Abstract
Bischofia javanica (Blume), an edible wild plant, has both prospective nutraceutical and therapeutic properties. Here, we intended to explore the pharmacological potentials of the methanol extract of B. javanica (MEBJ) through integrated approaches. Phytochemical screening revealed the presence of important phytoconstituents which were found to be safe during cytotoxicity analysis. The sedative potential of MEBJ (200 and 400 mg/kg) was determined by employing open field, hole cross, and thiopental sodium-induced sleeping time tests, where a significant reduction of the locomotor performance and an enhancement in the duration of sleeping have been observed, respectively. In addition, mice treated with MEBJ exhibited superior exploration during both elevated plus maze and hole board tests. In parallel, anti-diabetic potency was investigated via alpha-amylase inhibitory assay, where a dose-response increase in the percentage of inhibition has been marked. A similar response, such as an increased percentage of clot lysis, was observed during the thrombolytic test. Furthermore, molecular docking was performed with the identified compounds, demonstrated strong binding affinities to the target receptors of the experiments as mentioned above. Also, ADME/T and toxicological parameters verified the drug-like properties of the identified compounds. Collectively, these results indicate bioactivity of Bischofia javanica, which can be a potential candidate in the food and pharmaceutical industries.
Keywords: Bischofia javanica, Sedative and anxiolytic, Anti-diabetic, Thrombolytic, Cytotoxic, Molecular docking, Bioinformatics, Evidence-based medicine, Neuroscience, Pharmaceutical science, Pharmacology, Toxicology
Bischofia javanica; sedative and anxiolytic; Anti-diabetic; Thrombolytic; Cytotoxic; Molecular docking; Bioinformatics; Evidence-Based Medicine; Neuroscience; Pharmaceutical Science; Pharmacology; Toxicology
1. Introduction
Since primitive times, the discovery of medicinal herbs and their applications have been continued in the discipline of the traditional system of medicine. Every plant consists of some major phytochemical compounds which provide them with an intrinsic defensive benefit against pathogens [1]. These phytochemicals have some direct or indirect therapeutic potentials and can be used to synthesize and develop modern drugs [2]. Different parts of medicinal plants such as seeds, root, bark, fruits, leaves, flowers or even whole plant are used as a source of traditional medicine [3]. The World Health Organization (WHO) states that people can use natural plant materials as an alternative treatment for specific diseases at a local or regional scale [4]. Therefore, medicinal plants are being used in many countries (especially developing country) for thousands of years to mitigate the complications of numerous diseases [5].
At present, synthetic drugs have been broadly employed to treat several diseases, even though having various limitations, such as enormous side effects and lowest efficacy. For example, a lot of conventional drugs have been used to treat the following diseases like insomnia, anxiety, and depression where different classes of therapeutic agents like opioids, benzodiazepines, NSAID's are being prescribed for the treatment of neuropsychiatric disorders. However, these drugs have a limited use due to their additional side effects in conjunction with the tolerance and dependence produced [6]. On the other hand, diabetes is another condition in which insulin therapy is used despite its noxious effects, drug resistance, and toxicity [7]. As a result, there has been an increased demand for alternative treatment process of diabetes [8]. A Similar condition has been marked during the treatment of thromboembolic strokes, myocardial infarction, and deep vein thrombosis [9]. Well-known thrombolytic agent, streptokinase has been accepted less commonly because of its hemorrhagic complications through the degradation of circulating fibrinogen [10]. For this reason, exploration of medicinal plants, particularly attention on the development of a novel drug for the treatment of various diseases is increasing gradually because of their diverse chemical structure and best compatibility with human physiology. Furthermore, nowadays, computational methods are being considered the most suitable approaches to scrutinize or predict the biological activity of phytocompounds along with its safety and drug-likeliness properties since it saves not only the time but also the money [11, 12, 13, 14].
Bischofia javanica Blume, is belonging to the family Euphorbiaceae, broadly distributed in Bangladesh, India, Indonesia, China, Philippine and Vietnam [15]. The plant is regionally known by different names including Bengali (kanjail); English (bishop wood, Java cedar); Hindi (bhillar, paniala, kotsemla); Japanese (akagi); Vietnamese (nhoi) [16, 17]. The nutraceutical value of this plant is very prominent in East-Asia; particularly leaves are widely used in salad and condiment preparation [18]. Traditionally, this plant was used for the treatment of various chronic conditions like inflammation, tuberculosis, ulcer, fracture and dislocation [19, 20, 21]. Bark and leaves of B. javanica are used in the treatment of diarrhea, sore throat, and nervous disorder [22]. Also, several pharmacological activities have already been reported of this plant, such as antiparasitic [23], antimicrobial [24], anti-leukemic [25], anti-inflammatory, and anti-nociceptive activities [26]. Furthermore, previous reports on this plant suggested that the leaves extract of B. javanica yielded ten major phytochemicals viz. beta-amyrine, ursolic acid, betulinic acid, chrysoeriol, quercetin, friedelan-3-one, beta-sitosterol, fisetin, cynaroside and triacontane [27, 28].
Nevertheless, the plant has many beneficial traditional practices, as yet, no scientific investigation has been carried out to explore its effects against neuropsychiatric disorders (anxiety and insomnia), and for anti-diabetic and thrombolytic properties. Hence, this study was designed to evaluate the anxiolytic, sedative, anti-diabetic, and thrombolytic properties of the methanol extract of B. javanica leaves (MEBJ) in different experimental and computational models. In addition, a computational study (molecular docking, pharmacokinetics (ADME) and toxicological properties) was performed to reveal the mechanism(s) of the observed pharmacological activities and also to know its safety and drug-likeliness properties. The overall workflow is represented in Scheme 1.
Scheme 1.
Schematic representation of pharmacological potencies of Bischofia javanica Blume.
2. Materials and methods
2.1. Extract preparation
Fresh leaves of Bischofia javanica were collected from Bangladesh Forest Research Institute (BFRI), Chittagong, Bangladesh in august 2019. The collected leaves were authenticated by Dr. Shaikh Bokhtear Uddin, Professor, Department of Botany, University of Chittagong, Bangladesh, and a voucher specimen (accession: SBU 1629) has been deposited in the Herbarium center (University of Chittagong). The collected leaves were rinsed in clean water and kept on drying for 7 days. Dried leaves were then grinded in an electric blender. About 300 g of the pulverized leaves were immersed in 1.8 L of methanol and kept at room temperature along with continuous stirring for two weeks. Afterwards, the solution was filtered using a cotton plug and Whitman filter paper. Then the filtered was transferred on the water bath for evaporation for a period of 10 days at 55 °C temperature. The crude methanol extract of B. javanica (MEBJ) (yield 8.05 %) was secured in a vial and stored in 4 °C for further experiments.
2.2. Preliminary phytochemical screening
Qualitative phytoconstituent determination of MEBJ leaves was carried through standard methods described by Pandey et al [29].
2.3. Cytotoxicity test of MEBJ
Brine shrimp lethality bioassay was followed for assessing general toxicity of MEBJ [30, 31, 32]. The procedure was initiated by hatching the eggs of shrimp in unnatural brine formulated previously by the addition of sea salt (38 g/L). The plant extract was mixed with prepared brine by using DMSO (not more than 0.01%) and obtained different concentrations (31.25, 62.50, 125, 250 500 and 1000 μg/mL) of extract solution followed by the addition of 10 nauplii in each concentration. Vincristine sulphate was taken as standard, and validation of the process was done by using negative control DMSO. After 24 h of incubation at 25 °C-30 °C, the number of living nauplii was counted and calculated the percentage of mortality applying the following formula.
| % Mortality = (Nt/N0) × 100 |
where Nt represents the number of nauplii died after incubation and N0 represents the total number of nauplii transferred.
2.4. Management of experimental animals
Both male and female Swiss albino mice (weight: 26–34g) were collected from the animal lab, Department of Pharmacy, International Islamic University Chittagong, and kept in polypropylene boxes at 25 ± 2 °C room temperature and 55–60% humidity in a 12h day light cycle. The mice were given suitable drinking water and pellet diet and adapted for a week inside the laboratory environment prior to the experiment by following the “Guide for the Care and Use of Laboratory Animals, 8thed” USA [33]. All the test protocols (Pharm-P&D-61/08′19–128) followed in this study were authorized by the institutional animal ethics committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh.
2.5. Acute toxicity test
The test was carried out adopting standard laboratory conditions as stated in the guidelines of “Organization for Environmental Control Development” (OECD: Guidelines 420; Fixed-Dose Method). Twenty Swiss albino mice were divided into 2 groups and administered two doses (2000 and 4000 mg/kg) of MEBJ through oral route. The animals were then observed for general toxic symptoms including allergic reactions, behavioral distortions and mortality throughout 72h.
2.6. Experimental design
For each test, 24 experimental mice were randomly split up into four batches (control, standard and test groups) consisting of six mice in each batch. The two test groups received MEBJ 200 and 400 mg/kg, b.w, p.o, whereas 1% Tween-80 in water (10 mL/kg, p.o) was administered to the control group. Standard group of open field, hole cross, EPM, and hole board experiments received diazepam (1 mg/kg b.w.) intraperitoneally (i.p). In particular, thiopental sodium (40 mg/kg b.w, i.p) was administered to all groups during thiopental sodium-induced sleeping time test.
2.7. Locomotor activity of MEBJ
2.7.1. Open field (OP) test
The locomotor activity of MEBJ was evaluated by following the open field test method [34]. The open field apparatus consists of a sequence of squares which are painted black and white in preference and also covered by 4 walls of 40 cm high. Thirty min after the delivery of each dose (Mentioned in Section 2.6), the mice were observed for 3 min to record the number of squares crossed on 0, 30, 60, 90 and 120 min. For proper habituation, the animals were kept in the experiment room 1 h before the test.
2.7.2. Hole cross (HC) test
The experiment was performed as per the description of Takagi et al. [35] for assessing the locomotor performance of the experimental animals by MEBJ. Thirty min after the administration of each dose (Mentioned in Section 2.6), the mice were individually placed in a compartment which is divided by a wooden partition of 30 × 20 × 14 cm3. In the center of the compartment, a hole of 3 cm in diameter was made at the height of 7.5 cm. The number of times a mouse passed through that hole was observed and recorded for 5 min on 0, 30, 60, 90, and 120 min of dosing.
2.8. Sedative activity of MEBJ by thiopental sodium (TS) induced sleeping time test
Evaluation of the sedative activity of MEBJ was carried out employing a thiopental sodium-induced sleeping time test [36]. Twenty min after the administration of each dose (Mentioned in Section 2.6), thiopental sodium (40 mg/kg i.p.) was injected to all experimental mice in order to promote sleep. Afterwards, the mice were monitored for the dormant period (time between thiopental sodium administration and loss of righting reflex) and duration of sleep (time between loss and recovery of righting reflex).
2.9. Anxiolytic activity of MEBJ
2.9.1. Elevated plus maze (EPM) test
Handley and Mithani primarily proposed the procedure followed in this test with a slight modification [37]. The apparatus is composed of two closed arms (5 × 10 × 15 cm) and two open arms (5 × 10 cm) extended from the center (5 × 5 cm) to form plus sign resemblance. It was kept 40 cm upwards from the floor in a dimly illuminated room. The allocated mice were treated with desired doses (Mentioned in Section 2.6), and 30 min later, each mouse was put in the center of the apparatus facing towards the enclosed arms. The mice were observed for a total period of five min to calculate the percentage of entries into open arms and times passed in open arms.
2.9.2. Hole board (HB) test
The test procedure followed in this experiment observe exploratory behavior in mice using a hole board apparatus [38]. The experiment was carried out on a wooden board of 20 × 40 cm with 16 regularly arranged holes. The prepared doses (Mentioned in Section 2.6) were given to the individual mice groups, and 30 min later, each mouse was kept on the middle point of the board and counted the total number of head dipping for 5 min.
2.10. Quantitative phytochemical of MEBJ: total phenolic and flavonoid contents
Determination of total phenolic contents of MEBJ was done by following the Folin-Ciocalteu method [39] and demonstrated as mg of gallic acid equivalents. On the other hand, the determination of total flavonoid contents was done by Aluminium chloride (AlCl3) method [40] and demonstrated as mg of quercetin equivalents as we described previously [41].
2.11. Anti-diabetic activity of MEBJ by alpha amylase inhibitory assay
The alpha-amylase inhibitory test method was used to assess the anti-diabetic potential of MEBJ [42]. A solution was prepared by combining 0.3 mL of alpha-amylase solution, 0.3 mL of extract solution (various concentrations) and 0.6 mL of phosphate buffer and incubated for 15 min at 37 °C temperature. Later, 0.4 mL from test solution was added to a sample test-tube consisting of 3 mL of starch and 2 mL phosphate buffer. The mixture was further incubated for 45 min at 37 °C temperature. After incubation, the test solution (0.1 mL) was taken and added with iodine solution (10 mL), which was vortexed for the complete mixture. Finally, the absorbance was recorded at 565 nm. Percentage of inhibition was figured out using the formula given below.
| % of inhibition = [(A0-At) control – (A0-At) sample / (A0-At) control] × 100 |
where A0 and At are the values of absorbance at zero time and at the end time of incubation respectively.
2.12. Thrombolytic activity test of MEBJ
The thrombolytic test was performed following the method described by Prasad et al. [43]. Firstly, 2 mL of venous blood was withdrawn from each 10 healthy human participants with no record of anticoagulants or oral contraceptives. The Institutional Ethical Review Board endorsed the protocol followed in this experiment, and a consent paper was provided to each volunteer before the collection of the blood sample. The collected samples were transferred to pre-weighed Eppendorf tubes and incubated at 37 °C for 45 min to set up clots. After the formation of a clot, the serum from all the Eppendorf was withdrawn carefully without breaking the clot and weighed to measure (clot weight). A volume of 0.1 mL extract (different concentrations) was added to the clot and again incubated for 90 min at 37 °C. Commercially available streptokinase was taken as a positive control, and distilled water was taken as a negative control. After the final incubation period, the fluid of the upper layer from Eppendorf was removed, and the final weight was taken to calculate the difference in clot weight and measure the percentage of clot lysis.
2.13. Computational study of some isolated compounds of Bischofiajavanica: compound selection for computational study
Structures of Beta-amyrine (PubChem CID: 73145), Ursolic acid (PubChem CID: 64945), Betulinic acid (PubChem CID: 64971), Chrysoeriol (PubChem CID: 5280666), Quercetin (PubChem CID: 5280343), Friedelan-3-one (PubChem CID: 91472), Beta-sitosterol (PubChem CID: 222284), Fisetin (PubChem CID: 5281614), Cynaroside (PubChem CID: 5280637) and Triacontane (PubChem CID: 12535) were downloaded from PubChem database (www.pubchem.ncbi.nlm.nih.gov).
2.13.1. Molecular docking analysis
In this study, molecular docking experiment was accomplished using the methods and parameters as suggested previously [44]. Preparation of three-dimensional structure of downloaded compounds was operated in LigPrep module of Schrödinger maestro (LigPrep, Schrödinger, LLC, New York, NY, 2017). Conversely, the receptors used for molecular docking namely Human estrogen receptor alpha (PDB id: 3ERT) [45], Human gamma-aminobutyric acid receptor (PDB id: 4COF) [46], Potassium channel (PDB id: 4UUJ) [47], Human pancreatic alpha-amylase (PDB id: 4GQR) [48] and Human tissue plasminogen activator (PDB id: 1A5H) [49] were retrieved from RCSB Protein Data Bank [50] and prepared using protein preparation wizard in Schrödinger Maestro (Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2017). The active sites on target receptors were determined by PockDrug server, which estimates pocket druggability using ligand proximity method [51]. Finally, standard precision molecular docking was performed in Glide of Schrodinger maestro (v11.1) and expressed as glide score.
2.13.2. ADME/T analysis
The drug-like properties of docked compounds were determined based on Lipinski's rule of five [52]. As per the rule, acceptable physicochemical parameters of a compound include molecular weight ≤500, H-bond acceptor ≤10, H-bond donor ≤5, log P ≤ 5 and molar refractivity 40–130. Prediction of ADME characteristics of identified constituents from Bischofia javanica was carried out using QikProp module of Schrödinger maestro (v11.1). Furthermore, toxicity profiles including hepatotoxicity, carcinogenicity, immunotoxicity and mutagenicity of the selected compounds were evaluated using ProTox server (tox.charite.de/protox_II).
3. Results and discussion
3.1. Preliminary phytochemical screening
Plants have always been serving as an essential originator of drugs and lead compounds. A lot of potential drugs have been developed from the traditional history of medicinal plants. However, some of those plants may contain a wide range of chemical constituents, often with undetermined biological properties [53]. Therefore, the identification of active constituents in a plant can be initiated through phytochemical screening. In the current study, the qualitative phytochemical screening of MEBJ manifested the existence of carbohydrates, cholesterol, proteins, flavonoids, alkaloids, phenols, saponins, tannins, and fatty acids (data not shown).
3.2. In vitro cytotoxicity and in vivo oral acute toxicity test
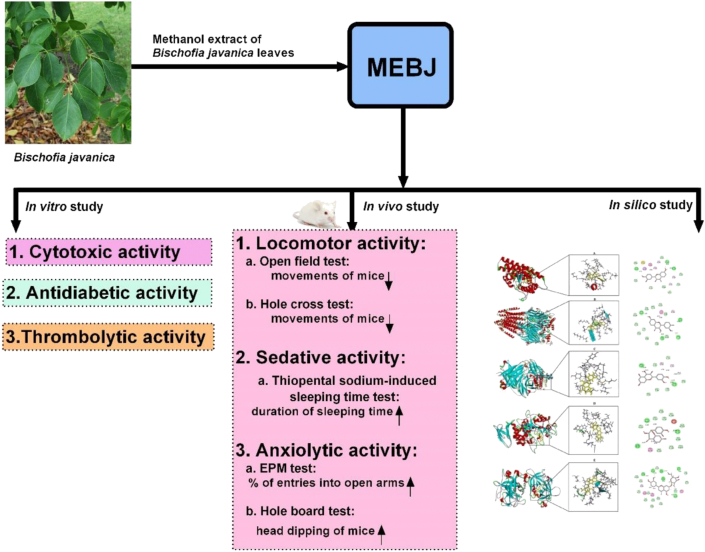
Although many phytochemicals possess extensive biological and pharmacological activities, some of the chemicals may contain mutagenic or genotoxic properties [54]. Hence, in favor of safe and effective treatment, it is of great importance to focus on toxicity evaluation [55]. One of the efficient tests for preliminary assessment of crude extract toxicity is brine shrimp lethality assay [56]. In our study, the cytotoxicity test of MEBJ exhibited an LC50 of 224.7 μg/mL, whereas the LC50 of standard drug vincristine sulphate was 39.25 μg/mL (Figure 1). According to the criteria of Clarkson [57], the outcomes from the brine shrimp lethality test revealed that the extract possesses moderate toxicity. Additionally, many studies have indicated a significant correlation between the results of the brine shrimp lethality test and acute toxicity test [55, 58]. As an alternative bioassay technique, oral acute toxicity test in mice is conducted for further verification of the toxic profile of plant extract. During acute toxicity assessment of MEBJ, no animals showed any sign of physical, behavioral or neurological changes, allergic reactions, or mortality at the highest dose of 4000 mg/kg. Therefore, the extract is safe in mice model up to the mentioned dose. Accordingly, these results signify the feasibility of the plant extract for further evaluation of its pharmacological potentials.
Figure 1.
Cytotoxic activity of MEBJ and standard drug on brine shrimp after 24 h incubation period. A) Percentage of mortality by MEBJ at different concentrations. B) Percentage of mortality by standard drug (Vincristine sulphate) at different concentrations.
3.3. Locomotor activity of MEBJ
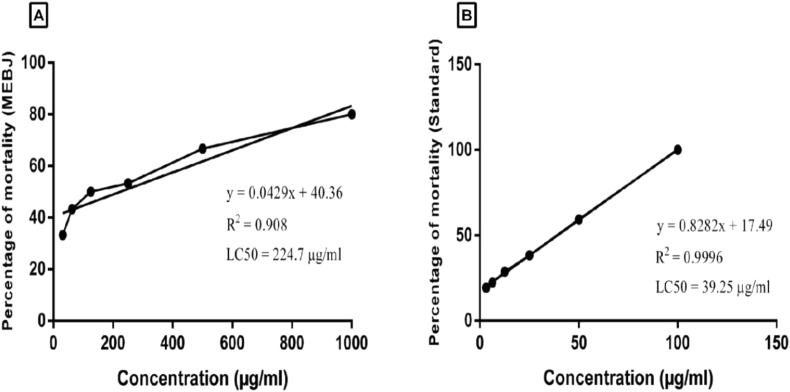
In order to assess the action of plant extract on the central nervous system, perception of locomotor activity is considered to be a remarkable method [59]. Locomotion of an experimental animal in a new environment represents its motor activity, and a downfall in the locomotor performance indicates CNS depressant action [60]. Because during active locomotion, excitatory feedback is supplied by sensory afferent neurons to spinal interneuron and motor neuron in regards to muscle constriction [59]. This feedback pathway is further inhibited by GABAergic interneuron through an allosteric moderation of GABA receptors and thus induces CNS depressant effect [61]. As reported by many researchers, open field and hole cross tests are the classic animal models to evaluate the instinctive activity of experimental mice [62]. In our study, the locomotor performance of each mouse has been decreased significantly due to the administration of high dose (400 mg/kg) of MEBJ at both open field and hole cross tests. In the open field test, MEBJ (200 and 400 mg/kg) exhibited a dose-dependent decrease in the movements of experimental animals during the total observation period (at all intervals over 120 min) (Figure 2A). Throughout the experiment, the exploration of mice was significantly (p < 0.001) reduced to 29.8 ± 1.07 and 20.6 ± 1.43 for 200 and 400 mg/kg respectively, in compared to the control (64.4 ± 1.08). In contrast, the number of movements for the standard drug (diazepam) was recorded at 13.2 ± 0.8.
Figure 2.
Effect of MEBJ on mice. A) Open field test. B) Hole cross. C) Onset of sleep on thiopental sodium-induced sleeping time test. D) Duration of sleep on thiopental sodium-induced sleeping time test. E) EPM test (percentage of entry into open arms). F) EPM test (time spent in open arms). G) Hole board test (number of head dipping). All the values are expressed as mean ± S.E.M. where ap< 0.001, bp< 0.01 significantly different from control; ANOVA followed by Dunnett's test (n = 6) per group.
In the same way, the hole cross test revealed that the number of holes crossed by the experimental mice was gradually decreased throughout the observation period (Figure 2B). The dose of 400 mg/kg was found statistically significant (P < 0.001) from 30 min to till the end when compared with the control group. Similarly, the dose of 200 mg/kg was significantly different from control at 60, 90, and 120 min. The overall number of hole cross reduced to 10.2 ± 1.16 and 2.8 ± 0.37 for 200 and 400 mg/kg respectively. This reduction in mobility is similar to the effect of CNS depressants. Therefore, it is predicted that MEBJ might function by enhancing GABAergic inhibition in the CNS through membrane hyperpolarization which eventually leads to a reduction in the firing rate of critical neurons in the brain [63, 64]. It might also be due to either increased affinity for the GABA receptor or owing to an increased GABA-gated channel opening duration [65].
3.4. Sedative activity of MEBJ by thiopental sodium (TS) induced sleeping time test
Previously, an interrelation has been reported between CNS depressant activity index and enhancement of hypnosis [66]. Drugs that hold CNS depressant effect either shorten the time for onset of sleeping or extend the total period of sleeping or both [67]. In this study, thiopental sodium induced sleeping time test was employed to evaluate the hypnosis effect of the extract on the mice. According to the results, the onset of sleeping decreased expressively after the administration of MEBJ 200 mg/kg (p < 0.01) and MEBJ 400 mg/kg (p < 0.001) (Figure 2C). However, a significant (P < 0.001) elevation in the duration of sleeping for both doses was recorded (Figure 2D). The duration of sleeping time was increased to 65.66 ± 1.65 and 101.3 ± 1.39 min at the dose of 200 and 400 mg/kg respectively when compared to the control (47.25 ± 1.94). Accordingly, it has been observed that the extract exerted its activity by reducing sleeping latency and increasing the duration of sleep. This occurrence corresponds to the features of sedative activity produced by benzodiazepines due to GABA mediated hyperpolarization of postsynaptic neurons [68].
3.5. Anxiolytic activity of MEBJ
Diazepam belongs to a class of benzodiazepine that exhibits anxiolytic-like effects along with its sedative action. In this regard, the anxiolytic potentials of MEBJ have been investigated using EPM and hole board tests. In the EPM test, drug possessing anxiolytic activity increases the percentage of entries into open arms and total time spent into open arms, whereas anxiogenic reduces in both parameters [69, 70]. In our study, both doses (200 and 400 mg/kg) of MEBJ increased the percentage of entries into open arms during the EPM test. The dose of 400 mg/kg was mostly observed as significant (p < 0.01) when compared with the control group (Figure 2E). Simultaneously, the time spent in open arms was also increased remarkably (p < 0.001) by both doses of MEBJ (Figure 2F).
The hole board test is another valuable paradigm to evaluate the anxiolytic response of mice under unfavorable conditions. The reluctance of the head dipping tendency is regarded as anxiogenic behavior of mice, whereas spontaneous exploration of visiting holes (head dipping) indicates the anxiolytic nature of plant extract [59]. During this study, the administration of MEBJ increased the tendency of head dipping significantly at the dose of 200 mg/kg (P < 0.01) and 400 mg/kg (P < 0.001) as compared with the control group (Figure 2G). The number of head dipping was noted 34.2 ± 1.85 for 200 mg/kg and 48.8 ± 1.6 for 400 mg/kg, while standard drug (diazepam) increased the head dipping to 68.2 ± 1.53. Therefore, the results from EPM and HB tests suggest that MEBJ possesses potential anxiolytic activity.
3.6. Quantitative phytochemicals: total phenolic and flavonoid content
It was reported that the sedative and anxiolytic activity of the plant extract result from its high alkaloids and flavonoids, which have a stronger binding affinity with GABA-ergic complex [71]. Binding of flavonoids to GABA type C receptors in the CNS leads to the activity of benzodiazepine-like molecules [72]. A study has found that the phenolic contents in plant extract are responsible for the CNS depressant effect [73]. During the quantitative phytochemical analysis of MEBJ, total phenolic content was found 718 ± 3.46 mg GAE/g, and the total flavonoid content was 116.67 ± 0.07 mg QE/g dried plant extract (Table 1). As our study reported high content of phenol and flavonoids in MEBJ, it is predicted that these phytoconstituents might be responsible for the observed activities in the CNS.
Table 1.
Total phenolic and flavonoid contents of MEBJ.
| Extract | Phenolic content (mg GAE/g dried extract) | Flavonoid content (mg QE/g dried extract) |
|---|---|---|
| MEBJ | 718 ± 3.46 | 116.67 ± 0.07 |
MEBJ: methanol extract of Bischofia javanica leaves; GAE: Gallic acid equivalent; QE: Quercetin equivalent.
3.7. Anti-diabetic activity by alpha amylase inhibitory assay
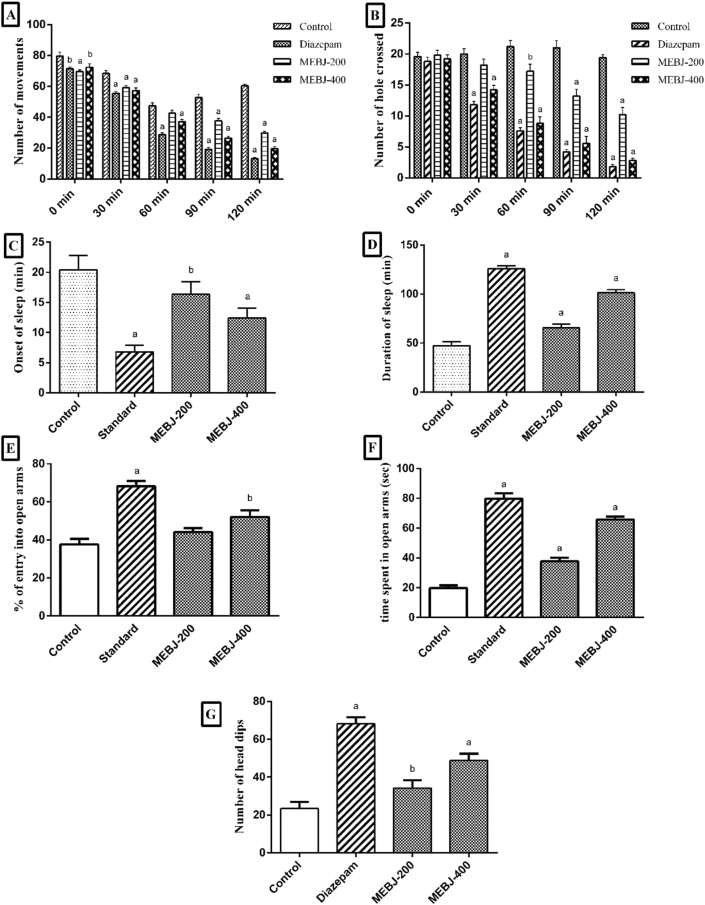
As per an investigation, it has been revealed that the symptoms of anxiety and depression are the considerable risk factors for the occurrence of type-2 diabetes [74]. Accordingly, our study aimed at exploring the anti-diabetic activity of MEBJ through alpha-amylase inhibitory assay. Alpha-amylase is an essential digestive enzyme that hydrolyzes starch into maltose, followed by breakdown into glucose [75]. After taking food with a high carbohydrate level, noticeable hyperglycemia leading to hyperinsulinemia is observed [76, 77]. Therefore, high postprandial blood glucose levels in diabetes can be reduced by inhibiting the alpha-amylase. In this study, MEBJ was found to inhibit alpha-amylase enzyme in a dose-dependent manner. Calculated IC50 of MEBJ was 24.56 μg/mL, and that of the standard drug (Acarbose) was 11.7 μg/mL (Figure 3). As the inhibition rate significantly increased along with the concentration, this result suggests that the extract possesses potential anti-diabetic activity.
Figure 3.
Inhibition of alpha amylase by MEBJ and Acarbose at different concentrations. A) Percentage of alpha amylase inhibition by MEBJ. B) Percentage of alpha amylase inhibition by standard drug (Acarbose).
3.8. Thrombolytic activity test
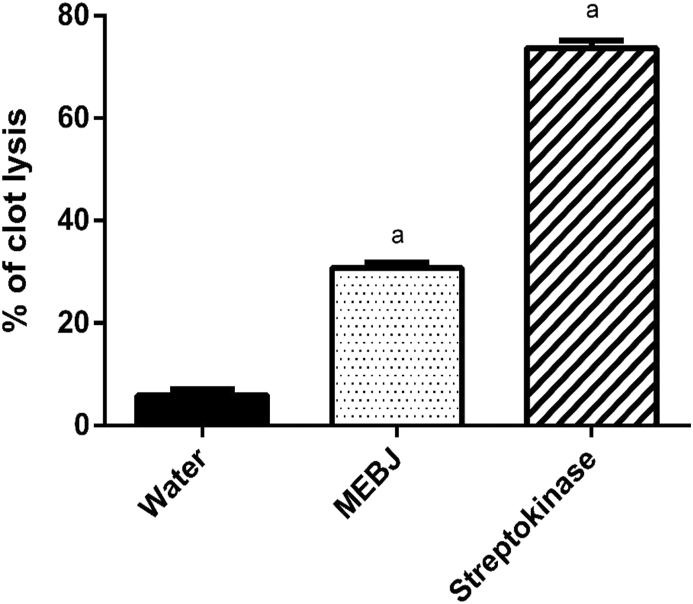
Thrombosis is a critical event caused by plaque generation in a blood vessel due to platelets, tissue factors and fibrin deposition [78]. Plaque formation and growth occur when activated platelets interact with leucocytes [79]. However, thrombolytic agents can disrupt the fibrinogen and fibrin in a clot, which leads to clot lysis. Although streptokinase is a well-known thrombolytic agent, it has several deleterious effects, including embolism and bleeding [80]. As a result, many studies have been conducted to overcome these complexities by discovering novel sources of thrombolytic agents with less adverse effects [81]. In our experiment, MEBJ showed a significant percentage of clot lysis when compared with the control. Streptokinase and MEBJ exhibited 73.71 ± 1.45% and 30.76 ± 1.01% clot lysis, respectively, significantly different from the negative control with negligible clot lysis of 5.93 ± 1.13% (Figure 4). As per the previous report, phytochemicals like alkaloids, saponins, and tannins might be engaged in the thrombolytic activity of plant extract [82]. Thus, the presence of the mentioned phytochemicals in MEBJ is thought to be responsible for its antithrombotic activity.
Figure 4.
Clot lysis percentage of water, MEBJ and streptokinase. Values are expressed as mean ± SEM (n = 6); ap< 0.0001, Tukey's test as compared with control.
3.9. Computational study
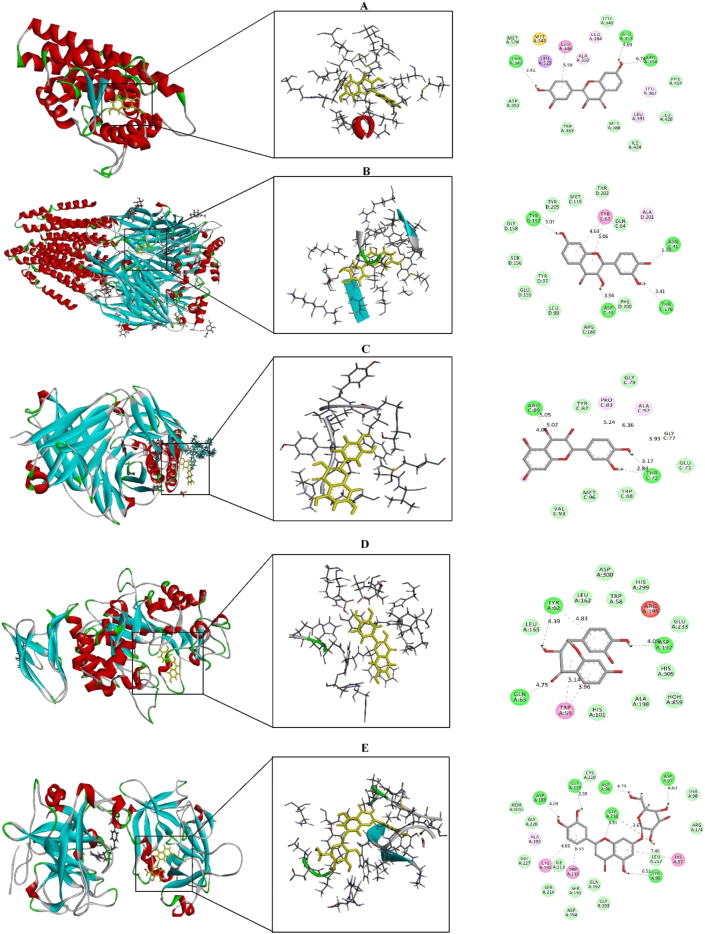
A computational study (molecular docking and ADME/T study) has been performed to understand the observed pharmacological activities of MEBJ thoroughly. In silico molecular docking provides information about the relative orientation of a ligand molecule when it is bound to a protein [83]. Such information can be used to discover novel compounds with more potency and selectivity. In this study, 10 chemical constituents of Bischofia javanica leaves were subjected to molecular docking study against human estrogen receptor alpha, human gamma-aminobutyric acid (GABA) receptor, potassium channel, human pancreatic alpha-amylase and human tissue plasminogen activator. The docking analysis revealed that fisetin has the highest binding affinity with three receptors that are responsible for cytotoxic (-8.262 kcal/mol), sedative (-8.013 kcal/mol) and anti-diabetic (-7.653 kcal/mol) activity. On the other hand, quercetin was found to possess the highest docking score (-5.751 kcal/mol) when bound with human pancreatic alpha-amylase enzyme regarding anti-diabetic activity. Lastly, cynaroside exhibits the best binding affinity (-8.438 kcal/mol) from the interaction with human tissue plasminogen activator. Docking scores of these compounds are higher than the standard drugs used that are Tamoxifen (-4.819 kcal/mol) as cytotoxic, Diazepam (-6.84 and -3.73 kcal/mol) for sedative and anxiolytic respectively, Acarbose (-7.206 kcal/mol) as antidiabetic and Streptokinase (-4.178 kcal/mol) as thrombolytic. All docking scores are presented in Table 2, and the best interaction figures are demonstrated in Figure 5. Interaction analysis of best-docked compounds, namely fisetin, quercetin and cymaroside, showed several hydrogen and hydrophobic bonds against the receptors used (Table 3). The binding analysis suggests that fisetin binds with human estrogen receptor alpha by forming three hydrogen bonds (Thr-347, Glu-353, and Arg-394) and a hydrophobic bond (Leu-346). Besides, fisetin also demonstrated four hydrogen bonds (Tyr-157, Asn-41, Thr-176, and Asp-43) and one hydrophobic interaction (Tyr-62) against human gamma-aminobutyric acid (GABA) receptor whereas, interactions of fisetin with human pancreatic alpha-amylase enzyme exhibited three hydrogen bond interactions (Tyr-62, Asp197, and Gln-63) and three hydrophobic bond interactions (Tyr-62 and Trp-59). Similarly, quercetin binds with the potassium channel by three hydrogen interactions to Arg-89 and Thr-72, along with four hydrophobic bonds to Pro-83, Ala-92, and Arg-89. Finally, against human tissue plasminogen activator, the best interacted compound cynaroside binds through six hydrogen bond interactions (Asp-189, Gly-219, Asp-96, Tyr-99, and Gly-216) and three hydrophobic interactions (Cys-191, Trp-215, and His-57). The interactions between ligand and receptor showed that the isolated compounds have relatively shorter and stronger bonds. These results suggest that fisetin, quercetin and cynaroside are the best compounds for the tested pharmacological activities.
Table 2.
Molecular docking scores of isolated compounds from MEBJ. The values with Bold represent highest score.
| Compounds | Docking Score (kcal/mol) |
||||
|---|---|---|---|---|---|
| 3ERT (Cytotoxic) | 4COF (Sedative) | 4UUJ (Anxiolytic) | 4GQR (Anti-diabetic) | 1A5H(Thrombolytic) | |
| Beta-amyrine | -5.067 | - | -2.734 | -5.683 | -4.306 |
| Ursolic acid | -4.578 | - | -1.593 | -4.435 | -4.385 |
| Betulinic acid | -5.096 | - | -2.986 | -4.774 | -3.588 |
| Chrysoeriol | -7.2 | -7.151 | -5.328 | -5.785 | -6.322 |
| Quercetin | -7.174 | -7.362 | -5.751 | -7.63 | -7.238 |
| Friedelan-3-one | -4.816 | - | -1.824 | -5.326 | -4.241 |
| Beta-sitosterol | - | - | -3.639 | -4.932 | -3.124 |
| Fisetin | -8.262 | -8.013 | -5.237 | -7.653 | -7.044 |
| Cynaroside | -6.431 | -7.026 | -4.057 | -6.868 | -8.438 |
| Triacontane | -3.879 | - | -2.865 | - | -2.031 |
| Standard (Tamoxifen /Diazepam/Acarbose/ Streptokinase) |
-4.819 | -6.84 | -3.73 | -7.206 | -4.178 |
Figure 5.
Molecular docking interaction between best docked compounds from MEBJ and selected receptors. (A) Fisetin against 3ERT, (B) Fisetin against 4COF, (C) Quercetin against 4UUJ, (D) Fisetin against 4GQR and (E) Cynaroside against 1A5H.
Table 3.
Ligand binding interactions of best docked compounds with selected receptors.
| Proteins | Ligands | Hydrogen bond interactions |
Hydrophobic interactions |
||
|---|---|---|---|---|---|
| Amino acid residue | Distance (Å) | Amino acid residue | Distance (Å) | ||
| 3ERT | Fisetin | Thr-347 | 3.41 | Leu-346 | 5.58 |
| Glu-353 | 4.69 | ||||
| Arg-394 | 6.74 | ||||
| 4COF | Fisetin | Tyr-157 | 3.01 | Tyr-62 | 4.63 |
| Asn-41 | 5.10 | 5.06 | |||
| Thr-176 | 3.41 | ||||
| Asp-43 | 3.94 | ||||
| 4UUJ | Quercetin | Arg-89 | 5.05 | Pro-83 | 5.24 6.36 5.02 4.06 |
| Thr-72 | 3.17 | Ala-92 | |||
| 2.84 | Arg-89 | ||||
| 4GQR | Fisetin | Tyr-62 | 4.39 | Tyr-62 | 4.83 |
| Asp-197 | 4.09 | Trp-59 | 5.14 | ||
| Gln-63 | 4.75 | 3.96 | |||
| 1A5H | Cynaroside | Asp-189 | 4.09 | Cys-191 | 4.66 |
| Gly-219 | 3.59 | Trp-215 | 6.53 | ||
| Asp-96 | 4.79 | His-57 | 7.46 | ||
| Tyr-99 | 6.51 | ||||
| Gly-216 | 3.91 | ||||
| 3.67 | |||||
In addition, pharmacokinetic (ADMET) and toxicological properties of a compound are essential parameters during the process of novel drug discovery. Our study demonstrated the physicochemical properties of the compounds from Bischofia javanica leaves are shown in Table 4. No compounds have been found to violate more than two parameters of Lipinski's rules of five. Moreover, three compounds (Figure 6), namely chrysoeriol, quercetin and fisetin, exhibit no violations. Additionally, toxicological characteristics of studied constituents were predicted (Table 5), which suggests that 9 out of all tested compounds are non-hepatotoxic and non-mutagenic. Particularly, chrysoeriol, fisetin, and triacontane showed no toxicity (hepatotoxicity, carcinogenicity, immunotoxicity and mutagenicity) at all while ursolic acid and betulinic acid were predicted to be carcinogenic and immunotoxic. The rest of the predicted compounds either showed carcinogenicity or immunotoxicity.
Table 4.
Physicochemical properties of identified compounds from MEBJ.
| Compounds | Lipinski's rules |
Lipinski's violations |
||||
|---|---|---|---|---|---|---|
| MW |
HBA |
HBD |
Log P |
MR |
||
| <500 | <10 | ≤5 | ≤5 | 40–130 | ≤1 | |
| Beta-amyrine | 426.72 | 1 | 1 | 7.18 | 134.88 | 2 |
| Ursolic acid | 456.70 | 3 | 2 | 5.94 | 136.91 | 2 |
| Betulinic acid | 456.70 | 3 | 2 | 6.11 | 136.91 | 2 |
| Chrysoeriol | 300.26 | 6 | 3 | 2.18 | 80..48 | 0 |
| Quercetin | 302.24 | 7 | 5 | 1.23 | 78.03 | 0 |
| Friedelan-3-one | 426.72 | 1 | 0 | 7.45 | 134.39 | 2 |
| Beta-sitosterol | 414.71 | 1 | 1 | 7.19 | 133.23 | 2 |
| Fisetin | 286.24 | 6 | 4 | 1.55 | 76.01 | 0 |
| Cynaroside | 448.38 | 11 | 7 | 0.16 | 108.13 | 2 |
| Triacontane | 422.81 | 0 | 0 | 11.51 | 146.32 | 1 |
MW, Molecular weight (g/mol); HBA, Hydrogen bond acceptor; HBD, Hydrogen bond donor; Log P, Lipophilicity; MR, Molar refractivity.
Figure 6.
Chemical structures of potential bioactive compounds identified in the MEBJ.
Table 5.
Toxicological properties of identified compounds from MEBJ.
| Compounds | Parameters |
|||
|---|---|---|---|---|
| Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | |
| Beta-amyrine | Inactive | Inactive | Active | Inactive |
| Ursolic acid | Active | Active | Active | Inactive |
| Betulinic acid | Inactive | Active | Active | Inactive |
| Chrysoeriol | Inactive | Inactive | Inactive | Inactive |
| Quercetin | Inactive | Active | Inactive | Active |
| Friedelan-3-one | Inactive | Inactive | Active | Inactive |
| Beta-sitosterol | Inactive | Inactive | Active | Inactive |
| Fisetin | Inactive | Active | Inactive | Inactive |
| Cynaroside | Inactive | Inactive | Inactive | Inactive |
| Triacontane | Inactive | Inactive | Inactive | Inactive |
Accordingly, toxicological analysis shows that all the compounds are at low risk of hepatotoxicity and mutagenicity. Thus, these compounds can be considered as potential lead compounds having acceptable oral bioavailability with minimal toxicity.
4. Conclusion
To summarize, our findings indicate that MEBJ possesses moderate cytotoxic properties with remarkable activity on the central nervous system leading to sedative and anxiolytic effects. These activities were thought to be due to the presence of phenol and flavonoid contents perceived through our experiments. Besides, in vitro tests suggest that the extract holds significant potentiality on inhibiting alpha-amylase enzyme as well as offering a notable percentage of clot lysis activity. Additionally, in silico evaluations of the identified compounds from MEBJ, three compounds, namely fisetin, quercetin, and cymaroside, might be responsible for the activities mentioned earlier as they exhibit effective binding to the target receptors. Overall, the observed potentialities can make this plant as a prospective candidate for the food and pharmaceutical industries. However, further investigations are required to elucidate the bioactive constituents’ mechanisms of in an animal model with a dose-response study.
Declarations
Author contribution statement
Md. Adnan and I. Mahmud: Analyzed and interpreted the data; Wrote the paper.
Md. R. Chowdhury: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
K.H. Chowdhury, N.B. Hanif and J. Mouah: Performed the experiments; Wrote the paper.
M.A. Sayeed and A.T.M. Mostafa Kamal: Contributed reagents, materials, analysis tools or data.
Md. N.U. Chy: Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are obliged to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh for providing all the laboratory facilities to complete this study. The authors are also thankful to Dr. Shaikh Bokhtear Uddin for his kind co-operation in the identification of this plant.
Contributor Information
Mohammed Abu Sayeed, Email: sayeed@iiuc.ac.bd.
Md. Adnan, Email: mdadnan@kangwon.ac.kr, mdadnan1991.pharma@gmail.com.
References
- 1.Mwonga K.B., waniki N.E., Dorcas Y.S., Piero N.M. Molluscicidal effects of aqueous extracts of selected medicinal plants from Makueni county, Kenya. Pharm. Anal. Acta. 2015 [Google Scholar]
- 2.Hassan B., Hassan R. 2012. Medicinal Plants (Importance and Uses) Pharmaceutica Analytica Acta. [Google Scholar]
- 3.Chy M.N.U., Adnan M., Rauniyar A.K., Amin M.M., Majumder M., Islam M.S., Afrin S., Farhana K., Nesa F., Sany M.A. Evaluation of anti-nociceptive and anti-inflammatory activities of Piper sylvaticum (Roxb.) stem by experimental and computational approaches. Orient. Pharm. Exp. Med. 2019:1–15. [Google Scholar]
- 4.Tilburt J.C., Kaptchuk T.J. Herbal medicine research and global health: an ethical analysis. Bull. World Health Organ. 2008;86:594–599. doi: 10.2471/BLT.07.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson B.P. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, Edited and Translated from the Second German Edition by Norman Grainger Bisset, London; German Edition Edited by Max Wichtl, Marburg. With a Foreword by J. David Phillip, Flavour Fragr. J. 1995. [Google Scholar]
- 6.Kimiskidis V.K., Triantafyllou N.I., Kararizou E., Gatzonis S.-S., Fountoulakis K.N., Siatouni A., Loucaidis P., Pseftogianni D., Vlaikidis N., Kaprinis G.S. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann. Gen. Psychiatr. 2007;6:28. doi: 10.1186/1744-859X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therapeutic and Pharmacological Potential of Foeniculum Vulgare Mill: a Review. 2020. [Google Scholar]
- 8.Ali H., Houghton P.J., Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006 doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Hasanat A., Kabir M.S.H., Ansari M.A., Chowdhury T.A., Hossain M.M., Islam M.N., Ahmed S., Chy M.N.U., Adnan M., Kamal A.T.M.M. Ficus cunia Buch.-Ham. ex Roxb. (leaves): an experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J. Basic Clin. Physiol. Pharmacol. 2019;30 doi: 10.1515/jbcpp-2016-0140. [DOI] [PubMed] [Google Scholar]
- 10.Mukhametova L.I., Aisina R.B., Zakharyan E.M., Karakhanov E.A., Gershkovich K.B., Varfolomeyev S.D. Thrombolytic and fibrinogenolytic properties of bioconjugate streptokinase-polyamidoamine dendrimers in vitro. Thromb. Res. 2017;154:50–52. doi: 10.1016/j.thromres.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 11.V Menon V., Fazal E., Mary Y.S., Panicker C.Y., Armaković S., Armaković S.J., Nagarajan S., Van Alsenoy C. FT-IR, FT-Raman and NMR characterization of 2-isopropyl-5-methylcyclohexyl quinoline-2-carboxylate and investigation of its reactive and optoelectronic properties by molecular dynamics simulations and DFT calculations. J. Mol. Struct. 2017;1127:124–137. [Google Scholar]
- 12.Sureshkumar B., Mary Y.S., Resmi K.S., Panicker C.Y., Armaković S., Armaković S.J., Van Alsenoy C., Narayana B., Suma S. Spectroscopic analysis of 8-hydroxyquinoline derivatives and investigation of its reactive properties by DFT and molecular dynamics simulations. J. Mol. Struct. 2018;1156:336–347. [Google Scholar]
- 13.Mary Y.S., Miniyar P.B., Mary Y.S., Resmi K.S., Panicker C.Y., Armaković S., Armaković S.J., Thomas R., Sureshkumar B. Synthesis and spectroscopic study of three new oxadiazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical potential. J. Mol. Struct. 2018;1173:469–480. [Google Scholar]
- 14.Auniq R., Chy M., Adnan M., Roy A., Islam M., Khan T., Hasan M., Ahmed S., Khan M., Islam N., Khan M., Hossain M., Kabir M., Mukut M., Islam S. Assessment of anti-nociceptive and anthelmintic activities of Vitex Peduncularis Wall. leaves and in silico molecular docking, ADME/T, and PASS prediction studies of its isolated compounds. J. Complement. Med. Res. 2019;10:170. [Google Scholar]
- 15.V.C.-H.M. Publisher, undefined . 1999. Dictionary of Vietnamese Medicinal Plants. [Google Scholar]
- 16.Sastri B.N.C. The wealth of India. A dictionary of Indian raw materials and industrial products. Raw materials., wealth India. A dict. Indian raw mater. Ind. Prod. Raw Mater. 1950 [Google Scholar]
- 17.Kanjilal U. volume I. 2013. (Flora of Assam). [Google Scholar]
- 18.Barwick M., Van der Schans A., Barwick Claudy J. Thames & Hudson; 2004. Tropical & Subtropical Trees : a Worldwide Encyclopaedic Guide. [Google Scholar]
- 19.Perry L.M., Metzger J. Medicinal plants of East and Southeast Asia: attributed properties and uses. Med. Plants East Southeast Asia Attrib. Prop. Uses. 1980 [Google Scholar]
- 20.George L. 1989. Tongan Herbal Medicine. [Google Scholar]
- 21.Sutharson L., Prasanna K., Lila K., Shila E., J.R.- Pharmacologyonline, undefined . 2009. Free Radical Scavenging Activity of Leaves of Bischofia Javanica Blume and Fraxinus Floribunda Wallich. [Google Scholar]
- 22.Ignacimuthu S., Ayyanar M., Sivaraman S.K. Ethnobotanical investigations among tribes in Madurai district of Tamil nadu (India) J. Ethnobiol. Ethnomed. 2006;2 doi: 10.1186/1746-4269-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.S.N.T.N. 3 , N.B., H.K. and K.K. Yohannes Alen, Antinematodal Activity of Some Tropical Rainforest Plants against the Pinewood Nematode, Bursaphelenchus xylophilus , (n.d.). [DOI] [PubMed]
- 24.Khan M.R., Kihara M., Omoloso A.D. Anti-microbial activity of Bidens pilosa, Bischofia javanica, Elmerillia papuana and Sigesbekia orientalis. Fitoterapia. 2001;72:662–665. doi: 10.1016/s0367-326x(01)00261-1. [DOI] [PubMed] [Google Scholar]
- 25.Lingadurai S., Roy S., Joseph R., Nath L. Antileukemic activity of the leaf extract of Bischofia javanica blume on human leukemic cell lines. Indian J. Pharmacol. 2011;43:143–149. doi: 10.4103/0253-7613.77348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingadurai S., Nath L., P.K.-A.J. of, undefined Antiinflammatory and antinociceptive activities of methanolic extract of leaves of Bischofia javanica Blume on experimental animals. Asian J. Chem. 2007 [Google Scholar]
- 27.Gupta D., Dhiman R., Naithani S., Pharmazie B.A., undefined . 1988. Chemical Investigation of Bischofia Javanica Blume., Cabdirect.Org. [Google Scholar]
- 28.Mai N.T. AN initial study ON chemical constituents OF bischofia javanica. J. Sci. Technol. 2017;55:188–194. [Google Scholar]
- 29.Pandey A., Kaushik A., S.T.-J.P.B. Sci, undefined . Evaluation of Antimicrobial Activity and Phytochemical Analysis of Citrus limon. 2011. [Google Scholar]
- 30.Chowdhury K.A.A., Hosen S.M.Z., Islam M.N., Huq I., Adnan M., Chy M.N.U., Kabir M.I., Auniq R.B.J., Uddin M.R., Shoibe M. Cytotoxic and thrombolytic activity of roots of Musa paradisiaca (Linn) Pharma Innov. 2016;5:97. [Google Scholar]
- 31.Chowdhury K.A.A., Huq M.E., Ali M.S., Huq I., Royhan M.J., Adnan M., Chy M.N.U., Kabir M.I., Auniq R.B.J., Uddin M.R. Antioxidant, cytotoxic and thrombolytic activity of leaves of Kalanchoe pinnata (LAM.) PERS. J. Pharmacogn. Phytochem. 2016;5:309. [Google Scholar]
- 32.Nichols D.E. Brine shrimp: a convenient general bioassay for active plant constituents. Thieme-Connect.Com. 1982;45:31–34. [PubMed] [Google Scholar]
- 33.Nih O., Oer O. Animals Institute for Laboratory Animal Research Division on …, Natl. Acad. Press; 2020. OF Eighth Edition Committee for the Update of the Guide for the Care and Use of Laboratory. [Google Scholar]
- 34.Kulkarni S. 2020. D.R.-M. And Findings in Experimental, Undefined 1996, Animal Behavioral Models for Testing Antianxiety Agents, Pascal-Francis.Inist.Fr. [PubMed] [Google Scholar]
- 35.Takagi K., Watanabe M., Saito H. Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Jpn. J. Pharmacol. 1971;21:797–810. doi: 10.1254/jjp.21.797. [DOI] [PubMed] [Google Scholar]
- 36.Ferrini R., F. R, M. G, T. B . 1974. Neuro-Pharmacological Studies on SB 5833, a New Psychotherapeutic Agent of the Benzodiazepine Class, Neuro-Pharmacological Stud. SB 5833, A New Psychother. Agent Benzodiazepine Cl. [PubMed] [Google Scholar]
- 37.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 38.Sonavane G.S., Sarveiya V.P., Kasture V.S., Kasture S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002;71:239–244. doi: 10.1016/s0091-3057(01)00660-8. [DOI] [PubMed] [Google Scholar]
- 39.Harborne A. 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 40.Aiyegoro O.A., Okoh A.I. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoibe M., Chy M.N.U., Alam M., Adnan M., Islam M.Z., Nihar S.W., Rahman N., Suez E. In vitro and in vivo biological activities of cissus adnata (roxb.) Biomedicines. 2017;5 doi: 10.3390/biomedicines5040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansawasdi C., Kawabata J. T.K.- Bioscience, Undefined biotechnology, undefined 2000, Bioscience, Biotechnology, and Biochemistry. Taylor Fr. 2014;64:1041–1043. doi: 10.1271/bbb.64.1041. [DOI] [PubMed] [Google Scholar]
- 43.Prasad S., Kashyap R.S., Deopujari J.Y., Purohit H.J., Taori G.M., Daginawala H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006;4:14. doi: 10.1186/1477-9560-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adnan M., Chy N.U., Mostafa Kamal A.T.M., Azad M.O.K., Paul A., Uddin S.B., Barlow J.W., Faruque M.O., Park C.H., Cho D.H. Investigation of the biological activities and characterization of bioactive constituents of ophiorrhiza rugosa var. prostrata (D. Don) & Mondal leaves through in vivo, in vitro, and in silico approaches. Molecules. 2019;24:1367. doi: 10.3390/molecules24071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiau A.K., Barstad D., Loria P.M., Cheng L., Kushner P.J., Agard D.A., Greene G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 46.Crystal structure of human GABAA receptor. Sci. Exch. 2014;7 doi: 10.1038/nature13293. 778–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenaeus M.J., Burdette D., Wagner T., Focia P.J., Gross A. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry. 2014;53:5365–5373. doi: 10.1021/bi500525s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams L.K., Li C., Withers S.G., Brayer G.D. Order and disorder: differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J. Med. Chem. 2012;55:10177–10186. doi: 10.1021/jm301273u. [DOI] [PubMed] [Google Scholar]
- 49.Renatus M., Bode W., Huber R., Stürzebecher J., Prasa D., Fischer S., Kohnert U., Stubbs M.T. Structural mapping of the active site specificity determinants of human tissue-type plasminogen activator: implications for the design of low molecular weight substrates and inhibitors. J. Biol. Chem. 1997;272:21713–21719. doi: 10.1074/jbc.272.35.21713. [DOI] [PubMed] [Google Scholar]
- 50.Berman H.M., Kleywegt G.J., Nakamura H., Markley J.L. The Protein Data Bank archive as an open data resource. J. Comput. Aided Mol. Des. 2014;28:1009–1014. doi: 10.1007/s10822-014-9770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussein H.A., Borrel A., Geneix C., Petitjean M., Regad L., Camproux A.-C. PockDrug-Server: a new web server for predicting pocket druggability on holo and apo proteins. Nucleic Acids Res. 2015;43 doi: 10.1093/nar/gkv462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Konan N.A., Bacchi E.M., Lincopan N., Varela S.D., Varanda E.A. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.) J. Ethnopharmacol. 2007;110:30–38. doi: 10.1016/j.jep.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Çelik T.A., Aslantürk Ö.S. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia (Bratisl) 2007;62:292–296. [Google Scholar]
- 55.Lagarto Parra A., Silva Yhebra R., Guerra Sardiñas I., Iglesias Buela L. Comparative study of the assay of Artemia salina L. And the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 56.Hamidi М.R., Jovanova B., Panovska K. 2014. Toxicоlogical Evaluation of the Plant Products Using Brine Shrimp (Artemia salina L.) Model. [Google Scholar]
- 57.Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Arslanyolu M., Erdemgil F.Z. Evaluation of the antibacterial activity and toxicity of isolated arctiin from the seeds of Centaurea sclerolepis. Ankara Univ. Eczac. Fak. Derg. 2006;35:103–109. [Google Scholar]
- 59.Adnan M., Chy M., Uddin N., Kama A.T.M., Azad M., Kalam O., Chowdhury K.A.A., Kabir M.S.H., Das Gupta S., Chowdhury M. Comparative study of piper sylvaticum roxb. Leaves and stems for anxiolytic and antioxidant properties through in vivo, in vitro, and in silico approaches. Biomedicines. 2020;8:68. doi: 10.3390/biomedicines8040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X.Y., Zhao J.L., Zhang M., Li F., Zhao T., Yang L.Q. Sedative, hypnotic and anticonvulsant activities of the ethanol fraction from Rhizoma Pinelliae Praeparatum. J. Ethnopharmacol. 2011;135:325–329. doi: 10.1016/j.jep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Sultana T., Mannan M.A., Ahmed T. Evaluation of central nervous system (CNS) depressant activity of methanolic extract of Commelina diffusa Burm. in mice. Clin. Phytosci. 2018;4:1–7. [Google Scholar]
- 62.Sousa F.C.F., Melo C.T.V., Monteiro A.P., Lima V.T.M., Gutierrez S.J.C., Pereira B.A., Barbosa-Filho J.M., Vasconcelos S.M.M., Fonteles M.F., Viana G.S.B. Antianxiety and antidepressant effects of riparin III from Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol. Biochem. Behav. 2004;78:27–33. doi: 10.1016/j.pbb.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 63.Adnan M., Chy M.N.U., Kamal A.T.M.M., Chowdhury K.A.A., Rahman M.A., Reza A.S.M.A., Moniruzzaman M., Rony S.R., Nasrin M.S., Azad M.O.K., Park C.H., Lim Y.S., Cho D.H. Intervention in neuropsychiatric disorders by suppressing inflammatory and oxidative stress signal and exploration of in silico studies for potential lead compounds from holigarna caustica (dennst.) oken leaves. Biomolecules. 2020;10:561. doi: 10.3390/biom10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gahlot K., Lal V.K., Jha S. Anticonvulsant potential of ethanol extracts and their solvent partitioned fractions from Flemingia strobilifera root. Pharmacogn. Res. 2013;5:265–270. doi: 10.4103/0974-8490.118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shams-Ud-Doha K.M., Al Mahmud Z., Bachar S.C., Qais N. Antinociceptive, anti-inflammatory, antimicrobial and central nervous system depressant activities of ethanolic extract of leaves and roots of Gomphostemma parviflorum var. parviflorum wall. Pharmacogn. Res. 2013;5:233–240. doi: 10.4103/0974-8490.118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujimori H. Potentiation of barbital hypnosis as an evaluation method for central nervous system depressants. Psychopharmacologia. 1965;7:374–378. doi: 10.1007/BF00403761. [DOI] [PubMed] [Google Scholar]
- 67.Nyeem M.A.B., Alam M.A., Awal M.A., Mostofa M., Uddin S.J., Islam N., Rouf R. 2006. CNS Depressant Effect of the Crude Ethanolic Extract of the Flowering Tops of Rosa Damascena. [Google Scholar]
- 68.Huang F., Xiong Y., Xu L., Ma S., Dou C. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. J. Ethnopharmacol. 2007;110:471–475. doi: 10.1016/j.jep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Adnan M., Chy M.N.U., Kamal A.T.M.M., Chowdhury M.R., Islam M.S., Hossain M.A., Tareq A.M., Bhuiyan M.I.H., Uddin M.N., Tahamina A., Azad M.O.K., Lim Y.S., Cho D.H. Unveiling pharmacological responses and potential targets insights of identified bioactive constituents of cuscuta reflexa roxb. Leaves through in vivo and in silico approaches. Pharmaceuticals. 2020;13:50. doi: 10.3390/ph13030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griebel G., Belzung C., Perrault G., Sanger D.J. Differences in anxiety-related behaviors and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 71.Kahnberg P., Lager E., Rosenberg C., Schougaard J., Camet L., Sterner O., Nielsen E.Ø., Nielsen M., Liljefors T. Refinement and evaluation of a pharmacophore model for flavone derivatives binding to the benzodiazepine site of the GABAA receptor. J. Med. Chem. 2002;45:4188–4201. doi: 10.1021/jm020839k. [DOI] [PubMed] [Google Scholar]
- 72.Hasan S.M.R., Hossain M.M., Akter R., Jamila M., Mazumder H., Rahman S. 2009. Sedative and Anxiolytic Effects of Different Fractions of the Commelina Benghalensis Linn. [PubMed] [Google Scholar]
- 73.Mombeini T., Gholami Pourbadie H., Kamalinejad M., Mazloumi S., Dehpour A.R. Anxiolytic-like and sedative effects of Alcea Aucheri (Boiss.) Alef. Flower extract in the laboratory rat. Iran. J. Pharm. Res. IJPR. 2017;16:1495–1508. [PMC free article] [PubMed] [Google Scholar]
- 74.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J. Psychosom. Res. 2007;62:31–38. doi: 10.1016/j.jpsychores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Mahomoodally M.F., Subratty A.H., Gurib-Fakim A., Choudhary M.I., Nahar Khan S. The cientificWorldJOURNAL research article traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo. Sci. World J. 2012;2012 doi: 10.1100/2012/285284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prashanth D., Padmaja R., Samiulla D.S. Effect of certain plant extracts on α-amylase activity. Fitoterapia. 2001;72:179–181. doi: 10.1016/s0367-326x(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 77.Youn J.Y., Park H.Y., Cho K.H. Diabetes Res. Clin. Pract. Elsevier; 2004. Anti-hyperglycemic activity of Commelina communis L.: inhibition of α-glucosidase; pp. S149–S155. [DOI] [PubMed] [Google Scholar]
- 78.Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 79.Das A., Dewan S.M.R., Ali M.R., Debnath P.C., Billah M.M. Investigation of in vitro thrombolytic potential of ethanolic extract of Momordica charantia fruits: an anti-diabetic medicinal plant. Der Pharm. Sin. 2013 [Google Scholar]
- 80.Bhowmick R., Sarwar M.S., Dewan S.M.R., Das A., Das B., Uddin M.M.N., Islam M.S., Islam M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res. 2014;47 doi: 10.1186/0717-6287-47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masudur S., Dewan R. 2013. Investigation of in Vitro Thrombolytic Potential and Phytochemical Nature of Crinum Latifolium L. Leaves Growing in Coastal Region of Bangladesh. [Google Scholar]
- 82.Chowdhury N.S., Alam M.B., Haque A.S.M.T., Zahan R., Ehsanul M., Mazumder H., Haque M.E. In vitro free radical scavenging and thrombolytic activities of Bangladeshi aquatic plant Aponogeton undulatus roxb. Glob. J. Pharmacol. 2011;5:27–32. [Google Scholar]
- 83.Taylor R.D., Jewsbury P.J., Essex J.W. A review of protein-small molecule docking methods. J. Comput. Aided Mol. Des. 2002;16:151–166. doi: 10.1023/a:1020155510718. [DOI] [PubMed] [Google Scholar]