transfection efficiency of miR-587 mimic (p = 0.001) and inhibitor (p = 0.037). The MTT assay showed that miR-587 upregulation (b) suppressed the cell viability of SMMC-7721 cells, whereas miR-587 downregulation (c) fostered the cell viability (both p < 0.05). Flow cytometry analysis showed that the number of SMMC-7721 cells at G1 phase increased and those at S phase decreased in the miR-587 mimic group (d) (p = 0.001, p = 0.044, respectively). The number of SMMC-7721 cells at G1 phase decreased and those at S phase increased in the miR-587 inhibitor group (e) (p = 0.028, p = 0.048, respectively). ∗p < 0.05, ∗∗p < 0.01. miR: microRNA.
Moreover, we explored changes in the cell cycle in miR-587-transfected SMMC-7721 cells by flow cytometry. The results showed that in comparison with NC, the number of SMMC-7721 cells significantly increased in the G1 phase of the cell cycle in the miR-587-overexpression group (p = 0.001), while the number markedly decreased in the S phase (p = 0.044) (Figure 2(d)). Besides, the number of SMMC-7721 cells significantly decreased in the G1 phase (p = 0.028) and increased in the S phase (p = 0.048) in the miR-587-downexpression group compared with the NC (Figure 2(e)). These results indicated that the overexpression of miR-587 induced cell-cycle arrest in the G1 phase and blocked cell division in SMMC-7721 cells after 48 h of transfection.
4.3. miR-587 Suppressed Cell Mobility and Invasion in HCC Cells
The wound healing migration assay showed that the migration rate of SMMC-7721 cells decreased in the miR-587 mimic group at 24 h compared with the mimic NC group (p < 0.001) (Figure 3(a)), and the rate increased in the miR-587 inhibitor group compared with the inhibitor NC group (p = 0.033) (Figure 3(b)). The Transwell assay showed that the overexpression of miR-587 curbed cell invasion—the number of cells on the lower side of the filter decreased—in the miR-587 mimic group compared with the mimic NC group (p = 0.034) (Figure 3(c)). In contrast, cell invasion in SMMC-7721 cells was triggered (or cells on the lower side of the filter increased) in the miR-587 inhibitor group compared with the inhibitor NC group (p < 0.001) (Figure 3(d)).
Figure 3.
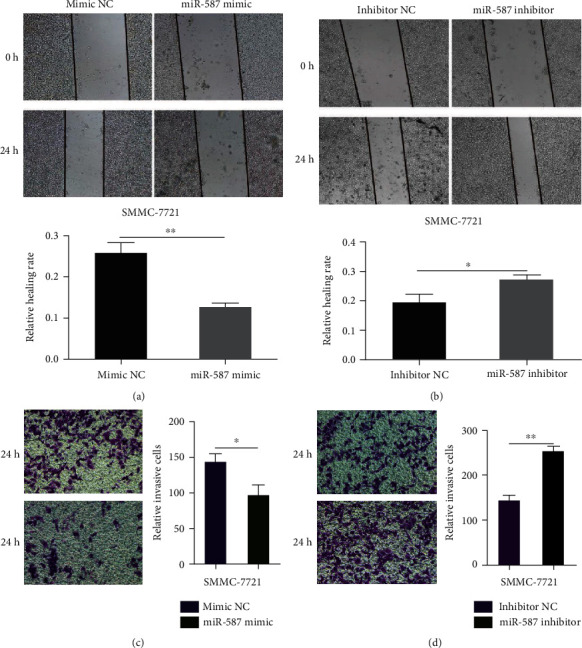
miR-587 curbed cell migration and invasion in HCC cells. The wound healing migration assay showed that miR-587 upregulation (a) depressed the migration rate of SMMC-7721 cells and miR-587 downregulation (b) elevated the migration rate of SMMC-7721 cells (p < 0.001, p = 0.033, respectively). The Transwell assay showed that miR-587 upregulation (c) suppressed the invasion and miR-587 downregulation (d) enhanced cell invasion of SMMC-7721 cells (p = 0.034, p < 0.001, respectively). ∗p < 0.05, ∗∗p < 0.01. miR: microRNA.
4.4. Bioinformatics Prediction Pinpointed RPSA as the Functional Target of miR-587 in HCC
The TargetScan Release 7.1 prediction algorithm was employed to explore potential miR-587 targets. The prediction algorithm screened out 81 target genes at the threshold of -0.4. As our results showed a downregulation of miR-587 in HCC tissues, significantly upregulated genes in HCC were selected based on the negative associations between miR-587 and its target genes. The analysis of TCGA data revealed that 4884 genes were upregulated in HCC (logFC = 1) (Figure 4(a)). Furthermore, we integrated the predicted target genes of miR-587 and the upregulated genes in HCC, and results showed that 13 differentially expressed target genes of miR-587 were found in HCC including OSR2, RAD54B, FAM81A, DKKL1, PTHLH, RPSA, ZNF781, ZNF92, CD163L1, ZNF138, PTPRR, TXNL4A, and ZNF124 (Figure 4(b)). Followed by literature retrieval in the National Center of Biotechnology Information (NCBI) database, only the gene RPSA in the intersection was eligible because it had been reported to be involved in the progression of several cancers, playing a vital role in the proliferation, invasion, and migration in cancer cells [16–18] and showing the inverse cellular function as miR-587.
Figure 4.
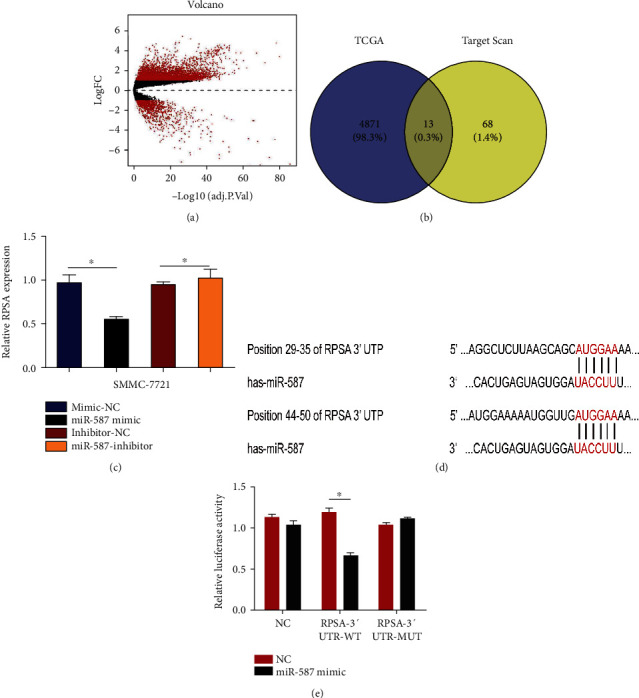
RPSA as a direct target of miR-587. (a) A total of 4884 overexpressed genes in HCC were screened out based on the TCGA database. (b) Thirteen potential target genes of miR-587 were included after intersecting outputs between TCGA database and TargetScan. (c) QRT-PCR assay revealed inverse expressions of RPSA and miR-587 in SMMC-7721 cells in both the mimic group (p = 0.014) and the inhibitor group (p = 0.024). (d) The 3′-UTR region of RPSA had two target sites interacting with miR-587. (e) Dual-luciferase reporter assay showed a specific interaction between miR-587 and the 3′-UTR of RPSA mRNA (p < 0.001). ∗p < 0.05, ∗∗p < 0.01. miR: microRNA.
4.5. The 3′-UTR of RPSA Was Directly Targeted by miR-587
Furthermore, we used qRT-PCR experiment to verify whether RPSA expression was regulated by miR-587, and it turned out that cells transfected with the miR-587 mimic showed a loss of 52.11% of the relative expression level of RPSA compared with those transfected with mimic NC (p = 0.014), and the RPSA level in cells transfected with the miR-587 inhibitor was 1.172 times higher than that in cells transfected with inhibitor NC (p = 0.024) (Figure 4(c)). Subsequently, the dual-luciferase reporter assay was conducted and results showed that the signal of RPSA-3′-UTR-WT+587 was significantly reduced by 44% compared with the control RPSA-3′-UTR-WT + NC (p < 0.001), which suggested the specific interaction between miR-587 and the mRNA 3′-UTR of RPSA (Figures 4(d) and 4(e)).
To further verify the negative regulation between miR-587 and RPSA, we surveyed the TCGA and GEO (GSE36376) databases over the expression of RPSA, and the elevated expression of RPSA was identified in HCC (p < 0.001) (Figures 5(a) and 5(b)). The qRT-PCR analysis of 86 paired clinical tissues confirmed that RPSA was significantly upregulated in HCC tissues compared with the adjacent noncancerous tissues (p < 0.001) (Figure 5(c)). Table 1 summarizes the associations between RPSA expression and clinicopathologic features of HCC patients. The results showed that RPSA was associated with alcoholism (p = 0.047) and early postoperative recurrence (p = 0.021).
Figure 5.
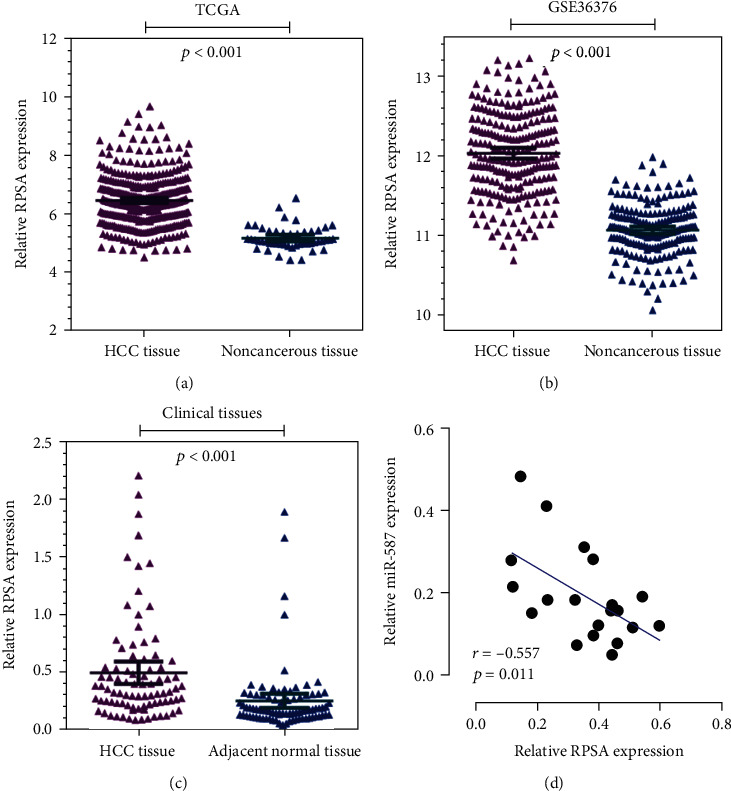
The negative regulation between miR-587 and RPSA in HCC. The gene expression analysis based on the (a) TCGA database, (b) GEO database (GSE36376), and (c) data from 86 paired HCC tissues showed that RPSA was overexpressed in HCC tissues compared with noncancerous tissues (all p < 0.001). (d) Pearson's analysis uncovered a negative correlation between miR-587 and RPSA expressions in HCC tissues (p = 0.011). miR: microRNA.
The relationship between miR-587 and RPSA was further assessed between the intersection of 20 clinic HCC tissues, and the results showed a significantly negative correlation (r = −0.557, p = 0.011, Figure 5(d)). These results accurately showed that RPSA was a direct target of miR-587, and it was negatively regulated by miR-587.
5. Discussion
Identifying the vital molecules in the tumorigenesis and progression of HCC is the prerequisite for a better treatment. Recently, numerous studies have focused on the mechanisms of how microRNAs participate in the process of HCC. Dysregulated miRNAs as oncogenes or tumor suppressors are proved to be involved in the cancer-related pathways in HCC [19–21]. The miR-199 family has emerged as tumor suppressors in HCC by targeting critical genes of MET, transmembrane glycoprotein, and mammalian target of rapamycin (mTOR) pathways [22]. miR-221 exacerbates HCC progression by facilitating the proliferation, migration, and invasion capability of HCC cells [23]. miR-21, the well-characterized oncogenic miRNA, regulates the downstream genes in hypoxia, inflammation, and TET/PTEN pathways [24]. Recent studies have revealed that miRNAs including the miR-200 family regulate the metastasis by enhancing E-cadherin expression and inhibiting EMT [25]. miR-155 fosters cell migration and invasion in HCC cells via targeting the Ras homolog gene family member A (RhoA) [26].
Our study demonstrates that miR-587 is downregulated in HCC tissues, revealing a tumor-suppressor role in HCC, despite its impacts on the progression and aggressiveness of the HCC cell line. Our results indicate that miR-587 inhibits the proliferation of HCC cells through cell-cycle arrest in G1 phase and the inhibition on cell division. Zhang et al. [11] show that miR-587-induced suppression of PPP2R1B triggers AKT activation (the downstream) to deliver an antiapoptotic signal, which weakens the efficiency of 5-FU-induced treatment and thereby brings about drug resistance in colorectal cancer cells. However, biofunctions of miR-587 in other cancers have not been fully explored. Herein, according to results of the dual-luciferase assay, we speculate that miR-587 may be involved in HCC progression by targeting RPSA—a well-established gene in cancers. Notably, miR-587 was downregulated, and RPSA was overexpressed in the HCC tissues, and the negative correlation was then confirmed. The results of the association between miR-587/RPSA expression and the clinicopathological features of HCC patients showed that low expression of miR-587 had a higher ratio in HCC patients with MVI, and high expression of RPSA had a higher ratio in HCC patients with early postoperative recurrence. The early postoperative recurrence rate after HCC resection is up to 30%, which is a major threat to the poor prognosis of HCC patients [27]. MVI is a pathological feature that can only be diagnosed by postoperative histological examination. MVI has been found to increase the risk of tumor recurrence by a factor of 4.4, which was also strongly associated with aggressive tumor behavior and poor prognosis [28]. These indicate that miR-587/RPSA may be considered as clinic biomarkers for the occurrence of HCC patients.
RPSA gene, located on the short arm of chromosome 3, consists of 1700 base pairs and encodes 295-amino-acid proteins. It has been found that RPSA protein as an important component of 40s ribosome subunits is necessary for cell survival [15]. Like many ribosomal proteins, RPSA has a variety of functions in vitro, most notably as a nonintegrin cell surface layer protein-1 receptor with high affinity for laminin. Therefore, RPSA is also called 37/67 kDa laminin receptor/high-affinity laminin receptor (LRP/LR) [15]. Numerous studies have elucidated that RPSA plays a pivotal role in tumorigenesis and cancer progression in breast cancer [17], esophageal cancer [29], Alzheimer's disease [30], colorectal cancer [31], and malignant melanoma [16]. RPSA has also been implicated in the metastasis and invasion in hematological malignancies, as well as apoptosis and cellular proliferation in tumor cells [16, 32–35]. Upon laminin engaging, RPSA promotes the production of extracellular matrix and releases of laminin-derived motility fragments [36]. All this ultimately facilitates tumor cell invasion and migration. RPSA positively regulates expressions of UPA and MMP-9 in gastric cancer, which is vital for the tumor matrix and basement membrane [37]. It is characterized that RPSA is a prominent factor for cell-laminin interaction in several signaling pathways. Li et al. [38] report that RPSA activates c-Myc via the MAPK-ERK pathway, leading to an increase of FASL expression in cholangiocarcinoma cells. The inhibition of 37LRP, a precursor of RPSA, represses glioma growth and invasion, and the resulting downregulation of RPSA suppresses p-ERK1/2 and p-p38 in U251 cells, suggesting the inhibitory effect of RPSA on glioma tumorigenesis [39]. Moreover, another study reports that RPSA acts as a H2O2 sensor in H2O2-dependent modulation of cell adhesion. RPSA oxidation not only enhances the cell adhesion efficiency to laminins but also promotes cell extravasation in vivo. The upregulation of RPSA has been found in both primary tumors and metastatic sites, together with elevated levels of H2O2 [40]. Our results highlight that miR-587 inhibits the proliferation, migration, and invasion of HCC cells by directly upregulating RPSA. However, the mechanisms of how RPSA participates in the progression of HCC have not fully unveiled. It has been proven that the level of LRP/LR is overexpressed on the membrane of liver cancer cell line HUH-7 cells on account of its interaction with laminin-1 [41]. Laminin-1 in the basement membrane of cells is found to promote cell adhesion, invasion, differentiation, growth, and migration in tumourigenic cells and biological processes [17, 42, 43]. The LRP/LR-laminin-1 interaction increases the sensitivity of proteolytic enzyme and hydrolyzes the collagen of basement membrane, leading to the degradation of basement membrane for the benefit of tumor cell invasion [44]. Moreover, the LRP/LR-laminin-1 interaction may promote tumor angiogenesis by delivering oxygen and nutrients to cancer cells [45]. These indicate that upregulated RPSA has the potential to be developed as a molecular marker for HCC. RPSA may promote the progression and aggressiveness of HCC cells through the LRP/LR-laminin-1 interaction. In this study, we for the first time report that the downexpression of miR-587 accelerates the process of HCC by targeting the mRNA RPSA in HCC. However, further experiments should be conducted to address some or all of the following: biofunctions of RPSA in HCC as reported in relevant literature needs to be confirmed; moreover, the conclusion drawn from in vitro experiments needs to be verified in in vivo experiments.
In summary, miR-587 overexpression inhibits tumor cell proliferation, migration, and invasion by directly downregulating RPSA mRNA in HCC. The miR-587/RPSA can be considered as a clinic biomarker for the occurrence of HCC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81860512) and the Key Research and Development Projects in Guangxi (2017AB48027).
Contributor Information
Danke Su, Email: sudanke@gxmu.edu.cn.
Hang Li, Email: lihang58@126.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors report no conflict of interest in this study.
Authors' Contributions
Miao Chen, Danke Su, and Hang Li designed the study and wrote the original manuscript. Duo Wang and Zhizhan Zhou conceived and conducted the research. Zhanling Ding and Lianfeng Liu collected and analyzed the data. Danke Su and Junjie Liu analyzed the data and interpreted the results. Miao Chen and Duo Wang contributed equally.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2018;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J., Sun H. C., Wang Z., et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition) Liver Cancer. 2018;7(3):235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragomir M. P., Knutsen E., Calin G. A. SnapShot: unconventional miRNA functions. Cell. 2018;174(4):1038–1038.e1. doi: 10.1016/j.cell.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Jiang M., Shi L., Yang C., et al. miR-1254 inhibits cell proliferation, migration, and invasion by down- regulating Smurf1 in gastric cancer. Cell Death & Disease. 2019;10(1):p. 32. doi: 10.1038/s41419-018-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moridikia A., Mirzaei H., Sahebkar A., Salimian J. MicroRNAs: potential candidates for diagnosis and treatment of colorectal cancer. Journal of Cellular Physiology. 2018;233(2):901–913. doi: 10.1002/jcp.25801. [DOI] [PubMed] [Google Scholar]
- 6.Braconi C., Henry J. C., Kogure T., Schmittgen T., Patel T. The role of microRNAs in human liver cancers. Seminars in Oncology. 2011;38(6):752–763. doi: 10.1053/j.seminoncol.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang N., Ekanem N. R., Sakyi C. A., Ray S. D. Hepatocellular carcinoma and microRNA: new perspectives on therapeutics and diagnostics. Advanced Drug Delivery Reviews. 2015;81:62–74. doi: 10.1016/j.addr.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Ji J., Rong Y., Luo C. L., et al. Upregulation of hsa-miR-210 promotes venous metastasis and predicts poor prognosis in hepatocellular carcinoma. Frontiers in Oncology. 2018;8:p. 569. doi: 10.3389/fonc.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Y., Huang Y., Sun H., Pan Y., Wu M., Zhang J. Deregulation of miR-520d-3p promotes hepatocellular carcinoma development via lncRNA MIAT regulation and EPHA2 signaling activation. Biomedicine & Pharmacotherapy. 2019;109:1630–1639. doi: 10.1016/j.biopha.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wei Q., Zhao L., Jiang L., et al. Prognostic relevance of miR-137 and its liver microenvironment regulatory target gene AFM in hepatocellular carcinoma. Journal of Cellular Physiology. 2018;234(7):11888–11899. doi: 10.1002/jcp.27855. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Talmon G., Wang J. Erratum: MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell Death & Disease. 2016;7(12, article e2525) doi: 10.1038/cddis.2016.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latchana N., DiVincenzo M. J., Regan K., et al. Alterations in patient plasma microRNA expression profiles following resection of metastatic melanoma. Journal of Surgical Oncology. 2018;118(3):501–509. doi: 10.1002/jso.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yerukala Sathipati S., Huang H. L., Ho S. Y. Estimating survival time of patients with glioblastoma multiforme and characterization of the identified microRNA signatures. BMC Genomics. 2016;17, article 1022(Supplement 13) doi: 10.1186/s12864-016-3321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolze A., Boisson B., Bosch B., et al. Incomplete penetrance for isolated congenital asplenia in humans with mutations in translated and untranslatedRPSAexons. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(34):E8007–E8016. doi: 10.1073/pnas.1805437115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiGiacomo V., Meruelo D. Looking into laminin receptor: critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biological Reviews of the Cambridge Philosophical Society. 2016;91(2):288–310. doi: 10.1111/brv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munien C., Rebelo T. M., Ferreira E., Weiss S. F. T. IgG1-iS18 impedes the adhesive and invasive potential of early and late stage malignant melanoma cells. Experimental Cell Research. 2017;351(2):135–141. doi: 10.1016/j.yexcr.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Khumalo T., Reusch U., Knackmuss S., Little M., Veale R. B., Weiss S. F. T. Adhesion and invasion of breast and oesophageal cancer cells are impeded by anti-LRP/LR-specific antibody IgG1-iS18. PLoS One. 2013;8(6, article e66297) doi: 10.1371/journal.pone.0066297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vania L., Rebelo T. M., Ferreira E., Weiss S. F. T. Knock-down of LRP/LR promotes apoptosis in early and late stage colorectal carcinoma cells via caspase activation. BMC Cancer. 2018;18(1):p. 602. doi: 10.1186/s12885-018-4531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lujambio A., Lowe S. W. The microcosmos of cancer. Nature. 2012;482(7385):347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung C. H., Chiu Y. C., Chen C. H., Hu T. H. MicroRNAs in hepatocellular carcinoma: carcinogenesis, progression, and therapeutic target. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/486407.486407 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Bandiera S., Pfeffer S., Baumert T. F., Zeisel M. B. miR-122 - A key factor and therapeutic target in liver disease. Journal of Hepatology. 2015;62(2):448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Lou Z., Gong Y. Q., Zhou X., Hu G. H. Low expression of miR-199 in hepatocellular carcinoma contributes to tumor cell hyper-proliferation by negatively suppressing XBP1. Oncology Letters. 2018;16(5):6531–6539. doi: 10.3892/ol.2018.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Q., Loya K., Rani B., et al. MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology. 2013;57(1):299–310. doi: 10.1002/hep.25984. [DOI] [PubMed] [Google Scholar]
- 24.Cao L. Q., Yang X. W., Chen Y. B., Zhang D. W., Jiang X. F., Xue P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol Cancer. 2019;18(1):p. 148. doi: 10.1186/s12943-019-1075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Yang F., Yuan J. H., et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34(3):577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- 26.Qian F., Hu Q., Tian Y., et al. ING4 suppresses hepatocellular carcinoma via a NF-κB/miR-155/FOXO3a signaling axis. International Journal of Biological Sciences. 2019;15(2):369–385. doi: 10.7150/ijbs.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X., Zhao H., Yang H., et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. Journal of Surgical Oncology. 2009;100(6):488–493. doi: 10.1002/jso.21354. [DOI] [PubMed] [Google Scholar]
- 28.Roayaie S., Blume I. N., Thung S. N., et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelosi G., Pasini F., Bresaola E., et al. High-affinity monomeric 67-kD laminin receptors and prognosis in pancreatic endocrine tumours. The Journal of Pathology. 1997;183(1):62–69. doi: 10.1002/(SICI)1096-9896(199709)183:1<62::AID-PATH1095>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Pinnock E. C., Jovanovic K., Pinto M. G., et al. LRP/LR antibody mediated rescuing of amyloid-beta-induced cytotoxicity is dependent on PrPc in Alzheimer's disease. Journal of Alzheimer's Disease. 2015;49(3):645–657. doi: 10.3233/JAD-150482. [DOI] [PubMed] [Google Scholar]
- 31.Vania L., Chetty C. J., Ferreira E., Weiss S. F. T. Anti-LRP/LR-Specific antibody IgG1-iS18 significantly impedes adhesion and invasion in Early- and Late-Stage colorectal carcinoma cells. Molecular Medicine. 2016;22(1):664–673. doi: 10.2119/molmed.2016.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moodley K., Weiss S. F. T. Downregulation of the non-integrin laminin receptor reduces cellular viability by inducing apoptosis in lung and cervical cancer cells. PLoS One. 2013;8(3, article e57409) doi: 10.1371/journal.pone.0057409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khumalo T., Ferreira E., Jovanovic K., Veale R. B., Weiss S. F. T. Knockdown of LRP/LR induces apoptosis in breast and oesophageal cancer cells. PLoS One. 2015;10(10, article e0139584) doi: 10.1371/journal.pone.0139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClintock S. D., Warner R. L., Ali S., et al. Monoclonal antibodies specific for oncofetal antigen--immature laminin receptor protein: effects on tumor growth and spread in two murine models. Cancer Biology & Therapy. 2015;16(5):724–732. doi: 10.1080/15384047.2015.1026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukamoto S., Huang Y., Kumazoe M., et al. Sphingosine kinase-1 protects multiple myeloma from apoptosis driven by cancer-specific inhibition of RTKs. Molecular Cancer Therapeutics. 2015;14(10):2303–2312. doi: 10.1158/1535-7163.MCT-15-0185. [DOI] [PubMed] [Google Scholar]
- 36.Sarnataro D., Pepe A., Altamura G., et al. The 37/67kDa laminin receptor (LR) inhibitor, NSC47924, affects 37/67kDa LR cell surface localization and interaction with the cellular prion protein. Scientific Reports. 2016;6(1):p. 24457. doi: 10.1038/srep24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesapane A., Ragno P., Selleri C., Montuori N. Recent advances in the function of the 67 kDa laminin receptor and its targeting for personalized therapy in cancer. Current Pharmaceutical Design. 2017;23(32):4745–4757. doi: 10.2174/1381612823666170710125332. [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Li D., Chen J., Wang S. A polysaccharide from Pinellia ternata inhibits cell proliferation and metastasis in human cholangiocarcinoma cells by targeting of Cdc42 and 67 kDa Laminin Receptor (LR) International Journal of Biological Macromolecules. 2016;93:520–525. doi: 10.1016/j.ijbiomac.2016.08.069. [DOI] [PubMed] [Google Scholar]
- 39.Wu H., Li J., Xu D., et al. The 37-kDa laminin receptor precursor regulates the malignancy of human glioma cells. Cell Biochemistry and Function. 2016;34(7):516–521. doi: 10.1002/cbf.3225. [DOI] [PubMed] [Google Scholar]
- 40.Vilas-Boas F., Bagulho A., Tenente R., et al. Hydrogen peroxide regulates cell adhesion through the redox sensor RPSA. Free Radical Biology & Medicine. 2016;90:145–157. doi: 10.1016/j.freeradbiomed.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Chetty C., Khumalo T., da Costa Dias B., et al. Anti-LRP/LR specific antibody IgG1-iS18 impedes adhesion and invasion of liver cancer cells. PLoS One. 2014;9(5, article e96268) doi: 10.1371/journal.pone.0096268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson J., McFerran N. V., Pivato G., et al. The 67 kDa laminin receptor: structure, function and role in disease. Bioscience Reports. 2008;28(1):33–48. doi: 10.1042/BSR20070004. [DOI] [PubMed] [Google Scholar]
- 43.Givant-Horwitz V., Davidson B., Reich R. Laminin-induced signaling in tumor cells. Cancer Letters. 2005;223(1):1–10. doi: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Fatehullah A., Doherty C., Pivato G., et al. Interactions of the 67 kDa laminin receptor and its precursor with laminin. Bioscience Reports. 2010;30(2):73–79. doi: 10.1042/BSR20090023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khusal R., da Costa Dias B., Moodley K., et al. In vitro inhibition of angiogenesis by antibodies directed against the 37kDa/67kDa laminin receptor. PLoS One. 2013;8(3, article e58888) doi: 10.1371/journal.pone.0058888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


