Abstract
The Langendorff-perfused model is a powerful tool to study biological responses in the isolated heart in the absence of confounders. The model has been adapted recently to enable study of the isolated mouse heart and the effects of genetic manipulation. Unfortunately, the small size and fragility of the mouse heart pose significant challenges, limiting application of the Langendorff model to the study of adult mice. Cardiac development is a complex and dynamic process that is incompletely understood. Thus, establishing an isolated-perfused heart model in the newborn mouse would be an important and necessary advance. Here we present a method to successfully cannulate and perfuse the isolated newborn murine heart. We describe the basic and fundamental physiological characteristics of the ex-vivo retrograde-perfused beating neonatal heart in wild-type C57Bl/6 male mice. Our approach will enable future study of the physiological and pharmacological responses of the isolated immature murine heart to enhance knowledge of how developmental cardiac biology impacts health and disease.
-
•
The Langendorff model is a powerful tool to study the heart without confounders.
-
•
An isolated-perfused newborn murine heart model has yet to be established.
-
•
We demonstrate the first successful isolated neonatal murine heart preparation.
Keywords: Cannulation, Newborn, Mouse, Langendorff, Isolated, Heart, Model
Graphical abstract
SPECIFICATIONS TABLE
| Subject Area: | Medicine and Dentistry |
| More specific subject area: | Isolated-perfused mouse heart model |
| Method name: | Cannulation of neonatal mouse heart and establishment of successful isolated neonatal murine heart preparation |
| Name and reference of original method: | O. Langendorff, Untersuchungen am uberlebenden Saugethierherzen, Pflügers Arch 61 (1895) 291–332. |
| Resource availability: | If applicable, include links to resources necessary to reproduce the method (e.g. data, software, hardware, reagent) |
*Method details
Brief history and rationale
Oskar Langendorff first established the isolated-perfused mammalian heart preparation in 1895 [1]. His approach was a modification of an existing isolated frog heart model and utilized retrograde perfusion of the aortic root via continuous hydrostatic pressure to perfuse the coronary circulation and myocardium [1]. With his technique, effluent returns to the right atrium via the coronary sinus and is ejected out of heart through an incised pulmonary artery following contraction of the right ventricle [1]. In order to quantify cardiac function, Langendorff measured the force of isometric ventricular contraction in the longitudinal axis using a suture connected between the apex of the heart and a force transducer [1]. The method has since been refined with the use of fluid-filled balloons to measure left ventricular performance, with utilization of various types of perfusate, different modes of perfusion, and pacing, and with the advent of the working heart preparation [2]. Today, the Langendorff model is recognized as a relevant and powerful tool to study physiological and pharmacological responses in the heart in the absence of confounding variables [1].
In his original work, Langendorff used large animals such as cats, rabbits, and dogs to study the mammalian heart in isolation [1]. Other investigators have since utilized pigs, sheep, cows, and monkeys [3]. Use of rodents became popular in the mid-to-late 1900s due to lower costs, shorter breeding times, and ease of manipulation [3,4]. Study of the isolated-perfused murine heart began only twenty-five years ago and since then, interest in ex-vivo assessment of mouse cardiac biology has steadily increased as investigators seek to understand the effects of genetic manipulation [1,5]. Although the Langendorff-perfused isolated heart preparation is elegant in its simplicity, the small size and fragility of the mouse heart pose both technical and logistical challenges. Thus, application of the Langendorff model and working heart preparation has largely been limited to the study of adult mice [6]. In fact, the youngest mice studied in this manner to date have been 4 week old juveniles [7].
Cardiac development is a complex and well-orchestrated process [8]. Transition to the extrauterine environment induces a number of dynamic biochemical, physiological, and anatomical responses in the immature postnatal heart [8]. These include a switch from cardiomyocyte proliferation to cellular hypertrophy, a change in cardiomyocyte substrate utilization, an increase in myocardial oxidative capacity, and a metabolic shift from anaerobic glycolysis to aerobic respiration [8,9]. Prior works using newborn rats, lambs, and rabbits have leveraged the Langendorff-perfused neonatal heart preparation to provide valuable insight into developmental cardiac biology and pathophysiology [10], [11], [12]. However, an isolated-perfused heart model in the newborn mouse has yet to be established. Because transgenic mice enable functional investigation of specific genes and gene products, development of a murine Langendorff-perfused newborn heart model would be an important and necessary advance. Here we present a method to successfully cannulate and perfuse the isolated newborn murine heart. We describe the basic and fundamental physiological characteristics of the ex-vivo retrograde-perfused beating neonatal heart in wild-type C57Bl/6 male mice.
Apparatus and approach
The isolated mammalian heart can be perfused using a variety of approaches. Methods generally differ based on mode of perfusion (i.e., retrograde versus antegrade, constant flow versus constant pressure) and type of perfusate (crystalloid, whole blood, or red blood cell augmented crystalloid) [2]. In order to maximize success, we have taken an approach that minimizes complexity: retrograde perfusion of the isolated newborn heart via constant flow using the mostly commonly employed buffered crystalloid perfusate [2]. Non-recirculating Krebs-Henseleit buffer (KHB), containing (mmol/L) NaCl 120, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.25, NaHCO3 25, and glucose 11 at pH 7.4, should be equilibrated with 95% O2–5% CO2 within the Langendorff apparatus and maintained at 37 °C. Flow rates are chosen based on weight-specific coronary flow. For example, coronary flow in the isolated perfused murine heart is ~75–80 mL/g·min and the weight of an average 10 day old (P10) mouse heart is ~30 mg [13,14]. Thus, retrograde perfusion of the newborn mouse heart should be maintained continuously at a flow rate of ~2.5 mL·min−1.
Cannula
With established mouse Langendorff preparations, the murine aorta is typically cannulated using a 20–22-gauge blunted needle [1]. Such cannulae are commercially available and some are machine-scored to maximize traction and minimize slippage. In our pilot work, we attempted to cannulate the P10 mouse aorta using a 22-gauge stainless steel blunted needle. Although the incised neonatal mouse aorta could be stretched to accommodate a 22-gauge cannula, cannulation was technically challenging, inconsistent, and the time to reperfusion was excessive. Therefore, we fabricated our own aortic cannula using a 26-gauge stainless steel needle.
The needle is first blunted by cutting off the beveled tip. Next, any sharp edges should be dulled by scraping the needle on the laboratory bench top surface. It is important to remove microscopic burs or sharp edges in this manner because they can easily tear the delicate tissue of the newborn mouse aorta or damage the aortic valve. Each 26-gauge cannula should then be assessed for resistance to flow. To do so, flow rate through the cannula should be measured with a timed collection of buffer and pressure differential should be quantified across the cannula. With our 26-gauge cannula, we detected a pressure differential of 40.0 ± 4.7 mmHg (mean ± S.D.) across the cannula at a constant buffer flow rate of 2.5 mL·min−1. Although flow was continuous and unimpeded, the 26-gauge cannula provided 16.0 ± 1.9 mmHg·min·mL−1 of resistance. The resistance of each cannula needs to be measured and accounted for when determining aortic perfusion pressure and calculating coronary resistance in each preparation. Because resistance is additive in series, these calculations can be made using Kirchhoff's and Ohm's law [15].
Cannulation
Time to cannulation is a critical variable that must be minimized in order to limit the effects of hypoxia and ischemia on the isolated heart [16]. Investigators have used a number of different approaches to establish reperfusion in a timely manner [16]. Our method was developed using 10 day old C57Bl/6 male mouse pups and our 26-gauge cannula. P10 was chosen to model a timepoint in human infancy [17], [18], [19]. Mice should be heparinized (10 kU/kg ip) to prevent microthrombus formation in the coronary circulation. Animals are then anesthetized with intraperitoneal pentobarbital (70 mg/kg) and a thoracotomy is performed through a transverse subxyphoid incision during spontaneous ventilation following loss of consciousness. The anterior portion of the diaphragm should be identified and incised. The rib cage is then cut bilaterally along the mid-axillary line and the sternum and ribs are reflected cranially to expose the intrathoracic organs. The inferior vena cava is identified and transected above the liver where it enters the thoracic cavity. With gentle traction applied to the inferior vena cava, the heart and lungs are then rapidly excised en bloc by dissecting posteriorly along the spine and the explanted organs are submerged briefly in ice-cold KHB.
Once the heart stops beating, it is positioned under a dissecting microscope. We find it useful to place the heart in an ice-cold petri dish, lined with moistened paper towel. The paper towel provides adequate friction to stabilize the heart during cannulation and moistening it with KHB prevents the heart from adhering to it. Care must be taken, however, to limit the amount of fluid in the petri dish, as the air-filled lungs can cause the excised organs to float and move. The pale thymus should be identified and oriented in the petri dish such that it is the most anterior and superior structure. With this orientation, the apex of the heart should project inferiorly. This approach will ensure that the heart is properly positioned under the microscope and that the investigator is viewing the anterior surface of the organs. Using forceps, the lobes of the thymus are bluntly separated and pulled apart to expose the great vessels. The right ventricle and main pulmonary artery are demarcated and can be identified by their relatively dark purple hue due to deoxygenated blood. The ascending aorta is located between the main pulmonary and the right atrium. Proper identification can be confirmed by recognizing the distinguishing features of aortic arch and its branches. The ascending aorta is then transected with sharp fine scissors proximal to the takeoff of the brachiocephalic artery. The incised aorta is cannulated with the blunted 26-gauge steel cannula and secured with a 5–0 silk suture using a two-person technique. Retrograde perfusion can then be initiated. The lungs, thymus, and excess tissue are trimmed after the heart is hung and the right atrium is incised to permit coronary sinus effluent to drip freely. Using this technique we can reliably establish retrograde perfusion in the newborn heart within 2–4 min.
Measurements
Continuous electrocardiogram (ECG) measurement can be established in the Langendorff-perfused newborn mouse heart using surface electrodes. Unfortunately, in pilot work we found the newborn heart to be too small and fragile to accommodate an intraventricular balloon for isovolumetric left ventricle pressure measurement. However, ventricular contractile force can be measured in the longitudinal axis using the method originally described by Langendorff [20]. With this technique, isometric tension is quantified with a 5–0 silk suture connected between the apex of the heart and a force displacement transducer. First, a knot is tied at the free end of a 5–0 suture (opposite end of the needle). Then, the apex of the beating heart is carefully punctured with the suture needle. Using forceps to apply gentle counter pressure, the tip of the needle is slowly advanced until is passes completely through the heart. Grasping the needle tip, the suture is then carefully pulled through heart until the knot rests on the free wall of the ventricle. Care must be taken to avoid twisting or torqueing the aorta or pulling the heart off of the cannula. The 5–0 suture is then attached to a calibrated force displacement transducer and diastolic tension adjusted to 1–2 g. Heart rate and rhythm, aortic pressure, and ventricular tension are then continuously recorded.
Metabolic status of the isolated heart can be assessed by measuring oxygen tension (PO2), glucose, and lactate and in the coronary inflow (affluent) and coronary sinus effluent. Percent O2 extraction and glucose uptake are calculated based on values obtained from perfusate and effluent. Myocardial O2 consumption is calculated as coronary flow x (PaO2 – PvO2) x O2 solubility at 760 mmHg (where O2 solubility is 24 µl/ml H2O at 37 °C). Coronary resistance is approximated by dividing aortic perfusion pressure by coronary flow.
Physiological parameters of the isolated retrograde-perfused newborn heart
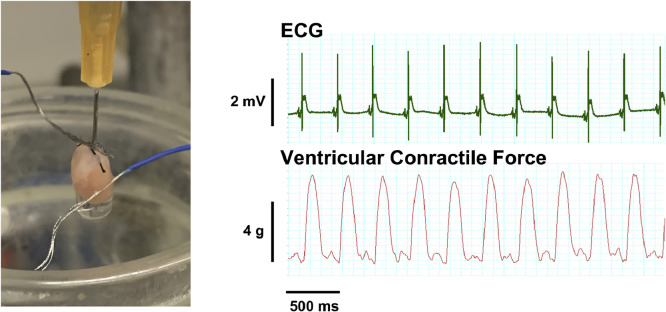
We evaluated 15 isolated P10 C57Bl/6 mouse hearts and calculated aortic perfusion pressure and coronary resistance in the model (Table 1 ). The perfusion pressure values were physiologically relevant given that mean arterial blood pressure and systolic left ventricular pressure are known to range between 40 and 60 mmHg in the P10 C57Bl/6 mouse [21,22]. With surface electrodes, we successfully captured and recorded a continuous ECG in each heart, quantified heart rate, and analyzed cardiac rhythm (Fig. 1). Hearts were permitted to beat spontaneously and we observed sinus rhythm in each (Fig. 1). As expected, the intrinsic rate of the ex-vivo P10 mouse heart was slower than in vivo newborn rates (418 ± 18 beats·min−1 from ref [23]) and relatively slower than that of the isolated adult murine heart (300–400 beats·min−1)(Table 1) [23,24]. Using the original approach described by Langendorff, we were able to successfully measure ventricular contractile force in the longitudinal axis (Table 1, Fig. 1, Video) [20].
Table 1.
Measurements in the isolated retrograde-perfused newborn heart.
| Parameter | ||||
|---|---|---|---|---|
| Cardiac Performance | ||||
| Aortic perfusion pressure, mmHg | 47.9 ± 6.9 | |||
| Coronary resistance, mmHg·min·mL−1 | 19.2 ± 2.8 | |||
| Heart rate, beats·min−1 | 226 ± 8.9 | |||
| Ventricular contractile force, g | 7.2 ± 1.2 | |||
| Metabolism | Affluent | Effluent | ||
| Oxygen extraction,% | 55.7 ± 2.3 | |||
| PO2, mmHg | 641 ± 7.9 | 295 ± 18.4 | ||
| Myocardial oxygen consumption, µL·min−1 | 28.2 ± 1.3 | |||
| Glucose uptake, mg·dL−1 | 1.4 ± 0.8 | |||
| Glucose, mg·dL−1 | 194.3 ± 3.8 | 193.0 ± 5.5 | ||
| Lactate, mmol·L−1 | < 0.3 ± 0.0 | < 0.3 ± 0.0 | ||
Values are expressed as means ± SE. PO2, partial pressure of oxygen.
Fig. 1.
The Isolated Retrograde-Perfused Newborn Mouse Heart. An image of a 10-day old mouse heart following successful cannulation with a 26-gauge blunt needle is depicted. Coronary effluent was permitted to freely drip from the heart through the incised right atrium. Stainless steel surface electrodes coated with blue polyethylene are seen contacting the heart. Surface ECG and ventricular contractile force were continuously measured and recorded and representative tracings are demonstrated. Heart rate in the depicted example is 194 beats·min−1 and rhythm is clearly sinus. The supplemental video demonstrates a 5–0 silk suture connected between the apex of the beating heart and a force displacement transducer to quantify isometric tension.
We next assessed the adequacy of our perfusion strategy (i.e., type of perfusion and flow rate) in meeting the metabolic demands of the isolated beating newborn heart. To do so, we measured oxygen tension, glucose, and lactate and in the aortic inflow (affluent) and coronary sinus effluent. Myocardial oxygen extraction and glucose uptake were quantified and myocardial oxygen consumption was calculated (Table 1). Results indicated that metabolic supply and demand were well matched in the ex-vivo beating P10 heart (Table 1). Evidence for this was found in the percent oxygen extraction, the relatively low amount of glucose uptake, and the lack of lactate produced by any heart (Table 1).
Discussion
Here we describe a method to cannulate and perfuse the isolated newborn murine heart and describe the ex-vivo physiological characteristics of the model. Success of our approach establishes a foundation for future study of the physiological and pharmacological responses of the isolated developing murine heart during health and disease. Importantly, our technique will also enable use of transgenic mice to investigate the role of specific genes and gene products in developmental cardiac biology in the absence of confounding variables. Thus, our work represents an important and necessary scientific advance.
As with any Langendorff-perfused heart preparation, investigators should define exclusion criteria prospectively and apply these to each heart [2]. Based on our findings we propose specific exclusion criteria for the isolated newborn murine heart (Table 2). These exclusions represent a slight modification of those previously established for the adult mouse [2]. Criteria for heart rate and aortic perfusion pressure were determined from our data using absolute deviation around the median [25]. Time to initiate reperfusion is an important variable in the Langendorff preparation because prolonged periods of ischemia can injure the myocardium or even precondition it [2]. As in the adult heart, we believe investigators should exclude hearts that necessitate more than 4 min to achieve reperfusion [2]. Flow rate and perfusion pressure are two more variables to consider (Table 2). High flow rates or low perfusion pressure are indicative of an aortic tear, leak from the innominate artery, or a damaged and incompetent aortic valve [2]. Low flow rates or high perfusion pressure, on the other hand, suggest microthrombi or air in the coronary circulation, a malpositioned aortic cannula, or obstructed coronary ostia [2]. Regardless of cause, flow rates and perfusion pressures that fall out of the pre-defined range suggest inadequate coronary perfusion and such hearts should be excluded from study (Table 2). Arrhythmogenic hearts and those that demonstrate intrinsic bradycardia or tachycardia should also be excluded (Table 2) [2]. This is because arrhythmias can interfere with quantification of ventricular function while extremes of heart rate affect force of contraction [2]. Furthermore, presence of arrhythmias often indicates an inadequately perfused heart [2]. Thus, heart rate and rhythm are important variables to consider for inclusion and exclusion.
Table 2.
Exclusion criteria for the isolated-perfused newborn murine heart.
| Parameter | |||
|---|---|---|---|
| Time to reperfusion, min | > 4 | ||
| Aortic perfusion pressure, mmHg | < 20 or > 75 | ||
| Heart rate, beats·min−1 | < 150 or > 300 | ||
| Arrhythmia duration, min | > 3 | ||
In our model, we opted to utilize the least complex approach in order to maximize the opportunity for success. Thus, we chose to perfuse the isolated newborn heart in a retrograde manner using the mostly commonly employed type of buffered perfusate [2]. Flow was continuous and the rate was selected based on published murine coronary flow rates [13]. We demonstrated that retrograde perfusion of the isolated P10 heart at rate of 2.5 mL·min−1 was adequate to meet the metabolic demands of the spontaneously beating heart. However, given that cardiac output in 10 day old C57Bl/6 mice has been calculated to be 5.3 mL·min−1, it is possible that our chosen flow rate provided luxury perfusion of the P10 heart [23]. Therefore, future studies will need to assess the dynamic response of the P10 heart to a range of coronary flow rates below 2.5 mL·min−1. With regard to metabolism, it is important to recognize that KHB is a glucose-based perfusate. Newborn cardiomyocytes are known to undergo a switch in substrate utilization from lactate and glucose to fatty acids in the postnatal period [8,9]. Although isolated P10 hearts efficiently utilized glucose for energy production in our model, alternative types of buffer, containing different metabolic substrates (i.e., fatty acids), will need to be tested in subsequent work. Finally, in future research, we will assess the effect of pacing and we will attempt to establish a working newborn heart model in P10 mice. We are hopeful that our technique will enable future study to the isolated-perfused newborn mouse heart to advance our understanding of developmental cardiac biology.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
None.
Source of funding
None.
NIH/NINDS R01NS112706 (R.L), NIH/NICHD HD082251 (G.G.).
Footnotes
Direct Submission or Co-Submission
Co-submissions are papers that have been submitted alongside an original research paper accepted for publication by another Elsevier journal.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2020.101058.
Appendix. Supplementary materials
Supplementary material and/or Additional information: Supplemental Video demonstrating isolated beating newborn mouse heart with 5–0 silk attached between apex and force transducer
References
- 1.Liao R., Podesser B.K., Lim C.C. The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H156–H167. doi: 10.1152/ajpheart.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell R.M., Mocanu M.M., Yellon D.M. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J. Mol. Cell. Cardiol. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Olejnickova V., Novakova M., Provaznik I. Isolated heart models: cardiovascular system studies and technological advances. Med. Biol. Eng. Comput. 2015;53:669–678. doi: 10.1007/s11517-015-1270-2. [DOI] [PubMed] [Google Scholar]
- 4.de Leiris J., Harding D.P., Pestre S. The isolated perfused rat heart: a model for studying myocardial hypoxia or ischaemia. Basic Res. Cardiol. 1984;179:313–321. doi: 10.1007/BF01908032. [DOI] [PubMed] [Google Scholar]
- 5.Marber M.S., Mestril R., Chi S.H., Sayen M.R., Yellon D.M., Dillmann W.H. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary K.R., Zordoky B.N., Edin M.L., Alsaleh N., El-Kadi A.O., Zeldin D.C., Seubert J.M. Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice. Prostaglandins Other Lipid Mediat. 2013;104-105:8–17. doi: 10.1016/j.prostaglandins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liaw N.Y., Hoe L.S., Sheeran F.L., Peart J.N., Headrick J.P., Cheung M.M., Pepe S. Postnatal shifts in ischemic tolerance and cell survival signaling in murine myocardium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1171–R1181. doi: 10.1152/ajpregu.00198.2013. [DOI] [PubMed] [Google Scholar]
- 8.Tan C.M.J., Lewandowski A.J. The Transitional Heart: from Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2020;47:373–386. doi: 10.1159/000501906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onay-Besikci Regulation of cardiac energy metabolism in newborn. Mol. Cell. Biochem. 2006;287:1–11. doi: 10.1007/s11010-006-9123-9. [DOI] [PubMed] [Google Scholar]
- 10.Ziyatdinova N.I., Kuptsova A.M., Faskhutdinov L.I., Galieva A.M., Zefirov A.L., Zefirov T.L. Effect of If Current Blockade on Newborn Rat Heart Isolated According to Langendorff. Bull. Exp. Biol. Med. 2019;167:424–427. doi: 10.1007/s10517-019-04541-w. [DOI] [PubMed] [Google Scholar]
- 11.Hickey P.R., Mayer J.E. Anti-CD18 attenuates myocardial stunning in the isolated neonatal lamb heart. J. Cardiothorac. Surg. 1993;8(2 Suppl):313–315. doi: 10.1111/j.1540-8191.1993.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 12.Cabigas E.B., Liu J., Boopathy A.V., Che P.L., Crawford B.H., Baroi G., Bhutani S., Shen M., Wagner M.B., Davis M.E. Dysregulation of catalase activity in newborn myocytes during hypoxia is mediated by c-Abl tyrosine kinase. J. Cardiovasc. Pharmacol. Ther. 2015;20:93–103. doi: 10.1177/1074248414533746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng B., Tilley S.L., Ledent C., Mustafa S.J. In vivo assessment of coronary flow and cardiac function after bolus adenosine injection in adenosine receptor knockout mice. Physiol. Rep. 2016;4:e12818. doi: 10.14814/phy2.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W., Barrientos T., Mao L., Rockman H.A., Sauve A.A., Andrews N.C. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015;13:533–545. doi: 10.1016/j.celrep.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z., Ambrosi E., Bricalli A., Ielmini D. Logistic computing with stateful neural networks of resistive switches. Adv. Mater. 2018;30 doi: 10.1002/adma.201802554. [DOI] [PubMed] [Google Scholar]
- 16.Motayagheni N. Modified Langendorff technique for mouse heart cannulation: improved heart quality and decreased risk of ischemia. Methods X. 2017;4:508–512. doi: 10.1016/j.mex.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klintsova A.Y., Helfer J.L., Calizo L.H., Dong W.K., Goodlett C.R., Greenough W.T. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin. Exp. Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 18.Clancy B., Finlay B.L., Darlington R.B., Anand K.J. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornig M., Chian D., Lipkin W.I. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol. Psychiatry. 2004;9:833–845. doi: 10.1038/sj.mp.4001529. [DOI] [PubMed] [Google Scholar]
- 20.Langendorff O. Untersuchungen am uberlebenden Saugethierherzen. Pflügers Arch. 1895;61:291–332. [Google Scholar]
- 21.Le V.P., Kovacs A., Wagenseil J.E. Measuring left ventricular pressure in late embryonic and neonatal mice. J. Vis. Exp. 2012;60:e3756. doi: 10.3791/3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Guo X., Kassab G.S. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H657–H664. doi: 10.1152/ajpheart.00803.2005. [DOI] [PubMed] [Google Scholar]
- 23.Wiesmann F., Ruff J., Hiller K.H., Rommel E., Haase A., Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H652–H657. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]
- 24.Reichelt M.E., Willems L., Hack B.A., Peart J.N., Headrick J.P. Cardiac and coronary function in the Langendorff-perfused mouse heart model. Exp. Physiol. 2009;94:54–70. doi: 10.1113/expphysiol.2008.043554. [DOI] [PubMed] [Google Scholar]
- 25.Leys C., Ley C., Klein O., Bernard P., Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013;49:764–766. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and/or Additional information: Supplemental Video demonstrating isolated beating newborn mouse heart with 5–0 silk attached between apex and force transducer