Abstract
Improved treatment of congenital heart defects (CHDs) has resulted in women with CHDs living to childbearing age. However, no US population-based systems exist to estimate pregnancy frequency or complications among women with CHDs. Cases were identified in multiple data sources from 3 surveillance sites: Emory University (EU) whose catchment area included 5 metropolitan Atlanta counties; Massachusetts Department of Public Health (MA) whose catchment area was statewide; and New York State Department of Health (NY) whose catchment area included 11 counties. Cases were categorized into one of 5 mutually exclusive CHD severity groups collapsed to severe versus not severe; specific ICD-9-CM codes were used to capture pregnancy, gestational complications, and nongestational co-morbidities in women, age 11 to 50 years, with a CHD-related ICD-9-CM code. Pregnancy, CHD severity, demographics, gestational complications, co-morbidities, and insurance status were evaluated. ICD-9-CM codes identified 26,655 women with CHDs, of whom 5,672 (21.3%, range: 12.8% in NY to 22.5% in MA) had codes indicating a pregnancy. Over 3 years, age-adjusted proportion pregnancy rates among women with severe CHDs ranged from 10.0% to 24.6%, and 14.2% to 21.7% for women with nonsevere CHDs. Pregnant women with CHDs of any severity, compared with nonpregnant women with CHDs, reported more noncardiovascular co-morbidities. Insurance type varied by site and pregnancy status. These US population-based, multisite estimates of pregnancy among women with CHD indicate a substantial number of women with CHDs may be experiencing pregnancy and complications. In conclusion, given the growing adult population with CHDs, reproductive health of women with CHD is an important public health issue.
Congenital heart defects (CHDs) vary in phenotypes and outcomes,1 with adults now comprising an estimated 2/3 of individuals with CHDs.2 Annual deliveries for women with CHDs increased 34.9% from 1998 to 2007, compared with 21.3% in the general population.3 Since pregnant women with CHDs face increased risk of maternal complications and mortality,4 data on pregnancies and their outcomes are needed to assist women with CHDs with reproductive decisions. Countries with national databases can prospectively track cohorts of pregnant women with CHDs,5 making it possible to estimate maternal risks in pregnancy.6–8 However, international databases do not reflect the diversity and healthcare patterns in the United States. Pregnancy outcomes are likely influenced by socioeconomic standing, healthcare access, provider knowledge, and chronic conditions.9–11 We analyzed data from 3 US surveillance sites to estimate the proportion of women with CHDs who became pregnant, their co-morbidities, insurance status, and gestational complications.
Methods
This analysis was a component of a larger CDC surveillance project of adolescents and adults with CHDs at 3 funded US sites: EU (Atlanta, GA), MA, and NY (Centers for Disease Control and Prevention, Grant/Award Number: CDC–RFA–DD12–1207). Overall project methods are described in a separate methods paper.12 For this analysis, cases were females aged 11 to 50 years (adolescents 11 to 19 years, adults 20 to 50 years), who resided in site-specific catchment areas: 5 metropolitan Atlanta counties for EU, 11 counties across NY state, and statewide for MA. All cases were presumed alive as of January 1, 2010, and had at least one healthcare encounter with an eligible CHD International Classification of Disease version 9.0 Clinical Modification (ICD-9-CM) code between January 1, 2008 (January 1, 2009 for MA) and December 31, 2010. Data sources included outpatient clinics, Medicaid claims, outpatient and hospital encounter data, and information from supplemental sources including state vital records (EU, MA), and birth defects registries (EU, NY). Participating sites had varying populations and data sources, completeness, and linkage methodologies.12
Cases identified as previously described12 were categorized into one of 5 mutually exclusive hierarchical groups based on anatomy: severe, shunt, valve, shunt plus valve, and other CHDs.12,13 The latter 4 categories were grouped as “not severe” for comparison to severe. The severe group included CHDs that typically require surgery in the first year of life such as pulmonary atresia, tetralogy of Fallot, or single ventricle defects.12 We excluded cases that had 745.5 code (secundum atrial septal defect or patent foramen ovale) without other specific CHD codes because 745.5 frequently includes persons without CHDs.14
We used Clinical Classification Software (CCS), developed by the Agency for Healthcare Research and Quality (AHRQ) to categorize non-CHD administrative codes. CCS collapses over 15,000 ICD-9-CM diagnostic and 3,900 procedure codes into clinical categories.15 We identified females who experienced pregnancies during the specified time period (‘pregnant women’) by the presence of at least one of 1,520 ICD-9-CM codes that were collapsed into 27 CCS categories (eTable A) occurring at any time during a woman’s 2008 to 2010 encounter history. Pregnancy onset and outcome were not available; therefore the timing and frequency of pregnancies for an individual woman could not be determined. Project clinicians identified, categorized and grouped non-CHD ICD-9-CM codes into maternal gestational complications based on individual ICD-9-CM codes for pregnancy or post-partum-specific conditions (eTable B) and into comorbidity categories not necessarily related to pregnancy (eTable C).15 For instance, ICD-9-CM codes 642.00–642.34 and 642.90–4 are used for gestational hypertension, and codes 401.1 and 401.9 are used for essential hypertension. Clinicians categorized gestational complications, and nongestational co-morbid conditions into cardiovascular and noncardiovascular categories for analysis. We assessed cases with a fetal echocardiogram Current Procedural Terminology (CPT) code (76825–8) for the presence of a CHD code outside of the fetal echocardiogram encounters to distinguish maternal CHD from potential CHD in their fetus.
Identified cases could have multiple healthcare encounters in several data sources. We combined, de-identified, and de-duplicated encounter and summary data to create a common collaborative analytic dataset across sites. We classified insurance status, based on primary health insurance type for the healthcare encounter nearest to January 1, 2010 into 4 categories: (1) self-pay/uninsured; (2) private/commercial; (3) government-based [Medicaid, Medicare/Social Security Income (SSI), Tricare]; and (4) other.
We conducted all analyses, except the age-adjusted proportion pregnant, using Statistical Analysis System (SAS) 9.4 TM Level IM5, w32_10Pro platform.16 Frequencies, percentages, and chi-square analyses of major categorical variables including CHD severity, age, pregnancy status (between 2008 and 2010), and insurance were conducted by site and pooled across sites. Chi-square analyses were used to compare frequencies of cardiovascular and noncardiovascular gestational complications and co-morbidities by CHD status, site and pregnancy status. To control for effects of maternal age on gestational complications and co-morbidities unrelated to pregnancy, sensitivity analyses were conducted constraining age to 20 to 35 year olds. Chi-square tests were computed to analyze the association of CHD severity with gestational cardiovascular and noncardiovascular complications. While crude pregnancy proportion is a useful summary measure comparing similar populations of different sizes, it is sensitive to differences in age distribution. Thus, we calculated age-adjusted proportion pregnant, which adjusts for the observed differences in age distributions across sites.
Results
During the project period, 26,655 females, ages 11 to 50 years, had at least 1 healthcare encounter with 1 or more CHD ICD-9-CM codes. Of these, 5672 (21.3%) had at least 1 pregnancy-related code (Table 1). Overall, EU had a higher proportion of females with severe CHDs (30.7%) compared with MA (17.8%) and NY (19.3%), regardless of pregnancy status (data not shown). Among women with severe CHD, NY had the lowest age-adjusted proportion pregnant and MA had the highest age-adjusted proportion (Table 1). EU had the same age-adjusted proportion pregnant irrespective of CHD severity. EU and NY sites had similar proportions of gestational cardiovascular complications including gestational hypertension, pre-eclampsia, and other cardiovascular complications, while MA had few pregnancy-related cardiovascular complications. Noncardiovascular complications among pregnant women varied across sites, including preterm labor, anemia, and hemorrhage (Table 2). Pooled across site and severity, pregnant women compared with nonpregnant women had significantly more nongestational cardiovascular co-morbidities including essential hypertension, hyperlipidemia, coronary artery disease, and venous disorder/phlebitis (Table 3). Differences in cardiovascular co-morbidities for pregnant compared with nonpregnant women varied by site (Table 3). While hypertension, hyperlipidemia, coronary artery disease, and venous disorder/phlebitis were more frequent among pregnant women compared with those who were not in MA and EU, this pattern was not seen in NY (Table 3). Pooled across site and severity, pregnant women compared with nonpregnant women also had significantly more nongestational noncardiovascular co-morbidities, including nongestational diabetes mellitus, hematologic conditions, gastrointestinal conditions, respiratory and pulmonary problems, infectious diseases, and genitourinary/gynecology problems (Table 4).
Table 1.
Pregnancy during project period, by age, congenital heart defect (CHD) severity, and surveillance site, including age-adjusted proportion pregnant, for women age 11–50 years with CHD^^, 2008 to 2010
| Pregnant | |||||
|---|---|---|---|---|---|
| TOTAL (n = 26,655) | Yes n = 5,672 (21.3%) | No 20,983 (78.7%) | |||
| Severe CHD 1,126 (19.8%) | Not Severe CHD 4,546 (80.2%) | Severe CHD 3,893 (18.5%) | Not Severe CHD 17,090 (81.5%) | ||
| EU* (n = 1,994)^ | |||||
| Pregnancy Status | n = 275 (13.8%) | n = 1,719 (86.2%) | |||
| Severe CHD | Not Severe CHD | Severe CHD | Not Severe CHD | ||
| Age-Adjusted Proportion Pregnant by CHD Severity | 14.2 | 14.2 | |||
| % Severe by Pregnancy Status | 84 (30.5%) | 191 (69.5%) | 529 (30.8%) | 1,190 (69.2%) | |
| CHD Severity by Pregnancy Status and Age | 11–19 | 10 (38.5%) | 16 (61.5%) | 261 (33.9%) | 508 (66.1%) |
| 20–29 | 47 (33.6%) | 93 (66.4%) | 148 (37.5%) | 247 (62.5%) | |
| 30–39 | 23 (24.5%) | 71 (75.5%) | 93 (32.6%) | 192 (67.4%) | |
| 40–50 | 4 (26.7%) | 11 (73.3%) | 27 (10.0%) | 243 (90.0%) | |
| MA* (n = 23,203)^ | |||||
| Pregnancy Status | n = 5,211 (22.5%) | n = 17,992 (77.5%) | |||
| Severe CHD | Not Severe CHD | Severe CHD | Not Severe CHD | ||
| Age-Adjusted Proportion Pregnant by CHD Severity | 24.6 | 21.7 | |||
| % Severe by Pregnancy Status | 1,028 (19.7%) | 4,183 (80.3%) | 3,097 (17.2%) | 14,895 (82.8%) | |
| CHD Severity by Pregnancy Status and Age | 11–19 | 198 (22.8%) | 670 (77.2%) | 1,014 (18.4%) | 4,486 (81.6%) |
| 20–29 | 381 (20.6%) | 1471 (69.8%) | 710 (18.7%) | 3,077 (81.3%) | |
| 30–39 | 254 (17.7%) | 1184 (82.3%) | 582 (16.3%) | 2,980 (83.7%) | |
| 40–50 | 195 (18.5%) | 858 (81.5%) | 791 (15.4%) | 4,352 (84.6%) | |
| NY* (n = 1,458)^ | |||||
| Pregnancy Status | n = 186 (12.8%) | n = 1,272 (87.2%) | |||
| Severe CHD | Not Severe CHD | Severe CHD | Not Severe CHD | ||
| Age-Adjusted Proportion Pregnant by CHD Severity | 10.0 | 21.5 | |||
| % Severe by Pregnancy Status | n = 14 (7.5%) | n = 172 (92.5%) | n = 267 (21.0%) | n = 1,005 (79.0%) | |
| CHD Severity by Pregnancy Status and Age | 11–19 | <10 | 43 (93.5%) | 202 (23.6%) | 653 (76.4%) |
| 20–29 | <10 | 73 (92.4%) | 35 (29.4%) | 84 (70.6%) | |
| 30–39 | <10 | 50 (92.6%) | 14 (14.3%) | 84 (85.7%) | |
| 40–50 | <10 | <10 | 16 (8.0%) | 184 (92.0%) | |
CHD = Congenital Heart Defect; see eTable D for CHD classification by ICD-9-CM codes using modified Marelli scheme.
Pregnant: experienced a pregnancy during 2008 to 2010; nonpregnant: did not experience a pregnancy during 2008 to 2010.
EU=Emory University; catchment area incl. 5 metro-Atlanta counties: Clayton, Cobb, DeKalb, Fulton and Gwinnett; MA=Massachusetts Dept. of Public Health; catchment area is state-wide; NY=New York State Dept. of Health; catchment area incl. 11 counties: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester & Wyoming
Percent of total by site: EU: 1,994/26,655 = 7.5%; MA: 23,203/26,655=87.0%; NY: 1,458/26,655=5.5%.
Individuals classified with ICD-9-CM code 745.5 in isolation are not included. Note. Cells with <10 count indicated as <10.
Table 2.
Prevalence of gestational complications for women with congenital heart defects (CHD)^^ at 3 surveillance sites, 2008 to 2010**
| EU* n = 275 (4.9%) | MA* n = 5,211 (91.9%) | NY* n = 186 (3.3%) | |
|---|---|---|---|
| Cardiovascular Complications | |||
| Hypertension in Pregnancy | 35 (12.7%) | 60 (1.2%) | 14 (7.5%) |
| Pre-Eclampsia | 20 (7.3%) | 44 (0.8%) | 12 (6.5%) |
| Other Cardiovascular Disorders | 60 (21.8%) | 46 (0.9%) | 48 (25.8%) |
| Non-Cardiovascular Complications | |||
| Preterm Labor | 98 (35.6%) | 142 (2.7%) | 19 (10.2%) |
| Gestational Diabetes | 33 (12.0%) | 130 (2.5%) | 11 (5.9%) |
| Anemia in Pregnancy | 56 (20.4%) | 45 (0.9%) | 31 (16.7%) |
| Hyperemesis | 39 (14.2%) | 66 (1.3%) | 14 (7.5%) |
| Infectious Disease in Pregnancy | 45 (16.4%) | 41 (0.8%) | 21 (11.3%) |
| Genitourinary / Gynecology in Pregnancy | 51 (18.6%) | 39 (0.8%) | 23 (12.4%) |
| Obesity in Pregnancy | 28 (10.2%) | 35 (0.7%) | <10 |
| Hemorrhage in Pregnancy | 60 (21.8%) | 120 (2.3%) | 36 (19.4%) |
| Prolonged Pregnancy | 23 (8.4%) | 42 (0.8%) | 24 (12.9%) |
| Malposition (malpresentation of fetus) | 35 (12.7%) | 40 (0.8%) | 14 (7.5%) |
| Smoking in Pregnancy | 13 (4.7%) | 22 (0.4%) | 15 (8.1%) |
| Mental Disorders in Pregnancy | 11 (4.0%) | 37 (0.7%) | 22 (11.8%) |
| Other Pregnancy Loss | 28 (10.2%) | 153 (2.9%) | 20 (10.8%) |
| Other Obstetrical Complications | 98 (35.6%) | 120 (2.3%) | 69 (37.1%) |
CHD = Congenital Heart Defect.
EU=Emory University; catchment area includes 5 metro-Atlanta counties: Clayton, Cobb, DeKalb, Fulton and Gwinnett; MA=Massachusetts Department of Public Health; catchment area is state-wide; and NY=New York State Department of Health; catchment area includes 11 counties: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester, and Wyoming
Data collapsed across CHD severity.
Individuals classified with ICD-9_CM code 745.5 in isolation are not included. Cells with <10 count indicated as <10.
Table 3.
Prevalence of cardiovascular co-morbidities for women with congenital heart defects (CHD)^^ by pregnancy during project period, overall and by surveillance site, 2008 to 2010**
| Hypertension | Hyperlipidemia | Coronary Artery Disease | Stroke, Thrombosis & Other Cardiovascular | Arrhythmias & Conduction Disorders | Venous Disorder / Phlebitis | Heart Failure | |
|---|---|---|---|---|---|---|---|
| Pooled Across Sites (n = 26,655) | |||||||
| Pregnant n = 5,672 (21.3%) | 31.1% | 18.7% | 10.3% | 39.4% | 25.0% | 6.2% | 10.8% |
| Not Pregnant n = 20,983 (78.7%) | 21.0% | 14.8% | 7.8% | 58.6% | 33.5% | 3.9% | 12.2% |
| p_value | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.01 |
| EU* (n = 1,994) | |||||||
| Pregnant n = 275 (13.8%) | 13.5% | 5.1% | 9.1% | 51.3% | 26.6% | 5.8% | 7.3% |
| Not Pregnant n = 1,719 (86.2%) | 9.5% | 4.9% | 5.2% | 47.8% | 21.5% | 3.0% | 4.3% |
| p_value | p <0.05 | p <0.918 | p <0.05 | p <0.279 | p <0.063 | p <0.05 | p <0.05 |
| MA* (n = 23,203) | |||||||
| Pregnant n = 5,211 (22.5%) | 32.9% | 20.0% | 10.6% | 38.4% | 25.0% | 6.4% | 11.2% |
| Not Pregnant n = 17,992 (77.5%) | 22.7% | 16.3% | 8.2% | 61.0% | 35.8% | 4.0% | 13.3% |
| p_value | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.001 | p <0.001 |
| NY * (n = 1,458) | |||||||
| Pregnant n = 186 (12.8%) | 8.6% | 1.1% | 3.8% | 49.5% | 21.5% | 1.6% | 5.9% |
| Not Pregnant n = 1,272 (87.2%) | 12.5% | 6.1% | 5.3% | 39.3% | 16.9% | 2.8% | 6.6% |
| p_value | p <0.127 | p <0.01 | p <0.362 | p <0.01 | p <0.123 | p <0.336 | p <0.722 |
CHD = Congenital Heart Defect.
EU=Emory University; catchment area incl. 5 metro-Atlanta counties: Clayton, Cobb, DeKalb, Fulton and Gwinnett; MA=Massachusetts Dept. of Public Health; catchment area is state-wide; NY=New York State Dept. of Health; catchment area incl. 11 counties: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester & Wyoming.
Data collapsed across CHD severity.
Individuals classified with ICD-9_CM code 745.5 in isolation are not included.
Table 4.
Prevalence of noncardiovascular co-morbidities for women with congenital heart defects (CHD) by pregnancy during project period, pooled across and by 3 surveillance sites, 2008 to 2010**
| Overall n = 26,655 | EU* n = 1,994 | MA n = 23,203 | NY* n = 1,458 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnant n = 5,672 (21.3%) | Not Pregnant n = 20,983 (21.3%) | p_value | Pregnant n = 275 (13.8%) | Not Pregnant n = 1,192 (86.2%) | p_value | Pregnant n = 5,211 (22.5%) | Not Pregnant n = 17,992 (21.3%) | p_value | Pregnant n = 186 (12.8%) | Not Pregnant n = 1,272 (87.2%) | p_value | |
| Diabetes Mellitus | 23.6% | 17.2% | <0.001 | 6.9% | 3.0% | 0.001 | 25.3% | 19.3% | <0.001 | <10 | 5.5% | ns |
| Other Endocrine | 16.5% | 12.0% | <0.001 | 6.2% | 4.0% | Ns | 17.5% | 13.1% | <0.001 | <10 | 6.5% | ns |
| Birth Defects | 20.4% | 15.8% | <0.001 | 8.7% | 12.3% | Ns | 21.5% | 16.1% | <0.001 | 5.9% | 15.3% | 0.001 |
| Hematologic | 22.5% | 17.6% | <0.001 | 27.3% | 10.7% | <0.001 | 22.2% | 18.7% | <0.001 | 24.7% | 12.0% | <0.001 |
| Neoplasms | 18.8% | 14.6% | <0.001 | 21.5% | 6.5% | <0.001 | 19.1% | 15.9% | <0.001 | <10 | 7.2% | ns |
| Gastrological | 36.4% | 31.3% | <0.001 | 33.8% | 15.3% | <0.001 | 36.7% | 33.2% | <0.001 | 31.7% | 25.2% | ns |
| Other Genitourinary | 7.7% | 9.3% | <0.001 | 6.2% | 2.8% | 0.01 | 7.9% | 10.4% | <0.001 | <10 | 3.8% | ns |
| CNS | 12.6% | 13.3% | ns | 5.5% | 5.0% | ns | 13.3% | 14.7% | 0.01 | 5.9% | 5.8% | ns |
| Immunologic/Rheumatologic/Allergy | 3.3% | 3.5% | ns | 3.6% | 1.5% | 0.01 | 3.4% | 3.7% | ns | <10 | 2.9% | ns |
| Respiratory/Pulmonary | 40.5% | 38.2% | 0.01 | 38.6% | 25.5% | <0.001 | 40.9% | 40.1% | ns | 30.7% | 29.3% | ns |
| Musculoskeletal | 43.6% | 36.4% | <0.001 | 28.4% | 14.5% | <0.001 | 44.9% | 39.6% | ns | 26.9% | 20.4% | 0.05 |
| Injury/Trauma | 16.9 % | 15.9% | ns | 17.5% | 8.0% | <0.001 | 16.5% | 16.1% | ns | 25.8% | 23.5% | ns |
| Infectious Disease | 29.7% | 21.8% | <0.001 | 45.5% | 17.9% | <0.001 | 28.6% | 21.6% | <0.001 | 37.1% | 30.4% | ns |
| Mental Health | 42.7% | 33.9% | <0.001 | 22.2% | 10.8% | <0.001 | 44.3% | 37.0% | <0.001 | 30.7% | 20.2% | 0.001 |
| Genitourinary / Gynecological | 38.7% | 29.4% | <0.001 | 78.9% | 16.1% | <0.001 | 36.2% | 31.4% | <0.001 | 48.9% | 18.7% | <0.001 |
CHD = Congenital Heart Defect.
EU=Emory University; catchment area incl. 5 metro-Atlanta counties: Clayton, Cobb, DeKalb, Fulton and Gwinnett; MA=Massachusetts Dept. of Public Health; catchment area is state-wide; and NY=New York State Dept. of Health; catchment area incl. 11 counties: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester &Wyoming.
Data collapsed across CHD severity.
Individuals classified with ICD-9_CM code 745.5 in isolation are not included.
A sensitivity analysis conducted to assess the effect of maternal age on co-morbidities with a constrained sample of 20 to 35 year olds mimicked findings from the full sample. Similar results to those in Table 3 were revealed for hypertension (28.6% vs 20.8%), hyperlipidemia (17.8% vs 14.5%), coronary artery disease (10.0% vs 8.2%), and venous disorder/phlebitis (5.9% vs 4.4%) with pregnant women with severe CHDs having significantly more nongestational cardiovascular co-morbidities compared with nonpregnant women with CHDs (data not shown). Pregnant women compared with nonpregnant women had significantly more nongestational, noncardiovascular co-morbidities, including diabetes mellitus, hematologic conditions, infectious diseases, and genitourinary/gynecology problems (Table 4). Pregnant women with severe CHD compared with those with not severe CHD more often had nongestational diabetes mellitus (28.3% vs 22.5%, p <0.0001), hematologic conditions (25.8% vs 21.7%, p <0.01), neurologic problems (14.7% vs 12.1%, p <0.05), and mental health problems (52.0% vs 40.5%, p <0.0001) (data not shown). Fewer than 2.5% of pregnancy cases had a CHD diagnosis code associated only with a fetal echocardiogram encounter.
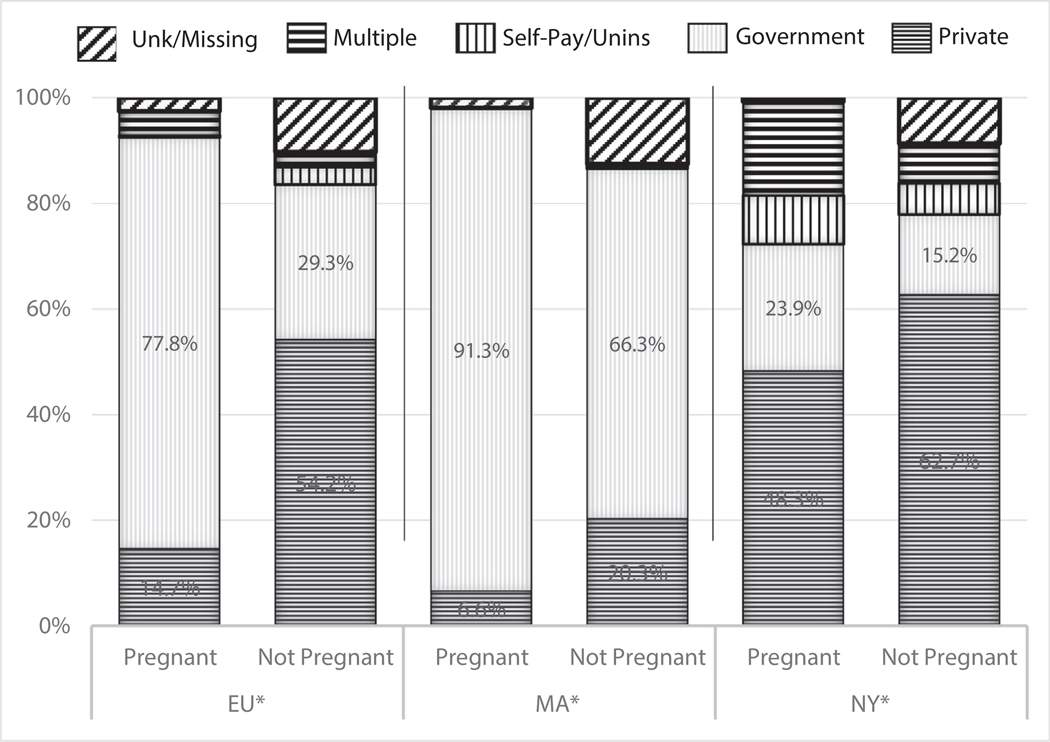
Figure 1 shows observed differences in insurance across sites for pregnant and nonpregnant women. While government-based insurance was more common among pregnant compared with nonpregnant women at both EU and MA, EU had more than a 2-fold higher difference between pregnant and nonpregnant women compared with MA. In MA, most women regardless of pregnancy status had government-based health insurance. In NY, private insurance was most common for both pregnant and nonpregnant women (Figure 1).
Figure 1.
Healthcare Insurance by pregnancy during project period for women age 11–50 years with congenital heart defects (CHD) at 3 surveillance sites, 2008 to 2010.**
CHD = Congenital Heart Defect
* EU=Emory University; catchment area includes 5 metro-Atlanta counties: Clayton, Cobb, DeKalb, Fulton and Gwinnett;
MA=Massachusetts Department of Public Health; catchment area is state-wide; and
NY=New York State Department of Health; catchment area includes 11 counties: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester, and Wyoming
** Data collapsed across CHD severity.
^^ Individuals classified with ICD-9_CM code 745.5 in isolation are not included.
Note. Government healthcare insurance includes Medicaid, Medicare, Tricare, and Military coverage.
Discussion
Through identification of pregnancy-related ICD-9-CM codes in clinical and administrative databases, we identified approximately 13% to 23% of females with CHD of childbearing age to have experienced a pregnancy from 2008 to 2010 surveillance data at 3 US sites. While our project methodology is not directly comparable to US population norms, our data suggest a substantial number of women with CHD are experiencing pregnancy. In this analysis, pregnant women with CHD experienced complications at a greater rate than previously reported by population norms.17–20 The proportion of pregnant women was greater in MA (22.5% in MA compared with 13% to 14% at the other 2 sites). Fewer gestational complications were found among those in MA compared with the other sites, and were below previously published US population norms,20–22 thus discussion focuses on EU and NY.
Pregnancy among women with severe CHDs more often involved several gestational complications compared with pregnancy among women without severe CHDs. However, women with CHD may have a higher risk for some pregnancy related complications such as anemia, hemorrhage, and hypertension when compared with women without CHD. The percentage of pregnant women who experienced hemorrhage was similar between NY and EU and higher than a US population estimate (4.2 per 1,000 deliveries in 2008 of severe postpartum hemorrhage, based on a definition of hemorrhage treated by blood transfusion, hysterectomy, and/or surgical repair of the uterus.18 Adults with CHDs treated with anticoagulants are at elevated risk of hemorrhage23 and those with certain CHDs (e.g., palliative single ventricle heart defects or mechanical valve) have elevated risk of hemorrhage due to coagulation disorders.24 Hypertensive disorders in the US occurred in 83.4 per 1,000 deliveries in 200619 compared with our observed prevalence of 75 to 127 per 1,000 pregnancies in NY and EU. Eclampsia or severe pre-eclampsia was 5 times the US prevalence (of 12.4 per 1,000 deliveries) among women with CHDs (65 to 73 per 1,000 in NY and EU), suggesting women with CHDs and gestational hypertension need close monitoring. Although our analysis could not distinguish anemia severity, the rate of anemia found in women with CHD was more than double the national average for all gestational anemia.21 The reason for this finding deserves further investigation, but may include anticoagulation and antiplatelet therapies. From 2008 to 2010, estimated gestational diabetes in the US was 5.6%.17 State-specific estimates of gestational diabetes varied from 3.5% to 7.2% in 2008, depending on sociodemographic and healthcare factors. Gestational diabetes estimates for pregnant women with CHDs in NY and EU were 5.9% and 12.0%, respectively, suggesting similar multifactorial and geographic variation of gestational diabetes for women with CHDs.
These data support a clinical concern that CHD-related issues such as need for anticoagulation, risk for thromboembolic complications, and factors predisposing to hypertension or heart failure may contribute to a higher risk of pregnancy complications compared with population norms. Women with CHDs contemplating pregnancy should receive preconception counseling regarding maternal, obstetric, fetal, and neonatal risk. Those with severe CHDs may be best served in a tertiary referral center with maternal fetal medicine expertise. The European Society of Cardiology 2018 Guidelines are a good resource for management of specific heart defects and cardiac complications in pregnancy.4
Pregnant women with CHDs had significantly more nongestational co-morbidities than nonpregnant women with CHDs. Clinically relevant co-morbidities may be exacerbated in pregnancy, such as pre-existing heart failure.25 Although we cannot discern why more nongestational co-morbidities appear in women who have been pregnant, it is possible this represents a more thorough medical history and/or more comprehensive coding in pregnant women.
Site-specific differences in insurance coverage for pregnant versus nonpregnant women with CHDs were evident in EU and MA, with proportionately more pregnant than nonpregnant women having government-based insurance; EU had the greater difference compared with MA, 48.5% pregnant versus 25.0% nonpregnant. This likely reflects state-based differences in insurance accessibility outside of pregnancy. Differences in access to insurance-paid healthcare among nonpregnant women might affect their susceptibility to complications when they do become pregnant. Substantial literature supports the need for preconception care for all women.26,27 As preconception care may be less accessible in Georgia when women lack insurance outside of pregnancy, those women may enter pregnancy at elevated risk of complications due to chronic conditions such as untreated diabetes and hypertension.28
Comparability of data across sites may be limited by variability and completeness in data sources. Under- or overreporting of pregnancies may occur due to data capture by administrative codes.6 We were unable to define pregnancy timing or frequency within the project period, thus temporal relation of nongestational co-morbidities to pregnancy cannot be determined. While the number of pregnant women with CHDs might have been over- or under-reported, so might the number of nonpregnant women with CHDs.
To our knowledge, this is the first US population-based, multi-site project estimating pregnancy among women with CHDs defined by coded healthcare encounters. Our findings suggest a substantial percent of women with CHDs may be experiencing gestational complications and other co-morbidities. Given the growing population of adults with CHDs, reproductive health of women of childbearing age with CHDs is an important public health issue warranting further investigation.
Supplementary Material
Acknowledgment (Funding/Support)
Centers for Disease Control and Prevention, Atlanta, GA USA, Grant/Award Number: CDC RFA DD12 1207.
Footnotes
DCDD Replication Statement
This analysis has undergone replication by Trenton Hoffman.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.12.001.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Mackie AS, Pilote L, Ionescu-Ittu R, Rahme E, Marelli AJ. Health care resource utilization in adults with congenital heart disease. Am J Cardiol 2007;99:839–843. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opotowsky AR, Siddiqi OK, D’Souza B, Webb GD, Fernandes SM, Landzberg MJ. Maternal cardiovascular events during childbirth among women with congenital heart disease. Heart 2012;98(2):145–151. [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, ESC SDG. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 5.Roos-Hesselink J, Baris L, Johnson M, De Backer J, Otto C, Marelli A, Jondeau G, Budts W, Grewal J, Sliwa K, Parsonage W, Maggioni AP, van Hagen I, Vahanian A, Tavazzi L, Elkayam U, Boersma E, Hall R. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019. March 25 10.1093/eurheartj/ehz136. [DOI] [PubMed] [Google Scholar]
- 6.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJM, Vliegen HW, van Dijk APJ, Voors AA, Yap SC, van Veldhuisen DJ, Pieper PG, Investigators ZAHARA. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31:2124–2132. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet V, Simonet T, Labombarda F, Dolley P, Milliez P, Dreyfus M, Hanouz JL. Neonatal and maternal outcomes of pregnancy with maternal cardiac disease (the NORMANDY study): years 2000–2014. Anaesth Crit Care Pain Med 2018;37:61–65. [DOI] [PubMed] [Google Scholar]
- 8.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, Wald RM, Colman JM, Siu SC. Pregnancy outcomes in women with heart disease: The CARPREG II Study. J Am Coll Cardiol 2018;71: 2419–2430. [DOI] [PubMed] [Google Scholar]
- 9.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation 2009;120:302–309. [DOI] [PubMed] [Google Scholar]
- 10.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012–2015. Obstet Gynecol 2018;132: 1158–1166. [DOI] [PubMed] [Google Scholar]
- 11.Bruce FC, Berg CJ, Joski PJ, Roblin DW, Callaghan WM, Bulkley JE, Bachman DJ, Hornbrook MC. Extent of maternal morbidity in a managed care population in georgia. Paediatr Perinat Epidemiol 2012;26: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glidewell J, Book W, Raskind-Hood C, Hogue C, Dunn JE, Gurvitz M, Ozonoff A, McGarry C, Van Zutphen A, Lui G, Downing K, Riehle-Colarusso T. Population-based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res 2018;110:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163–172. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez FH, Ephrem G, Gerardin JF, Raskind-Hood C, Hogue C, Book W. The 745.5 issue in code-based, adult congenital heart disease population studies: relevance to current and future ICD-9-CM and ICD-10-CM studies. Congenit Heart Dis 2018;13:59–64. [DOI] [PubMed] [Google Scholar]
- 15.HCUP CCS. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; March 2017. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [PubMed] [Google Scholar]
- 16.Institute SAS. Base SAS 9.3 Procedures Guide Statistical Procedures. Cary, N.C.: SAS Institute; 2011. Available at: http://www.books24×7.com/marc.asp?bookid=44484. Accessed November 14, 2018. [Google Scholar]
- 17.Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, Correa A. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care 2013;36:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, Joseph KS. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013;209:449.e1–449. e7. [DOI] [PubMed] [Google Scholar]
- 19.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009; 113:1299–1306. [DOI] [PubMed] [Google Scholar]
- 20.Bardenheier BH, Imperatore G, Devlin HM, Kim SY, Cho P, Geiss LS. Trends in pre-pregnancy diabetes among deliveries in 19 U.S. states, 2000–2010. Am J Prev Med 2015;48:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le CH. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003–2012). PLoS One 2016;11: e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U. S. women continue to drop. NCHS Data Brief 2013:1–8. [PubMed] [Google Scholar]
- 23.Yang H, Heidendael JF, de Groot JR, Konings TC, Veen G, van Dijk APJ, Meijboom FJ, Sieswerda GT, Post MC, Winter MM, Mulder BJM, Bouma BJ. Oral anticoagulant therapy in adults with congenital heart disease and atrial arrhythmias: implementation of guidelines. Int J Cardiol 2018;257:67–74. [DOI] [PubMed] [Google Scholar]
- 24.Monteiro RS, Dob DP, Cauldwell MR, Gatzoulis MA. Anaesthetic management of parturients with univentricular congenital heart disease and the Fontan operation. Int J Obstet Anesth 2016;28: 83–91. [DOI] [PubMed] [Google Scholar]
- 25.Anthony J, Sliwa K. Decompensated heart failure in pregnancy. Card Fail Rev 2016;2:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins C, Boulet SL, Morgan I, D’Angelo DV, Zapata LB, Morrow B, Sharma A, Kroelinger CD. Disparities in preconception health indicators - behavioral risk factor surveillance system, 2013–2015, and pregnancy risk assessment monitoring system, 2013–2014. MMWR Surveill Summ 2018;67:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witt WP, Mandell KC, Wisk LE, Cheng ER, Chatterjee D, Wakeel F, Park H, Zarak D. Infant birthweight in the US: the role of preconception stressful life events and substance use. Arch Womens Ment Health 2016;19:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgia Department of Human Services. Right from the Start Medicaid (RSM): The RSM Medicaid Program available through the Division of Family and Children Services (DFCS). Avail-able at: http://dhs.georgia.gov/sites/dhs.georgia.gov/files/related_files/document/DFCS.RSM%20Medicaid%205.12.pdf. Accessed September 2, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.