Abstract
Aims:
Worsening heart failure (HF) is associated with shorter left ventricular systolic ejection time (SET), but there are limited data describing the relationship between SET and clinical outcomes. Thus, the objective was to describe the association between SET and clinical outcomes in an ambulatory HF population irrespective of ejection fraction (EF).
Methods and Results:
We identified ambulatory patients with heart failure with a reduced EF (HFrEF) and HF with a preserved EF (HFpEF) who had an outpatient transthoracic echocardiogram (TTE) performed between August 2008 and July 2010 at a tertiary referral center. Multivariable logistic regression was used to evaluate the association between SET and 1-year outcomes. A total of 545 HF patients (171 HFrEF, 374 HFpEF) met eligibility criteria. Compared with HFpEF, HFrEF patients were younger (median age 60 (50, 69)) (value (25th, 75th)) vs. 64 (53, 74), with fewer females (30% vs. 56%) and a similar percentage of African Americans (35% vs. 35%). Median (25th, 75th) EF with HFrEF was 30% (25%, 35%) and with HFpEF was 54% (48%, 58%). Median SET was shorter (280 vs. 315ms, p<0.001), median pre-ejection period was longer (114 vs. 89ms, p<0.001), and median relaxation time was shorter (78.7ms vs. 93.3ms, p<0.001) among patients with HFrEF versus HFpEF. Death or HF hospitalization occurred in 26.9% (N=46/171) HFrEF and 11.8% (N=44/374) HFpEF patients. After adjustment, longer SET was associated with lower odds of the composite of death or HF hospitalization at 1 year among HFrEF but not HFpEF patients.
Conclusion:
Longer SET is independently associated with improved outcomes among HFrEF patients but not HFpEF patients, supporting a potential role for normalizing SET as a therapeutic strategy with systolic dysfunction.
Keywords: Heart Failure, Ejection Fraction, Systolic Function, Systolic Ejection Time
INTRODUCTION
Patients with heart failure (HF) have a number of hemodynamic changes that can be evaluated using non-invasive testing, including derangements in systolic time intervals that can be reliably quantified by conventional echocardiography and/or arterial tonometry. In particular, early studies of HF patients demonstrated shorter left ventricular systolic ejection times (SET) and longer pre-ejection periods (PEP) compared with normal controls at any given heart rate (1, 2). A shortening of SET among patients with HFrEF likely reflects an impairment in LV contractility. This corresponds to a longer isovolumic contraction time required to begin systolic ejection and possible compensatory increases in heart rate which cause an obligate shortening of systolic time intervals. Indeed, systolic time intervals expressed as the Tei index are a well-studied marker of left ventricular function (3).
Shorter SET is associated with other clinical variables suggestive of poorer prognosis in heart failure, including lower ejection fraction, cardiac index, and global longitudinal strain and a more rapid heart rate (1, 4). In addition, shorter SET has been associated with poor prognosis in patients with cardiac amyloid while SET has a U shaped relationship for mortality among patients with coronary artery disease (5, 6). SET is also associated with incident HF (7). Nevertheless, the prognostic impact of SET on outcomes for a broad population of ambulatory HF patients has not been well-investigated.
In this study, we assessed the relationship between SET and outcomes among a broad population of ambulatory HF patients with HFrEF and HFpEF treated at a large tertiary medical center. We further describe systolic time intervals among patients with HF and subjects without cardiovascular disease.
METHODS
STUDY DESIGN AND POPULATION
This was a single-center, retrospective, observational cohort study using a prospectively maintained digital archive of all clinical transthoracic echocardiograms (TTEs) performed since 1995 at Duke University Medical Center with corresponding linked clinical data (Duke Echocardiography Laboratory Database [DELD]). This study was approved by the institutional review board. We identified patients with HF who underwent an echocardiogram from August 1, 2008 to July 31, 2010, including patients with HF with reduced ejection fraction (HFrEF) (i.e., defined as EF ≤40%) and HF with preserved ejection fraction (HFpEF) (i.e., defined as EF >40%). We also identified a separate group of patients without known cardiovascular disease for comparison of baseline systolic time intervals. All patients were ≥18 years of age, and HF patients had an ICD-9 code for HF within the 18 months prior to the index echocardiogram, specifically codes 428.x, 402.x1, 404.x1, or 404.x3. We included only patients that had ≥2 outpatient visits within the Duke University Health System prior to the index echocardiogram in order to enhance the likelihood that patients maintained longitudinal follow-up at Duke University and to optimize event capture. We excluded patients without an ECG in the prior 18 months, presence of paced rhythm or atrial arrhythmia on most recent ECG, severe aortic stenosis, severe mitral stenosis, prosthetic heart valves, primary pulmonary hypertension, hypertrophic obstructive cardiomyopathy, congenital heart defects including atrial septal defect, ventricular septal defect, and inter-atrial flow communications, history of heart transplant, history of other solid organ transplant in the prior 18 months, history of end stage renal disease or hemodialysis, patients on inotropic support, and missing blood pressure value within 24 hours of the index echocardiogram. We also excluded studies where the TTE could not be interpreted. Patients without known cardiovascular disease met all criteria above and additionally could not have greater than mild valvular regurgitation or stenosis, enlarged cardiac chambers, left ventricular hypertrophy, diastolic dysfunction, or history of hypertension (Figure 1).
Figure 1. Consort Diagram.
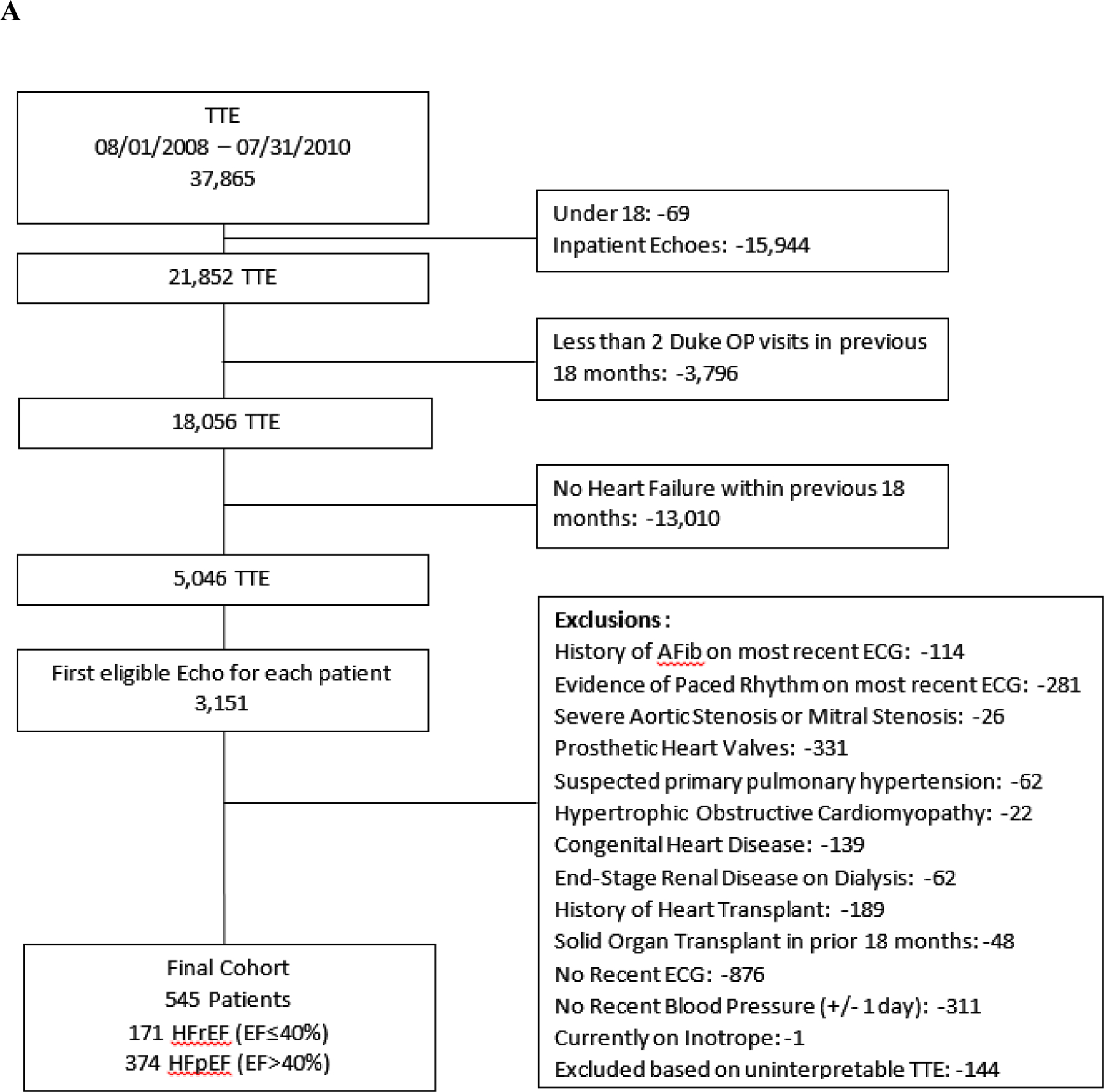
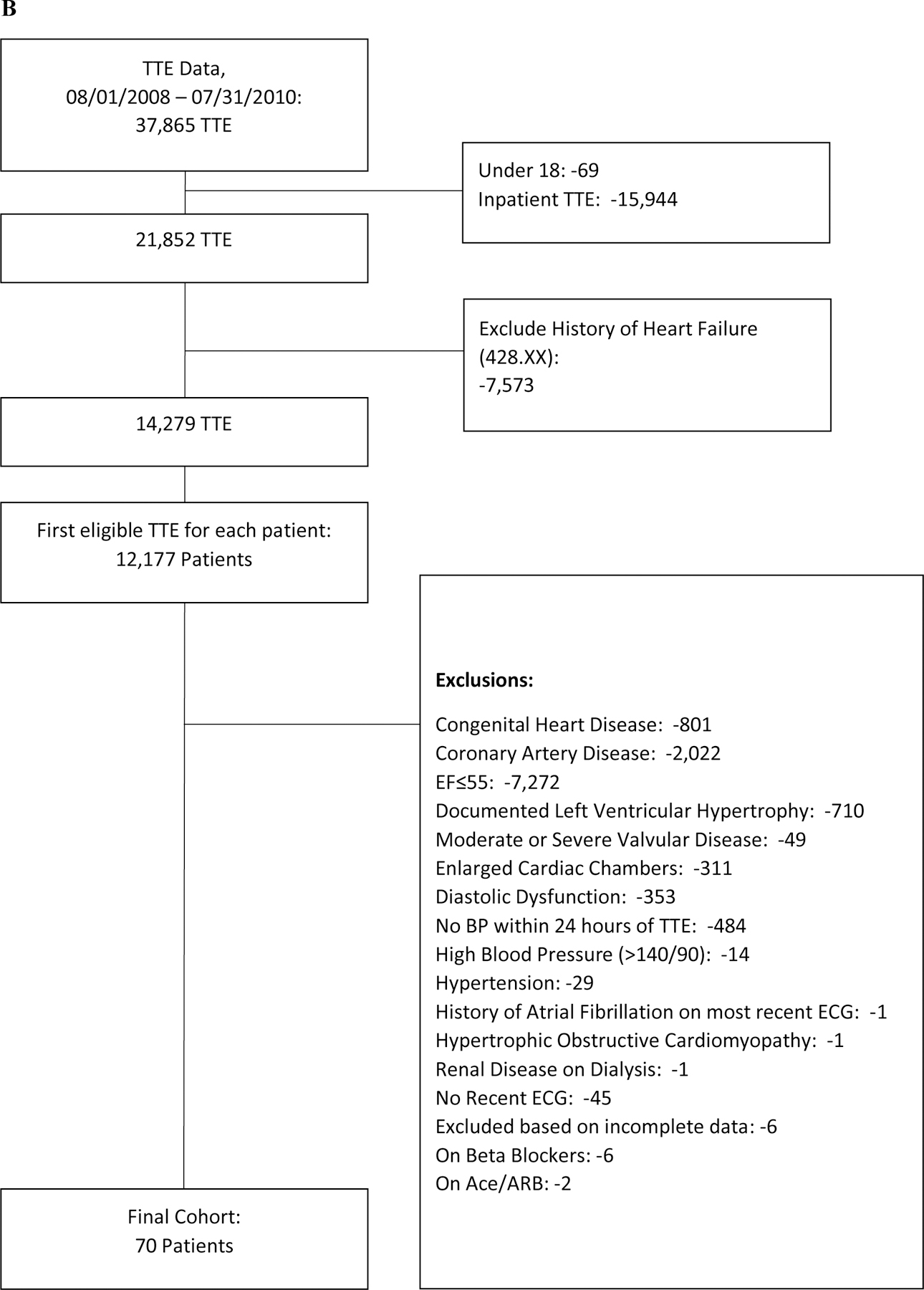
The cohort assembly for the (A) HF patients and (B) healthy controls without cardiovascular disease.
For outcomes, hospitalization and cause were ascertained from Duke University hospital administrative & billing records. HF hospitalization was identified by primary diagnosis of HF (i.e., ICD9 of 428.x). Death was ascertained from Duke University hospitalization records and the Social Security Administration Death Master File.
ECHOCARDIOGRAPHIC MEASURMENTS
All two-dimensional TTE examinations were acquired using standard clinical protocols and transferred in DICOM (Digital Imaging and Communications in Medicine) format to an independent software package for review (Digisonics’s; DigiView 3.8.4). Three experienced echocardiogram readers (PAP, FA, JT) blinded to clinical status performed all echocardiographic measurements. The measures of SET, pre-ejection period (PEP), R to E time, diastolic filling time, ejection fraction, left ventricular dimensions, left atrial dimensions, and diastolic function parameters were performed in triplicate according to guidelines set forward by the American Society of Echocardiography (Figure 2) (8, 9). R to E time was defined as the amount of time from the onset of R wave, or Q wave if present, to the start to next E wave. The diastolic filling time was defined as the time from onset of E wave to the start of the next QRS complex. The average value for three measurements was used for analyses.
Figure 2. Systolic and Diastolic Time Interval Measurements.
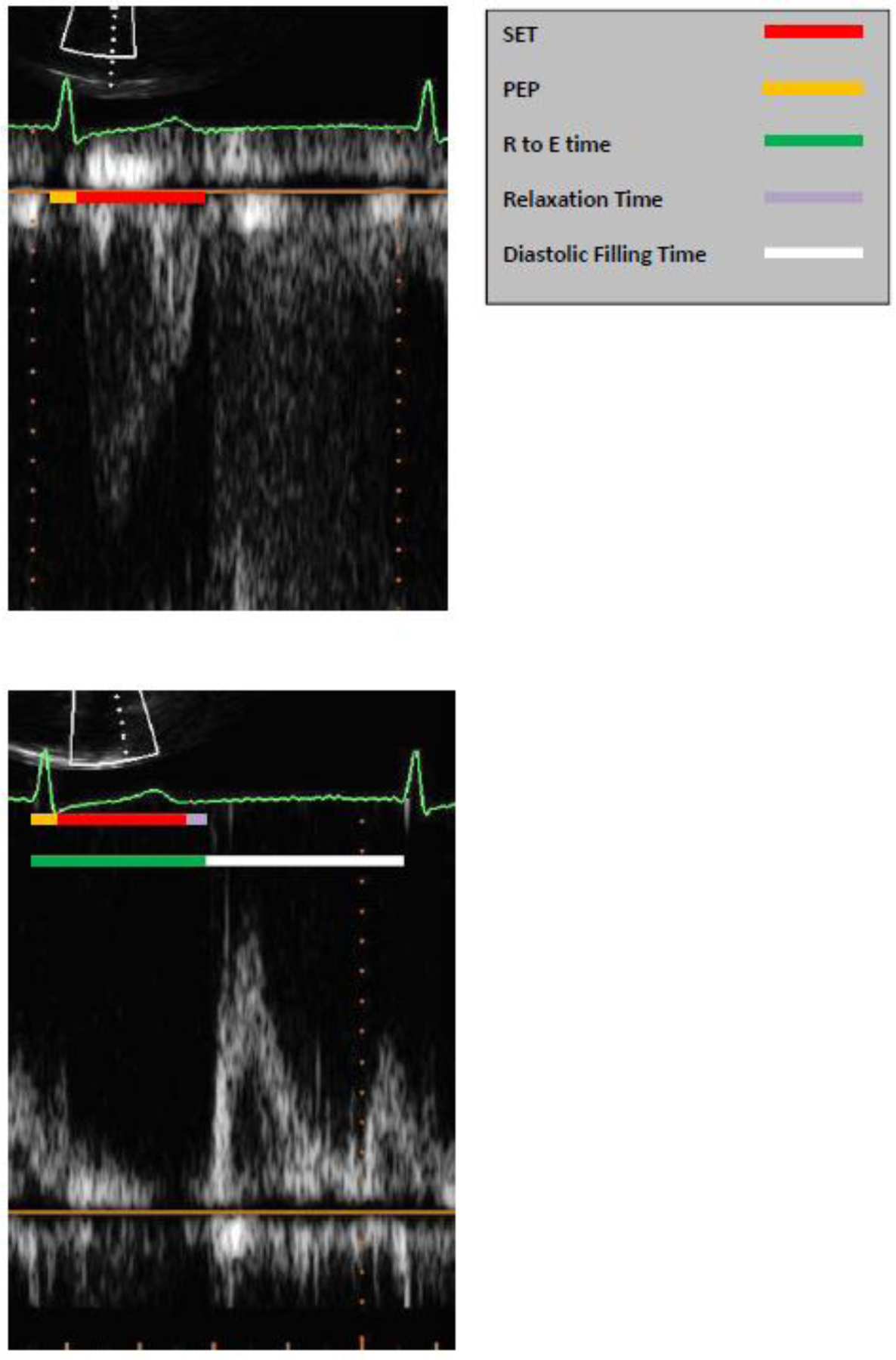
The pre-ejection period was defined at the onset of QRS complex to the initiation of ventricular systole on pulse wave Doppler of the left ventricular outflow tract (LVOT). The SET was measured as the length of time during systole where ventricular ejection occurred, and was automatically calculated from tracings of the LVOT time-velocity integral (TVI). The R to E time was a measure of total duration of electrical and mechanical systole, measured as the time from initiation of QRS complex to start of mitral E wave on the pulse wave Doppler tracing of mitral inflow velocities. The relaxation time was calculated as R to E time minus PEP minus SET. The diastolic filling time was time from onset of E wave to start of subsequent QRS complex.
Inter and Intra-rater reliability was estimated by having the three raters measure key variables of interest including mitral valve E wave velocity, LV end-diastolic dimension, and LVOT time-velocity integral (from which SET was automatically calculated) on TTE images from 20 randomly selected patients on three different occasions. Coverage probabilities were calculated based on published acceptable differences and were ≥0.85 for all variables, suggesting good inter-rater reliability (10). In addition, intraclass correlations for pre-ejection period and LVOT TVI were above 0.94, suggesting good intra-rater reproducibility.
STATISTICAL ANALYSIS
We assessed the relationship between SET and 1-year outcomes including mortality, heart failure hospitalization, all-cause hospitalization, and the composite of death or heart failure hospitalization. Patient demographics, medical history, laboratory findings, and echocardiography variables were summarized as frequencies and percentages for categorical variables and by medians (25th and 75th percentiles and interquartile range [IQR]) for continuous variables, and stratified by type (HFrEF, HFpEF, no prior CV disease). Baseline characteristics were compared between groups using the Wilcoxon rank-sum tests for continuous variables, and Pearson chi-square or exact tests for categorical variables as appropriate.
To assess the association between echocardiographic and clinical parameters and SET, multivariate linear regressions were modeled treating SET as the response variable. These analyses were performed in the entire population and additionally stratified by heart failure type, (Supplemental Table 1). In addition to echocardiographic parameters selected by investigators based on their clinical importance and low missingness, models were adjusted for demographic factors (age, sex, race) and heart rate.
We assessed the relationship between SET and 1-year outcomes including mortality, HF hospitalization, and 1-year death/HF hospitalization using multivariable logistic regression models. Among the cohort, 92% of patients had follow up through 1 year and 8% died within 1 year. Adjustment variables included age, sex, race, BMI, ejection fraction, heart rate, serum creatinine, BUN, serum sodium, pulse pressure (PP), beta-blocker use, and angiotensin converting-enzyme inhibitor (ACEI) use, and E/E’. Odds ratios (ORs) for outcomes were calculated with corresponding 95% confidence intervals (CIs), where an OR represents a 10 ms increase in SET. Linearity assumptions were assessed for SET and continuous covariates. Single imputation at the median was used for covariates with missing values. These variables included creatinine, BUN, sodium, systolic BP and heart rate. The maximum missing rate for these variables was 3.2%. In addition, unadjusted Kaplan-Meier (KM) curves were generated for the primary composite endpoint of 1-year death/HF hospitalization, stratified by quartiles of SET, to illustrate the timing of outcomes relative to index echocardiogram in the HFrEF cohort.
All analyses used 2-tailed α = 0.05 to establish statistical significance and we reported 95% confidence intervals, and no adjustment was made for multiple comparisons. All statistical computations were generated using SAS version 9.3 or higher (SAS Institute Inc., Cary, NC).
RESULTS
STUDY POPULATION
We identified 171 HFrEF, 374 HFpEF, and 70 patients without known cardiovascular disease who met inclusion criteria (Figure 1). Baseline characteristics are shown in Table 1. Median EF among HFrEF patients was 30% (IQR 10), among HFpEF was 54% (IQR 10), and among patients without cardiovascular disease was 57% (IQR 9). HFrEF patients were younger than HFpEF patients (median age 60 vs. 64), with a lower proportion of females (30.4% vs. 56.4%) and a similar proportion of African Americans (35.7% vs 27.8%). The rate of background neurohormonal therapy was similar between HFpEF and HFrEF, as was renal function (median eGFR 72 vs. 69.6 mg/dL/1.72m2).
Table 1.
Baseline Clinical Characteristics.
| Variable | HFrEF n=171 |
HFpEF n=374 |
No Prior CV Disease n=70 |
P value for HFrEF vs HFpEF |
|---|---|---|---|---|
| Age (yrs.) | 60 (50 – 69) | 64 (53 – 74) | 33 (26 – 41) | 0.002 |
| Female | 30.4% | 56.4% | 68.6% | <0.001 |
| Race | 0.026 | |||
| White or Caucasian | 59.1% | 62.8% | 60.0% | |
| Black or African American | 35.7% | 35.0% | 28.6% | |
| Biplane EF | 30 (25 – 35) | 54 (48 – 58) | 57 (53 – 62) | NA |
| Clinical Characteristics | ||||
| Atrial fibrillation | 25.7% | 32.9% | 0.0% | 0.093 |
| COPD | 7.0% | 11.5% | 0.0% | 0.107 |
| Hypertension | 81.3% | 80.7% | 0.0% | 0.882 |
| Type II Diabetes | 38.0% | 38.2% | 2.8% | 0.960 |
| Prior PCI/CABG | 53.8% | 48.4% | 0.0% | 0.242 |
| Prior CVA/TIA | 16.4% | 18.7% | 0.0% | 0.509 |
| ACE-I/ARB use | 65.5% | 61.8% | 0.0% | 0.403 |
| Beta Blocker use | 67.3% | 58.8% | 0.0% | 0.061 |
| BUN (mg/dL) | 17.0 (13.0 – 24.0) | 16.0 (12.0 – 23.0) | 10.0 (8.00 – 12.0) | 0.552 |
| Sodium (mmol/L) | 139 (137 – 141) | 139 (137 – 140) | 139 (137 – 140) | 0.655 |
| Creatinine (mg/dL) | 1.10 (0.90 – 1.40) | 1.00 (0.80 – 1.40) | 0.80 (0.70 – 0.90) | 0.093 |
| eGFR (mL/min/1.73m2) | 72.0 (56.6 – 91.9) | 69.6 (51.2 – 89.6) | 99 (83.3 – 111) | 0.426 |
| NT-proBNP (pg/mL) | 1073 (425 – 3117) | 428 (170 – 1296) | 21.0 (20.0 – 49.0) | <0.001 |
| MAP (mmHg) | 88 (80 – 100) | 90 (82 – 101) | 86 (79 – 92) | 0.187 |
| SBP (mmHg) | 120 (105 – 136) | 128 (116 – 142) | 116 (106 – 122) | <0.001 |
| DBP (mmHg) | 73 (67 – 81) | 71 (64 – 80) | 72 (65 – 80) | |
| Heart rate (beats/min) | 72 (63 – 84) | 66 (59 – 76) | 71 (62 – 78) | <0.001 |
NT-proBNP = n-terminal pro-brain natriuretic peptide, COPD=chronic obstructive pulmonary disease, PCI = percutaneous coronary intervention, CABG=coronary artery bypass surgery, CVA=cerebrovascular accident, TIA=transient ischemic attack, ACE-I=angiotensin converting enzyme inhibitor, ARB=angiotensin receptor blocker, BUN=blood urea nitrogen, eGFR=estimated glomerular filtration rate, MAP=mean arterial pressure, SBP=systolic blood pressure. No p-value is reported for EF comparison because the groups are expected to be different.
SET AND PATIENT OUTCOMES
Median SET was shorter (280ms, IQR 55ms) and PEP was longer (114ms, IQR 34ms) among patients with HFrEF compared with HFpEF and patients without cardiovascular disease (Table 2). Diastolic function parameters including deceleration time, relaxation time, and diastolic filling time were also impaired in patients with HFrEF versus patients with HFpEF or patients without cardiovascular disease. As would be expected, atrial and ventricular dimensions were larger among patients with HFrEF compared with HFpEF patients and patients without cardiovascular disease.
Table 2.
Echocardiographic Measurements.
| Variable | HFrEF (n=171) | HFpEF (n=374) | No Prior CV Disease (n=70) | P values for HFrEF vs. HFpEF |
|---|---|---|---|---|
| SET (ms) | 280 (251 – 306) | 315 (288 – 339) | 309 (291 – 317) | <0.001 |
| PEP (ms) | 114 (96 – 130) | 89 (75 – 105) | 78 (71 – 90) | <0.001 |
| PEP/SET | 0.4 (0.3 – 0.5) | 0.3 (0.2 – 0.3) | 0.3 (0.2 – 0.3) | <0.001 |
| LVEDD (cm) | 5.86 (5.30 – 6.50) | 4.81 (4.29 – 5.27) | 4.62 (4.35 – 4.98) | <0.001 |
| LA Volume index (mL/m2) | 39 (33 – 49) | 36 (28 – 47) | 28 (25 – 36) | 0.060 |
| Deceleration Time (ms) | 164.0 (127.7 – 206.0) | 196.0 (164.0 – 230.7) | 173.3 (153.7 – 203.3) | <0.001 |
| Diastolic Filling Time (ms) | 351 (253 – 455) | 411 (329 – 519) | 394 (340 – 519) | <0.001 |
| Relaxation Time (ms) | 93.3 (67.3 – 122.0) | 78.7 (58.0 – 104.7) | 64.0 (49.7 – 78.3) | <0.001 |
SET AND OUTCOMES
Among HFrEF patients, the rate of all-cause hospitalization was 44%, HF hospitalization was 20.4%, death was 9.9%, and death/HF hospitalization was 26.9% at 1 year. For HFpEF patients, the rate of all-cause hospitalization was 34.8%, HF hospitalization was 5.9%, death was 7.0%, and death/HF hospitalization was 11.8% at 1 year.
In unadjusted analyses for HFrEF patients, longer SET was associated with lower odds of 1-year HF hospitalization and reductions of 1-year death/HF hospitalization (Table 3). After multivariable adjustment, the association with 1-year death/HF hospitalization remained statistically significant. There was also an association with reduction in all-cause mortality at 1 year after multivariable adjustment (Table 3), OR (95% CI): 0.752 (0.596, 0.949). To further explore the association between SET and outcomes among HFrEF patients, we created Kaplan-Meier Plots of SET by quartile which demonstrate that patients in the lowest two quartiles of SET compared to highest two quartiles had worse outcomes, with divergence in curves within the first 100 days (Figure 3).
Table 3.
Unadjusted and Adjusted Odds Ratios for 1-year Outcomes.
| Heart Failure Reduced EF (n=171) | Heart Failure Preserved EF (n=374) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||
| Odds Ratio Per 10ms increase in SET (95% CI) |
p-value | Odds Ratio Per 10ms increase in SET (95% CI) |
p-value | Odds Ratio Per 10ms increase in SET (95% CI) |
p-value | Odds Ratio Per 10ms increase in SET (95% CI) |
p-value | |
| 1 Year All Cause Hospitalization (HFrEF n=75; HFpEF n=130) |
0.955 (0.890 – 1.025) |
0.205 | 0.957 (0.839 – 1.091) |
0.551 | 1.004 (0.951 – 1.060) |
0.880 | 1.003 (0.921 – 1.093) |
0.942 |
| 1 Year HF Hospitalization (HFrEF n=35; HFpEF n=22) |
0.913 (0.835 – 0.997) |
0.044 | 0.890 (0.758 – 1.046) |
0.157 | 0.965 (0.865 – 1.077) |
0.527 | 1.138 (0.936 – 1.384) |
0.196 |
| 1 Year Death (HFrEF n=17; HFpEF n=26) |
0.907 (0.806 – 1.021) |
0.107 | 0.746 (0.594 – 0.936) |
0.011 | 0.947 (0.855 – 1.048) |
0.293 | 0.967 (0.821 – 1.139) |
0.687 |
| 1 Year Death/HF Hospitalization (HFrEF n=46; HFpEF n=44) |
0.895 (0.824 – 0.973) |
0.009 | 0.829 (0.712 – 0.965) |
0.015 | 0.962 (0.888 – 1.043) |
0.352 | 1.043 (0.913 – 1.192) |
0.536 |
The n in the first column indicates number of patients with outcome event.
Adjustment variables: Age, sex, race, BMI, Ejection Fraction, Heart Rate, Creatinine, BUN, Sodium, PP, Beta Blocker Use, ACE-I Use, and E/E’
Figure 3. Kaplan-Meier Curve for Outcomes.
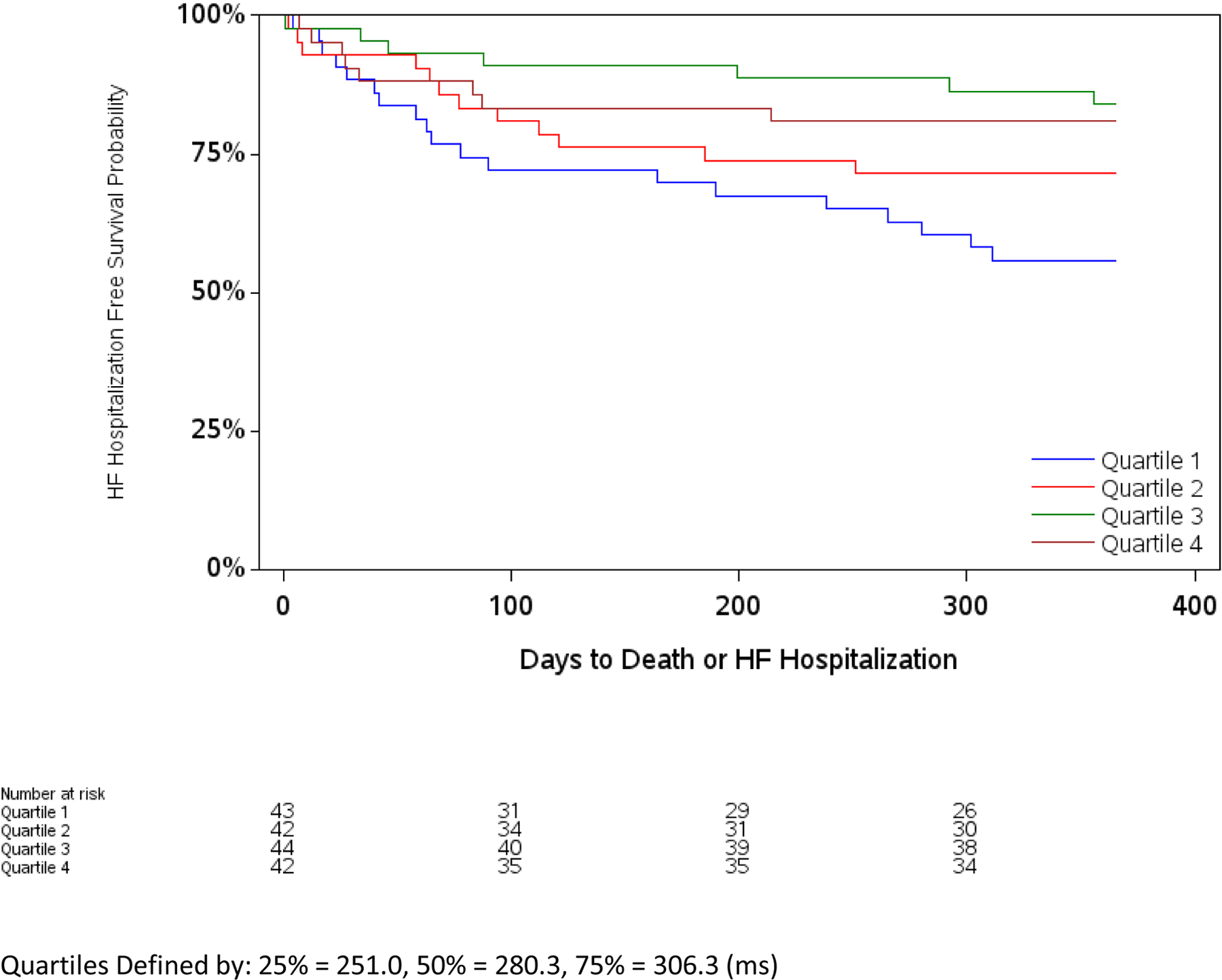
These event plots show death or HF hospitalization by SET quartile among patients with heart failure and reduced ejection fraction.
In contrast, longer SET was not associated with improved outcomes among patients with HFpEF. We further explored the relationship between SET and outcomes among HFpEF patients and performed a sensitivity analysis separating out patients with HF with a mid-range ejection fraction (HFmrEF) (i.e., defined as a EF 41–49%) from a more stringently defined HFpEF patient population with a frankly preserved EF ≥50% (Supplemental Table 2). In short, the directionality of the results and the inferences of the findings were fundamentally unchanged.
DISCUSSION
To the best of our knowledge, this is the first comprehensive analysis of SET in patients with ambulatory HF compared to healthy controls without known cardiovascular disease. In general, HFrEF patients were younger and more likely to be male, had a lower incidence of cardiac and non-cardiac comorbidities, and were more likely to be prescribed an ACEI/ARB and/or β-blocker at baseline compared to HFpEF patients. HFrEF patients also tended to have dilated cardiac chambers (i.e. LVEDD and LAVI) and marked abnormalities in echocardiographic parameters of both systolic (i.e. shorter SET) and diastolic function (i.e. rapid deceleration time). After adjusting for potential confounders, shorter SET was independently associated with increased risk of death or hospitalization for HF in patients with HFrEF but not HFpEF.
The echocardiographic parameters of systolic and diastolic function assessed in the present analysis may improve our collective understanding of the natural history and pathophysiology of HFrEF and HFpEF compared with healthy controls. Although EF is the most widely used surrogate marker of systolic function and has important clinical implications for the diagnosis, prognosis, and treatment of HFrEF, there is substantial interobserver variability in measuring EF (11, 12). In addition, EF is dependent on loading conditions and there may be a temporal lag between unfavorable changes in other echocardiographic measures of systolic function such as global longitudinal strain and clinically discernible decreases in EF or signs and symptoms of HF (13). This study clearly demonstrates that SET can be measured in an accurate and reproducible manner and is independently associated with an increased risk of morbidity and mortality among HFrEF patients even after adjusting for LVEF and heart rate, suggesting that SET is clinically useful to measure as part of a comprehensive assessment of global left ventricular function. Our data although promising, highlight a need for additional real-world prospective cohort studies to identify the natural history of SET changes among patients with HF with progressive systolic dysfunction, and to further characterize normal versus abnormal SET values as well as the added prognostic value of the ratio of PEP/SET, which although more technically challenging to measure in clinical practice may be the single most useful measure of LV systolic function (1, 2). Patients with HFrEF and the lowest SET values had the greatest clinical event rates, suggesting a hypothesis that progressive systolic dysfunction leads to shortening of SET as stroke volume falls and cardiac output becomes heart rate-dependent.
In contrast to patients with HFrEF, we found that there were relatively few clinically meaningful differences in echocardiographic parameters between patients with HFpEF and healthy controls without cardiovascular disease. This finding highlights the challenges of diagnosing HFpEF and underscores our poor understanding of HFpEF as a clinical entity (14–16). Traditionally, the prototypical HFpEF patient has been described as having a small ventricle with marked concentric hypertrophy and diastolic dysfunction. However, we now know that this classic phenotype is present in only one-third of HFpEF patients (17, 18). This is consistent with the present analysis which found that the LVEDD of patients with HFpEF was marginally greater than healthy controls. It also noteworthy that we found the median deceleration time was approximately 200 ms, suggesting that the majority of patients had normal or grade I diastolic dysfunction and presumably normal left-sided filling pressures at rest. However, these patients likely have diminished diastolic reserve and with activity experience an increase in left-sided filling pressures, a hypothesis which is consistent with the finding that the majority of HFpEF patients had moderate-severe left atrial enlargement (19–21). It also notable that SET was comparable between HFpEF patients and healthy controls and SET was not independently associated with clinical outcomes among HFpEF patients. It has long been recognized that HFpEF patients have impairments in systolic function that are not captured by EF; nonetheless, these data do not support an incremental role for routinely measuring SET as a surrogate of systolic function in HFpEF patients (22).
In addition to having a putative role in the assessment of systolic function in HFrEF patients, there has also been recent interest in SET as a therapeutic target for emerging and novel pharmacotherapies. Although the SHIFT trial demonstrated that ivabradine, a selective sinus node inhibitor, reduced heart rate and improved the composite of cardiovascular mortality or hospital admission for worsening HF, the mechanism of benefit remains unknown (23–25). It has been speculated that HF is characterized by impairments in substrate utilization and cellular metabolism and that ivabradine may reduce oxygen consumption in the energy-starved myocardium leading to improved clinical outcomes. However, a complementary hypothesis is that by reducing heart rate, ivabradine may also increase SET and facilitate improvements in overall myocardial performance. In addition, data from the omecamtiv mecarbil program have raised the hypothesis that SET may also be a mediator of improved clinical outcomes (26, 27). The results of the ATOMIC and COSMIC trials demonstrate that omecamtiv mecarbil decreases LV dimensions and normalizes SET (26, 27). GALACTIC is an ongoing cardiovascular outcomes trial designed to test the hypothesis that improvements in cardiac performance seen with omecamtiv mecarbil in phase II trials translate into benefits on cardiovascular morbidity and mortality.
There are several limitations of the data that should be acknowledged. First, this was a retrospective, single-center study and the findings presented should be interpreted as hypothesis-generating. Duke is a tertiary referral center and therefore the study population may not necessarily represent an unselected community-based cohort of ambulatory HF patients. Second, SET was measured in a blinded fashion by the study team, and the accuracy and reproducibility of the measurements was only assessed in a subset of the overall study cohort. However, this suggests that the measurements are reliable and the results generalizable to everyday clinical practice. Third, it is possible that hospitalizations may have occurred outside of the Duke University Health System and not captured. We attempted to minimize this risk by including only those patients that had multiple clinical visits in the 18 months prior to index TTE who presumptively received care within the health system. Finally, although the healthy control group free of cardiovascular disease provide a convenient baseline estimate of the reference range for normal values for SET in healthy patients free of cardiovascular disease undergoing a transthoracic echocardiogram in our laboratory, there are profound between-group differences in demographics and clinical characteristics that preclude potential inferences regarding the relative contribution of HF in and of itself to differences in measurements of SET.
Conclusions
In conclusion, we found that patients with symptomatic HFrEF had a shorter SET compared to both HFpEF patients and patients free of cardiovascular disease. In addition, SET was independently associated with increased risk of cardiovascular morbidity and mortality in HFrEF. These data suggest that SET, when measured accurately, has an important role in the global assessment of systolic function for HFrEF patients. Additional research is required to better understand normal ranges for SET and to explore the natural history of SET with progressive systolic dysfunction. Ongoing and future clinical trials may further validate the importance of SET as a surrogate endpoint for early phase drug development programs.
Supplementary Material
Acknowledgements:
The authors thank Michael MacKenzie for his role in data extraction and aggregation and John Clement for his input on statistical methods.
Funding sources: This work was funded in part by a research grant from Amgen to EJV. PAP was funded by NIH grant T32-HL007101 and Amgen. APA was funded by NIH grant 5T32HL069749.
Disclosures: PAP received research support from Amgen. EJV has research grants from NHLBI, Alnylam Pharmaceuticals, Amgen, Inc., Novartis Pharmaceutical Corp. and Pfizer. Consulting services for ABIOMED, Amgen, Inc., Merck & Co., Novartis Pharmaceutical Corp., and Philips Ultrasound, Inc. NH is an Amgen employee and stockholder. MKV was employed by Amgen when this research was conducted.
Abbreviations
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- SET
systolic ejection time
- PEP
pre-ejection period
- TTE
transthoracic echocardiogram
- LVOT
left ventricular outflow tract
- TVI
time-velocity integral
- eGFR
estimated glomerular filtration rate
- LVEDD
left ventricular end-diastolic dimension
- LAVI
left atrial volume index
REFERENCES
- 1.Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation. 1968. February;37(2):149–159. [DOI] [PubMed] [Google Scholar]
- 2.Weissler AM, Peeler RG, Roehll WH Jr. Relationships between left ventricular ejection time, stroke volume, and heart rate in normal individuals and patients with cardiovascular disease. American heart journal. 1961. September;62:367–378. [DOI] [PubMed] [Google Scholar]
- 3.Sasao H, Noda R, Hasegawa T, Endo A, Oimatsu H, Takada T. Prognostic value of the Tei index combining systolic and diastolic myocardial performance in patients with acute myocardial infarction treated by successful primary angioplasty. Heart and vessels. 2004. March;19(2):68–74. [DOI] [PubMed] [Google Scholar]
- 4.Reant P, Dijos M, Donal E, Mignot A, Ritter P, Bordachar P, Dos Santos P, Leclercq C, Roudaut R, Habib G, Lafitte S. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two-dimensional strain. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2010. December;11(10):834–844. [DOI] [PubMed] [Google Scholar]
- 5.Migrino RQ, Mareedu RK, Eastwood D, Bowers M, Harmann L, Hari P. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009. December;22(12):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haiden A, Eber B, Weber T. U-shaped relationship of left ventricular ejection time index and all-cause mortality. American journal of hypertension. 2014. May;27(5):702–709. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sorensen T, Querejeta Roca G, Hegde SM, Shah AM, Claggett B, Mosley TH Jr., Butler KR Jr., Solomon SD. Left ventricular ejection time is an independent predictor of incident heart failure in a community-based cohort. European journal of heart failure. 2017. September 4. [DOI] [PMC free article] [PubMed]
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2015. March;16(3):233–270. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent, Liege B, Cleveland O, Novara I, Rochester M, Bucharest R, Louis M St.. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2016. December;17(12):1321–1360. [DOI] [PubMed] [Google Scholar]
- 10.Daubert MA, Yow E, Barnhart HX, Rabineau D, Crowley AL, Douglas PS. Quality Improvement Implementation: Improving Reproducibility in the Echocardiography Laboratory. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015. August;28(8):959–968. [DOI] [PubMed] [Google Scholar]
- 11.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ, American Society of E. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004. October;17(10):1086–1119. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. January;28(1):1–39 e14. [DOI] [PubMed] [Google Scholar]
- 13.Khouri MG, Hornsby WE, Risum N, Velazquez EJ, Thomas S, Lane A, Scott JM, Koelwyn GJ, Herndon JE, Mackey JR, Douglas PS, Jones LW. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res Treat. 2014. February;143(3):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O’Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2014. April;2(2):97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaduganathan M, Michel A, Hall K, Mulligan C, Nodari S, Shah SJ, Senni M, Triggiani M, Butler J, Gheorghiade M. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail. 2016. January;18(1):54–65. [DOI] [PubMed] [Google Scholar]
- 16.Butler J, Hamo CE, Udelson JE, Pitt B, Yancy C, Shah SJ, Desvigne-Nickens P, Bernstein HS, Clark RL, Depre C, Dinh W, Hamer A, Kay-Mugford P, Kramer F, Lefkowitz M, Lewis K, Maya J, Maybaum S, Patel MJ, Pollack PS, Roessig L, Rotman S, Salsali A, Sims JJ, Senni M, Rosano G, Dunnmon P, Stockbridge N, Anker SD, Zile MR, Gheorghiade M. Exploring New Endpoints for Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016. November;9(11). [DOI] [PubMed] [Google Scholar]
- 17.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD, Investigators T. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014. January;7(1):104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014. September;7(5):740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011. July;4(4):444–455. [DOI] [PubMed] [Google Scholar]
- 20.Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005. January;18(1):63–68. [DOI] [PubMed] [Google Scholar]
- 21.Oh JK, Kane GC. Diastolic stress echocardiography: the time has come for its integration into clinical practice. J Am Soc Echocardiogr. 2014. October;27(10):1060–1063. [DOI] [PubMed] [Google Scholar]
- 22.DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, Anstrom KJ, Hernandez AF, Velazquez EJ. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017. July;19(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teerlink JR. Ivabradine in heart failure--no paradigm SHIFT…yet. Lancet. 2010. September 11;376(9744):847–849. [DOI] [PubMed] [Google Scholar]
- 24.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators S. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010. September 11;376(9744):875–885. [DOI] [PubMed] [Google Scholar]
- 25.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators S. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010. September 11;376(9744):886–894. [DOI] [PubMed] [Google Scholar]
- 26.Teerlink JR, Felker GM, McMurray JJ, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JG, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, Investigators A-A. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J Am Coll Cardiol. 2016. March 29;67(12):1444–1455. [DOI] [PubMed] [Google Scholar]
- 27.Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF, Jr., Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsanyi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, Investigators C-H. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016. December 10;388(10062):2895–2903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


