Abstract
Acute bronchiolitis is one of the most common lower respiratory tract infections in children with less than 2 years of age. Nowadays, molecular methods provide an opportunity to better understand the etiology of bronchiolitis. Several viral agents including Respiratory syncytial virus (RSV), Rhinovirus, Parainfluenza and Human bocavirus (HBoV) are responsible for acute bronchiolitis. There are growing studies on the prevalence of HBoV in patients with bronchiolitis. The present systematic review and meta-analysis were conducted to determine the pooled prevalence of HBoV in the respiratory samples of children with acute bronchiolitis.
A literature search was conducted in the databases of PubMed, Scopus and Web of Science to recruit studies reporting the frequency of HBoV in <2-year-old children with acute bronchiolitis from 2005 to 2019. Only studies that used polymerase chain reaction (PCR)-based methods to detect the virus in nasopharyngeal samples were included. A total of 22 studies assessing 6751 cases were analyzed. According to the meta-analysis based on the random-effects model, the overall prevalence of HBoV in children with <2 years old was obtained 13% (95% CI: 0.09-0.17). Additionally, the rates of single (as the sole organism) and mixed (in combination with other viruses) HBoV infections were 4% and 9%, respectively. This study showed a high rate of HBoV detection in children with acute bronchiolitis. This should be considered as part of a diagnostic test panel for respiratory infections in children with bronchiolitis.
Keywords: Bronchiolitis, Children, Human bocavirus (HBoV), Prevalence, Respiratory virus
Highlights
-
•
The overall prevalence of HboV was 13% (95% CI: 0.09-0.17) in children under two years old.
-
•
The rate of HBoV infection as a mono-infection was 4% and 9% as mixed infection.
-
•
This study showed a high prevalence of Bocavirus in children with acute bronchiolitis.
Introduction
Bronchiolitis is a common respiratory tract infection in children. It is associated with inflammation, edema, increased mucus production, and the necrosis of the epithelial cells of bronchioles [1] Bronchiolitis primarily presents with the symptoms of upper respiratory tract infections. These include nasal congestion, fever, and cough progressing to wheezing and tachypnea. Because the lungs and immune system of young children are not fully maturated, acute bronchiolitis can be a serious clinical condition leading to the blockage of airways in these children. Acute bronchiolitis is generally diagnosed based on clinical and chest X-ray (CXR) examinations; however, complete blood cell count (CBC) can also be helpful [2].
Several respiratory viruses are known to cause bronchiolitis, among these, respiratory syncytial virus (RSV) is responsible for 70% of the cases. Other causative agents include human rhinovirus (HRV), type A and B influenza viruses, parainfluenza, adenovirus, human coronavirus, and human bocavirus (HBoV) which is a relatively new etiology of bronchiolitis.
Studies on the pathogenic role of HBoV in humans are on the rise [3]. HBoV is a non-enveloped DNA virus that belongs to the Parvoviridae family, the subfamily of Parvovirinae, and the genus of Bocavirus. This virus was first discovered by Allander et al., and phylogenetically, it reveals similarities with parvoviruses from bovine and canine [4]. HBoV, which has been detected in respiratory, fecal, urine, saliva, and blood specimens, shows a global distribution [5,6]. After the identification of HBoV, many studies have reported this virus as a cause of acute respiratory diseases in children. However, the frequent co-detection of HBoV with other viruses in patients' samples has debated its role as a true pathogen, and some suggested the virus as a passenger agent [7,8]. On the other hand, although HBoV persistence is not fully understood, HboV DNA remains in the tissues of children's respiratory tract for a long time after the primary infection, it may be one reason for identifying HBoV as a co-infection in some studies [7,9,10].
So far, there has been one fatality reported in an immunocompetent child due to severe acute bronchiolitis caused by HBoV [11]. Some studies have described the HBoV as the second or third most prevalent cause of bronchiolitis [3,12]. The seasonal distribution of HBoV is similar to that of RSV leading to a prominent coincidence between these two infections [13]. Overall, the prevalence of HBoV has been widely variable in patients with bronchiolitis in different studies [14].
Here, we conducted a systematic review and meta-analysis to estimate the frequency of HBoV in < 2-year-old children with acute bronchiolitis.
Materials and methods
Searching and selection of related articles
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [15], we searched various databases including PubMed, Scopus and Web of Science for related articles using the Medical Subject Heading (MeSH) terms of “bocavirus”, “HBoV”, “prevalence”, “frequency”, “epidemiology”, “acute bronchiolitis”, “lower respiratory tract infection”, and “acute respiratory tract infection” either alone or in combination with each other applying the Boolean operators (AND, OR).
All the related papers published from January 2005 to October 2019 were retrieved. After removing duplicates, the abstracts and full texts of the studies were reviewed. In addition, to make sure that the search was complete, the reference lists of eligible articles were also manually searched for related works. Furthermore, the links to “similar articles” on the PubMed had been opening to find other relevant papers.
Inclusion and exclusion criteria
All English-language research articles published on the prevalence of HBoV in children with acute bronchiolitis were enrolled. Other types of articles including reviews and letters to editors were excluded. In addition, studies that used non-molecular methods for detecting the virus were excluded. Studies performed on children older than 2 years were removed from the assessment.
Data extraction
The following data was collected and recorded into a checklist: first authors' names, year of publication, the country of study conduction, the number of cases, the number of male and female subjects, sample types, patients' mean age, the number and percentage of positive samples, and finally the rates of single (only HBoV) and mixed (HBoV along with other pathogen) infections.
Statistical analysis
To analyze and combine the results of different studies, a binomial distribution was considered for the prevalence of the virus in each study. The standard errors were calculated based on this distribution. Heterogeneity was assessed using the Cochran Q test and I2 index. Based on the high heterogeneity rate among the studies, the random effects model was used for meta-analysis. Meta-regression analysis was applied to investigate the sources of heterogeneity and the relationship between the virus prevalence and each of sample size and year of publication. Sensitivity analysis was also used to investigate the impact of each study on the calculation of the final outcome. All the analyses were performed in STATA 11 software, and P values less than 0.05 were considered significant in all the statistical tests.
Results
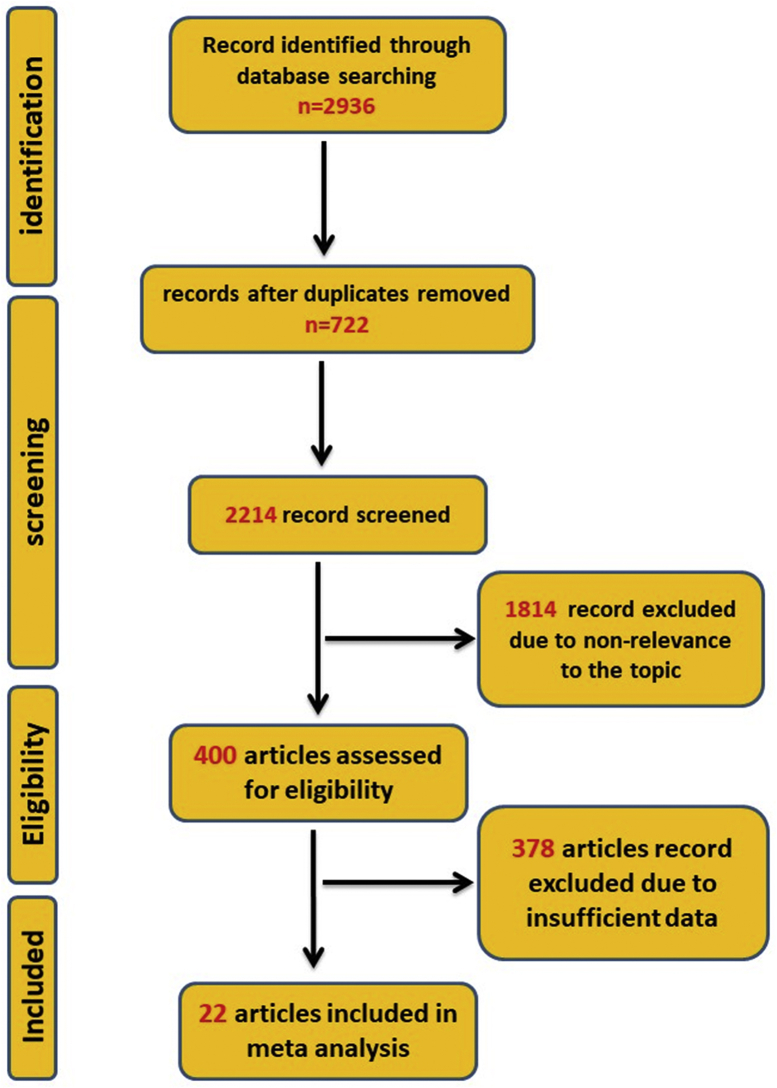
A total of 2936 articles were collected upon the initial search in the electronic databases. Of these, 722 duplicate studies were removed. By subsequent screenings, 1814 additional articles were excluded according to our exclusion criteria. After that, the full texts of 400 articles were carefully assessed. Of these, 378 studies met at least one exclusion criterion. Finally, 22 articles were included in the meta-analysis process (Fig. 1).
Fig. 1.
The flowchart of study design and process.
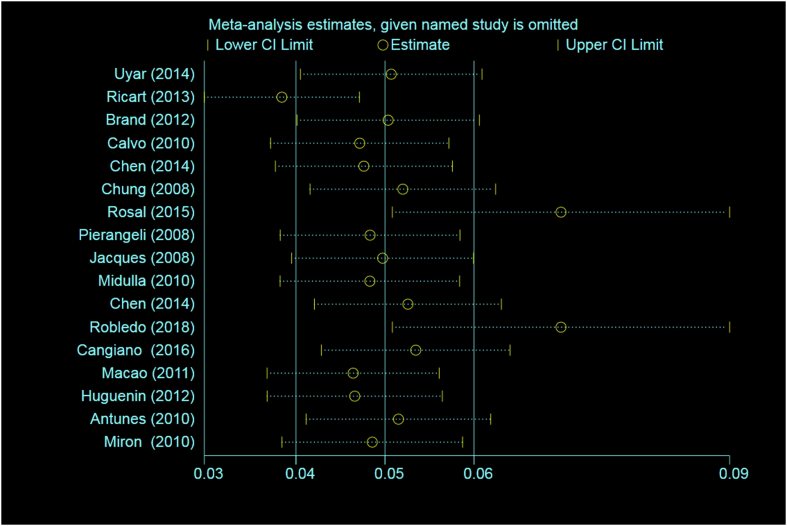
In this study, a total of 22 articles comprising of 6751 children with a mean age of 6.7 months (95% CI: 5.1-8.3) were included in the meta-analysis process. The details of the reviewed articles [12,13,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] have been shown in Table 1. The rate of heterogeneity among the studies was high (97.4%), and therefore the random effects model was used for meta-analysis. Sensitivity analysis was also used to investigate the impact of each study on the calculation of the final outcome (Fig. 2).
Table 1.
The characteristics of the studies included in this review
| Author | Year | Country | Numer of case | Male | Female | Sample type | Mean age (months) | Number of positive | Percent | Coinfection | Single infection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zappa A et al. [14] | 2011 | Italy | 22 | NA | NA | Pharyngeal Swabs | 5 | 3 | 12.4 | NA | NA |
| Uyar M et al. [15] | 2014 | Turkey | 62 | 45 | 17 | Nasopharyngeal Aspirate | N | 3 | 4.8 | 2 | 1 |
| Ricart S et al. [16] | 2013 | Spain | 404 | NA | NA | Nasopharyngeal Aspirate | 3.6 | 118 | 18.8 | 101 | 17 |
| Brand HK et al. [17] | 2012 | Netherlands | 142 | NA | NA | Nasopharyngeal Aspirate | 4.5 | 6 | 4.22 | 6 | 0 |
| Calvo C et al. [18] | 2010 | Spain | 318 | 175 | 193 | Nasopharyngeal Aspirate | 5.6 | 42 | 11.4 | 28 | 14 |
| Chen yw et al. [10] | 2014 | Taiwan | 113 | 77 | 36 | Nasopharyngeal Aspirate | 9 | 22 | 19.5 | 16 | 6 |
| Chung JY et al. [10] | 2008 | Republic of Korea | 308 | NA | NA | Nasopharyngeal Aspirate | 11.4 | 37 | 12 | 8 | 29 |
| Rosal TD et al. [19] | 2015 | Spain | 684 | NA | NA | Nasopharyngeal Aspirate | 9.1 | 31 | 4.5 | 0 | 31 |
| Pierangeli A et al. [20] | 2008 | Italy | 204 | 102 | 102 | Nasal Washings | N | 22 | 10.7 | 16 | 6 |
| Jacques J et al. [21] | 2008 | French | 192 | NA | NA | Nasopharyngeal Aspirate | 8.6 | 24 | 12.5 | 10 | 14 |
| Midulla F et al. [11] | 2010 | Italy | 182 | NA | NA | Nasal Washings | 2.5 | 22 | 12.5 | 15 | 7 |
| Ricart S et al. [16] | 2013 | Spain | 484 | 235 | 173 | Nasopharyngeal Aspirate | 12 | 119 | 24.58 | NA | NA |
| Wang k et al. [22] | 2009 | China | 51 | NA | NA | Nasal/throat Swabs | N | 11 | 21.6 | NA | NA |
| Chen ZR et al. [10] | 2014 | China | 998 | 672 | 326 | Nasopharyngeal Aspirate | 8.5 | 82 | 11.6 | 24 | 58 |
| Robledo MA et al. [23] | 2018 | Mexico | 134 | 81 | 53 | Nasopharyngeal Aspirate | 6.6 | 9 | 5.9 | 0 | 9 |
| Janahi I et al. [24] | 2017 | Qatar | 369 | 247 | 122 | Nasopharyngeal Aspirate | N | 15 | 4.1 | NA | NA |
| Cangiano G et al. [25] | 2016 | Italy | 723 | 395 | 201 | Nasopharyngeal Aspirate | 2.13 | 13 | 1.8 | 13 | 0 |
| Praznik A et al. [26] | 2018 | Slovenia | 473 | NA | NA | Nasopharyngeal Swabs | 10 | 87 | 18.4 | NA | NA |
| Macao P et al. [27] | 2011 | Portugal | 78 | 47 | 30 | Nasopharyngeal Aspirate | 8.5 | 29 | 37.1 | 26 | 3 |
| Huguenin A et al. [28] | 2012 | France | 138 | 81 | 57 | Nasopharyngeal Aspirate | 4 | 37 | 27 | 24 | 13 |
| Antunes H et al. [29] | 2010 | Portugal | 207 | NA | NA | Nasopharyngeal Aspirate | N | 8 | 3.8 | 6 | 2 |
| Miron D et al. [30] | 2010 | Israel | 465 | 279 | 186 | Sputum or Nasal Wash | 3 | 31 | 6.7 | 28 | 3 |
NA: Not Available.
Fig. 2.
Sensitivity analysis of the included studies.
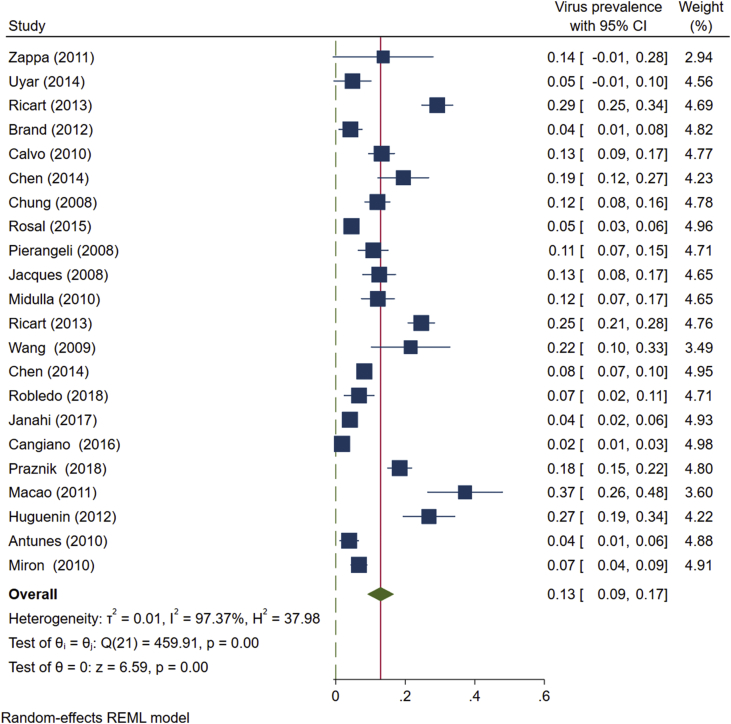
Based on studies conducted from 2005 to 2019, the overall prevalence of HBoV in the airway samples of <2-year-old children with bronchiolitis was estimated as 13% (95% CI: 0.09-0.17) (Fig. 3).
Fig. 3.
The prevalence of HBoV in nasopharyngeal aspirate specimens of < 2-year-old children with bronchiolitis based on the random effects model. The points represent percentages, and the lengths of lines show 95% confidence interval in each study. The rhombic symbol indicates the overall prevalence of HBoV in all the studies.
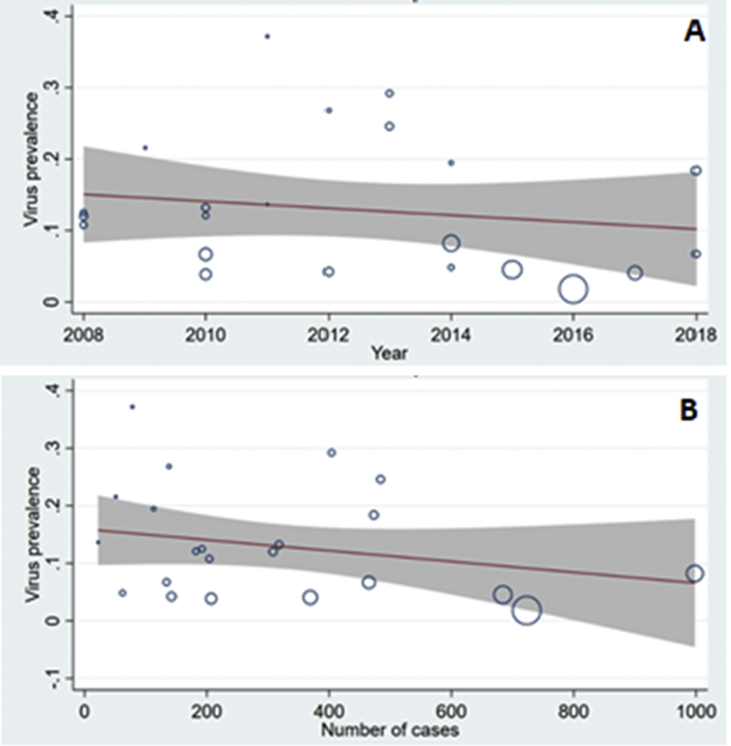
The lowest and highest frequencies of HBoV were reported by Cangiano et al. (1.8%) and Macao et al. (37.1%), respectively (Fig. 4) [27,30].
Fig. 4.
The prevalence of human bocavirus in <2-year-old children with bronchiolitis. Considering the year of study (A) and sample size(B).
A meta-regression model was used to investigate the relationship between the prevalence of HBoV in children and each of the year of study and sample size. Fig. 4 shows the relationships between the prevalence of HBoV and the year of study conduction (Fig. 4A) and sample size (Fig. 4B). Although there were decreasing trends in the prevalence of HBoV by elevations in the year and sample size, with respect to the obtained p values and regression coefficients (0.444 and 0.235, respectively), these associations were not statistically significant.
Prevalence of HBoV as either a mono- or mixed-infection
For calculating the prevalence of single HBoV infection in <2-year-old children with bronchiolitis, a total of 15 articles were included in the meta-analysis process. The total number of children in these studies was 4487 with an average age of 6.7 months (95% CI: 5.1-8.3). As the rate of heterogeneity among the studies was high (83.2%), the random effects model was used for meta-analysis.
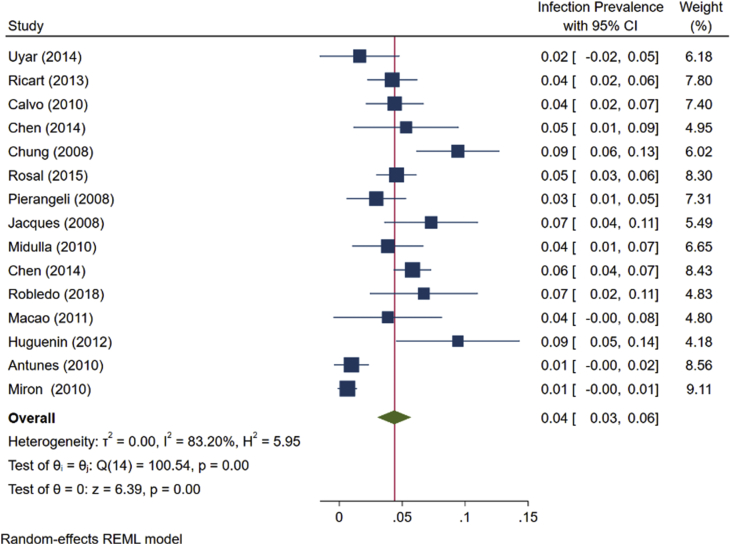
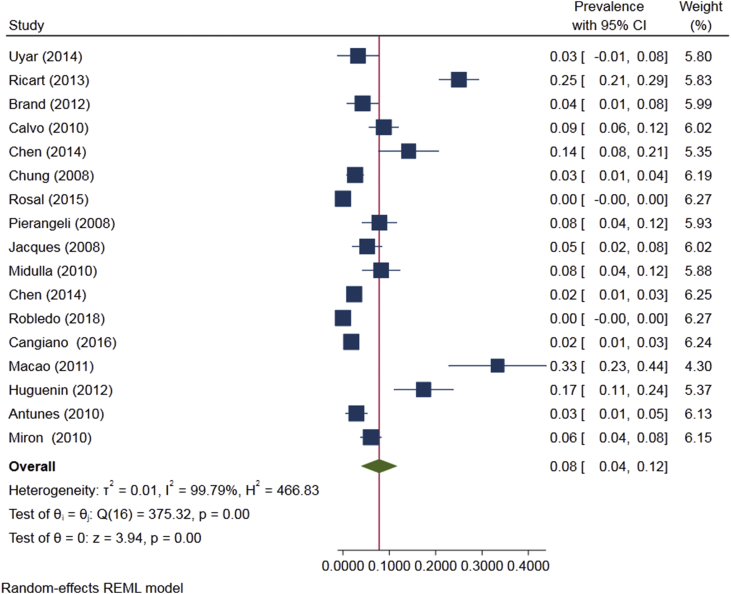
As shown in Fig. 5, the prevalence of HBoV as a single infection in <2-year-old children with bronchiolitis was 4.4% (95% CI: 3.0-5.7, Fig. 5). The prevalence of HBoV as a co-infection in these children, which was estimated based on studies on 6751 children with an average age of 6.7 months, was 8% (95%CI: 0.04-0.12, Fig. 6).
Fig. 5.
The prevalence of Human bocavirus as a single infection in < 2-year-old children with bronchiolitis based on the random effects model. The points estimate the frequencies, and the lengths of lines show 95% confidence intervals in each study.
Fig. 6.
The prevalence of Human bocavirus as a co-infection in <2-year-old children with bronchiolitis based on the random effects model. The points represent percentages, and the lengths of lines display 95% confidence intervals in each study.
Discussion
Bronchiolitis is a major cause of hospitalization due to respiratory infections among children with the age of <6 months. Several viral agents including respiratory syncytial virus, HRV, influenza, and human metapneumovirus have been associated with bronchiolitis in children. In addition to the above-mentioned viruses, HBoV has also been reported as a cause of bronchiolitis; however, this association is still controversial. In fact, some studies have described a considerably wide range of HBoV infection in patients with bronchiolitis [36,37].
The findings of our study estimated an overall prevalence of 13% for HBoV infection in <2-year-old children with bronchiolitis. These results support previous studies in which HBoV has been frequently detected in the samples of patients with bronchiolitis, sometimes even as the second or third most common viral agent [12].
The rate of HBoV infection in children with bronchiolitis varies from 1.8% to 37% in different countries [27,30]. Among the studies assessed here, the lowest and highest frequencies of HBoV were reported by Cangiano et al. (1.8%) in Italy and Macao et al. in Portugal (37.1%). This indicates that geographical location may be one of the factors contributing to the heterogeneity observed among the analyzed studies. In accordance, the prevalence of HBoV has been variable between different countries, and even within countries [27,30].
Most of the assessed studies had been conducted in Spain and Italy. Four studies in Italy from 2005 to 2016 reported frequencies from 1.8% to 12.5% [13,27]. Our findings also suggested that the prevalence of HBoV may be associated with the year of study conduction. Another study conducted in 2016 reported a HBoV prevalence of 1.8% in children with a mean age of 2.1 months [27]. According to previous studies, the prevalence of HBoV infection is generally higher within the ages of 3 to 6 months [2].
Based on the meta regression model, HBoV prevalence showed a decreasing trend over time. Although this observation was not statistically significant, this declining trend may be related to improved health conditions, educations, and also increasing awareness about the transmission ways of respiratory infections.
Although the overall prevalence of HBoV infection was estimated as 12.9%, the incidence of the virus as a single agent was 4%. This supports previous reports noting that HBoV is more commonly identified as a mixed infection in association with other viruses [8].
Although HBoV infection is diagnosed throughout the year, but it peaks during winter and spring [38]. Nevertheless, the seasonal occurrence of HBoV is still a subject of debate, and there are increasing evidences suggesting the higher frequencies of the viral infection in the cold months of the year, especially in January and February [36]. So, sampling time can also be a factor contributing to the heterogeneity among the studies. In fact, in studies with a longer sampling time (e.g. 1-year period), the prevalence of respiratory infections may be underestimated compared to studies performed during the months showing a peak activity of respiratory viruses. This can actually be a source of bias in these studies. Therefore, this factor should be considered in meta-analysis studies to investigate the prevalence of respiratory viruses.
In this study, although inclusion criteria were applied to select studies on a similar population (i.e. children younger than 2 years) and with same detection methods (i.e. molecular assays), there was still a high heterogeneity among the studies. It seems that several factors such as seasonal distribution, age spectrum, year of study, geographical location, and sampling time may be responsible for the heterogeneity observed among the studies.
From the limitations of this study was a high heterogeneity among the studies. Furthermore, there were no reports in many countries limiting our results to certain geographical locations.
Conclusion
The data obtained here shows that HBoV infection is relatively frequent in children with bronchiolitis aged <2 years old. HBoV may be either a cause or a risk factor for bronchiolitis in these children. Therefore, this virus should be considered when determining the etiology of bronchiolitis in young children. It is also recommended to incorporate HBoV testing in bronchiolitis diagnostic panels.
Conflict of interest
The authors declare no conflict of interest.
Funding sources
There is no funding for this study.
References
- 1.Pavia A.T. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Inf Dis. 2011;52(Suppl. l_4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soleimani G., Shahri E.S., Rashidi S., Salari Z., Moghadam A.A. Epidemiology, clinical, and laboratory characteristics of bronchiolitis in hospitalized children. J Comp Pediatr. 2014;5(3) [Google Scholar]
- 3.Praznik A., Vinsek N., Prodan A., Erculj V., Pokorn M., Mrvic T. Risk factors for bronchiolitis severity: a retrospective review of patients admitted to the university hospital from central region of Slovenia. Inf Other Resp Viruses. 2018;12(6):765–771. doi: 10.1111/irv.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De R., Liu L., Qian Y., Zhu R., Deng J., Wang F. Risk of acute gastroenteritis associated with human bocavirus infection in children: a systematic review and meta-analysis. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monavari S.H., Noorbakhsh S., Mollaie H., Fazlalipour M., Kiasari B.A. Human Bocavirus in Iranian children with acute gastroenteritis. Med J Islam Rep Iran. 2013;27(3):127. [PMC free article] [PubMed] [Google Scholar]
- 6.Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., üsterback R. Human bocavirus and acute wheezing in children. Clin Inf Dis. 2007;44(7):904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guido M., Tumolo M.R., Verri T., Romano A., Serio F., De Giorgi M. Human bocavirus: current knowledge and future challenges. World J Gastroenterol. 2016;22(39):8684. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schildgen O., Müller A., Allander T., Mackay I.M., Völz S., Kupfer B. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagasi A.A., Howson-Wells H.C., Clark G., Tarr A.W., Soo S., Irving W.L. Human Bocavirus infection and respiratory tract disease identified in a UK patient cohort. J Clin Virol. 2020;129:104453. doi: 10.1016/j.jcv.2020.104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning A., Willey S.J., Bell J.E., Simmonds P. Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J Inf Dis. 2007;195(9):1345–1352. doi: 10.1086/513280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziemele I., Xu M., Vilmane A., Rasa-Dzelzkaleja S., Hedman L., Hedman K. Acute human bocavirus 1 infection in child with life-threatening bilateral bronchiolitis and right-sided pneumonia: a case report. J Med Case Rep. 2019;13(1):1–6. doi: 10.1186/s13256-019-2222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y.-W., Huang Y.-C., Ho T.-H., Huang C.-G., Tsao K.-C., Lin T.-Y. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Inf. 2014;47(2):116–121. doi: 10.1016/j.jmii.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midulla F., Scagnolari C., Bonci E., Pierangeli A., Antonelli G., De Angelis D. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Childhood. 2010;95(1):35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y., Gu X., Zhao X., Luo J., Luo Z., Wang L. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knobloch K., Yoon U., Vogt P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillofacial Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Zappa A., Canuti M., Frati E., Pariani E., Perin S., Ruzza M.L. Co-circulation of genetically distinct human metapneumovirus and human bocavirus strains in young children with respiratory tract infections in Italy. J Med Virol. 2011;83(1):156–164. doi: 10.1002/jmv.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyar M., Kuyucu N., Tezcan S., Aslan G., Tasdelen B. [Determination of the frequency of human bocavirus and other respiratory viruses among 0-2 years age group children diagnosed as acute bronchiolitis] Mikrobiyol Bul. 2014;48(2):242–258. doi: 10.5578/mb.7575. [DOI] [PubMed] [Google Scholar]
- 18.Ricart S., Garcia-Garcia J.J., Anton A., Pumarola T., Pons M., Muñoz-Almagro C. Analysis of human metapneumovirus and human bocavirus viral load. Pediatr Inf Dis J. 2013;32(9):1032–1034. doi: 10.1097/INF.0b013e3182932f4f. [DOI] [PubMed] [Google Scholar]
- 19.Brand H.K., de Groot R., Galama J.M., Brouwer M.L., Teuwen K., Hermans P.W. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47(4):393–400. doi: 10.1002/ppul.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo C., Pozo F., García-García M., Sanchez M., Lopez-Valero M., Pérez-Breña P. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99(6):883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Rosal T., García-García M., Calvo C., Gozalo F., Pozo F., Casas I. Recurrent wheezing and asthma after bocavirus bronchiolitis. Allergologia et immunopathologia. 2016;44(5):410–414. doi: 10.1016/j.aller.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Pierangeli A., Scagnolari C., Trombetti S., Grossi R., Battaglia M., Moretti C. Human bocavirus infection in hospitalized children in Italy. Inf Other Resp Viruses. 2008;2(5):175–179. doi: 10.1111/j.1750-2659.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacques J., Moret H., Renois F., Lévêque N., Motte J., Andréoletti L. Human Bocavirus quantitative DNA detection in French children hospitalized for acute bronchiolitis. J Clin Virol. 2008;43(2):142–147. doi: 10.1016/j.jcv.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., Wang W., Yan H., Ren P., Zhang J., Shen J. Correlation between bocavirus infection and humoral response, and co-infection with other respiratory viruses in children with acute respiratory infection. J Clin Virol. 2010;47(2):148–155. doi: 10.1016/j.jcv.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robledo-Aceves M., de Jesús Moreno-Peregrina M., Velarde-Rivera F., Ascencio-Esparza E., Preciado-Figueroa F.M., Caniza M.A. Risk factors for severe bronchiolitis caused by respiratory virus infections among Mexican children in an emergency department. Medicine. 2018;97(9) doi: 10.1097/MD.0000000000010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janahi I., Abdulkayoum A., Almeshwesh F., Alkuwari M., Alameri M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Inf Dis. 2017;17(1):139. doi: 10.1186/s12879-017-2225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cangiano G., Nenna R., Frassanito A., Evangelisti M., Nicolai A., Scagnolari C. Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol. 2016;51(12):1330–1335. doi: 10.1002/ppul.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Praznik A., Vinšek N., Prodan A., Erčulj V., Pokorn M., Mrvič T. Risk factors for bronchiolitis severity: a retrospective review of patients admitted to the university hospital from central region of Slovenia. Inf Other Resp Viruses. 2018;12(6):765–771. doi: 10.1111/irv.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mação P., Dias A., Azevedo L., Jorge A., Rodrigues C. Acute bronchiolitis: a prospective study. Acta Medica Portuguesa. 2011;24:407–412. [PubMed] [Google Scholar]
- 30.Huguenin A., Moutte L., Renois F., Leveque N., Talmud D., Abely M. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012;84(6):979–985. doi: 10.1002/jmv.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes H., Rodrigues H., Silva N., Ferreira C., Carvalho F., Ramalho H. Etiology of bronchiolitis in a hospitalized pediatric population: prospective multicenter study. J Clin Virol. 2010;48(2):134–136. doi: 10.1016/j.jcv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miron D., Srugo I., Kra-Oz Z., Keness Y., Wolf D., Amirav I. Sole pathogen in acute bronchiolitis: is there a role for other organisms apart from respiratory syncytial virus? Pediatr Inf Dis J. 2010;29(1):e7–e10. doi: 10.1097/INF.0b013e3181c2a212. [DOI] [PubMed] [Google Scholar]
- 33.Ricart S., Marcos M.A., Sarda M., Anton A., Muñoz-Almagro C., Pumarola T. Clinical risk factors are more relevant than respiratory viruses in predicting bronchiolitis severity. Pediatr Pulmonol. 2013;48(5):456–463. doi: 10.1002/ppul.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z.-R., Ji W., Wang Y.-Q., Yan Y.-D., Shao X.-J., Zhang X.-L. Etiology of acute bronchiolitis and the relationship with meteorological conditions in hospitalized infants in China. J Form Med Assoc. 2014;113(7):463–469. doi: 10.1016/j.jfma.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung J.-Y., Han T.H., Kim J.-S., Kim S.W., Park C.-G., Hwang E.-S. Th1 and Th2 cytokine levels in nasopharyngeal aspirates from children with human bocavirus bronchiolitis. J Clin Virol. 2008;43(2):223–225. doi: 10.1016/j.jcv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Gökçe Ş., Kurugöl Z., Koturoğlu G., Çiçek C., Aslan A. Etiology, seasonality, and clinical features of viral respiratory tract infections in children hospitalized with acute bronchiolitis: a single-center study. Global Pediatr Health. 2017;4 doi: 10.1177/2333794X17714378. 2333794X17714378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansbach J.M., Camargo C.A. Respiratory viruses in bronchiolitis and their link to recurrent wheezing and asthma. Clin Lab Med. 2009;29(4):741–755. doi: 10.1016/j.cll.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang C.-Y., Kao C.-L., Huang L.-M., Lu C.-Y., Shao P.-L., Lee P.-I. Human bocavirus as an important cause of respiratory tract infection in Taiwanese children. J Microbiol Immunol Infect. 2011;44(5):323–327. doi: 10.1016/j.jmii.2011.01.036. [DOI] [PubMed] [Google Scholar]