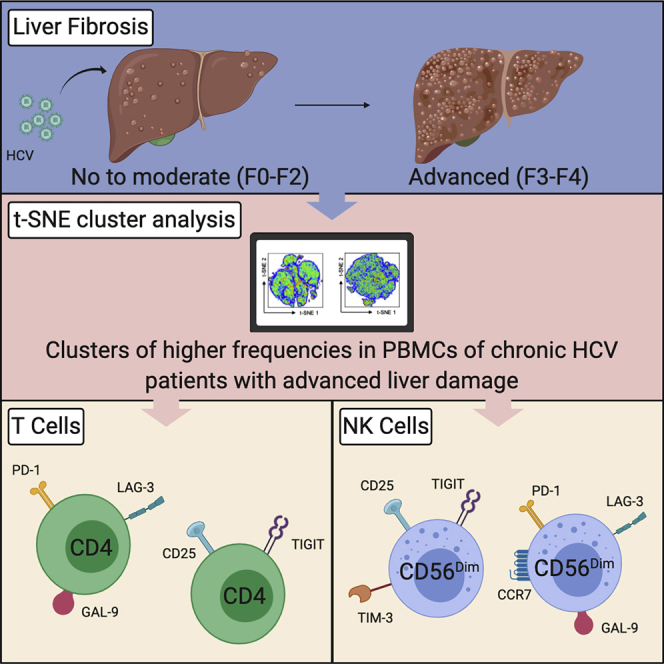
Summary
Chronic HCV can result in advanced liver disease, including cirrhosis. Patients with advanced fibrosis experience poor clinical outcomes and increased risk for hepatocellular carcinoma (HCC). These outcomes are, in part, a consequence of immune dysfunction. Increased inhibitory receptor and Galectin-9 (GAL-9) expression is a possible mechanism promoting lymphocyte dysfunction. In this study, we measured the expression of inhibitory receptors and GAL-9 on T/NK cells of patients with chronic HCV with no to moderate fibrosis (F0-F2) and advanced fibrosis (F3-F4). To analyze their co-expression, we employed t-SNE analysis. Notably, we found that F3-F4 patients had higher frequencies of >3 inhibitory receptor co-expression on NK cells. Moreover, F3-F4 patients manifest a higher frequency of NK cells co-expressing TIGIT and TIM-3, and CD4/NK cells co-expressing LAG-3 and GAL-9. In conclusion, we identified phenotypes of immune dysregulation that could explain the increased susceptibility to infection and HCC in patients with chronic HCV with advanced fibrosis.
Subject Areas: Virology, Immunology
Graphical Abstract
Highlights
-
•
We compared T/NK cell phenotypes of HCV patients in different fibrosis stages
-
•
t-SNE showed co-expression clusters of high frequency in patients with advanced fibrosis
-
•
T/NK cluster co-expressing GAL-9, LAG-3, and PD-1 of high frequency in these patients
-
•
NK cluster co-expressing TIM-3 and TIGIT is also of high frequency in these patients
Virology; Immunology
Introduction
Untreated, acute hepatitis C virus (HCV) progresses to chronic infection in 50%–80% of infected individuals (Blackard et al., 2008). Chronic HCV affects ∼70 million people worldwide and is the leading infectious cause of cirrhosis, hepatocellular carcinoma (HCC), liver transplantation, and liver-related deaths (Chhatwal et al., 2016; Gane et al., 2015). Chronic HCV infection results in liver fibrosis through hepatocellular damage and inflammation. TGF-β production activates hepatic stellate cells that produce and deposit collagen, and continual scar tissue accumulation causes patients with chronic HCV to develop cirrhosis, portal hypertension, and HCC (Friedman, 2010; Kawada, 2011). Interestingly, patients with advanced liver fibrosis have a poor response to vaccination, recurrent infections, and an increased risk of developing HCC (Aggeletopoulou et al., 2017; Bonnel et al., 2011; Gurtsevitch, 2008).
Lymphocytes play a crucial role in anti-viral responses and cancer surveillance at both the innate and adaptive immune levels. Natural killer (NK) cells are innate immune cells vital to defense against tumors and virus-infected cells. Through the recognition of infected cells and cancerous cells by activating receptors, NK cells are able to perform their effector function by releasing cytokines and cytotoxic granules (Lanier, 2005; Lee et al., 2007; Ljunggren and Karre, 1990). CD8+ T cells or cytotoxic T lymphocytes (CTLs) play a role in the adaptive immune response to tumors and viral infections, as they recognize viral and tumor antigens presented on MHC class I molecules and release perforin and granzymes to induce apoptosis of the target cell (Farhood et al., 2019). CD4+ T cells also act to maintain and boost immune cell functions, including those of CTLs, in order to orchestrate an effective response to infections. During optimal immune responses, expression of both activating and inhibitory receptors is homeostatically maintained on T and NK cells to ensure that they are adequately activated to perform their effector or helper function.
Inhibitory receptors have a critical role in regulating immune responses to infections, thereby limiting autoimmunity and/or immunopathology (Bi and Tian, 2019; Joller and Kuchroo, 2017). However, increased and sustained expression of inhibitory receptors, often found in patients with chronic viral infections and malignancies, is a principle mechanism priming lymphocytes to be dysfunctional. The suppressive role of some inhibitory receptors, programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin and mucin-domain containing-3 (TIM-3), and, more recently, T cell immunoglobulin and ITIM domain (TIGIT), is relatively well understood (Bi and Tian, 2019; Golden-Mason and Rosen, 2013; Joller and Kuchroo, 2017; Lee et al., 2010). With chronic antigen availability, the surface expression of the inhibitory receptor is significantly upregulated and maintained on T and NK cells (Bi and Tian, 2019; Joller and Kuchroo, 2017). It leads to T and NK cell dysfunction, which manifests as an inability to effectively perform their effector function. Moreover, the sustained expression of these inhibitory receptors creates an environment permitting the development of advanced stages of cancer as dysfunctional immune cells are unable to conduct tumor immunosurveillance. Notably, T cells highly expressing inhibitory receptors showed impaired effector functions (Singer et al., 2016). For this reason, the targeting of inhibitory receptors is actively being explored in cancer immunotherapies (Pauken and Wherry, 2015).
Inhibitory receptor expression has also been implicated in chronic virus infections. Studies have shown that the progression of acute to chronic HCV infection is associated with high PD-1 expression on HCV-specific CTLs (Rutebemberwa et al., 2008). During chronic HCV infections, HCV-specific CTLs in the liver have also been shown to co-express PD-1 and CTLA-4, but this phenotype was not observed in the peripheral blood lymphocytes (Nakamoto et al., 2009). Additionally, prior to the revolutionary impact of direct-acting anti-viral (DAA) therapy on the treatment of chronic HCV infection, anti-PD-1 therapy showed positive potential as a means to persistently suppress HCV replication in chronic patients (Gardiner et al., 2013). Importantly, the upregulation of PD-1 and LAG-3 expression is observed on not only antigen-specific CTLs but also the bulk CD8+ T cell population in mice chronically infected with lymphocytic choriomeningitis virus (LCMV) (Blackburn et al., 2009).
More recently, the role of Galectin-9 (GAL-9) surface expression in immune cell dysfunction has been identified. GAL-9 is a TIM-3 ligand and is widely expressed in tissues but is abundant in the liver (Wada and Kanwar, 1997). During chronic viral infections, specifically HCV, the circulating levels of GAL-9 and its expression in the liver are increased, primarily by GAL-9 producing macrophages and monocytes (Golden-Mason and Rosen, 2013; Mengshol et al., 2010). Studies have shown that the presence of soluble GAL-9 during NK cell stimulation impairs their cytokine production and cytotoxic ability (Golden-Mason and Rosen, 2013). Most recently, NK and T cells with surface expression of GAL-9 (GAL-9+ cells) in patients with HIV were shown to have reduced cytotoxicity with reduced expression of granzyme B and perforin (Motamedi et al., 2019; Shahbaz et al., 2020). Studies have also shown that HCC tumors express GAL-9, and currently, the potential use of GAL-9 expression as a biomarker of HCC is being explored (Sideras et al., 2019).
As previously mentioned, the introduction of DAAs transformed the HCV treatment landscape as most therapies show an over 95% cure rate in all HCV genotypes (Martinello et al., 2018). However, patients with chronic HCV with advanced liver fibrosis face immune dysregulation that persists after viral clearance. We have previously shown that bulk CD8+ T cells of HCV-infected individuals with advanced liver disease were dysregulated and showed sustained activated status compared with those with minimal liver fibrosis, and this persisted post DAA treatment (Vranjkovic et al., 2019). Importantly, this differential nature of immune suppression is present not only in patients with chronic HCV relative to healthy individuals but specifically in patients with advanced fibrosis compared with those with minimal or no fibrosis. Therefore, it is imperative to characterize the immune dysregulation associated with advanced liver fibrosis to develop targeted strategies for restoring the effective immune function of T and NK cells in order to treat or reduce the risk of adverse clinical outcomes of cirrhosis in HCV+ individuals such as increased HCC risk. To do this, we examined the patterns of GAL-9 expression alongside inhibitory receptors on bulk T cells and NK cells and identified immune phenotypes associated with immune dysregulation in patients with chronic HCV with advanced liver fibrosis.
Results
Proportions of T and NK Cell Subsets in Patients with HCV with Liver Fibrosis
To characterize the nature of immune dysregulation associated with advanced liver fibrosis during chronic viral infection, we recruited patients with chronic HCV and placed them into two groups based on their stage of fibrosis. The characteristics of study participants are summarized in Table 1 with F0-F2 patients having no to moderate fibrosis (n = 15) and F3-F4 patients having advanced fibrosis, including cirrhosis (n = 15). F3-F4 patients are older than F0-F2 patients, presumably owing to the years required to develop liver fibrosis. As expected, higher levels of AST and ALT are observed in F3-F4 patients, indicating advanced liver damage.
Table 1.
Characteristics of Patients with Chronic HCV
| Characteristicsa | F0-F2b | F3-F4c |
|---|---|---|
| Age (years) | 46.8 ± 2.0 | 55.3 ± 2.4 |
| Male/Female (N) | 11/3 | 13/2 |
| Race (N)-Caucasian | 15 | 15 |
| LSMd (kPa) | 6.05 ± 0.5 | 27.8 ± 4.9 |
| ASTe (U/L) | 48 ± 8 | 97 ± 19 |
| ALTf (U/L) | 75 ± 10 | 104 ± 19 |
| HCV genotype (N) | ||
| -1a | 10 | 6 |
| -2 | 0 | 3 |
| -3 | 2 | 5 |
| -4 | 1 | 1 |
| Metavir fibrosis (N) | ||
| -F0 | 4 | – |
| -F1 | 6 | – |
| -F2 | 3 | – |
| -F3 | – | 3 |
| -F4 | – | 11 |
All data are presented as mean ± SEM.
Two patients within this group could not be identified in the clinic post analysis but at the time of sample collection were placed in this group by the clinician. Thus, we are lacking AST, ALT, HCV genotype and Metavir stage for the two patients and the gender of one patient.
One patient within this group did not have a definitive Metavir classification owing to lack of liver stiffness measurement or liver biopsy but was placed in this group after evaluation by the clinician.
Five patients were excluded owing to lack of LSM data.
AST, aspartate transaminase.
ALT, alanine transaminase.
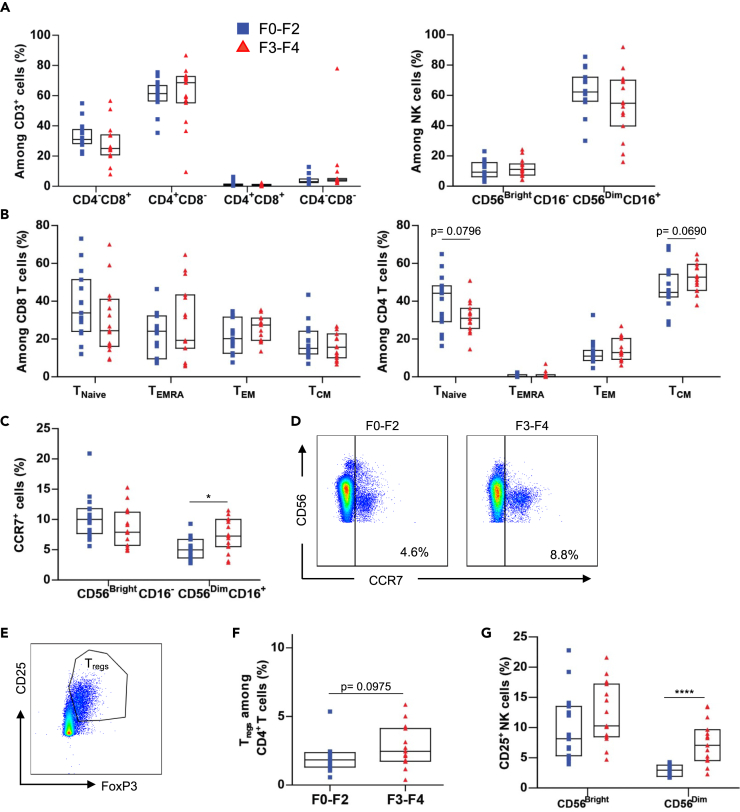
Given the pivotal role that T and NK cells play in immunosurveillance, we examined T cells and NK cells in PBMC samples to detect any changes in subset frequencies associated with liver fibrosis severity. Advanced liver fibrosis was not associated with altered frequencies of CD8+CD4- (CD8+), CD8-CD4+ (CD4+), CD8+CD4+ (double-positive), and CD8-CD4- (double-negative) T cell or CD56Bright and CD56Dim NK cell frequencies compared with frequencies in minimal fibrosis (Figures S1 and 1A). T cell subsets were identified using CD45RO and CCR7 markers with CD45RO- CCR7+ denoting naive cells (TNaive), CD45RO- CCR7-, terminal effector cells (TEMRA), CD45RO+ CCR7-, effector memory cells (TEM), and CD45RO+ CCR7+, central memory cells (TCM). Although CD8 and CD4 subsets were not significantly different between the HCV-infected groups, there was a trend showing a lower TNaive frequency and a higher TCM frequency in CD4 subsets of F3-F4 patients when compared with F0-F2 patients (Figure 1B). Lastly, the expression of CCR7 on CD56Dim NK cells was higher in the F3-F4 group compared with the F0-F2 group (Figures 1C and 1D).
Figure 1.
Proportions of T and NK Cell Subsets in Patients with HCV with Liver Fibrosis
Freshly thawed PBMCs of F0-F2 (n = 15) and F3-F4 (n = 15) chronically infected patients with HCV were stained for surface and intracellular expression of T and NK cell population markers.
(A) Cumulative data showing the percentage of T cell subpopulations (CD4-CD8+, CD4+CD8-, CD4+CD8+, and CD4-CD8-) and NK cell subpopulations (CD56DimCD16+ and CD56BrightCD16-) of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(B) Cumulative data showing the percentage of CD8 and CD4 T cell subsets of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(C) Cumulative data comparing the percentage of CD56BrightCD16- and CD56DimCD16+ NK cells expressing CCR7 of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(D) Representative plot showing the expression of CCR7 on CD56Dim NK cells of F0-F2 and F3-F4 patients.
(E) Representative plot showing the Treg (CD25+FoxP3+) population.
(F) Data comparing the frequency of Tregs between CD4 T cells of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(G) Cumulative data comparing CD25 expression on NK cells of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4. Terminal effector (EMRA), Effector memory (EM), Central memory (CM) and T Regulatory cells (Treg). Data are represented as median with interquartile range (IQR) and each point represents an individual. ∗p < 0.05, ∗∗∗∗p < 0.0001.
The regulatory T cells (Tregs) play an important role in downregulating immune responses and are defined in part by the expression of IL-2 receptor-α chain, CD25High, and the transcriptional factor FoxP3. We found no significant difference in Treg frequency between F0-F2 patients and F3-F4 patients (Figures 1E and 1F). Notably, the F3-F4 patients showed a significantly higher proportion of CD25+ CD56Dim NK cells compared with F0-F2 patients (Figure 1G).
Higher Frequencies of T and NK Cells Expressing Multiple Inhibitory Receptors in Patients with Advanced Liver Fibrosis
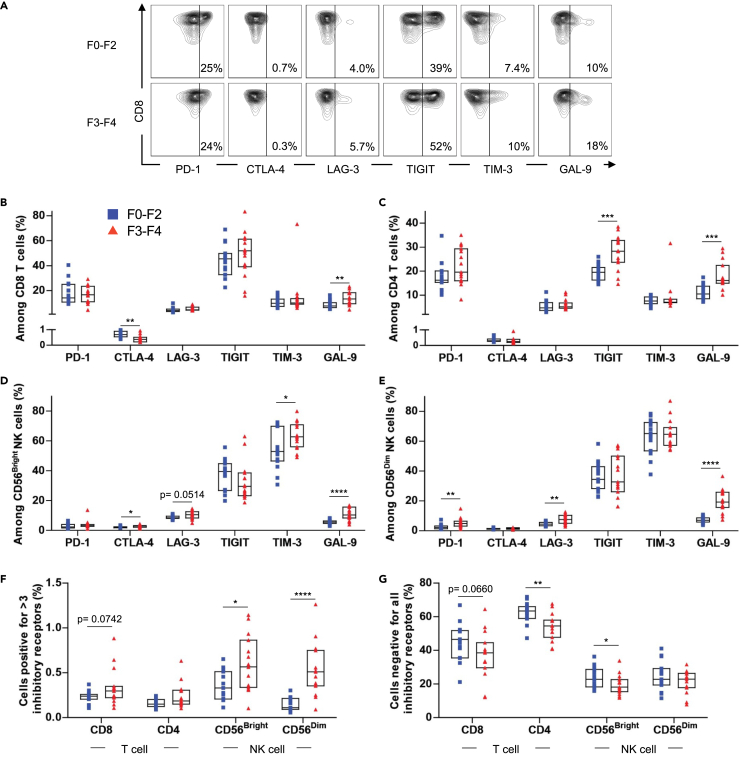
Given the critical role of inhibitory receptors and their ligands in modulating T and NK cell function, we measured the surface expression of PD-1, CTLA-4, LAG-3, TIGIT, TIM-3, and GAL-9 on CD8+/CD4+ T cells and CD56Bright/CD56Dim NK cells. In the CD8+ T cell population, F0-F2 and F3-F4 patients showed no differences in frequencies of PD-1+, LAG-3+, TIGIT+, and TIM-3+ cells (Figures 2A and 2B). F0-F2 patients had higher frequencies of CTLA-4+ cells than F3-F4 patients. F3-F4 patients had a higher frequency of GAL-9+ cells than F0-F2 patients. In the CD4+ T cell population, F0-F2 and F3-F4 patients showed no differences in frequencies of PD-1+, CTLA-4+, LAG-3+, and TIM-3+ cells (Figure 2C). Notably, F3-F4 patients had a higher frequency of TIGIT+ and GAL-9+ cells than F0-F2 patients.
Figure 2.
The Expression of Inhibitory Receptors and GAL-9 on Lymphocytes of Patients with HCV with Liver Fibrosis
Freshly thawed PBMCs of F0-F2 (n = 15) and F3-F4 (n = 15) chronically infected patients with HCV were stained for surface expression of PD-1, CTLA-4, LAG-3, TIGIT, TIM-3, and GAL-9.
(A) Representative plot of PD-1, CTLA-4, LAG-3, TIGIT, TIM-3, and GAL-9 expression on CD8+ T cells of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(B–E) Cumulative data comparing PD-1, CTLA-4, LAG-3, TIGIT, TIM-3, and GAL-9 expression on CD8 T cells (B), CD4 T cells (C), CD56Bright NK cells (D) and CD56Dim NK cells (E) of patients with chronic HCV in fibrosis stages F0-F2 and F3-F4.
(F) Cumulative data comparing Boolean analysis of the frequency of T and NK cells expressing >3 inhibitory receptors (PD-1, CTLA-4, LAG-3, TIGIT, TIM-3).
(G) Cumulative data comparing Boolean analysis of the frequency of T and NK cells expressing none of the five inhibitory receptors (PD-1, CTLA-4, LAG-3, TIGIT, TIM-3). Data are represented as median with IQR and each point represents an individual. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
On the CD56Bright NK cell population, F0-F2 and F3-F4 patients showed no differences in frequencies of PD-1+ and TIGIT+ cells (Figure 2D). Interestingly, F3-F4 patients had a higher frequency of TIM-3+, CTLA-4+, and GAL-9+ cells on CD56Bright NK cells than F0-F2 patients (Figure 2D). We also observed a trend toward a higher frequency of LAG-3+ cells, although this did not reach the level of statistical significance (p = 0.0514). Lastly, on the CD56Dim NK cell population, F0-F2 and F3-F4 patients showed no differences in frequencies of CTLA-4+, TIM-3+, and TIGIT+ cells (Figure 2E). F3-F4 patients had a higher frequency of PD-1+, LAG-3+, and GAL-9+ cells on CD56Dim NK cells than F0-F2 patients. Notably, the higher expression of GAL-9 was consistent on all tested T and NK cells of F3-F4 patients. The CTLA-4 surface expression was found minimal on all tested subsets, presumably owing to its rapid internalization in the absence of ligand binding (Qureshi et al., 2012).
Extensive lymphocyte exhaustion is characterized by the simultaneous expression of multiple inhibitory receptors, and likewise, functionally superior lymphocytes express limited inhibitory receptors (Blackburn et al., 2009; Wherry and Kurachi, 2015). Thus, using Boolean analysis, we compared the frequency of T and NK cells simultaneously expressing more than three inhibitory receptors, PD-1, CTLA-4, LAG-3, TIGIT, and TIM-3 (Figure 2F), and the frequency of T and NK cells negative for all five receptors (Figure 2G). We chose to measure the expression of >3 receptors as the surface expression of CTLA-4 on both T and NK cells was minimal.
Notably, we observed a lower frequency of both CD56Bright and CD56Dim NK cells positive for >3 (i.e., 4 or 5) inhibitory receptors in F0-F2 patients compared with F3-F4 patients (Figure 2F). No difference was observed in the CD4+ and CD8+ T cell populations. Conversely, there was a clear trend showing a lower frequency of cells negative for all inhibitory receptors in F3-F4 patients, even though it only reached a significance on the CD4+ T cell and CD56Bright NK cell populations (Figure 2G). Altogether, the results suggest that a higher frequency of extensively exhausted NK cells and a lower frequency of functionally superior T and NK cells are characteristics of patients with advanced liver fibrosis.
Higher Frequencies of CD25+TIGITmed-hi CD4+ T Cells and PD-1med CD4+ T Cells Co-expressing LAG-3 and GAL-9 in Patients with Advanced Liver Fibrosis
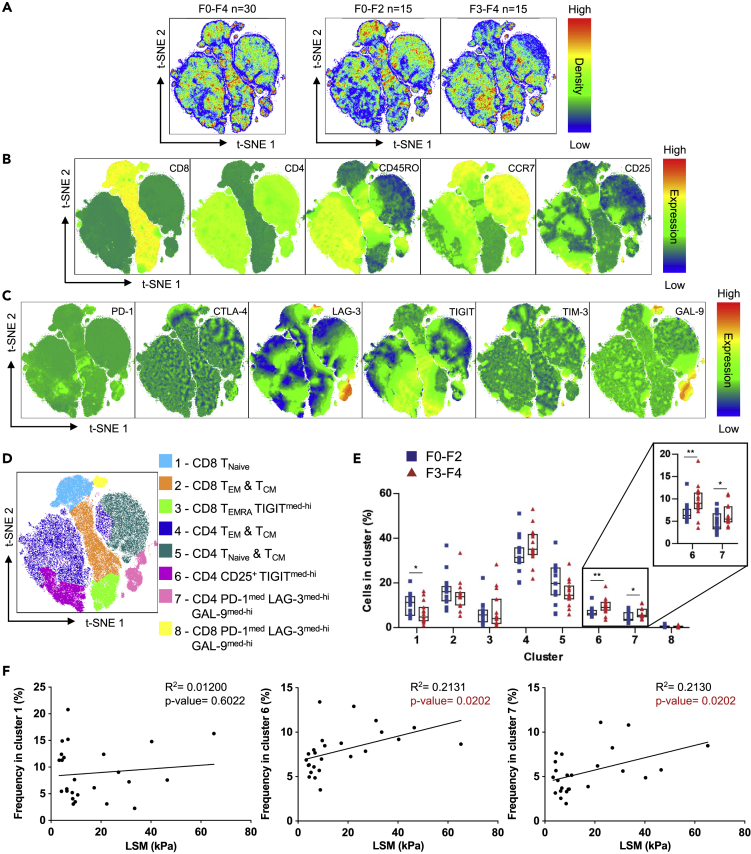
We have shown that the expression of multiple inhibitory receptors is a characteristic of patients with advanced liver fibrosis. Thus, we decided to investigate the co-expression of inhibitory receptors on the surface of T cells. In addition, we included GAL-9 expression for the co-expression analysis. The t-SNE analysis was employed to dimensionally reduce the expression data of cell surface markers, inhibitory receptors, and GAL-9 from all 30 patients with HCV and to visualize expression patterns in two dimensions (Belkina et al., 2019). t-SNE analysis of all 30 patients (F0-F4) revealed several clusters, and separating the plot based on the fibrosis scores showed the distribution of cell densities (Figure 3A). We analyzed the expression of T cell subset markers (CD8, CD4, CD45RO, CCR7, and CD25) to characterize T cell clusters (Figure 3B). To visualize the co-expression patterns of the inhibitory receptors and GAL-9, we classified their expressions as low, medium (med), and high (hi) (Figure S3). Through this classification, T cells were found to be PD-1low or PD-1med; CTLA-4low or CTLA-4med; LAG-3low, LAG-3med, or LAG-3hi; TIGITlow, TIGITmed, or TIGIThi; TIM-3low, TIM-3med, or TIM-3hi ;and GAL-9low, GAL-9med, or GAL-9hi (Figure 3C).
Figure 3.
t-SNE Analysis of T Cells of Patients with Chronic HCV
CD3+ cells excluding CD4-CD8- cells from F0-F2 (n = 15) and F3-F4 (n = 15) chronically infected patients with HCV were combined to conduct t-SNE analysis.
(A) Merged T cell t-SNE analysis of 30 patients with chronic HCV followed by the analysis of F0-F2 and F3-F4 patients separately.
(B) Expression of T cell subset markers (CD8, CD4, CD45RO, CCR7, and CD25) in t-SNE analysis.
(C) Expression of inhibitory receptors (PD-1, CTLA-4, LAG-3, TIGIT, TIM-3) and GAL-9 in t-SNE analysis.
(D) Clustering of t-SNE analysis based on subset markers, inhibitory receptor, and GAL-9 expression.
(E) Cumulative data comparing the proportion of F0-F2 and F3-F4 patients T cells found in each cluster.
(F) Linear regression analysis of cluster 1, 6, and 7 frequencies against LSM of patients with chronic HCV (n = 25). Liver Stiffness Measurement (LSM). R2 is the coefficient of determination. Cluster analysis data are represented as median with IQR and each point represents an individual. ∗p < 0.05, ∗∗p < 0.01. Statistically significantly regression p values are identified in red.
Prior to cluster analysis, t-SNE plots revealed a high co-expression of GAL-9hi and LAG-3hi on both CD8+ and CD4+ T cells with PD-1med and TIM-3low expression (Figure 3C). The t-SNE cluster analysis further generated eight clusters based on the expression of cell surface markers and inhibitory receptors as follows: (1) naive CD8 T cells, (2) TEM and TCM CD8 T cells, (3) TEMRA TIGITmed-hi CD8 T cells, (4) TEM and TCM CD4 T cells, (5) TNaive and TCM CD4 T cells, (6) CD25+ TIGITmed-hi CD4 T cells, (7) LAG-3med-hiGAL-9med-hi PD-1med CD4 T cells, and (8) LAG-3med-hiGAL-9med-hi PD-1med CD8 T cells (Figure 3D). We next compared the T cell frequency of F0-F2 patients and F3-F4 patients in each cluster (Figure 3E). Notably, we found that F3-F4 patients had less of their T cells found in cluster 1 (naive CD8 T cells), but more found in clusters 6 (CD25+ TIGITmed-hi CD4 T cells) and cluster 7 (Lag-3med-hiGal-9med-hi PD-1med CD4 T cells) when compared with F0-F2 patients. These data indicate that more advanced stages of fibrosis are associated with a higher frequency of CD25+ CD4 T cells with high TIGIT expression. Interestingly, TIGIT is highly expressed on Tregs compared with Teffs, CD25+FoxP3- CD4 T (Figure S2). In addition, it indicates that GAL-9 and LAG-3 are highly co-expressed on PD-1med cells, and this co-expression is found in both CD8+ T and CD4+ T cells. Lastly, F3-F4 patients have an increased frequency of these LAG-3med-hiGAL-9med-hi CD4+ T cells.
To correlate the identified cell clusters with liver damage, we conducted linear regression analyses between patients' Liver Stiffness Measurements (LSM) and the frequency of cells found in significantly different clusters (clusters 1, 6, and 7). We found a significant correlation of cluster 6 and 7 frequencies with LSM, but not cluster 1 (Figure 3F). Taken together, using the t-SNE analysis, we identified high TIGIT expression on CD25+ CD4+ T cells and high LAG-3 and GAL-9 co-expression on PD-1med CD4+ T cells and these expression patterns are associated with advanced liver fibrosis.
Higher Frequencies of CD56Dim NK Cells Co-expressing LAG-3 and GAL-9 as well as TIGIT and TIM-3 in Patients with Advanced Liver Fibrosis
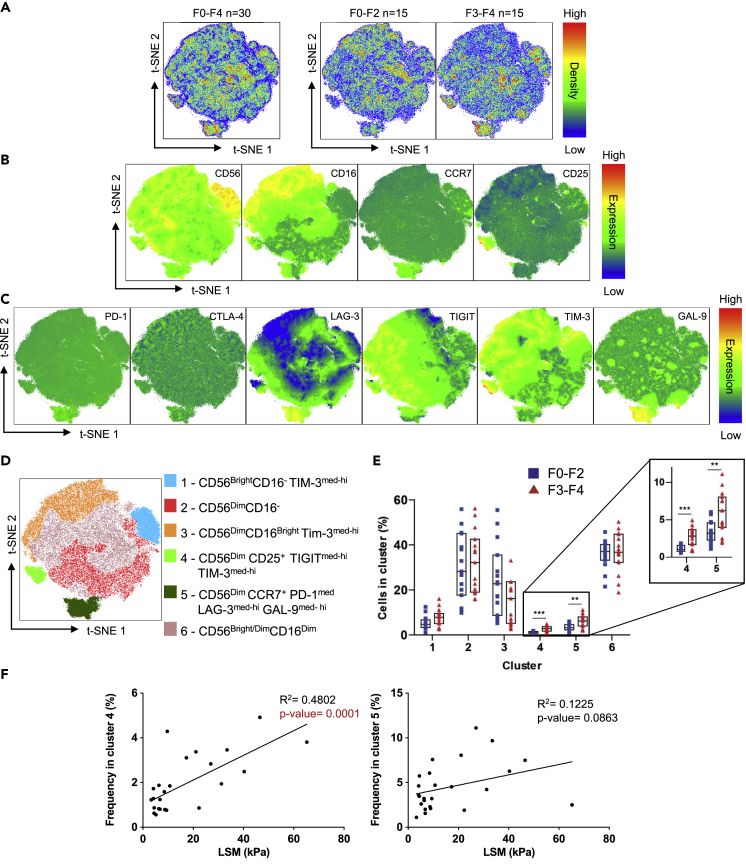
t-SNE analysis of T cells revealed high co-expression of GAL-9 and LAG-3 on CD4 and CD8 T cells. To determine co-expression patterns of inhibitory receptors on NK cells, we employed t-SNE analysis on CD56+ NK cells, first combining the plots derived from all 30 patients irrespective of fibrosis stage and then separating plots based on the stage of fibrosis, F0-F2 and F3-F4 (Figure 4A). The expression of subset markers on NK cells was classified as CD56Dim or CD56Bright; CD16-, CD16Dim, or CD16Bright; CCR7- or CCR7+; and CD25- or CD25+ (Figure 4B). The expression of inhibitory receptors was classified as PD-1low or PD-1med; CTLA-4low or CTLA-4med, LAG-3low, LAG-3med, or LAG-3hi; TIGITlow, TIGITmed, or TIGIThi, TIM-3low, TIM-3med, or TIM-3hi and GAL-9low, GAL-9med, or GAL-9hi (Figure 4C). Using this classification, NK cells were divided into six clusters (Figure 4D) as follows: (1) CD56BrightCD16- TIM-3med-hi cells, (2) CD56DimCD16- cells, (3) CD56DimCD16Bright TIM-3med-hi cells, (4) CD56Dim CD25+TIGITmed-hiTIM-3med-hi cells, (5) CD56Dim CCR7+PD-1medLAG-3med-hiGAL-9med-hi cells, and (6) CD56Bright/DimCD16Dim cells.
Figure 4.
t-SNE Analysis of NK Cells of Patients with Chronic HCV
CD56+ NK cells from F0-F2 (n = 15) and F3-F4 (n = 15) chronically infected patients with HCV were combined to conduct t-SNE analysis.
(A) Merged NK cell t-SNE analysis of 30 patients with chronic HCV followed by the analysis of F0-F2 and F3-F4 patients separately.
(B) Expression of NK cell subset markers (CD56, CD16, CCR7, and CD25) in the t-SNE analysis.
(C) Expression of inhibitory receptors (PD-1, CTLA-4, LAG-3, TIGIT, TIM-3) and GAL-9 in the t-SNE analysis.
(D) Clustering of t-SNE analysis based on subset markers, inhibitory receptor, and GAL-9 expression.
(E) Cumulative data comparing the proportion of F0-F2 and F3-F4 patients NK cells found in each cluster.
(F) Linear regression analysis of cluster 4 and 5 frequencies against LSM of patients with chronic HCV (n = 25). Liver Stiffness Measurement (LSM). R2 is the coefficient of determination. Cluster analysis data are represented as median with IQR and each point represents an individual. ∗∗p < 0.01, ∗∗∗p < 0.001. Statistically significantly regression p values are identified in red.
We compared the frequency of patients' NK cells in each cluster (Figure 4E) and found that F3-F4 patients had a significantly higher frequency of their NK cells found in cluster 4 (CD56Dim CD25+TIGITmed-hiTIM-3med-hi cells) and cluster 5 (CD56Dim CCR7+PD-1medLAG-3med-hiGAL-9med-hi cells). Interestingly, the higher expression on CCR7+ NK cells in F3-F4 patients was observed in Figure 1C, and the t-SNE analysis identified the co-expression of LAG-3, GAL-9, and PD-1 on the CCR7+ NK cells. Linear regression analysis was employed correlating patients' LSM with the frequency of cells found in clusters 4 and 5. We found a significant correlation of cluster 4 frequencies with LSM and a correlation in cluster 5, although this did not rise to the level of statistical significance, p = 0.0863 (Figure 4F). Taken together, using t-SNE analysis, we identified CD25+TIGITmed-hiTIM-3med-hi cells and CCR7+PD-1medLAG-3med-hiGAL-9med-hi cells, and these co-expression patterns on CD56Dim NK cells are associated with advanced liver fibrosis.
Discussion
NK and T cells play a vital role in the anti-viral and anti-tumor responses. Patients with chronic HCV with advanced stages of fibrosis have an increased susceptibility to infection and an increased risk of HCC post-viral clearance with DAAs (Aggeletopoulou et al., 2017; Bonnel et al., 2011; Gurtsevitch, 2008; Vranjkovic et al., 2019). Thus, it is imperative to understand the immune dysfunction in these patients to improve their immune function after they are cured by DAAs. Previously, it has been shown that the expression of inhibitory receptors is a mechanism contributing to immune cell suppression of the NK cell and bulk T cell population (Blackburn et al., 2009; da Silva et al., 2014; Motamedi et al., 2019; Woo et al., 2012; Xu et al., 2015). Therefore, there is a need to understand the differences in immune dysregulation between patients with minimal liver fibrosis and those in the advanced stages to create targeted strategies to improve immune function.
Although the expression of only one inhibitory receptor does not suggest lymphocyte exhaustion, the expression of multiple inhibitory receptors is a clear indicator of extensively exhausted immune cells (Blackburn et al., 2009; Wherry and Kurachi, 2015). On the other hand, immune cells not expressing any inhibitory receptors are known to exhibit superior effector function (Blackburn et al., 2009). We observed that F3-F4 patients had a higher frequency of NK cells, which expressed more than 3 (i.e., 4 or 5) inhibitory receptors, suggesting the extensive exhaustion of these cells. Conversely, these patients showed a lower frequency of T and NK cells that did not express any of the measured inhibitory receptors. Therefore, our data demonstrate that patients with advanced liver fibrosis contain higher proportions of NK cells expressing multiple inhibitory receptors and lower proportions of T and NK cells free from inhibitory receptors expression.
As CD25 is the IL-2 receptor α-chain, its expression plays a crucial role in the response of NK cells in patients with chronic HCV. Co-culturing of NK cells with HCV-infected hepatocytes has been shown to increase their CD25 surface expression (Pollmann et al., 2018). CD56Dim NK cells with increased expression of CD25 have enhanced responsiveness to low doses of IL-2, thus improving their production of IFNγ and cytotoxicity in these conditions (Leong et al., 2014). Here we showed that patients with advanced stages of fibrosis have a higher frequency of CD25+ CD56Dim NK cells than patients with minimal fibrosis. The t-SNE analysis revealed that these CD25+ cells also highly co-express TIM-3 and TIGIT. TIM-3 expression denotes functionally exhausted CD56Dim NK cells with impeded cytokine production and cytotoxicity (da Silva et al., 2014). Similarly, TIGIT engagement on NK cells directly inhibits their cytotoxicity and cytokine secretion by prematurely terminating the activity of NF-κB (nuclear factor-κB), PI3K (phosphoinositide 3-kinase), and MAPK (mitogen-activated protein kinase) (Li et al., 2014; Liu et al., 2013). TIM-3 expression has also been observed on HCV-specific CD8 T cells, but this increased expression was restricted to the cells in the intrahepatic compartment and not the peripheral blood (Golden-Mason et al., 2009). Knowing TIM-3 and TIGIT are co-expressed on NK cells, which are already primed for enhanced function (CD25+), a combined immune blockade therapy can be employed to unleash these exhausted cells, thus conferring protection to the host against other infections and HCC. Tumor infiltrating lymphocytes (TILs) of B16F10 melanoma in mice highly co-express TIM-3 and TIGIT, and their effector function is only restored in TIGIT-/- mice treated with anti-TIM-3 antibodies (Kurtulus et al., 2015). Blockade of TIM-3 or TIGIT has separately been shown to restore NK cell function (Xu et al., 2015; Zhang et al., 2018). Thus, our data suggest that high co-expression of TIM-3 and TIGIT on CD25+ NK cells hinders their function, and a combined blockade therapy using anti-TIGIT and anti-TIM-3 antibodies could provide a potential therapeutic benefit by improving NK cell function in patients with chronic HCV with advanced liver fibrosis.
Our t-SNE analysis also revealed a higher frequency of TIGITmed-hi CD25+ CD4+ T cells (Tregs and Teffs) in F3-F4 patients when compared with F0-F2 patients. Tregs begin to express IL-10 and Fgl2 after TIGIT engagement, and Fgl2 expression allows Tregs to selectively suppress Th1 and Th17 pro-inflammatory responses (Joller et al., 2014). TIGIT expression on Tregs is also implicated in their lineage stability (Fuhrman et al., 2015). Altogether, our data reveal that patients with advanced liver fibrosis have an increase in the frequency of TIGIT+ CD25+ CD4 T cells, which we found to be approximately 50% TIGIT+ Tregs, and evidence suggests TIGIT+ Tregs could possess increased immunosuppressive capabilities and increased stability.
GAL-9 is one of the ligands of TIM-3, and the role of TIM-3/GAL-9 interactions on modulating immune cell functions has been well studied (Elsegood et al., 2017; Mengshol et al., 2010). We found that CD8+ and CD4+ T cells as well as CD56Dim and CD56Bright NK cells in patients with advanced liver fibrosis had higher frequencies of GAL-9+ cells when compared with F0-F2 patients. GAL-9+ NK cells in patients with HIV had reduced cytotoxicity with lower expression of perforin and granzyme B (Motamedi et al., 2019). Furthermore, GAL-9 binding to TIM-3 has been shown to trigger cell death in Th1 and Tc1 cells (Zhu et al., 2005). t-SNE analysis of patients with chronic HCV showed that the T cells co-expressing LAG-3 and GAL-9 were PD-1+ cells. It also showed that F3-F4 patients had a higher frequency of CD4+ T cells with this expression pattern. Given the vital role of the TIM-3/GAL-9 interactions in modulating T/NK cell function, the higher frequency of T and NK cells expressing GAL-9 in patients with advanced liver fibrosis when compared with F0-F2 patients is a phenotype potentially denoting immune dysregulation.
Lastly, we observed a higher frequency of CCR7 expressing CD56DimCD16+ NK cells in patients with advanced fibrosis than in those with less fibrosis (Figure 1D). CCR7 is a receptor vital for the migration of immune cells into secondary lymphoid organs such as the lymph nodes, and CD56Dim NK cells in the peripheral blood lacking CCR7 have been shown to be cytotoxic effector cells (Berahovich et al., 2006; Campbell et al., 2001). The increase in CD56Dim CCR7+ NK cells in F3-F4 patients suggests that the frequency of cytotoxic effector NK cells is reduced. The phenotype was accompanied by the close co-expression of LAG-3, GAL-9, as well as PD-1. Studies have previously shown LAG-3 and PD-1 are co-expressed on TILs in various human tumors as well as LCMV chronically infected mice, and LAG-3 blockade was not sufficient to reverse the CTL exhausted phenotype (Blackburn et al., 2009; Matsuzaki et al., 2010; Woo et al., 2012). Notably, combined blockade of LAG-3 and PD-1 vastly improves CTL function to control tumor growth (Woo et al., 2012). The combined blockade of LAG-3 and PD-L1 was also shown to improve viral control in LCMV chronically infected mice (Blackburn et al., 2009). Thus, our findings reason that combined blockade of LAG-3, GAL-9, and PD-1 could potentially improve the function of T/NK cells in patients with advanced liver fibrosis.
In conclusion, we investigated PBMCs from patients with chronic HCV with minimal to no fibrosis and chronic HCV with advanced fibrosis by analyzing the co-expression of five inhibitory receptors and GAL-9 on T/NK cells. A recent meta-analysis found that PD-1 inhibitor monotherapy and anti-CTLA-4 monotherapy had 18.6% efficacy when used for treating liver cancer in patients with chronic HBV and HCV (Pu et al., 2020). Ultimately, they concluded that immune checkpoint inhibitors are safe for these patients, but virus reactivation remains a possibility. Our findings revealed multiparametric phenotypes that characterize immune suppression in patients with chronic HCV with advanced liver fibrosis. Mechanisms underlying these phenotypes would provide a better understanding of immune dysregulation associated with advanced liver damage and increased susceptibility to infections and HCC and will lead to the development of targeted therapies to improve their immune function.
Limitations of the Study
Although we utilize an extensive number of inhibitory receptors, there are other inhibitory receptors at play, which were not included in this study, but could be critical in inducing and maintaining immune dysregulation. Owing to the limited sample size, we acknowledge that statistically significant differences do not necessarily translate into biological significance; thus, we are looking forward to our results being reproduced in other studies with larger sample sizes. In addition, our data represent the status of circulating immune cells and may not reflect the phenotypic and functional picture of their counterparts in the liver. Nonetheless, information regarding the immunological status of T and NK cells in the peripheral blood should be vital to understand the susceptibility to other infections and HCC in patients. Lastly, we did not include healthy controls in the study. Thus, our data itself might not be sufficient to distinguish phenotypes of immune dysregulation associated with advanced liver damage from those found in healthy controls. Nonetheless, there is ample literature that has compared the expression of inhibitory receptors in healthy controls with that in patients with chronic HCV.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Seung-Hwan Lee (seunglee@uottawa.ca).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets or codes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the Canadian Network on Hepatitis C (CanHepC) for their generous funding of C.I.A.O. through a CanHepC trainee fellowship. This work was supported by funding from the Canadian Institutes of Health Research (MOP-130385, PJT-156106) to S.-H.L.. We would also like to thank all HCV-infected individuals who participated in this study.
Author Contributions
Conceptualization, C.I.A.O. and S.-H.L.; Methodology, C.I.A.O., J.S.O., and S.-H.L.; Investigation, C.I.A.O.; Formal Analysis, C.I.A.O.; Visualization, C.I.A.O.; Resources, C.L.C. and J.S.O.; Writing – Original Draft, C.I.A.O. and S.-H.L.; Writing – Review & Editing, C.I.A.O., S.-H.L., C.L.C., and A.M.C.; Funding Acquisition, S.-H.L.; Supervision, S.-H.L.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101513.
Supplemental Information
References
- Aggeletopoulou I., Davoulou P., Konstantakis C., Thomopoulos K., Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev. Med. Virol. 2017;27 doi: 10.1002/rmv.1942. [DOI] [PubMed] [Google Scholar]
- Belkina A.C., Ciccolella C.O., Anno R., Halpert R., Spidlen J., Snyder-Cappione J.E. Automated optimized parameters for T-distributed stochastic neighbor embedding improve visualization and analysis of large datasets. Nat. Commun. 2019;10:5415. doi: 10.1038/s41467-019-13055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berahovich R.D., Lai N.L., Wei Z., Lanier L.L., Schall T.J. Evidence for NK cell subsets based on chemokine receptor expression. J. Immunol. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- Bi J., Tian Z. NK cell dysfunction and checkpoint immunotherapy. Front. Immunol. 2019;10:1999. doi: 10.3389/fimmu.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard J.T., Shata M.T., Shire N.J., Sherman K.E. Acute hepatitis C virus infection: a chronic problem. Hepatology. 2008;47:321–331. doi: 10.1002/hep.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Wherry E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel A.R., Bunchorntavakul C., Reddy K.R. Immune dysfunction and infections in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Qin S., Unutmaz D., Soler D., Murphy K.E., Hodge M.R., Butcher E.C. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- Chhatwal J., Wang X., Ayer T., Kabiri M., Chung R.T., Hur C., Kanwal F. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016;64:1442–1450. doi: 10.1002/hep.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva I.P., Gallois A., Jimenez-Baranda S., Khan S., Anderson A.C., Kuchroo V.K., Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsegood C.L., Tirnitz-Parker J.E., Olynyk J.K., Yeoh G.C. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin. Transl. Immunol. 2017;6:e161. doi: 10.1038/cti.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhood B., Najafi M., Mortezaee K. Cancer-associated fibroblasts: secretions, interactions, and therapy. J. Cell Biochem. 2019;120:2791–2800. doi: 10.1002/jcb.27703. [DOI] [PubMed] [Google Scholar]
- Friedman S.L. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- Fuhrman C.A., Yeh W.I., Seay H.R., Saikumar Lakshmi P., Chopra G., Zhang L., Brusko T.M. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 2015;195:145–155. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane E., Kershenobich D., Seguin-Devaux C., Kristian P., Aho I., Dalgard O., Estes C. Strategies to manage hepatitis C virus (HCV) infection disease burden - volume 2. J. Viral Hepat. 2015;22(Suppl 1):46–73. doi: 10.1111/jvh.12352. [DOI] [PubMed] [Google Scholar]
- Gardiner D., Lalezari J., Lawitz E., DiMicco M., Ghalib R., Reddy K.R., Lowy I. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L., Palmer B.E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Rosen H.R. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L., Rosen H.R. Natural killer cells: multifaceted players with key roles in hepatitis C immunity. Immunol. Rev. 2013;255:68–81. doi: 10.1111/imr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtsevitch V.E. Human oncogenic viruses: hepatitis B and hepatitis C viruses and their role in hepatocarcinogenesis. Biochemistry (Mosc) 2008;73:504–513. doi: 10.1134/s0006297908050039. [DOI] [PubMed] [Google Scholar]
- Joller N., Kuchroo V.K. Tim-3, Lag-3, and TIGIT. Curr. Top. Microbiol. Immunol. 2017;410:127–156. doi: 10.1007/82_2017_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N., Lozano E., Burkett P.R., Patel B., Xiao S., Zhu C., Kuchroo V.K. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada N. Evolution of hepatic fibrosis research. Hepatol. Res. 2011;41:199–208. doi: 10.1111/j.1872-034X.2011.00776.x. [DOI] [PubMed] [Google Scholar]
- Kurtulus S., Sakuishi K., Ngiow S.F., Joller N., Tan D.J., Teng M.W., Anderson A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lanier L.L. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lee J., Suh W.I., Shin E.C. T-cell dysfunction and inhibitory receptors in hepatitis C virus infection. Immune Netw. 2010;10:120–125. doi: 10.4110/in.2010.10.4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Miyagi T., Biron C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Leong J.W., Chase J.M., Romee R., Schneider S.E., Sullivan R.P., Cooper M.A., Fehniger T.A. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transpl. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Xia P., Du Y., Liu S., Huang G., Chen J., Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J. Biol. Chem. 2014;289:17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang H., Li M., Hu D., Li C., Ge B., Fan Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20:456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H.G., Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Martinello M., Hajarizadeh B., Grebely J., Dore G.J., Matthews G.V. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat. Rev. Gastroenterol. Hepatol. 2018;15:412–424. doi: 10.1038/s41575-018-0026-5. [DOI] [PubMed] [Google Scholar]
- Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P., Beck A., Miller A., Tsuji T., Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol J.A., Golden-Mason L., Arikawa T., Smith M., Niki T., McWilliams R., Rosen H.R. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi M., Shahbaz S., Fu L., Dunsmore G., Xu L., Harrington R., Elahi S. Galectin-9 expression defines a subpopulation of NK cells with impaired cytotoxic effector molecules but enhanced IFN-gamma production, dichotomous to TIGIT, in HIV-1 infection. Immunohorizons. 2019;3:531–546. doi: 10.4049/immunohorizons.1900087. [DOI] [PubMed] [Google Scholar]
- Nakamoto N., Cho H., Shaked A., Olthoff K., Valiga M.E., Kaminski M., Chang K.M. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann J., Gotz J.J., Rupp D., Strauss O., Granzin M., Grunvogel O., Cerwenka A. Hepatitis C virus-induced natural killer cell proliferation involves monocyte-derived cells and the OX40/OX40L axis. J. Hepatol. 2018;68:421–430. doi: 10.1016/j.jhep.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Pu D., Yin L., Zhou Y., Li W., Huang L., Cai L., Zhou Q. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine (Baltimore) 2020;99:e19013. doi: 10.1097/MD.0000000000019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Kaur S., Hou T.Z., Jeffery L.E., Poulter N.S., Briggs Z., Sansom D.M. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J. Biol. Chem. 2012;287:9429–9440. doi: 10.1074/jbc.M111.304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutebemberwa A., Ray S.C., Astemborski J., Levine J., Liu L., Dowd K.A., Cox A.L. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J. Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaz S., Dunsmore G., Koleva P., Xu L., Houston S., Elahi S. Galectin-9 and VISTA expression define terminally exhausted T cells in HIV-1 infection. J. Immunol. 2020;204:2474–2491. doi: 10.4049/jimmunol.1901481. [DOI] [PubMed] [Google Scholar]
- Sideras K., de Man R.A., Harrington S.M., Polak W.G., Zhou G., Schutz H.M., Bruno M.J. Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels. Sci. Rep. 2019;9:10677. doi: 10.1038/s41598-019-47235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Wang C., Cong L., Marjanovic N.D., Kowalczyk M.S., Zhang H., Anderson A.C. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166:1500–1511 e1509. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic A., Deonarine F., Kaka S., Angel J.B., Cooper C.L., Crawley A.M. Direct-acting antiviral treatment of HCV infection does not resolve the dysfunction of circulating CD8(+) T-cells in advanced liver disease. Front. Immunol. 2019;10:1926. doi: 10.3389/fimmu.2019.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J., Kanwar Y.S. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J. Biol. Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J., Vignali D.A. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Huang Y., Tan L., Yu W., Chen D., Lu C., Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W., Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J., Kuchroo V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets or codes.