Abstract
Background
Considering the structural and functional complexity of the craniofacial tissues, 3D bioprinting can be a valuable tool to design and create functional 3D tissues or organs in situ for in vivo applications. This review aims to explore the various aspects of this emerging 3D bioprinting technology and its application in the craniofacial bone or cartilage regeneration.
Method
Electronic database searches were undertaken on pubmed, google scholar, medline, embase, and science direct for english language literature, published for 3D bioprinting in craniofacial regeneration. The search items used were ‘craniofacial regeneration’ OR 'jaw regeneration' OR 'maxillofacial regeneration' AND ‘3D bioprinting’ OR 'three dimensional bioprinting' OR 'Additive manufacturing' OR 'rapid prototyping' OR 'patient specific bioprinting'. Reviews and duplicates were excluded.
Results
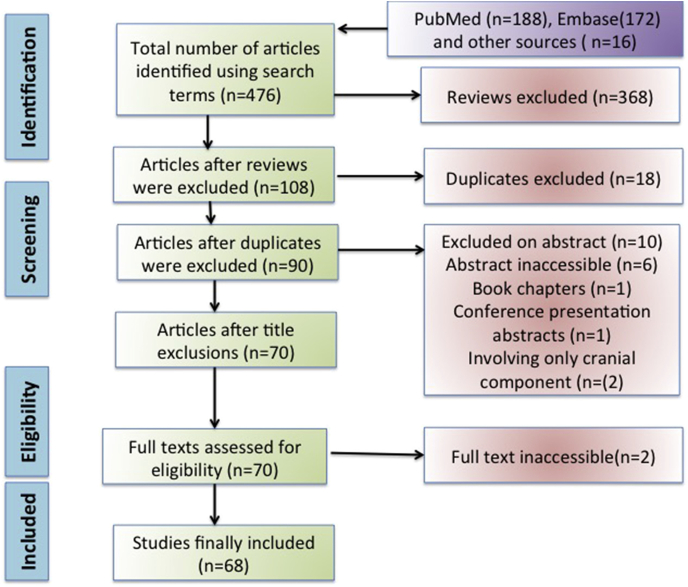
Search with above described criteria yielded 476 articles, which reduced to 108 after excluding reviews. Further screening of individual articles led to 77 articles to which 9 additional articles were included from references, and 18 duplicate articles were excluded. Finally we were left with 68 articles to be included in the review.
Conclusion
Craniofacial tissue and organ regeneration has been reported a success using bioink with different biomaterial and incorporated stem cells in 3D bioprinters. Though several attempts have been made to fabricate craniofacial bone and cartilage, the strive to achieve desired outcome still continues.
Keywords: Bioprinting, Craniofacial regeneration, Scaffolds, Bioink
1. Introduction
The craniofacial tissues are well arranged in a complex 3-dimensional (3D) architecture comprising 14 facial and 8 cranial bones that provide essential structural support and projection for the overlying soft tissues. Histologically these bones consist of an inorganic/organic matrix and are formed either by intramembranous or endochondral ossification. Mature bone is osteonal with haversian systems having concentric lamellae of matrix containing osteocytic lacunae. The cartilaginous part on the contrary, is formed from chondroblasts and has chondrocytes.1,2
Mimicking this 3D complexity and its multicellular interactions, is one of the greatest challenges, especially for functional reconstruction of the craniofacial defects. Although autogenous grafts have served the gold standard, their limited availability is a major drawback. Biofabrication implies manufacturing of tissues and/or organs employing living cells, molecules, extracellular matrices and biomaterials, using bioprinters and may serve as a good solution.
Bioprinting is simultaneous writing of living cells and biomaterial with layer-by-layer stacking using a computer-aided fabrication of bioengineered constructs.3 3D bioprinting is still in its infancy, and is being used mainly in research than in clinical application, because of its ability to produce simple homogenous tissues only. However, it does have the potential to fabricate heterogenous tissues as well.
The aim of this review is to explore the various aspects of this emerging 3D-bioprinting technology and its application in the craniofacial reconstruction.
Method: Electronic database searches were undertaken on pubmed, google scholar, medline, embase, and science direct for english language literature, published for 3D bioprinting in craniofacial regeneration. The search items used were ‘craniofacial regeneration’ OR 'jaw regeneration' OR 'maxillofacial regeneration' AND ‘3D bioprinting’ OR 'three dimensional bioprinting' OR 'Additive manufacturing' OR 'rapid prototyping' OR 'patient specific bioprinting'. Reviews and duplicates were excluded.
Results: Search with above described criteria yielded 476 articles, which reduced to 108 after excluding reviews. Further screening of individual articles led to 77 articles to which 9 additional articles were included from references, and 18 duplicate articles were excluded. Finally we were left with 68 articles to be included in the review (Fig. 1).
Fig. 1.
Prisma flowchart detailing the database searches, the number of abstracts screened and the full texts retrieved.
2. 3 D bioprinting: Applications and advantages
3D bioprinting technology can be applied in tissue engineering and regenerative medicine, tissue or organ transplantation sciences, screening of drugs and cancer research.4, 5, 6 3D bioprinting of various tissues like epithelial, connective, muscular and nervous tissues has been attempted in labs, using cell laden scaffolds and constructs which closely mimic the anatomy.7
The advantages of 3D bioprinting include capacity to modulate internal and external 3D architecture of scaffold systems, ease in fabrication, high precision patient matched scaffolds, potential to print multiple materials for fabrication of scaffolds, and capability of controlling cell behavior and mechanical response of the construct by predefining the scaffold architecture.8,9
3D bioprinting overcomes the shortcomings of the traditional 3D printers due to its ability to precisely place the cells in desired patterns and allow fabrication of native-like tissues of heterogenous origin. In vivo implantation of 3D bioprinted tissues to various sites has been documented.10 As human trials for transplantation are still awaiting approvals, the progress is being made in advances in biomaterials and cells, as well as technology of transplantation. Hence, the transition of 3D bioprinting into clinical practice from bench to bedside is still awaited. However, its use in drug and pharmaceutics is quite popular, and is being used for fabrication of tissues for drug testing and high-throughput assays. 3D bioprinted tissue models of liver have been fabricated by incorporating multiple cell types and appropriate physiological environment, and are being used for drug screening.11 In the field of cancer research 3D bioprinting has been used for development of in vitro cancer models as well as personalized medicines focused on hydrogels and therapeutic implants.12
3. 3 D bioprinting tools
Bioprinting, although a relatively new technology, demonstrates appreciable reproducibility and automated precise control over fabricated constructs. Although the term 3D printing, also referred as additive manufacturing or rapid prototyping, is often used interchangeably with 3D bioprinting, they differ in material used and printing potential. 3D printed inert or bioactive scaffolds lack living cells and are known as acellular scaffolds,13 whereas 3D bioprinting includes cell-laden biomaterial, with printing of both cells and scaffold or scaffold free dense aggregates of cells.8
3.1. Bioink
The process of 3D bioprinting incorporates bioink, a solution or a blend/mixture of biomaterial preferably hydrogel that encapsulates the desired cell types, and creates tissue constructs. The bioink is stabilized or crosslinked either during the process of bioprinting or immediately post-printing to provide accurate structural shape and architecture to the construct designed.
Appropriate chemical, mechanical, rheological and biological properties are desired in an ideal bioink to ensure functional and structural accuracy in bioprinted tissues and organs.14 This biomaterial may be natural, synthetic, or hybrid and are the potential alternatives to the standard autologous/allogenic grafts to attain clinically efficacious bone regeneration. Cell aggregates without any biomaterial can also be used as a bioink.
3.2. Cellular component for the bioink
The major sources of stem cells that are most commonly used in bioink for tissue engineering include embryonic, mesenchymal and induced pluripotent stem cells.
Embryonic stem cells (ESC) - exhibit optimal degree of multipotency but difficulty in procurement, ethical issues, and issues with immunogenicity.15
Mesenchymal stem cells (MSC) - easy to obtain, stimulate immunotolerance in target tissue, but degree of multipotency is much inferior to ESCs.16
Induced pluripotent stem cells (iPSC) - possess enhanced multipotency but studies also claim their role in promoting tumorigenesis.17
Scaffold-free cell aggregates-cellular aggregates forming spheroid structures, positioned and architecturally oriented to create tubular or ring-like structures.18,19 These scaffold-free constructs are free from the potentially toxic or immunogenic scaffold material, and exhibit the ability to create a high cell density construct.8,20 Although primarily these constructs are scaffold-free, the cells are encapsulated within a biocompatible and biodegradable hydrogel to aid in survival of the cell and provide mechanical support to construct. Hydrogel also eliminates the risk of tissue fusion while the cells are maintained in the suspension reservoir of the 3D bioprinter.21 This cell aggregation can be minimized by incorporating cross-linking solutions containing CaCl2 or gelatin.22,23 Cellular survival and scaffold integrity is maintained by the pH of bioink, and addition of NaOH stabilizes the pH of hydrogel containing modified biopolymers.24
Though quite beneficial, the cell aggregate approach exhibits certain limitations like amount of time consumed by cellular spheroids to create larger tissue structures that may also become non-uniform.21 Advances in this field include development of multicellular cylinders, which can be shaped as desired.19 Most of the research studies demonstrating 3D bioprinting using cell aggregate are performed in-vitro. Keeping in mind the in-vivo potential, further studies are required to study the safety and integrity of the scaffold free construct.
4. 3 D bioprinting modalities
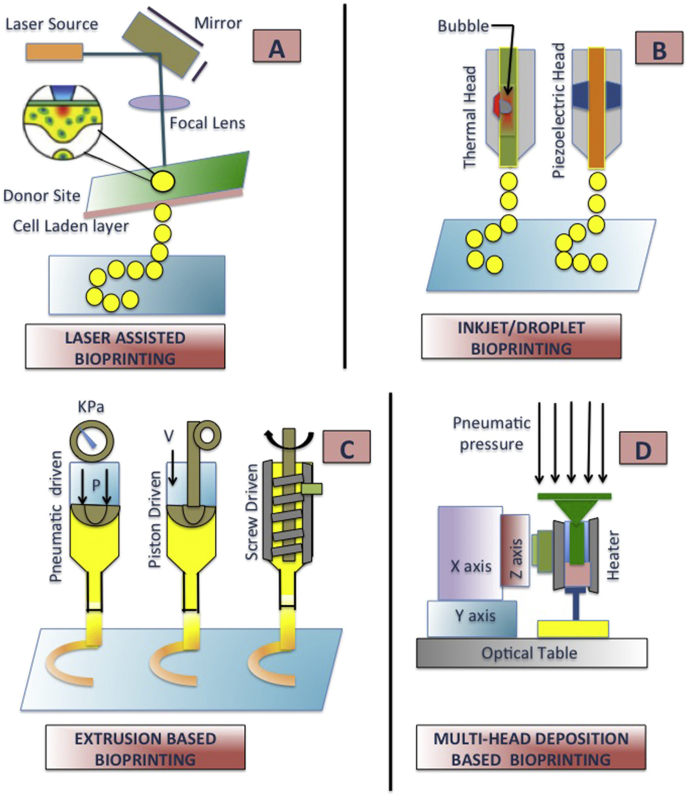
The past five years have witnessed an immense increase in the availability of bioprinting methods. Amongst these the more prevailing and well established 3D bioprinting modalities include: laser-assisted, inkjet/droplet bioprinting and extrusion-based bioprinting. In addition, several other like multi-head deposition systems, 4D bioprinting technology, custom-made bioprinting systems exist where the programming is done in a computer-aided design/computer - aided manufacturing (CAD/CAM) system to design the 3D construct.25, 26, 27, 28, 29, 30, 31, 32, 33, 34 The different types of techniques are described in detail. Table 1.
Table 1.
Description of various techniques of 3D Bioprinting.
| Type of 3D Bioprinting technology | Basic Principle Involved | Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| Laser-Assisted Printing |
Laser pulse stimulates a small area of the target. | Bone tissue engineering Creation of complex scaffolds for guided tissue regeneration. | High degree of precision and resolution, ability to use high viscosity bio-ink print high cell density >95% cell viability |
Time consuming, high cost |
| Ink-jet Printing | Pressure change in the upstream of nozzle Results in a downstream droplet ejection. | Printing of complex ceramic-like structures to support guided tissue regeneration. Drop-by-drop bioprinting of live cells for cell aggregate approach. |
High speed, availability, low cost >85% cell viability |
Lack of precision in droplet placement and size, need for low viscosity bio-ink |
| Extrusion based printing | Material fuses together at room temperature after leaving the nozzle. | Can be used with many material for creation of simple biocompatible biodegradable scaffolds for guided tissue regeneration | Affordability, high-speed printing, and potential of printing multiple materials at the same time | Only thermoplastic materials can be used Inability to embed cells in the material |
| Multi head deposition system | Extrusion of components via multiple mixing nozzles, exposing each layer to UV light or heat | Can be used with wider range and forms of material for creation of biocompatible and biodegradable scaffolds for guided tissue regeneration | Wide range of flexibility in the material that can be used | Subdued resolution and speed |
5. Laser assisted bioprinting (LaAB)
The laser bioprinting technology has emerged from LIFT (Laser induced forward transfer) technology as a promising method for tissue engineering. Though less popular, this technique has prominent advantages. The major components of LaAB include: (1) A pulsed laser source, (2) Target in the form of a transparent glass slide or ribbon that serves as a support for the bioprinting material and (3) Substrate that receives or collects the material. The bioprinting procedure initiates when a focused laser pulse stimulates a small area on the target, which comprises of an energy-absorbing layer on the surface and bioink solution underneath followed by evaporation of a portion of the energy-absorbing layer resulting in the formation of a droplet that is collected by the receiving substrate to be cross-linked thereafter.35,36 (Fig. 2a).
Fig. 2.
Different 3D bioprinting modalities. a). Laser Assisted Bioprinting- A focused laser pulse stimulates an area on the target, comprising an energy-absorbing surface layer and bioink solution underneath followed by evaporation of a portion of the energy-absorbing layer resulting in the formation of a droplet that is collected by the receiving substrate to be cross-linked. b). Inkjet/Droplet Bioprinting- Volume change occurring upstream in the nozzle, creates pressure change causing downstream droplet ejection. c) Extrusion Based Bioprinting- Thermoplastic polymer filament gets melted at the nozzle into a semi-fluid state and then extruded on a platform which after solidification fuses together to create a continuous structure. d) Multiple Deposition System- Multiple cartridges mounted in XYZ stage along with regulation of pressure and temperature. Pneumatic or mechanical pressure causes extrusion of viscous material from the cartridge nozzle onto the platform.
Laser-assisted bioprinters do not have nozzles, as observed in inkjet bioprinters thus abstaining the direct contact between the bioink and dispenser, and also material/cells clogging is prevented. Therefore LaABs have demonstrated high-resolution products and are compatible with several high viscosity material, (1–300 mPa/s), have a capability to maintain >95% cell viability.37 These benefits render laser-assisted bioprinting technology to be used for tissue engineering with reasonable assurance.
Selective laser sintering (SLS), stereolithography (SLA) and LIFT are among the few popular variations of laser-assisted 3D bioprinting. SLS technology was first developed by Carl Deckard in 1989, who used high-powered carbon dioxide laser beam to create structures on the basis of sectional data from CAD by fusing the powder layer-by-layer with the underlying powder as support. After creation of one layer, the powder bed recedes and subsequent layer is rolled over it. Such process is repeated until the scaffold is completed. Since then SLS has been extensively used for regeneration of complex anatomical tissue like craniofacial bone or cartilage.38,39A variety of powder material can be employed by SLS bioprinters to produce tissue engineering scaffolds like metals, bio-ceramics, and synthetic polymers like polylactic acid (PLA), polycaprolactone (PCL), poly-ethyl-ether-ketone (PEEK), and polyether-ketone-ketone (PEKK). Incorporation of HA powders in the polymers have demonstrated increased osteo-induction in bone scaffolds.40, 41, 42 However, in SLS high temperature generated by the laser during bioprinting, limits the use of natural polymers in blended scaffolds.
Of all the synthetic polymers, PCL is successfully and advantageously used in SLS bioprinters due to its low melting (59–64 °C) and glass-transition temperatures (−60 °C) that facilitates the prototyping process. SLS-printed PCL scaffolds have demonstrated biocompatibility and adequate strength in periodontal repair, craniofacial bone, or osteochondral defects.43, 44, 45, 46 Another polymer popularly used in SLS bioprinting is PEEK, added in the category of high-performance polymers. Though the manufacturing strategy does not differ much from that of PCL scaffolds, PEEK exhibits improved mechanical strength particularly compressive strength than PCL,41 and has exhibited successful application in the craniofacial region.47, 48, 49, 50
Considering the efficiency of fabrication, flexibility and complexity of the 3D constructs prepared, none of other bioprinting technology can compete with the SLS. However, complicated control of laser bioprinting system hinders further advancement of laser-assisted technologies. Also laser exposure induced side effects cannot be overlooked.
5.1. Inkjet bioprinting
In inkjet or droplet bioprinting, a small volume change is introduced upstream of the nozzle, which creates a pressure change causing downstream droplet ejection. The droplet ejection is performed by either piezo-electrically induced inkjet head system or thermally induced system. Piezoelectric heads induce change in the volume of a piezoelectric biomaterial by applying a voltage pulse. In thermal induced heads, current is passed through a resistor that vaporizes the fluid in contact to form a bubble that subsequently expands in the reservoir to cause increase in pressure. The increased pressure causes droplet ejection through the nozzle.51,52 Several powder biomaterials can be processed using inkjet or droplet ejection technology such as polymers, ceramics, proteins, and cells. However, to avoid continuous flow of the material or high ejection pressures, the ink's viscosity is limited to range of 5–20 Pa. Apart from high-speed printability and low cost, the feasibility to encapsulate cells in the biomaterial is the chief advantage of this technique.53 (Fig. 2b).
Cooper et al. demonstrated calvarial bone formation by using inkjet-based biopattern demonstrating osteoblast differentiation controlled by bone morphogenetic protein-2 (BMP-2). Microporous scaffolds made from dermamatrix, containing extracellular molecules such as collagen and fibronectin incorporating BMP-2, showed in vitro cell differentiation and subsequent tissue formation in-vivo.54 Inzana et al. demonstrated fabrication of composite calcium phosphate and collagen scaffolds using inkjet bioprinting, where calcium phosphate scaffold was coated with collagen. The resultant implanted scaffolds were biodegradable and exhibited appreciable osteo-conductivity.30
5.2. Extrusion-based bioprinting
Extrusion based bioprinters are also known as fused deposition modeling (FDM) printers and are the most popular. There is a thermoplastic polymer in the form of continuous filament that gets melted at the nozzle into a semi-fluid state and is extruded either on a platform or over the initially deposited layer. After solidification at room temperature, the material fuses together to create a continuous structure. Alterations in the bioprinting speed, thickness of layer and orientation of the construct can bring about the desired modification in the quality of printing the tissue construct.55 (Fig. 2c).
Compared to other bioprinters, extrusion 3D bioprinters are able to rapidly fabricate large-scale constructs, can be tailored to dispense a variety of material with proven osteo-inductive capacity, such as calcium phosphate injectable pastes, ceramic bases, cell-laden hydrogel, or PCL.14,34 Moreover, cost efficacy and the potential to print multiple material simultaneously with a multi-nozzle bioprinter are the major advantages.
For FDM printing, the biomaterial must exhibit low melting points such as polycarbonate (PC), acrylonitrile butadiene styrene (ABS), PCL, or PLA.56 Hence only thermoplastic material can be used with this technology. Since the melting temperature of thermoplastic biomaterials >37 °C, the cells become nonviable limiting the cell incorporation within the material.
Kim et al. implanted FDM bioprinted anatomically shaped molar scaffolds composed of PCL and hydroxyapatite (HA) with 200-μm diameter interconnecting microchannels perfused with stromal-derived factor-1 (SDF1) and BMP-7 in rats. Increase in endogenous cells and subsequent angiogenesis was observed in the experimental group as compared to control group.57 Li et al. implanted PCL scaffolds in rats along with freeze-dried platelet-rich plasma and exhibited osteogenic differentiation of dental pulp stem cells with subsequent induction of bone formation.58
5.3. Multi-head deposition systems (MHDS)
Another technique resembling FDM to a great extent is the 3D plotting technique or the multi-head deposition system. It comprises of multiple cartridges mounted in an XYZ stage such that the position of each cartridge, pressure and temperature of the biomaterial can be regulated. Pneumatic or mechanical pressure causes the viscous material from the cartridge to extrude out from the nozzle on a specific place on the platform.56 Similar to FDM, the MHDS technique also allows multi material printing of heterogeneous structures. For bioprinting and curing of the material, extrusion of the reactive components occurs via mixing the nozzles, followed by exposure of individual layer to UV light or by heating the stage to stabilize the material post printing resulting in the desired heterogenous construct.59 (Fig. 2d).
The major advantage of this technique is the wide range of flexibility in the material ranging from hydrogel, plastics, pastes, to solutions. These biocompatible material allow cell encapsulation prior to bioprinting. The major disadvantage of this method is the low resolution and speed as compared to FDM technique.
For bone tissue regeneration, multiple bioinks suitable for MHDS technique have been proposed. Ma et al. used bioink comprising of different ratios of gelatin methacrylate (Gel MA) and poly-ethylene glycol (PEG) dimethacrylate with encapsulated periodontal cells.60 Wenz et al. used methacrylate gel and HA modified methacrylated hyaluronic acid as bioink along with encapsulate human adipose-derived stem cells.61
5.4. 4D bioprinting
Now a ‘‘four-dimensional (4D) bioprinting” technology is emerging wherein the designed and bioprinted constructs can change form and function post-printing in response to an environmental stimuli, thus possessing capability for performance-driven applications. This technology is dependent on evolution of novel biomaterials that interact with the environmental factors (e.g., humidity, temperature, or chemicals) that may cause change in their form and function post fabrication.62, 63, 64
Miao and colleagues developed renewable soyabean oil epoxidized acrylate liquid resin to be used in multidimensional SLA for fabrication of scaffolds for biomedical regeneration. The scaffolds exhibited excellent shape memory dependent on the glass transition temperature (GTT) of the polymerized acrylate resin. When the temperature < GTT, cross-linker became frozen and shape of construct got fixed. When the temperature > GTT, the scaffold recovered to its original shape.65
To date, 4D bioprinting of scaffolds is mainly based on use of polymers with few attempts of ceramic-based functionally graded material.66, 67, 68, 69, 70 However limited applications in fabrication of scaffolds for bone tissue engineering have been demonstrated. In future, 4D bioprinting might generate geometrically programmable implantable scaffolds for bone regeneration.
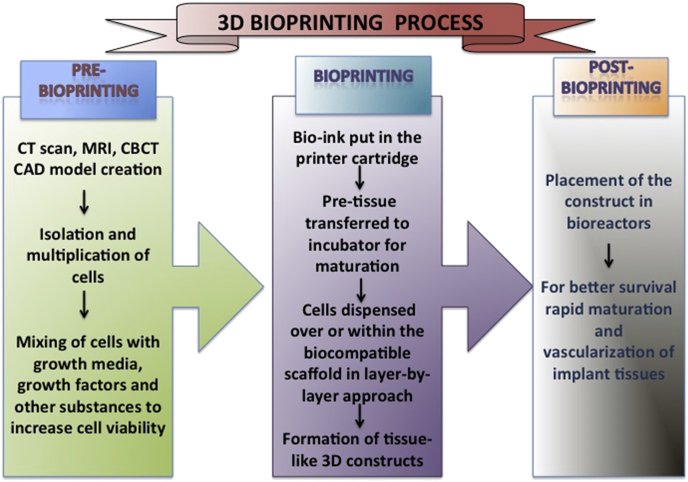
6. 3D bioprinting process
The basic process behind 3D bioprinting can be classified as pre-bioprinting, bioprinting, and post-bioprinting.10,71 (Fig. 3).
Fig. 3.
Basic processes behind 3D bioprinting.
6.1. Pre-bioprinting
Pre-bioprinting is the process of creating a CAD model of the desired tissue and choosing appropriate biomaterial. Computed tomography (CT) or cone beam CT in Digital Imaging and Communications in Medicine (DICOM) format and magnetic resonance imaging of the desired tissue is obtained and fed to the bioprinter in Standard Triangle Language (STL) format.71,72
However, before initiating the bioprinting, isolation and multiplication of desired cells is done. These cells are mixed with growth media containing growth factors like FGF (Fibroblast growth Factor), PDGF (Platelet derived Growth Factor), BMP (Bone morphogenetic proteins), IGF (Insulin-like growth factor), TGFb (Transforming growth factor beta), VEGF (Vascular endothelial growth factor) along with other substances like antioxidants, dexamethasone, ascorbic acid, and rosiglitazone, and nutrients to maintain and improve the viability of cells.73 Sometimes cell aggregates without scaffolds are used wherein the cells are encapsulated in cellular spheroids about 500 μm in diameter.74
6.2. Bioprinting
It is the bioprinting process per se, wherein the bioink comprising cells, matrix, and nutrients is put in the printer cartridge and is used to print the image following a layer by layer approach.75 For maturation of tissues the bioprinted cell based pre-tissue is transferred to an incubator.76 The cells are then dispensed over or within the biocompatible scaffold using a successive layer-by-layer approach to generate tissue-like three-dimensional biological constructs.77
6.3. Post-bioprinting
In order to create a stable structure and maintain the mechanical integrity and function of the 3D construct, proper post-bioprinting process is necessary.71 The mechanical and chemical stimulations send signals to the cells to control the remodeling and growth of tissues thus responsible for maintenance of the construct.
Latest advancements include bioreactors that possess ability for better survival of transplants and also aid in rapid maturation and vascularization of tissues.10,78 Bioreactors work on different modules such as providing convective nutrient transport, creating microgravity environments, changing the pressure causing solution to flow through the cells, or add compression for dynamic or static loading. For cartilaginous tissues, compression bioreactors are considered ideal.79
6.4. Biomaterial for 3D bioprinting of craniofacial tissues
3D bioprinters are technically designed to fabricate bony and cartilaginous scaffolds customized according to the specific defects with high fidelity.80 High incidence of bone loss due to trauma, osteoporosis, tumors etc renders bone to be one of the most frequently transplanted tissue apart from cartilage. For such replacements to be a success, the biomaterial should be biocompatible, printable, osteo-conductive, osteo-inductive, and possess comparable mechanical properties; such as bio-ceramics, polymers, their composites and hydrogel.81
6.5. Bio-ceramics
Bio-ceramic composed of calcium and phosphate mineral phases are the most commonly selected biomaterial. These include HA, β-tricalcium phosphate (TCP), or bioactive glasses (BGs). 3D bioprinted ceramics have been demonstrated to promote osteogenesis by creating a bioactive ion-rich cellular micro-environment and promote cell proliferation by close cell-cell interactions.82 Saijo and colleagues demonstrated that the bio-ceramic scaffolds implanted in load bearing areas of the craniofacial region were too brittle for implantation. Specifically in the maxilla-mandibular defects, HA/αTCP composite scaffolds lacked the strength and dimensional stability.83 Shao found that the flexural strength of ∼10% Mg-substituted wollastonite was much higher (∼31 MPa) than βTCP and other bioceramics containing calcium-silicate. Kargozar observed that by addition of metallic ions like Cu2+ and Co2+ into BGs, the angiogenic activity can be initiated in-vivo, which can aid in rapid wound healing.84,85 Additionally, addition of bio-ceramics enhances the osteogenesis and osteo-inductivity.
6.6. Polymers
Superior printability and efficient promotion of osteogenesis makes polymers as material of choice for bioprinting craniofacial tissues. However, they lack adequate stiffness and exhibit poor cellular interaction. Though quite popular earlier, PLA and PGA (Polyglycolic acid) due to their low compressive strength and osteo-conductivity, are rarely the material of choice for bone scaffolds. However, co-polymer PLGA was found to exhibit remarkable osteo-conductivity and better mechanical properties. Another polymer PCL has demonstrated more frequent use in bone tissue engineering for its easy availability, cost efficacy and suitability for modification ie. adjustable physio-chemical state, biological properties and mechanical strength. This imparts PCL capability to withstand physical, chemical and mechanical insults without significant loss of properties. Also since its rate of degradation is quite low subsequently denser tissues can be generated using PCL. The low melting points (62 °C) renders PCL to be a material of choice for bone scaffolds in extrusion-based 3D-bioprinting/FDM.
The newer SLS 3D bioprinters are more precise and can therefore control the porosity desired for growth and proliferation of the cell. A spectrum of thermoplastic materials including high performance plastics are now available for SLS technique. Nyberg and colleagues fabricated PCL scaffolds by SLS and observed the stiffness of PCL scaffolds (range ∼15–300 MPa), was much superior to conventional 3D polymers but still lower than human trabecular bone within the condyle (120–450 MPa) or mandibular body (112–910 MPa). On the other hand, metal-based scaffolds exhibit enough stiffness but possess a higher Young's modulus, which leads to stress shielding causing implant failure.50
A major limitation of using PCL alone is its relative bio-inertness. It is suggested that a composite scaffold containing bio-inert PCL framework with biological active components like βTCP, HA, de-cellularized trabecular bone, or growth factors should be incorporated into 3D bioprinting system.14,43,86, 87, 88 Other limitations in using PCL include its hydrophobic nature and acidic environment caused by its degradation. To overcome this, hydrophilic polymers like PEG may be added or a surface coating of natural polymers like chitosan can be beneficial.89,90
High-performance polymers like Polyaryletherketones (PEAKs) are gaining popularity as craniofacial tissue substitutes since their Young's modulus is comparable and compatible to natural bone, as desired for load bearing bone and craniofacial implants. In PEAK family, PEKK has more remarkable performance in terms of mechanical strength and biocompatibility. Adamzyk and colleagues fabricated implants for craniofacial bone defect model using PEKK in SLS bioprinters. They exhibited improved mechanical strength, biocompatibility and appreciable osteointegration in-vivo. Similar findings were observed by Lin et al. wherein mesenchymal stem cells derived from TMJ synovial fluid were used.40,49,50
6.7. Hydrogel
Hydrogel exhibit capability to synthesize extracellular matrix. Fabrication of hydrogel encapsulating chondrocytes or MSCs involves micro-extrusion technique. The spectrum of cell-laden hydrogels includes natural polymers like alginate, collagen and synthetic like Gel MA, polyethylene glycol dimethacrylate (PEGDMA).91,92
The basic challenge associated with the use of hydrogel is the dilemma of using appropriate polymer concentration. Higher polymer concentration is desirable to improve the mechanical properties, and viscosity of the construct. While for proliferation of cells and their chondrogenic differentiation, lower polymer concentration is more effective.
To address this issue, Lee suggested employing stiff thermoplastic polymer such as PCL to cell-laden hydrogels via hybrid strategies. PCL acts as a frame to reinforce the constructs by modifying the polymer percentage, attain equilibrium of the compressive modulus as desired for fabrication of articular cartilage.93,94 However, slower rate of degradation of PCL is a potential limitation because the residual filaments cause hindrance by acting as a barrier to tissue formation. To overcome this, polymers with higher degradation rate like poly hydroxymethylglycolide-co-caprolactone (PHMGCL) or PLGA are employed in multicomponent biomaterial. Yet the acidic by-products of PLGA causing adverse inflammatory response, still makes it a concern for its future applications.95
A region-variant TMJ disc scaffold was fabricated comprising specifically-aligned PCL along with PLGA microspheres encapsulating TGFβ3. Upon seeding with MSCs, multiphase fibrocartilaginous tissues were formed. This resulted in significant improvement of the healing process of the perforated disc and restoration of the dynamic function with no arthritic changes on the condyle four weeks post-implantation.96,97 Visser demonstrated yet another way of reducing the residual PCL material by increasing its porosity using melt-electro writing (MEW) technique. This technique was similar to FDM but comprised of a voltage gated nozzle tip. Thus very thin (diameter apporox. 0.8 μm) PCL could be fabricated by MEW with 93–98% porosity along with stiffness and yielding strains comparable to native cartilage.98
Kang et al., demonstrated fabrication of 3D bioprinted multi-typic tissue construct with cell-laden hydrogel for regeneration of large mandibular bone defect. The resultant scaffold had well gauged porosity to stimulate new vasculature formation.9 Another example includes 3D bioprinted scaffolds using medical-grade PCL with TCP combined with rhBMP-7, for bone regeneration of a 30 mm long bone defect in a sheep model. Post-surgical evaluation by biomechanical and microcomputed tomography analyses after 12 months revealed significantly greater bone formation with better quality of bone formed. Thus bridging of the gap by regenerated bone was more remarkable than the autologous bone.99
Recently Anada et al. developed a 3D hydrogel based bone scaffold with cells loaded within it. A two-step digital light processing technique employing Gel MA hydrogels, octa-calcium phosphate and cell aggregates of human umbilical vein endothelial cells in the form of spheroids was attempted. It was observed osteoblastic differentiation of mesenchymal stem cells was stimulated with formation of capillary-like structures.100
When a customized PCL scaffold was 3D-printed as per the requirements of the defect to prevent its mechanical failure, and ensure integration, Zamani et al. found that their mechanical properties differed in scaffold building direction compared with the side direction.101
Atala et al. also demonstrated fabrication of soft hydrogel scaffolds with incorporated firm polymers and stem cells using an “integrated tissue-organ bioprinter”. For bone bioprinting, a bio-ink comprising gelatin and hyaluronic acid along with TCP was used.9,102
Description of constructs used for craniofacial regeneration in animals and human studies are provided in Table 2.
Table 2.
Description of constructs used for craniofacial regeneration in animals and human studies.
| LASER ASSISTED BIOPRINTING | |||||
|---|---|---|---|---|---|
| S No. | Studied by | Type of construct | Model used | Outcome | |
| 1 | Williams et al. (2005)45 | Porous PCL mandibular condyle scaffold | Pigs | Compressive modulus and yield strength values ranged from 52 to 67 MPa, 2.0–3.2 MPa. Bone formation in-vivo observed. | |
| 2 | Smith et al. (2007)46 | PCL based condylar ramus unit scaffold for TMJ reconstruction with BMP7. | Pigs | Cartilaginous tissue regeneration along the articulating surface with exuberant osseous tissue formation. Significant new bone growth interior and exterior to the scaffold seen. | |
| 3 | Zhang et al. (2015)42 | HA/epoxide acrylate maleic artificial implants for craniomaxillofacial bone defects. | Human | Improved aesthetic Results and functional recovery after reconstruction. | |
| 4 | Zopf et al. (2016)92 | Subcutaneous PCL auricular and nasal scaffolds | Porcine | Excellent appearance and complete soft tissue ingrowth. In-vitro histologic analysis of scaffolds showed native appearing cartilaginous growth respecting the boundaries of scaffold | |
| 5 | Adamzyk et al. (2016)49 | High performance thermoplastic PEKK scaffolds along with Humans and sheep MSCs in calvarial defects | Sheep | 3D PEKK scaffolds were cyto- and bio-compatible and exhibited adherence, growth and osteogenic differentiation with newly formed bone, a fibrous capsule around implants. | |
| 6 | Roskies et al. (2017)40 | 3D-printed PEKK scaffolds combined with Adipose-derived stem cells in critical-sized mandibular defect | Rabbits | Improved bone-implant interface and increased resistance to forces of mastication after mandibular reconstruction. | |
| 7 |
Lin et al. (2019)50 |
3D-printed PEKK scaffolds along with Human synovial fluid MSCs (hSF-MSCs) for calvarial critical-sized bone defects |
Rabbits |
In-vitro, hSF-MSCs attached, proliferated, and were osteogenic on PEKK In-vivo twice the amount of newly formed bone when compared to PEKK seeded with osteogenically-induced hSF-MSCs or PEKK scaffolds alone. |
|
| INKJET/DROPLET BIOPRINTING | |||||
| 1 | Lee et al. (2005)104 | Anatomically shaped Zygoma scaffolds of PCL/HA | In-vitro | Intestinal epithelial cell attached to scaffolds uniformly, grew preferentially in villi region. | |
| 2 | Saijo et al. (2009)78 | Alpha-tricalcium phosphate powder scaffolds for maxillofacial deformities. | Human | Partial union between the artificial bones and host bone tissues was seen. | |
| 3 | Cooper et al. (2010)54 | Circular Derma Matrix human allograft scaffold constructs with BMP2 for calvarial defect model. | Mice | Patterns of bone formation in vivo were comparable with patterned responses of osteoblastic differentiation in vitro | |
| 4 |
Lee et al. (2013)103 |
Custom scaffolds with orthogonal interconnected channels to mimic human mandibular condyle using PCL, chitosan with HA coating for osteochondral tissue engineering |
In vitro |
Bone marrow stromal cells (BMSC) showed good viability in the scaffolds, and the apatite coating further enhanced cellular spreading and proliferation. |
|
| EXTRUSION BASED BIOPRINTING | |||||
| 1 | Kuss et al. (2017)105 | Bone constructs of PCL/HA and SVF derived cell (SVFC)-laden hydrogel bioink | Mice | Microvessel formation in vitro and in vivo, integration with existing host vasculature along with osteogenic differentiation of SVFC. | |
| 2 | Kang et al. (2016)9 | Cell laden hydrogels to construct mandible, calvarial scaffolds. | In vitro | Successful fabrication of mandible and calvarial bone, cartilage and skeletal muscle. | |
7. 3D bioprinting: Limitations
The major limitations can be assembled into three groups pertaining to material, manufacturing or vascularity. The most crucial aspect for satisfactory bioprinting of clinically useful tissue construct is selection of appropriate biomaterial. Many of the traditionally used biomaterial are biologically active enough to cause undesired cellular interactions and therefore cause premature or unwanted stem cell differentiation.106 The novel biopolymers and hydrogel lack the structural integrity and are unfit for conventional bioprinting methods despite close nanostructural resemblance with native tissue and similarity in ECM and other components.107
Hence it is recommended to combine two different biomaterial to harness their benefits and obtain a firm scaffold with better mechanical properties and use a softer substance exhibiting better proliferative and cytocompatible effects on the construct.
Other drawbacks include large time span required for bioprinting, inability to consistently deliver the number of cells needed for tissue regeneration, alteration in cellular geometry or even cell death. Hence the overall efficiency of the bioprinting process needs to be improved.108
The other greatest challenge in bioprinting functional tissues involves the formulation of vasculature needed for survival of the tissue. In-vivo growth of tissues beyond 100–200 μm, requires a vascular network as oxygen can diffuse within this limit only.109 In absence of vasculature the newly formed tissue constructs lack nutrients leading to incomplete tissue formation or necrosis.110 It is noteworthy that the vasculature must develop at an early developmental stage so as to prevent tissue death and for attachment and growth of endothelium. Later the vasculature needs to take over all the functions like maintenance of a selective barrier for waste products, inflammatory functions, coagulation and other homeostatic functions occurring during normal development.111
Bioprinting of vasculature is challenging because of the limitations in printing resolution in the current bioprinters. The desired resolution is 3 μm diameter for capillaries but the highest resolution obtained by laser-based bioprinters is 20 μm. To overcome these challenges, incorporation of angiogenic growth factors is suggested within the bioink to restore growth of host vasculature after in-vivo implantation. Also vascular networks of synthetic origin can be used. Bioprinting of larger diameter vessels has been successful, however in small-caliber vascular grafts (<5 mm) patency rates are quite poor. Accomplishment of functional vasculature in the construct for supporting the development of tissue and preventing tissue death is still to be achieved.
8. Future perspectives
Various 3D bioprinting techniques have been attempted to fabricate tissues and organs of varied origin. Though literature review cites few examples of 4D bioprinted grafts, the actual endeavor lies in development of smart polymers that could change their form and function like change in volume or other properties post printing in response to environmental stimuli such as pH, temperature, or magnetic field for fabrication of new generation smart or programmable tissue constructs.112 Also, miRNAs could be spatially incorporated into 3D bioprinted constructs that may potentially initiate or regulate vascularization and bone formation in 3D bioprinted bone tissue constructs.113
Scaffolds can also incorporate anti-bacterial or anti-cancer drugs for improved treatment of infection/bone tumor resection-induced defects.114 However, the use of these materials for bone tissue engineering requires more evidence of success for future applications. In view of the exponential research on the latest 3D bioprinting technologies, bioprinted patient specific surgical implants for craniofacial reconstruction, can be vascularized and rapidly integrated with supporting host tissue. Replicating different microniches in bone with the cell types, bioprinted constructs may encourage normal balance between osteogenesis and remodeling.115 Biofabricated tissue scaffolds could be transferred directly to the surgical table for implantation, thereby increasing the therapeutic and logistical benefits for craniofacial regeneration.116 Suitable bio-inks exhibiting viscoelasticity and nonlinear behavior, similar to native cartilage of the temporo-mandibular joint disc, auricular or nasal cartilage may also be bioprinted.
9. Conclusion
Various 3D bioprinting techniques have been attempted to fabricate different tissues and organs in the craniofacial region using advanced engineered composite biomaterial incorporated with stem cells in the bioink. Though several studies reporting success exist in the literature, however considering either of these materials as an ideal for craniofacial bone or cartilage tissue engineering requires more evidence of success for future applications.
Funding source
ICMR Research Grant No. ∗IRIS No. 2015–0331∗.
Declaration of competing interest
Authors have no conflicts of interest to declare.
Acknowledgements
Department of Health Research Multispecialty Research Unit, King George's Medical University, Lucknow.
References
- 1.Hardiman R., Kujan O., Kochaji N. Normal variation in the anatomy, biology, and histology of the maxillofacial region. In: Farah C., Balasubramaniam R., McCullough M., editors. Contemporary Oral Medicine. Springer; Cham: 2019. [DOI] [Google Scholar]
- 2.Visscher D.O., Farré-Guasch E., Helder M.N., Gibbs S., Forouzanfar T. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016;34(9):700–710. doi: 10.1016/j.tibtech.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ozbolat I.T. Scaffold-based or scaffold-free bioprinting: competing or complementing approaches? J Nanotechnol Eng Med. 2015;6(2) [Google Scholar]
- 4.Ozbolat I.T., Peng W., Ozbolat V. Application areas of 3D bioprinting. Drug Discov Today. 2016;21(8):1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Moroni L., de Wijn J.R., van Blitterswijk C.A. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27:974–985. doi: 10.1016/j.biomaterials.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Perkins J.D. Are we reporting the same thing? Liver Transplant. 2007;13:465–466. [PubMed] [Google Scholar]
- 7.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:22001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obregon F., Vaquette C., Ivanovski S., Hutmacher D.W., Bertassoni L.E. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res. 2015;94:143s–152s. doi: 10.1177/0022034515588885. [DOI] [PubMed] [Google Scholar]
- 9.Kang H.W., Lee S.J., Kap Ko I., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 10.Ozbolat I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33(7):395–400. doi: 10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Snyder J.E., Hamid Q., Wang C. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication. 2011;3(3) doi: 10.1088/1758-5082/3/3/034112. [DOI] [PubMed] [Google Scholar]
- 12.Serrano D.R., Terres M.C., Lalatsa A. Applications of 3D printing in cancer. J 3D Print Med. 2018;2(3) doi: 10.2217/3dp-2018-0007. [DOI] [Google Scholar]
- 13.Ghorbani F., Li D., Ni S., Zhou Y., Yu B. 3D printing of acellular scaffolds for bone defect regeneration: a review. Mat Tod Comm. 2020;22 doi: 10.1016/j.mtcomm.2020.100979. 100979. [DOI] [Google Scholar]
- 14.Dwivedi R., Kumar S., Pandey R. Polycaprolactone as biomaterial for bone scaffolds: review of literature. J Oral Biol Craniofac Res. 2020;10:361–388. doi: 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10(6):678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Gao F., Chiu S.M., Motan D.A. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura K., Okada Y., Aoi T. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 18.Mironov V., Visconti R.P., Kasyanov V., Forgacs G., Drake C.J., Markwald R.R. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norotte C., Marga F.S., Niklason L.E., Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama K. Chapter 1. In vitro biofabrication of tissues and organs. In: Forgacs G., Sun W., editors. 1–21. William Andrew Publishing; Boston, MA, USA: 2013. (Biofabrication). [Google Scholar]
- 21.Munaz A., Vadivelu R.K., John J St, Barton M., Kamble H., Nguyen N.-T. Three-dimensional printing of biological matters. J Sci Adv Mater Devices. 2016;1:1–17. [Google Scholar]
- 22.Chung J.H.Y., Naficy S., Yue Z. Bio-ink properties and printability for extrusion printing living cells. Biomat Sci. 2013;1:763–773. doi: 10.1039/c3bm00012e. [DOI] [PubMed] [Google Scholar]
- 23.Tan Y., Richards D.J., Trusk T.C. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/2/024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozano R., Stevens L., Thompson B.C. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials. 2015;67:264–273. doi: 10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Wüst S., Müller R., Hofmann S. Controlled positioning of cells in biomaterials—approaches towards 3D tissue printing. J Funct Biomater. 2011;2:119–154. doi: 10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dababneh A.B., Ozbolat I.T. Bioprinting technology: a current state-of-the-art review. J Manufac Sc Eng, Transa. ASME. 2014;136(6) [Google Scholar]
- 27.Khalil S., Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng. 2009;131(11):111002. doi: 10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z., Abdulla R., Parker B., Samanipour R., Ghosh S., Kim K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/4/045009. [DOI] [PubMed] [Google Scholar]
- 29.Trombetta R., Inzana J.A., Schwarz E.M., Kates S.L., Awad H.A. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng. 2017;45(1):23–44. doi: 10.1007/s10439-016-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inzana J.A., Olvera D., Fuller S.M. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do A.V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv Healthcar Mat. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim J.H., Won J.Y., Sung S.J. Comparative efficacies of a 3D-printed PCL/PLGA/β-TCP membrane and a titanium membrane for guided bone regeneration in beagle dogs. Polymer. 2015;7(10):1500. [Google Scholar]
- 33.Wu Z., Su X., Xu Y., Kong B., Sun W., Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016;6:24474. doi: 10.1038/srep24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 35.Schiele N.R., Corr D.T., Huang Y., Raof N.A., Xie Y., Chrisey D.B. Laser-based Ngo direct-write techniques for cell printing. Biofabrication. 2010;2 doi: 10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colina M., Serra P., Fernandez-Pradas J.M., Sevilla L., Morenza J.L. DNA deposition through laser induced forward transfer. Biosens Bioelectron. 2005;20:1638–1642. doi: 10.1016/j.bios.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Mandrycky C., Wang Z., Kim K., Kim D.H. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzoli A. Selective laser sintering in biomedical engineering. Med Biol Eng Comput. 2013;51:245–256. doi: 10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 39.Brunello G., Sivolella S., Meneghello R. Powder-based 3D printing for bone tissue engineering. Biotechnol Adv. 2016;34:740–753. doi: 10.1016/j.biotechadv.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Roskies M.G., Fang D., Abdallah M.N. Three-dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical-sized mandibular defects. Laryngoscope. 2017;127:E392–E398. doi: 10.1002/lary.26781. [DOI] [PubMed] [Google Scholar]
- 41.Roskies M., Jordan J.O., Fang D. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl. 2016;31:132–139. doi: 10.1177/0885328216638636. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Shen S., Yu H., Shen S.G., Wang X. Computer-aided design and computer-aided manufacturing hydroxyapatite/epoxide acrylate maleic compound construction for craniomaxillofacial bone defects. J Craniofac Surg. 2015;26:1477–1481. doi: 10.1097/SCS.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 43.Rasperini G., Pilipchuk S.P., Flanagan C.L. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94:153s–157s. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- 44.Nyberg E.L., Farris A.L., Hung B.P. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45:45–57. doi: 10.1007/s10439-016-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams J.M., Adewunmi A., Schek R.M. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 46.Smith M.H., Flanagan C.L., Kemppainen J.M. Computed tomography-based tissue-engineered scaffolds in craniomaxillofacial surgery. Int J Med Robot Comput Assist Surg. 2007;3:207–216. doi: 10.1002/rcs.143. [DOI] [PubMed] [Google Scholar]
- 47.Peyre P., Rouchausse Y., Defauchy D., Regnier G. Experimental and numerical analysis of the selective laser sintering (SLS) of PA12 and PEKK semi-crystalline polymers. J Mater Process Technol. 2015;225:326–336. [Google Scholar]
- 48.Converse G.L., Conrad T.L., Merrill C.H., Roeder R.K. Hydroxyapatite whisker-reinforced polyetherketoneketone bone ingrowth scaffolds. Acta Biomater. 2010;6:856–863. doi: 10.1016/j.actbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Adamzyk C., Kachel P., Hoss M. Bone tissue engineering using polyetherketoneketone scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model. J Cranio-Maxillo-Fac Surg. 2016;44:985–994. doi: 10.1016/j.jcms.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y., Umebayashi M., Abdallah M.N. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci Rep. 2019;9:472. doi: 10.1038/s41598-018-36778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar A.V., Dutta A., Fay J.E. Electrophotographic printing of part and binder powders. Rapid Prototyp J. 2004;10:7–13. [Google Scholar]
- 52.Noguera R., Lejeune M., Chartier T. 3D fine scale ceramic components formed by ink-jet prototyping process. J Eur Ceram Soc. 2005;25:2055–2059. [Google Scholar]
- 53.Shirazi S.F.S., Gharehkhani S., Mehrali M. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16 doi: 10.1088/1468-6996/16/3/033502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper G.M., Miller E.D., DeCesare G.E. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng. 2010;16:1749–1759. doi: 10.1089/ten.tea.2009.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngo T.D., Kashani A., Imbalzano G., Nguyen K.T.Q., Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B Eng. 2018;143:172–196. [Google Scholar]
- 56.Wang X., Jiang M., Zhou Z., Gou J., Hui D. 3D printing of polymer matrix composites: a review and prospective. Compos Part B Eng. 2017;110:442–458. [Google Scholar]
- 57.Kim K., Lee C.H., Kim B.K., Mao J.J. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89:842–847. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Chen M., Wei X., Hao Y., Wang J. Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials. 2017;10:831. doi: 10.3390/ma10070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 60.Ma Y., Ji Y., Huang G., Ling K., Zhang X., Xu F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/4/044105. [DOI] [PubMed] [Google Scholar]
- 61.Wenz A., Borchers K., Tovar G.E.M., Kluger P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa91ec. [DOI] [PubMed] [Google Scholar]
- 62.Gladman A.S., Matsumoto E.A., Nuzzo R.G., Mahadevan L., Lewis J.A. Biomimetic 4D printing. Nat Mater. 2016;15(4):413–418. doi: 10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- 63.Li Y.C., Zhang Y.S., Akpek A., Shin S.R., Khademhosseini A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1) doi: 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- 64.Ge Q., Sakhaei A.H., Lee H., Dunn C.K., Fang N.X., Dunn M.L. Multimaterial 4D printing with tailorable shape memory polymers. Sci Rep. 2016;6:31110. doi: 10.1038/srep31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao S., Zhu W., Castro N.J. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:27226. doi: 10.1038/srep27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Z., Yuan C., Peng X., Wang T., Qi H.J., Dunn M.L. Direct 4D printing via active composite materials. Sci Adv. 2017;3(4) doi: 10.1126/sciadv.1602890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang L., Jiang R., Wu J. Ultrafast digital printing toward 4D shape changing materials. Adv Mater. 2017;29(7):1–6. doi: 10.1002/adma.201605390. [DOI] [PubMed] [Google Scholar]
- 68.Kuang X., Chen K., Dunn C.K., Wu J., Li V.C.F., Qi H.J. 3D printing of highly stretchable, shape-memory, and self-healing elastomer toward novel 4D printing. ACS Appl Mater Interfaces. 2018;10(8):7381–7388. doi: 10.1021/acsami.7b18265. [DOI] [PubMed] [Google Scholar]
- 69.Su J.W., Tao X., Deng H. 4D printing of a self morphing polymer driven by a swellable guest medium. Soft Matter. 2018;14(5):765–772. doi: 10.1039/c7sm01796k. [DOI] [PubMed] [Google Scholar]
- 70.Scheithauer U., Weingarten S., Johne R. Ceramic-Based 4D components: additive manufacturing (AM) of ceramic-based functionally graded materials (FGM) by thermoplastic 3D printing (T3DP) Materials. 2017;10(12):1368. doi: 10.3390/ma10121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashkan S., Anthony A. Printing technologies for medical applications. Trends Mol Med. 2016;22(3):254–265. doi: 10.1016/j.molmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Mironov V., Boland T., Trusk T. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 73.Phillippi J.A., Miller E., Weiss L., Huard J., Waggoner A., Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle-and bone-like subpopulations. Stem Cell. 2008;26(1):127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 74.Phadke A., Chang C.W., Varghese S. Springer; 2010. Biomaterials as Stem Cell Niche; pp. 19–44. Functional biomaterials for controlling stem cell differentiation. [Google Scholar]
- 75.Cooper-White M. Huffpost Science. The Huffington Post.com, Inc; 2015. How 3D Printing Could End the Deadly Shortage of Donor Organs. Retrieved 17 February 2016. [Google Scholar]
- 76.Thomas D.J. Could 3D bioprinted tissues offer future hope for microtia treatment? Int J Surg. 2016;32:43–44. doi: 10.1016/j.ijsu.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 77.Harmon K. A sweet solution for replacing organs. Sci Am. 2013;308(4):54–55. [Google Scholar]
- 78.Singh D., Thomas D. Advances in medical polymer technology towards the panacea of complex 3D tissue and organ manufacture. Am J Surg. 2018;217(4):807–808. doi: 10.1016/j.amjsurg.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Murphy S., Anthony A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 80.Taz O., Mascort J.K., Lin Y. The applications of 3D printing for craniofacial tissue engineering. Micromachines. 2019;10(7):480–498. doi: 10.3390/mi10070480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L., Yang G., Johnson B.N., Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/j.actbio.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 82.Lobo S.E., Glickman R., da Silva W.N., Arinzeh T.L., Kerkis I. Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tissue Res. 2015;361:477–495. doi: 10.1007/s00441-015-2116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saijo H., Igawa K., Kanno Y. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J Artif Organs. 2009;12:200–205. doi: 10.1007/s10047-009-0462-7. [DOI] [PubMed] [Google Scholar]
- 84.Shao H., Sun M., Zhang F. Custom repair of mandibular bone defects with 3D printed bioceramic scaffolds. J Dent Res. 2018;97:68–76. doi: 10.1177/0022034517734846. [DOI] [PubMed] [Google Scholar]
- 85.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Park S.H., Park D.S., Shin J.W. Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J Mater Sci Mater Med. 2012;23:2671–2678. doi: 10.1007/s10856-012-4738-8. [DOI] [PubMed] [Google Scholar]
- 87.Shim J.H., Yoon M.C., Jeong C.M. Efficacy of rhBMP-2 loaded PCL/PLGA/beta-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed Mater. 2014;9 doi: 10.1088/1748-6041/9/6/065006. [DOI] [PubMed] [Google Scholar]
- 88.Hung B.P., Naved B.A., Nyberg E.L. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater Sci Eng. 2016;2:1806–1816. doi: 10.1021/acsbiomaterials.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fedore C.W., Tse L.Y.L., Nam H.K., Barton K.L., Hatch N.E. Analysis of polycaprolactone scaffolds fabricated via precision extrusion deposition for control of craniofacial tissue mineralization. Orthod Craniofac Res. 2017;20(Suppl. 1):12–17. doi: 10.1111/ocr.12159. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-Méndez I., Fernández-Gutiérrez M., Rodríguez-Navarrete A. Bioactive Sr(II)/Chitosan/Poly(ε-caprolactone) scaffolds for craniofacial tissue regeneration. In vitro and in vivo behavior. Polymer. 2018;10:279. doi: 10.3390/polym10030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daly A.C., Cunniffe G.M., Sathy B.N., Jeon O., Alsberg E., Kelly D.J. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater. 2016;5:2353–2362. doi: 10.1002/adhm.201600182. [DOI] [PubMed] [Google Scholar]
- 92.Zopf D.A., Mitsak A.G., Flanagan C.L., Wheeler M., Green G.E., Hollister S.J. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg. 2015;152:57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J.S., Hong J.M., Jung J.W., Shim J.H., Oh J.H., Cho D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 94.Park S.H., Yun B.G., Won J.Y. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127:1036–1043. doi: 10.1002/lary.26400. [DOI] [PubMed] [Google Scholar]
- 95.Seyednejad H., Gawlitta D., Kuiper R.V. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(epsilon-caprolactone) Biomaterials. 2012;33:4309–4318. doi: 10.1016/j.biomaterials.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Tarafder S., Koch A., Jun Y., Chou C., Awadallah M.R., Lee C.H. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/2/025003. [DOI] [PubMed] [Google Scholar]
- 97.Legemate K., Tarafder S., Jun Y., Lee C.H. Engineering human TMJ discs with protein-releasing 3D-printed scaffolds. J Dent Res. 2016;95:800–807. doi: 10.1177/0022034516642404. [DOI] [PubMed] [Google Scholar]
- 98.Visser J., Melchels F.P., Jeon J.E. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 99.Reichert J.C., Cipitria A., Epari D.R. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4(141):93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 100.Anada T., Pan C.C., Stahl A.M. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci. 2019;20(5):1096. doi: 10.3390/ijms20051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zamani Y., Amoabediny G., Mohammadi J. 3D-printed poly(?-caprolactone) scaffold with gradient mechanical properties according to force distribution in the mandible for mandibular bone tissue engineering. Mech Behav Biomed Mater. 2020;104:103638. doi: 10.1016/j.jmbbm.2020.103638. [DOI] [PubMed] [Google Scholar]
- 102.Skardal A., Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43:730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- 103.Lee J.Y., Choi B., Wu B., Lee M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication. 2013;5(4) doi: 10.1088/1758-5082/5/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee M., Dunn J.C.Y., Wu B.M. Scaffold fabrication by indirect three-dimensional printing. Biomaterials. 2005;26(20):4281–4289. doi: 10.1016/j.biomaterials.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 105.Kuss M.A., Harms R., Wu S. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017;7(47):29312–29320. doi: 10.1039/c7ra04372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin I., Simmons P.J., Williams D.F. Manufacturing challenges in regenerative medicine. Sci Transl Med. 2014;6(232):232fs216. doi: 10.1126/scitranslmed.3008558. [DOI] [PubMed] [Google Scholar]
- 107.Hinton T.J., Jallerat Q., Palchesko R.N. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9) doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clark E.A., Brugge J.S. Integrins and signal transduction pathways: the road taken. Science. 1995;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 109.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 110.Malda J., Woodfield T.B., van der Vloodt F. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials. 2004;25(26):5773–5780. doi: 10.1016/j.biomaterials.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 111.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196(3):430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 112.Xia L.W., Xie R., Ju X.J. Nano-structured smart hydrogels with rapid response and high elasticity. Nat Commun. 2013;4:2226. doi: 10.1038/ncomms3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang C., Geng J., Jiang S. MicroRNAs in regulation of osteogenic differentiation of mesenchymal stem cells. Cell Tissue Res. 2017;368(2):229–238. doi: 10.1007/s00441-016-2462-2. [DOI] [PubMed] [Google Scholar]
- 114.Wang C., Huang W., Zhou Y. 3D printing of bone tissue engineering scaffolds. Bioactive Mater. 2020;5:82–91. doi: 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ibrahim A. 2018. 3D Bioprinting Bone. [DOI] [Google Scholar]
- 116.Datta P., Ozbolat V., Ayan B., Dhawan A., Ozbolat I.T. Bone tissue bioprinting for craniofacial reconstruction. Biotechnol Bioeng. 2017 Nov;114(11):2424–2431. doi: 10.1002/bit.26349. [DOI] [PubMed] [Google Scholar]