Abstract
Purpose
Statins are widely prescribed medications for treatment of dyslipidemia and prevention of cardiovascular disease. Beyond their lipid-lowering property, statins exhibit multiple pleiotropic and antimicrobial effects. We aimed to investigate the effect of statins on the long-term risk of pneumonia after acute ischemic stroke.
Methods
This retrospective observational research was performed using South Korean National Health Insurance Service claim data, which consist of population-based random sampling. We included patients discharged with acute ischemic stroke (I63 in the ICD10) and no prior history of pneumonia. The primary outcome measure was the occurrence of pneumonia determined based on ICD10 code J09–J18. Treatment with statins during follow-up was collected as a time-dependent variable based on prescription records.
Results
A total of 7,001 subjects with acute ischemic stroke and no prior history of pneumonia were included. During the mean 3.96-year follow-up, pneumonia occurred in 1,715 subjects (24.5%). On multivariate time-dependent Cox proportional hazard–regression analyses, significant preventive benefit of treatment with statins against pneumonia was noted (adjusted HR 0.86, 95% CI 0.77–0.97). Compared to no use of statin, adjusted HRs (95% CIs) for current use of low–intermediate high-intensity statins were 0.88 (0.78–0.99) and 0.49 (0.27–0.87), respectively.
Conclusion
Our retrospective national cohort study found reduced risk of poststroke pneumonia with statin therapy after acute ischemic stroke. Our study suggests that treatment with statins may have a preventive effect against the common complication of poststroke pneumonia.
Keywords: statin, pneumonia, infection, stroke
Introduction
Stroke victims are at particularly increased risk of acquiring pneumonia, a critical cause of death after stroke.1 Stroke victims frequently experience dysphagia leading to aspiration of food and development of pneumonia. Additionally, not only older age but also malnutrition and limited physical activity due to stroke are well-known risk factors for developing pneumonia.2,3 Because poststroke pneumonia is fatal and a marker of worse prognosis,4 identification of risk factors and development of preventive strategies for poststroke pneumonia are clinically important in stroke victims.5,6
Statins are widely prescribed medications for treatment of dyslipidemia and prevention of cardiovascular disease. Beyond their lipid-lowering property, statins exhibit multiple pleiotropic and antimicrobial effects. Statins can affect a variety of immuno responses, including immune-cell activation, migration, cytokine production, stimulation of antigen-presenting cells, and immune-cell metabolism and survival, which are related to protection against infection or inflammation.7 Owing to statins’ immunomodulating effects, prior studies have suggested that treatment with them may lower the risk of pneumonia.8,9 There have been studies on whether treatment with statins before admission or during the acute phase of stroke might reduce the development of pneumonia.10,11 However, the findings were inconsistent and unclear, and so failed to establish a definitive relationship between long-term risk of pneumonia and exposure to statins beyond the acute phase of stroke.11 In clinical practice, the use of drugs is dynamic and may be frequently changed. The influence of statin intensity on the risk of developing pneumonia is not well known. Therefore, the current retrospective study was conducted using a Korean national sample cohort data, which include longitudinal data regarding the occurrence of pneumonia and records of prescription, to evaluate the effects of treatment with statins on the long-term risk of poststroke pneumonia.
Methods
Data Sources
This was a retrospective study based on the random-sampling dataset of the South Korean National Health Insurance Service claim database.12 This dataset is composed of 1,025,340 subjects randomly sampled and stratified by age, sex, and earning level. Because the National Health Insurance Service in South Korea is a single-payer system, overall health claims comprising demographics, hospitals visits, receipt of surgery or procedures, prescriptions, diagnosis, and mortality information of the participants included were available. Diagnostic codes at each hospital visit were recorded based on the ICD10. The National Health Insurance database is fully anonymized and does not contain any personal identification information. The Institutional Review Board of Bundang CHA Medical Center (IRB CHAMC 2017–12-043) approved the study and waived the requirement for informed consent.
Study Subjects
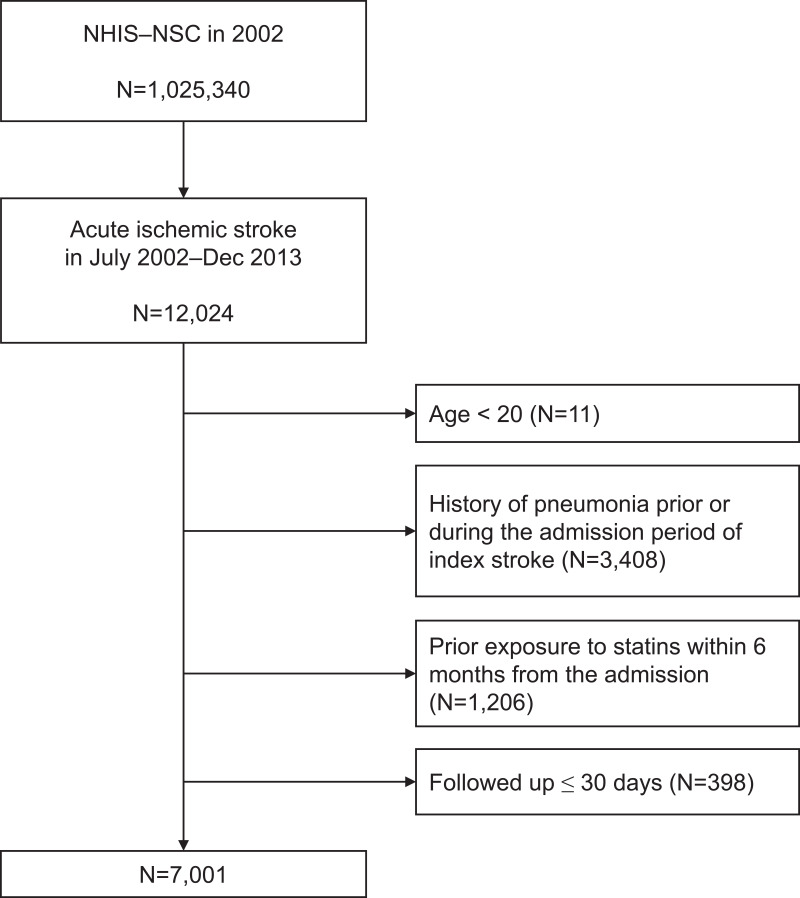
Patients ≥20 years of age who had been hospitalized (visited emergency medical center or admitted to hospital) between July 2002 and December 2013 with a main diagnosis of ischemic stroke (I63 in ICD10) were included. In order to enroll subjects with acute ischemic stroke, participants who had undergone brain magnetic resonance imaging or computed tomography during hospital admission were selected, considering that subjects with acute ischemic stroke would have had brain imaging performed.13 Patients who had ICD10 code of J09–J18 (pneumonia) prior to discharge date of the index stroke were excluded. In South Korea’s health-claim system, the previously registered diagnostic code may be automatically registered in the latter visit. Therefore, once the pneumonia ICD10 code has been assigned to a patient, it is not possible to determine clearly whether the pneumonia has been cured or remains in the NHIS-NSC data set. When a patient revisits the same hospital, it is ambiguous whether the revisit is due to recurrence of pneumonia or another medical problem. Therefore, to exclude the potency of false positives due to prior diagnosis, we included only patients who had not had a prior diagnosis of pneumonia (excluding patients with a history of pneumonia). Because inclusion of prevalent statin users in the observational study may introduce selection bias, patients who had exposure to statins within 6 months before the admission of acute ischemic stroke were excluded.14,15 To detect only patients who newly developed pneumonia after stroke as outcome, patients who had less than a month of follow-up after discharge were also excluded.16 Flowchart of the inclusion and exclusion criteria of patients is shown in Figure 1.
Figure 1.
Flowchart of patients included.
Abbreviation: NHIS-NSC, National Health Insurance Service national sample cohort.
Outcome and Follow-Up
The primary outcome measure was the time of occurrence of pneumonia, which was considered from hospital-visit records with diagnostic codes J09–J18.17,18 Detection of pneumonia from ICD10 codes has been reported to be reliable and widely used in research data.19,20 All subjects were followed up until the occurrence of pneumonia, death, loss of eligibility for the South Korean National Health Insurance Service, or 31 December, 2013, whichever occurred first.
Drug-Exposure Assessment
On each day of follow-up after discharge from the index stroke, data on treatment with statins was accessed using the prescription records of each patient, which were collected as time-dependent variables. To evaluate the dose–response relationship, statin intensity was grouped into low– intermediate intensity (atorvastatin <20 mg, rosuvastatin <10 mg, pravastatin ≤80 mg, lovastatin ≤40 mg, simvastatin ≤40 mg, fluvastatin ≤80 mg, pitavastatin ≤4 mg) and high intensity (atorvastatin ≥40 mg, rosuvastatin ≥20 mg) according to the type and daily dose of the statins in accordance with the low-density lipoprotein–lowering potency of each agent.21
Other Covariates
Other covariates were age, sex, earning level, and comorbidities: hypertension, diabetes mellitus, atrial fibrillation, myocardial infarction, and chronic obstructive pulmonary disease. In the NHIS-NSC dataset, age is recorded at 5-year intervals for privacy protection. Earning level is stratified into tertile groups. Use of thrombolysis (653,500,660, 653,500,670, E04260071, E04260161 for intravenous and M6631, M6632, M6633 for intra-arterial) for acute ischemic stroke was determined by the procedure codes in the NHIS-NSC. The presence of hypertension (I10–I15), diabetes mellitus (E08–E11, E13–E14), atrial fibrillation (I48), myocardial infarction (I21), and chronic obstructive pulmonary disease (J42, J43 [not J43.0], J44) were defined by diagnostic and procedure codes before or during hospitalization for the index stroke.13,22 Hypertension and diabetes mellitus were recognized as relevant only if subjects had received antihypertensive or antidiabetic medications at the time of diagnosis.23 Stroke severity was considered a possible marker of prognosis; however, data were unavailable in the NHIS-NSC, with limitations in health insurance–claim data. Instead, length of hospital stay following index stroke was considered an alternative marker, which has proven to be reliably associated with baseline stroke severity and stroke-related disability.24 Participants were divided into two groups (≤16 days, >16 days) based on median length of hospital stay following index stroke. Based on the health-care resources of the hospital at index stroke, classification was “general hospital”, which referred to large-scale hospitals, and “clinic or hospital”.
Statistical Analyses
Age recorded at 5-year intervals was treated as a continuous variable in the statistical analysis and expressed as medians and IQRs. Comparison of characteristics between the two groups was performed using χ2 tests for categorical variables and Mann–Whitney U tests for continuous variables (age-groups). The use of statins on each day of follow-up was considered the time-dependent variable (unit of time is day). Plots for estimated pneumonia-free probability according to treatment with statins were done considering the nature of the time-dependent variable.25 HRs and 95% CI were calculated by time-dependent Cox proportional hazard–regression models. During follow-up, patients who died without pneumonia were treated as censoring events. To investigate associative factors for occurrence of pneumonia, adjustments were performed for age, sex, earning level, history of hypertension, diabetes mellitus, atrial fibrillation, myocardial infarction, chronic obstructive pulmonary disease, use of thrombolysis, and type of hospital at index stroke. Linear trends in statin intensity and clinical outcome were estimated by regarding the statin-intensity groups as a continuous variable (no statins 0, low–intermediate intensity statins 1, and high-intensity statins 2). The proportional-hazard assumption for the model was tested by calculating Schoenfeld residuals and found to be satisfactory. Data management and statistical analyses were undertaken with R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) and PostgreSQL version 10.6 (PostgreSQL Global Development Group; https://www.postgresql.org). Two-sided p<0.05 was considered statistically significant.
Results
Characteristics
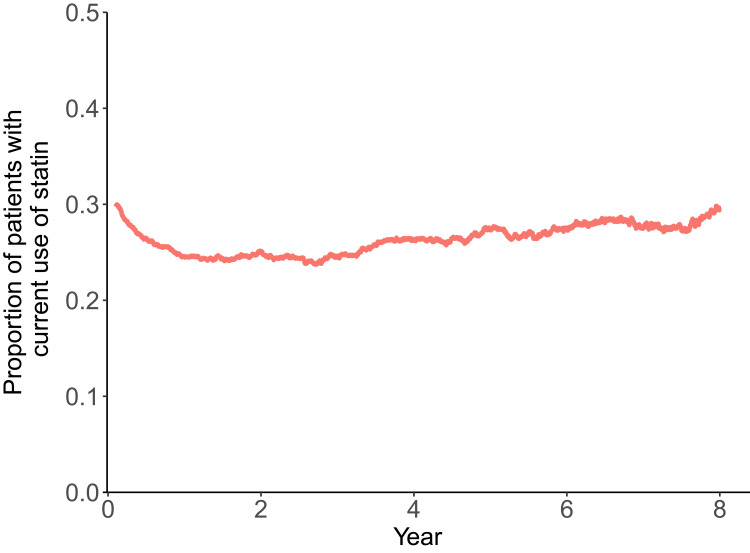
The current study included 7,001 subjects discharged after acute ischemic stroke without a history of pneumonia (Figure 1). The median age was 65–69 years (IQR 55–59 to 75–79 years), and 55.7% were men (Table 1). Figure 2 shows the proportions of participants with current use of statins during the follow-up period after stroke. In the figure, the proportion of subjects with current use of statins was relatively consistent (about 30%) throughout the poststroke period. At 1 year after discharge from index stroke, 5,640 subjects were at risk of developing pneumonia (patients remained and had no pneumonia until the time point). Among the 5,640 subjects at 1 year postdischarge, 1,386 (24.6%) were current users of statins. To identify the difference in characteristics of subjects who received statins with those who did not, clinical characteristics were compared between the groups (Table 2). Subjects with current use of statins were more likely to be male, younger, have hypertension and/or diabetes mellitus, have received thrombolysis and been hospitalized in a general hospital with longer length of stay in hospital for index stroke.
Table 1.
Baseline Characteristics of Patients with Acute Ischemic Stroke
| n=7,001 | |
|---|---|
| Sex, male | 3,902 (55.7) |
| Age, years | 65–69 (55–59 to 75–79) |
| Hypertension | 5,083 (72.6) |
| Diabetes mellitus | 1,862 (26.6) |
| Myocardial infarction | 473 (6.8) |
| Atrial fibrillation | 688 (9.8) |
| Chronic obstructive pulmonary disease | 1,383 (19.8) |
| Thrombolysis | 230 (3.3) |
| Household income | |
| Low | 2,314 (33.1) |
| Middle | 2,551 (36.4) |
| High | 2,136 (30.5) |
| Hospital type at index stroke | |
| General hospital | 5,844 (83.5) |
| Hospital or clinic | 1,157 (16.5) |
| Duration of hospital stay following index stroke | |
| ≤16 days | 3,690 (52.7) |
| >16 days | 3,311 (47.3) |
Note: Data presented as n (%) or medians (IQRs).
Figure 2.
Proportion of patients with current of statin use throughout the poststroke period. The proportion was calculated by dividing the number of patients with current statin use by the number of patients still at risk of pneumonia at each time point.
Table 2.
Comparison of Characteristics Between Patients with Current Use of Statin and Those Without at 1-Year After Acute Ischemic Stroke
| No use of statins (n=4,254) | Current use of statins (n=1,386) | p-value | |
|---|---|---|---|
| Sex, male | 2323 (54.6) | 817 (58.9) | 0.005 |
| Age, years | 65–69 (55–59 to 75–79) | 65–69 (55–59 to 70–74) | <0.001 |
| Hypertension | 2,965 (69.7) | 1,046 (75.5) | <0.001 |
| Diabetes mellitus | 982 (23.1) | 435 (31.4) | <0.001 |
| Myocardial infarction | 268 (6.3) | 94 (6.8) | 0.567 |
| Atrial fibrillation | 369 (8.7) | 129 (9.3) | 0.505 |
| Chronic obstructive pulmonary disease | 784 (18.4) | 236 (17.0) | 0.255 |
| Thrombolysis | 114 (2.7) | 61 (4.4) | 0.002 |
| Household income | 0.152 | ||
| Low | 1,409 (33.1) | 421 (30.4) | |
| Middle | 1,540 (36.2) | 515 (37.2) | |
| High | 1,305 (30.7) | 450 (32.5) | |
| Hospital type at index stroke | <0.001 | ||
| General hospital | 3,468 (81.5) | 1,264 (91.2) | |
| Hospital or clinic | 786 (18.5) | 122 (8.8) | |
| Duration of hospital stay following index stroke | <0.001 | ||
| ≤16 days | 2,389 (56.2) | 624 (45.0) | |
| >16 days | 1,865 (43.8) | 762 (55.0) |
Note: Data presented as n (%) or medians (IQRs).
Risk of Pneumonia
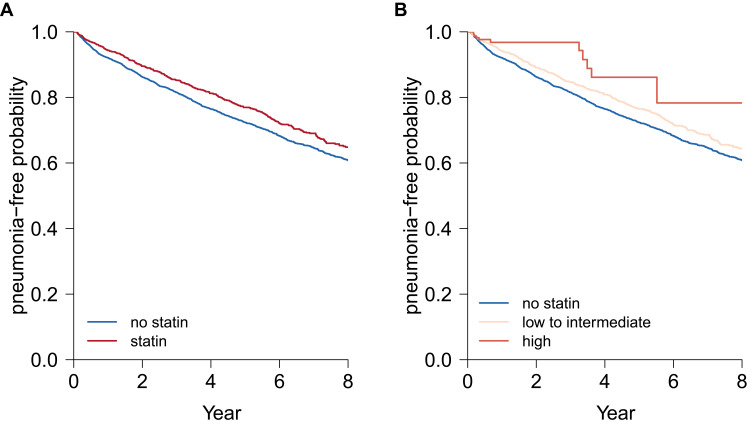
During 3.96±2.99 years of follow-up after discharge from index stroke, there were 1,715 subjects that developed pneumonia (24.5%). Censored patients without pneumonia numbered 5,286 (1,218 who had died, 152 with loss of eligibility for national health insurance, and 4,068 subjects who had survived without pneumonia until the study end date). Figure 3 demonstrates the estimated pneumonia-free survival curves according to current treatment with statins during the follow-up period. In the plot, subjects who received statins had lower risk of pneumonia compared to those who did not (unadjusted HR 0.81, 95% CI 0.73–0.91; p<0.001; Figure 3A). When subdivision was based on statin intensity (low– intermediate and “high”), use of both low– intermediate (unadjusted HR (0.83, (95% CI 0.74–0.94; p=0.002) and high (unadjusted HR (0.45, (95% CI 0.25–0.81; p=0.008)–intensity statins was significantly associated with lower risk of pneumonia than no use of statins (Figure 3B). In the multivariate time-dependent Cox proportional hazard–regression model, there was significantly lower risk of developing pneumonia with current use of statins (adjusted HR 0.86, 95% CI 0.77–0.97; p=0.013) than no use of statins (Table 3). Other significant risk factors for poststroke pneumonia were male sex, old age, diabetes mellitus, chronic obstructive pulmonary disease, and admission to a hospital or clinic at index stroke.
Figure 3.
Estimated pneumonia-free probability according to treatment with statins (A) and according to intensity of statins (B) during follow-up. HRs for pneumonia and 95% CIs were derived from the univariate time-dependent Cox proportional hazard–regression model.
Table 3.
Effects of Treatment with Statins on Risk of Poststroke Pneumonia
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Sex, male | 0.95 (0.86–1.04) | 1.18 (1.07–1.31) |
| Age, per 5-year interval | 1.22 (1.19–1.24) | 1.21 (1.18–1.23) |
| Hypertension | 1.41 (1.26–1.58) | 1.12 (1.00–1.25) |
| Diabetes mellitus | 1.28 (1.15–1.42) | 1.23 (1.11–1.37) |
| Myocardial infarction | 1.17 (0.97–1.41) | 1.09 (0.91–1.32) |
| Atrial fibrillation | 1.40 (1.20–1.63) | 1.17 (1.00–1.37) |
| Chronic obstructive pulmonary disease | 1.63 (1.45–1.82) | 1.29 (1.15–1.45) |
| Thrombolysis | 1.02 (0.76–1.37) | 1.10 (0.81–1.48) |
| Household income | ||
| Low | 1 (ref) | 1 (ref) |
| Middle | 0.95 (0.84–1.06) | 0.96 (0.86–1.08) |
| High | 1.09 (0.97–1.22) | 0.95 (0.84–1.07) |
| Hospital type at index stroke | ||
| General hospital | 1 (ref) | 1 (ref) |
| Hospital or clinic | 1.41 (1.25–1.59) | 1.20 (1.06–1.35) |
| Duration of hospital stay following index stroke | ||
| ≤16 days | 1 (ref) | 1 (ref) |
| >16 days | 0.95 (0.87–1.05) | 0.92 (0.84–1.01) |
| Time-dependent variables | ||
| No use of statins | 1 (ref) | 1 (ref) |
| Current use of statins | 0.81 (0.73–0.91) | 0.86 (0.77–0.97) |
Note: Data derived from Cox proportional hazard–regression model for the development of poststroke pneumonia.
Risk of Pneumonia According to Statin Intensity
In the multivariate model considering statin intensity (Table 4), both statin-intensity groups were significantly associated with lower risk of poststroke pneumonia. Compared with no statin, the risk of pneumonia (adjusted HR [95% CI]) with low- to intermediate-intensity statins and high-intensity statins was 0.88 (0.78–0.99) and 0.49 (0.27–0.87), respectively. There was a significant dose–response relationship between statin intensity and risk of poststroke pneumonia (p for trend=0.004).
Table 4.
Risk of Poststroke Pneumonia According to Statin Intensity
| Statin therapy | Adjusted HR (95% CI) | p-value | p-value for trend |
|---|---|---|---|
| No use of statins | 1 (ref) | — | 0.004 |
| Current use of low- to intermediate-intensity statins | 0.88 (0.78–0.99) | 0.038 | |
| Current use of high-intensity statins | 0.49 (0.27–0.87) | 0.015 |
Note: Data derived from multivariate time-dependent Cox proportional hazard–regression model adjusted for the variables listed in Table 3.
Discussion
Using the national sample cohort based on health insurance–claim data in South Korea, we investigated the risk of occurrence of pneumonia according to treatment with statins throughout the long-term follow-up period after acute ischemic stroke. Poststroke pneumonia was common in the current study during the mean follow-up of 3.96 years, wherein 24.5% of patients suffered it. The chief finding of this study was that treatment with statins was significantly associated with lower risk of poststroke pneumonia. The amplitude of risk reduction with statins (adjusted HR 0.86) was comparable with data from prior epidemiological studies suggesting a preventive role of statins against pneumonia.26 In this real-world study on acute ischemic stroke, the proportion of patients who received statin therapy was suboptimal. Suboptimal use of statins in the real world, even after major cardiovascular disease, has been consistently reported and remains a major challenge in clinical practice.13 As poststroke pneumonia is common and associated with short-term and long-term disability, attempts should be made to prevent development of the condition.10 Data from the current study suggest that enhancement of guideline-recommended statin therapy after ischemic stroke may further reduce the risk of poststroke pneumonia beyond the established cardiovascular risk reduction.13
The exact mechanism of action of statins on pneumonia remains uncertain. Multiple antimicrobial and immunomodulating mechanisms of statins have been suggested.27 Experimental and animal studies have indicated that treatment with statins results in a protective immunoresponse against pathogens and attenuation of tissue injury by proinflammatory pathways, independently of lipid-lowering properties.28 There are cellular and molecular protective mechanisms of statins in cytokine synthesis, adhesive molecules, Toll-like receptors, B lymphocytes, and helper and regulatory T lymphocytes.29,30 In acute inflammatory states induced by exotoxin, pretreatment with simvastatin significantly attenuates expression of P-selectin in endothelia, leukocyte rolling, adherence, and transmigration of leukocytes.31 The statin-mediated interaction between leukocytes and endothelial cells may be involved in both inflammatory pathways and tissue injury in infectious diseases. In lipopolysaccharide (LPS) stimulation, the protein secretion and mRNA expression of IL6 and IL8 are significantly inhibited by treatment with pitavastatin and pravastatin.32 Atorvastatin attenuates TNFα expression and production in LPS-stimulated macrophages.33 Administration of atorvastatin reduces levels of serum IFNγ and IL4 in Candida albicans–infected mice.34 Proliferation and activation of T lymphocytes is inhibited by multiple statin-mediated mechanisms.35,36 There is evidence of CD4:CD8 ratio and shift from type 1 to type 2 helper T cells with simvastatin.37 These altered immuno responses from statins are predominantly anti-inflammatory and immunosuppressive, which might be predisposing factors in infection.38 However, numerous studies have shown that statins also can induce strong proinflammatory responses when costimulatory signals are provided.39 In addition, there are experimental data that statins can enhance and modulate innate immunity.40,41 In macrophages, statins promote autophagy pathways by inducing endosome maturation and fusion of autophagosomes with lysosomes, resulting in degradation of sequestered bacteria.42 Statins have been shown to increase plasma high-density lipoprotein, which can act as an antioxidative and anti-inflammatory molecule that impedes monocyte chemotaxis.43 Lipoproteins play an important role in immunoresponse.44 Furthermore, statins may exhibit antibacterial activity that interrupts bacterial cell functions via binding and disruption of cell-wall structures such as teichoic acids, lipoteichoic acids, LPSs, and surface proteins.45 Modulation of gene expression in immune cells and cell-adhesion molecules by statins may reduce replication of viruses, including influenza.46 By promotion of the host-defense mechanism and inhibition of pathological inflammation, statins may play a beneficial protective role against infectious diseases.27 Along with the theoretical background, many epidemiological studies have reported that therapy with statins may reduce the risk of pneumonia, infection, and mortality in the general population and various disease groups, including stroke.8,10,26,47,48 As supporting data for the antibacterial and immunoprotective effects of statins, treatment with statins confers a survival advantage to patients with a diagnosis of pneumonia.49 Among thrombolyzed stroke patients, continued treatment with statins is negatively associated with pneumonia after stroke.10 In cohort studies using Taiwan’s National Health Insurance Research Database, statin use exhibited lower risk of hospitalization and vital-organ failure in COPD patients.50,51
Despite the promising reports from animal and observational studies, there are conflicting data from clinical trials on the relationship between statin therapy and risk of pneumonia. A meta-analysis that included eleven randomized trials on use of statins found no significant preventive effect of the drug on the risk of infection.52 Similarly, a recent meta-analysis that included five clinical trials with 8,791 stroke or transient ischemic–attack patients did not report a significant effect of statins on reduction in risk of infection.53 Based on these negative findings, there is a concern for potential bias in observational studies suggesting the preventive effect of statins in pneumonia.54,55 Treatment with statins may reflect the willingness of the patient to follow treatment and better functional status (healthy-user effects), rather than the true biological preventive effect of the drug.10 However, caution must be exercised in interpretation of the negative findings of the clinical trials, because none of the trials set pneumonia as the primary outcome measure. The randomized, double-blind, placebo-controlled JUPITER trialdocumented that the rosuvastatin group had a lower risk of pneumonia than the placebo group.26
In the present study, the preventive effect of statins remained unchanged after additional adjustment for treatment with antihypertensive medications. This finding suggested that the preventive effect of treatment with statins was not completely explained by compliance with medical treatment. The conflicting data among studies might be related to heterogeneous regimens, timing of treatment with statins, variability in definition of pneumonia, and study duration.56 Patients with acute ischemic stroke in the present study, who were particularly at great risk for pneumonia, may be more likely to benefit from treatment with statins than the general population. To date, no large randomized clinical study has been conducted evaluating the effect of statins on the development of pneumonia as the primary outcome measure. Further evidence based on well-designed prospective randomized trials is needed to address the key question of the beneficial role of statins in pneumonia.8
In accordance with the initial expectation, we found a dose-dependent relationship between risk of pneumonia and intensity of statins. Along with lipid-lowering potency, the beneficial pleiotropic effects of statins, such as the antibacterial and immunomodulatory properties, may act in a dose-dependent fashion.57 To maximize the potential benefits of statins, further experimental and clinical investigations are warranted to elucidate the underlying biological mechanism of action of statins in pneumonia.
The main strength of the current study is the use of information from the population-based random-sampling database, which enabled inclusion of >7,000 subjects with acute ischemic stroke. Based on the national database, longitudinal follow-up of the participants to assess the occurrence of pneumonia was possible. Unlike prior studies, which have focused mainly on exposure to statins before or during the early phase of admission following a stroke, data on treatment with statins throughout follow-up after discharge from hospital were collected in the current study, reflecting dynamic changes in medications in clinical practice. The potential limitations of this study should also be acknowledged. Clinical data regarding stroke severity, functional disability, dysphagia, smoking, and lipid levels were not obtained, owing to unavailability of such information from the health-insurance database. Due to the retrospective observational design of the study, causality between statin use and risk of pneumonia could not be established. Data on statin use was obtained from the prescription records of each subject. There may be a gap between prescribed medication and actual medicine-taking behaviors of subjects. Previous research has shown a good association between values calculated from prescription records and real intake of medications.58 In the current study, detection of pneumonia outcome was based on ICD10 codes, which may be associated with loss of subjects with pneumonia by error of registration in clinical practice or by them missing hospital visits. Moreover, the ICD10 codes J09–J18 can overestimate pneumonia diagnosis, since suspected diagnoses can also be labeledICD10 J09–J18 in real-world practice. The current study was performed using data of Asian subjects with acute ischemic stroke. Further studies must be conducted to evaluate the general applicability of the results in different ethnicities and disease populations.
Conclusion
Using a national health-insurance database, the current study found reduced risk of poststroke pneumonia with statin therapy after acute ischemic stroke. The preventive effect of statins on pneumonia did not differ based on statin intensity. Encouragement of statin treatment after ischemic stroke, the guideline-recommended treatment, may reduce poststroke pneumonia, which is a common and critical illness in stroke subjects.
Acknowledgments
This study used NHIS-NSC data (NHIS-2019-2-059) from the NHIS. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03033382 to JK). This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1D1A1B07040959 to T-JS). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Armstrong JR, Mosher BD. Aspiration pneumonia after stroke: intervention and prevention. Neurohospitalist. 2011;1(2):85–93. doi: 10.1177/1941875210395775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104(4):350–360. [PMC free article] [PubMed] [Google Scholar]
- 3.Salive ME, Satterfield S, Ostfeld AM, Wallace RB, Havlik RJ. Disability and cognitive impairment are risk factors for pneumonia-related mortality in older adults. Public Health Rep. 1993;108(3):314–322. [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. 2012;21(1):61–67. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masiero S, Pierobon R, Previato C, Gomiero E. Pneumonia in stroke patients with oropharyngeal dysphagia: a six-month follow-up study. Neurol Sci. 2008;29(3):139–145. doi: 10.1007/s10072-008-0925-2 [DOI] [PubMed] [Google Scholar]
- 6.Rocco A, Fam G, Sykora M, Diedler J, Nagel S, Ringleb P. Poststroke infections are an independent risk factor for poor functional outcome after three-months in thrombolysed stroke patients. Int J Stroke. 2013;8(8):639–644. doi: 10.1111/j.1747-4949.2012.00822.x [DOI] [PubMed] [Google Scholar]
- 7.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69–75. doi: 10.1111/imm.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chopra V, Flanders SA. Statins and pneumonia. BMJ. 2011;342(apr06 2):d1907. doi: 10.1136/bmj.d1907 [DOI] [PubMed] [Google Scholar]
- 9.Kwok CS, Yeong JK, Turner RM, Cavallazzi R, Singh S, Loke YK. Statins and associated risk of pneumonia: a systematic review and meta-analysis of observational studies. Eur J Clin Pharmacol. 2012;68(5):747–755. doi: 10.1007/s00228-011-1159-4 [DOI] [PubMed] [Google Scholar]
- 10.Scheitz JF, Endres M, Heuschmann PU, Audebert HJ, Nolte CH. Reduced risk of poststroke pneumonia in thrombolyzed stroke patients with continued statin treatment. Int J Stroke. 2015;10(1):61–66. doi: 10.1111/j.1747-4949.2012.00864.x [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez de Antonio LA, Martinez-Sanchez P, Martinez-Martinez MM, et al. Previous statins treatment and risk of post-stroke infections. Neurologia. 2011;26(3):150–156. doi: 10.1016/j.nrl.2010.07.030 [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. doi: 10.1093/ije/dyw317 [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Lee HS, Nam CM, Heo JH. Effects of statin intensity and adherence on the long-term prognosis after acute ischemic stroke. Stroke. 2017;48(10):2723–2730. doi: 10.1161/STROKEAHA.117.018140 [DOI] [PubMed] [Google Scholar]
- 14.Franklin JM, Schneeweiss S. When and how can real world data analyses substitute for randomized controlled trials? Clin Pharmacol Ther. 2017;102(6):924–933. doi: 10.1002/cpt.857 [DOI] [PubMed] [Google Scholar]
- 15.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–262. doi: 10.1093/aje/kwr301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song T-J, Kim J, Katsanos AH. Risk of post-stroke pneumonia with proton pump inhibitors, H2 receptor antagonists and mucoprotective agents: a retrospective nationwide cohort study. PLoS One. 2019;14(5):e0216750. doi: 10.1371/journal.pone.0216750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RE, Dougherty E, McArthur C, Huang QS, Baker MG. Cold, dry air is associated with influenza and pneumonia mortality in Auckland, New Zealand. Influenza Other Respir Viruses. 2016;10(4):310–313. doi: 10.1111/irv.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniecka-Bryla I, Paciej-Golebiowska P, Dziankowska-Zaborszczyk E, Bryla M. Lost life years due to premature mortality caused by diseases of the respiratory system. Adv Clin Exp Med. 2018;27(6):743–748. doi: 10.17219/acem/69227 [DOI] [PubMed] [Google Scholar]
- 19.Azmi S, Aljunid SM, Maimaiti N, et al. Assessing the burden of pneumonia using administrative data from Malaysia, Indonesia, and the Philippines. Int J Infect Dis. 2016;49:87–93. doi: 10.1016/j.ijid.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 20.Kim SA, Kilgore PE, Lee SY, Nyambat B, Ki M. Trends in pneumonia and influenza-associated hospitalizations in South Korea, 2002–2005. J Health Popul Nutr. 2011;29(6):574–582. doi: 10.3329/jhpn.v29i6.9894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46(5):658–664. doi: 10.4070/kcj.2016.46.5.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YM, Kim YJ, Park JY, Lee WJ, Jung CH. Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovasc Diabetol. 2016;15(1):131. doi: 10.1186/s12933-016-0451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang KC, Tseng MC, Weng HH, Lin YH, Liou CW, Tan TY. Prediction of length of stay of first-ever ischemic stroke. Stroke. 2002;33(11):2670–2674. doi: 10.1161/01.STR.0000034396.68980.39 [DOI] [PubMed] [Google Scholar]
- 25.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended kaplan-meier estimator. Am Stat. 2005;59(4):301–307. doi: 10.1198/000313005X70371 [DOI] [Google Scholar]
- 26.Novack V, MacFadyen J, Malhotra A, Almog Y, Glynn RJ, Ridker PM. The effect of rosuvastatin on incident pneumonia: results from the JUPITER trial. CMAJ. 2012;184(7):E367–E372. doi: 10.1503/cmaj.111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol. 2019;19(2):104–117. doi: 10.1038/s41577-018-0094-3 [DOI] [PubMed] [Google Scholar]
- 28.Bjorkhem-Bergman L, Bergman P, Andersson J, Lindh JD, Ratner AJ. Statin treatment and mortality in bacterial infections – a systematic review and meta-analysis. PLoS One. 2010;5(5):e10702. doi: 10.1371/journal.pone.0010702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro-Vornhagen A, Liebig T, von Bergwelt-baildon M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood. 2008;112(4):1544–1545. doi: 10.1182/blood-2008-04-149609 [DOI] [PubMed] [Google Scholar]
- 30.El-Achkar GA, Mrad MF, Mouawad CA, et al. Heme oxygenase-1-dependent anti-inflammatory effects of atorvastatin in zymosan-injected subcutaneous air pouch in mice. PLoS One. 2019;14(5):e0216405. doi: 10.1371/journal.pone.0216405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruefer D, Makowski J, Schnell M, et al. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106(16):2104–2110. doi: 10.1161/01.CIR.0000034048.38910.91 [DOI] [PubMed] [Google Scholar]
- 32.Iwata A, Shirai R, Ishii H, et al. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol. 2012;168(2):234–240. doi: 10.1111/j.1365-2249.2012.04564.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XQ, Luo NS, Salah ZQ, Lin YQ, Gu MN, Chen YX. Atorvastatin attenuates TNF-alpha production via heme oxygenase-1 pathway in LPS-stimulated RAW264.7 macrophages. Biomed Environ Sci. 2014;27(10):786–793. doi: 10.3967/bes2014.114 [DOI] [PubMed] [Google Scholar]
- 34.Rahal EA, Constantin WN, Zeidan N, Abdelnoor AM. Atorvastatin reduces the survival of Candida albicans-infected BALB/c mice. Front Microbiol. 2015;6:1474. doi: 10.3389/fmicb.2015.01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank N, Schiller M, Krienke S, et al. Atorvastatin inhibits T cell activation through 3-hydroxy-3-methylglutaryl coenzyme A reductase without decreasing cholesterol synthesis. J Immunol. 2007;179(6):3613–3621. doi: 10.4049/jimmunol.179.6.3613 [DOI] [PubMed] [Google Scholar]
- 36.Brumeanu TD, Goldstein R, Casares S. Down-regulation of autoreactive T-cells by HMG CoA reductase inhibitors. Clin Immunol. 2006;119(1):1–12. doi: 10.1016/j.clim.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Kanda H, Yokota K, Kohno C, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol. 2007;17(5):364–368. doi: 10.3109/s10165-007-0589-4 [DOI] [PubMed] [Google Scholar]
- 38.Becker K, Tanzi P, Kalil A, Shibata D, Cain K. Early statin use is associated with increased risk of infection after stroke. J Stroke Cerebrovasc Dis. 2013;22(1):66–71. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18(13):3524–3531. doi: 10.1158/1078-0432.CCR-12-0489 [DOI] [PubMed] [Google Scholar]
- 40.Walton GM, Stockley JA, Griffiths D, Sadhra CS, Purvis T, Sapey E. Repurposing treatments to enhance innate immunity. Can statins improve neutrophil functions and clinical outcomes in COPD?. J Clin Med. 2016;5(10):89. doi: 10.3390/jcm5100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow OA, von Kockritz-blickwede M, Bright AT, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8(5):445–454. doi: 10.1016/j.chom.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao WC, Huang MZ, Wang ML, et al. Statin decreases helicobacter pylori burden in macrophages by promoting autophagy. Front Cell Infect Microbiol. 2016;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JD. Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipidol. 2010;4(5):382–388. doi: 10.1016/j.jacl.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaysen GA, Grimes B, Dalrymple LS, et al. Associations of lipoproteins with cardiovascular and infection-related outcomes in patients receiving hemodialysis. J Clin Lipidol. 2018;12(2):481–487.e414. doi: 10.1016/j.jacl.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko HHT, Lareu RR, Dix BR, Hughes JD. In vitro antibacterial effects of statins against bacterial pathogens causing skin infections. Eur J Clin Microbiol Infect Dis. 2018;37(6):1125–1135. doi: 10.1007/s10096-018-3227-5 [DOI] [PubMed] [Google Scholar]
- 46.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99(3):417–435. doi: 10.1016/j.antiviral.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 47.Tariq R, Mukhija D, Gupta A, Singh S, Pardi DS, Khanna S. Statin use and the risk of clostridium difficile infection: a systematic review with meta-analysis. Infect Drug Resist. 2018;11:405–416. doi: 10.2147/IDR.S156475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia DK, Hu ZG, Tian YF, Zeng FJ. Statin use and prognosis of lung cancer: a systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des Devel Ther. 2019;13:405–422. doi: 10.2147/DDDT.S187690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ. 2011;342(apr06 2):d1642. doi: 10.1136/bmj.d1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeh JJ, Syue SH, Lin CL, Hsu CY, Shae Z, Kao CH. Statin use and vital organ failure in patients with asthma-chronic obstructive pulmonary disease overlap: a Time-Dependent Population-Based Study. Front Pharmacol. 2019;10:889. doi: 10.3389/fphar.2019.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang CC, Chan WL, Chen YC, et al. Statin use and hospitalization in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study in Taiwan. Clin Ther. 2011;33(10):1365–1370. doi: 10.1016/j.clinthera.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 52.van den Hoek HL, Bos WJ, de Boer A, van de Garde EM. Statins and prevention of infections: systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ. 2011;343(nov29 1):d7281. doi: 10.1136/bmj.d7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin S-P, Long Y-M, Chen X-H, Kirchmair R. The effects of statins on infections after stroke or transient ischemic attack: a meta-analysis. PLoS One. 2015;10(7):e0130071. doi: 10.1371/journal.pone.0130071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin JY, Eberg M, Ernst P, Filion KB. Statin potency and the risk of hospitalization for community-acquired pneumonia. Br J Clin Pharmacol. 2017;83(6):1319–1327. doi: 10.1111/bcp.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yende S, Milbrandt EB, Kellum JA, et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 2011;39(8):1871–1878. doi: 10.1097/CCM.0b013e31821b8290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang J-H, Kao L-T, Lin H-C, Wang T-J, Yang T-Y, Aalto-Setala K. Do outpatient statins and ACEIs/ARBs have synergistic effects in reducing the risk of pneumonia? A population-based case-control study. PLoS One. 2018;13(6):e0199981. doi: 10.1371/journal.pone.0199981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Meij E, Koning GG, Vriens PW, et al. A clinical evaluation of statin pleiotropy: statins selectively and dose-dependently reduce vascular inflammation. PLoS One. 2013;8(1):e53882. doi: 10.1371/journal.pone.0053882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JK, Grace KA, Foster TG, et al. How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag. 2007;3(4):685–690. [PMC free article] [PubMed] [Google Scholar]