Abstract
Introduction
Seasonal malaria chemoprevention (SMC), with sulphadoxine–pyrimethamine plus amodiaquine (SP+AQ) is effective but does not provide complete protection against clinical malaria. The RTS,S/AS01E malaria vaccine provides a high level of protection shortly after vaccination, but this wanes rapidly. Such a vaccine could be an alternative or additive to SMC. This trial aims to determine whether seasonal vaccination with RTS,S/AS01E vaccine could be an alternative to SMC and whether a combination of the two interventions would provide added benefits.
Methods and analysis
This is an individually randomised, double-blind, placebo-controlled trial. 5920 children aged 5–17 months were enrolled in April 2017 in Mali and Burkina Faso. Children in group 1 received three priming doses of RTS,S/AS01E vaccine before the start of the 2017 malaria transmission season and a booster dose at the beginning of two subsequent transmission seasons. In addition, they received SMC SP+AQ placebo on four occasions each year. Children in group 2 received three doses of rabies vaccine in year 1 and hepatitis A vaccine in years 2 and 3 together with four cycles of SMC SP+AQ each year. Children in group 3 received RTS,S/AS01E vaccine and four courses of SMC SP+AQ. Incidence of clinical malaria is determined by case detection at health facilities. Weekly active surveillance for malaria is undertaken in a randomly selected subset of children. The prevalence of malaria is measured in surveys at the end of each transmission season. The primary endpoint is the incidence of clinical malaria confirmed by a positive blood film with a minimum parasite density of 5000 /µL. Primary analysis will be by modified intention to treat defined as children who have received the first dose of the malaria or control vaccine.
Ethics and dissemination
The protocol was approved by the national ethics committees of Mali and Burkina Faso and the London School of Hygiene and Tropical Medicine. The results will be presented to all stakeholders and published in open access journals.
Trial registration number
Keywords: immunology, tropical medicine, epidemiology
Strengths and limitations of this study.
One major strength of this trial is that it is exploring a novel approach of seasonal vaccination to malaria control in an area where the burden of malaria remains very high despite a high coverage of currently recommended interventions.
The study is adequately powered to test the non-inferiority hypotheses, which requires a large sample size.
A third strength of the trial is that the clinical impact of the two interventions is supported by laboratory studies, which will measure the impact of seasonal malaria chemoprevention on drug resistance and of RTS,S/AS01E on potential vaccine escape parasites.
Despite establishment of an intensive surveillance system, some episodes of malaria and some serious adverse events may be missed, but because the trial is individually randomised these should be distributed equally among study groups.
To apply the trial results to areas where the seasonal malaria transmission duration and pattern is different, modelling may be needed.
Introduction
The RTS,S/AS01E malaria vaccine is a recombinant protein vaccine that contains a component of the circumsporozoite protein of Plasmodium falciparum fused to hepatitis B surface antigen (HBsAg), co-expressed in yeast together with a free HBsAg (S) to form a virus-like particle (RTS,S). It is given with the powerful adjuvant AS01E.1 A phase 3 trial of RTS,S/AS01E conducted in 15 439 children in 7 countries in Africa showed that three doses of RTS,S/AS01E given at monthly intervals, followed by a fourth dose 18 months later, gave 36.5% [95% CI 31% to 41%] protection against clinical attacks of malaria in children aged 5–17 months who were followed for 48 months.2 RTS,S/AS01E provides a high level of protection during the first 3 months after vaccination,3 estimated to be 68% in the phase 3 trial.4 The main safety issues related to administration of RTS,S/AS01E detected in the phase 3 trial were (1) an unexplained, statistically significant, increase in cases of meningitis observed in children given the vaccine at the age of 5–17 months, (2) a possible increase in the proportion of severe cases of malaria that were classified as cerebral malaria and (3) a gender imbalance in the small number of deaths recorded during the trial.5
Seasonal malaria chemoprevention (SMC) involves monthly administration of a full therapeutic dose of an antimalarial drug or drug combination to children on three or four occasions during the period of highest risk of malaria infection. Studies undertaken in several countries in West Africa have shown that SMC with a combination of sulphadoxine/pyrimethamine (SP) and amodiaquine (AQ) (SP+AQ) is highly effective in reducing the incidence of severe and uncomplicated malaria in areas where the transmission of malaria is markedly seasonal.6–8 SMC with SP+AQ is very safe.9 Many countries in the Sahel and sub-Sahel region of West Africa have now incorporated SMC into their national malaria control programme achieving high levels of coverage10 but malaria continues to be a major cause of mortality and morbidity in these countries and additional control tools are needed. Adding azithromycin to the antimalarial drugs used for SMC did not reduce hospital admissions or deaths from non-traumatic causes.11 Thus, determining whether adding RTS,S/AS01E would provide valuable additional protection is important. The primary objectives of the trial are to determine (1) whether seasonal vaccination following priming with the RTS,S/AS01E malaria vaccine is non-inferior in preventing malaria to SMC with SP+AQ in children living in the areas of the Sahel and sub-Sahel region of West Africa where malaria transmission is highly seasonal and (2) whether RTS,S/AS01E can provide additional, useful and cost-effective protection against malaria if given together with SMC in areas with high seasonal malaria transmission.
Methods
This is an individually randomised, double-blind, placebo-controlled trial with three groups. The trial will be conducted and reported according to Consolidated Standards of Reporting Trials guidelines.12
Study setting
The trial is being conducted in Houndé district, Burkina Faso, and in Bougouni district, Mali (figure 1). Malaria, due, predominantly, to P. falciparum, is highly seasonal in both districts. The prevalence of P. falciparum malaria in school-age children in December 2016 was 53% in Bougouni and 50% in Houndé.11 The main malaria vector in both study areas is Anopheles gambiae ss. In 2016, a high proportion of children slept under an insecticide-treated bednet (ITN) in Bougouni (95%) but the percentage was slightly lower in Houndé (79.9%). The first-line treatment for malaria in the public health system in both districts is artemether–lumefantrine. The incidence of parasite confirmed malaria was 1068 per 1000 child years at risk during the transmission season among 3–59-month-old children who were allocated to the SMC group in a trial conducted in Bougouni and Houndé districts in 2014–2016.11 Malaria is seasonal, with 79.6% of annual cases occurring over 5 months of the year from July to November in Bougouni and 81.0% between July and November in Houndé.
Figure 1.
Study districts in Mali and Burkina Faso.
Study population
All households within the study areas with children 5–17 months of age on 1 April 2017 were enumerated in February–March 2017 and children were screened for their eligibility to enter the trial. A child was considered eligible if (a) the child was a permanent resident of the study area and likely to remain a resident for the duration of the trial or (b) the child was 5–17 months of age on 1 April 2017. A child was deemed to be ineligible if (a) the child was a transient resident; (b) the age of the child was outside the stipulated range; (c) the child had a history of an adverse reaction to SP or AQ; (d) the child had a serious underlying illness (self-reported or obtained from health records), including known HIV infection, unless this was well controlled by treatment, or severe malnutrition (weight for age or mid arm circumference Z scores <3 SD); (e) the child was known to have an immune deficiency disease or was receiving an immunosuppressive drug; (f) the child had previously received a malaria vaccine or (g) the child was enrolled in another malaria intervention trial. Written, informed consent (online supplemental file 1) was obtained by study staff from their caretakers of eligible children prior to their inclusion in the trial.
bmjopen-2019-035433supp001.pdf (115.4KB, pdf)
Each eligible child was assigned a unique census identification number (ID), and their demographic data (date of birth and/or age, and gender), use of ITNs and history of receiving SMC during the last transmission season were collected. The census data were updated in April/May 2018 and 2019 prior to the administration of the booster doses of vaccine.
The study started on 1 February 2017 and is expected to be completed on 30 June 2020 (figure 2).
Figure 2.
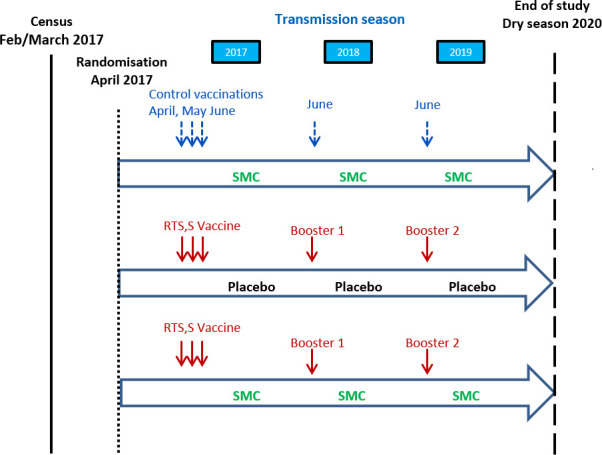
Study design.
Patient and public involvement
No patients are involved in this trial. Healthy children were enrolled with the consent of their caretakers. Caretakers were not involved in the development of the research question or study design.
Randomisation
Eligible children who were consented soon after the census were allocated randomly to one of the three study groups (table 1). The randomisation list was prepared using permuted blocks after sorting the list of eligible children by age, gender, area of residence and prior receipt of SMC, to ensure these characteristics would be balanced between the three groups. An independent information technology consultant loaded the randomisation list on four tablet computers and handed over two tablet computers each to the chief pharmacist in Mali and Burkina Faso. The chief pharmacists used these tablet computers to scan the QR code containing the child’s name and study ID printed on the ID cards of children to find out the vaccine allocated to each child. These tablet computers were locked in the vaccine room and were accessible to the chief pharmacists only.
Table 1.
Study groups and the interventions that they received
| Group 1 | Group 2 | Group 3 | |
| RTS,S/AS01E alone | SMC alone | SMC+RTS,S/AS01E | |
| Year 1 | |||
| April–June | RTS,S/AS01E×3 | Rabies vaccine×3 | RTS,S/AS01E×3 |
| July–Oct | SMC placebo*×4 | SMC*×4 | SMC*×4 |
| Year 2 | |||
| June | RTS,S/AS01E×1 | Hepatitis A vaccine×1 | RTS,S/AS01E×1 |
| July–Oct | SMC placebo*×4 | SMC*×4 | SMC*×4 |
| Year 3 | |||
| June | RTS,S/AS01E×1 | Hepatitis A vaccine×1 | RTS,S/AS01E×1 |
| July–Oct | SMC placebo*×4 | SMC*×4 | SMC*×4 |
*SMC or placebo will be given at monthly intervals on four occasions during the malaria transmission season.
SMC, seasonal malaria chemoprevention.
Interventions
RTS,S/AS01E vaccine: three doses of RTS,S/AS01E were given to children allocated to the RTS,S or RTS,S+SMC groups at approximately monthly intervals (window: 3–8 weeks) in April–June 2017 followed by a fourth and fifth dose given in June 2018 and June 2019 prior to the malaria transmission seasons.
Control vaccines: rabies vaccine Rabipur®, produced by GlaxoSmithKline (GSK), was used as the control vaccine for the primary series of vaccinations during the dry season of 2017 for those allocated to the SMC alone group. Hepatitis A vaccine (Havrix), a licensed inactivated hepatitis A vaccine produced by GSK, was used for the booster dose in years 2 and 3.
SMC: four courses of SMC with SP+AQ were given at monthly intervals during the malaria transmission season in line with WHO’s recommendation and national policy. A course of SMC for a child over the age of 1 year comprises a single treatment of SP (500 mg sulphadoxine/25 mg pyrimethamine) and AQ 150 mg on day 1 and AQ 150mg on days 2 and 3. Infants receive half of these doses. Children in the RTS,S alone group received a matching SP+AQ placebo. Dispersible forms of SP and AQ and matching placebo were obtained from Guilin Pharmaceuticals, Shanghai, a Good Manufacturing Practice certified supplier. All doses of SMC were given under observation.
Implementation of the interventions
Vaccines: syringes containing RTS,S/AS01E or the control vaccine were prepared by the chief pharmacist who took no other part in the trial. Loading of syringes with vaccines and masking with tape to blind the person administering the vaccine was done by a member of the study staff who also took no further part in the trial. Vaccines were administered by study health staff trained to give vaccines.
Vaccination was undertaken in fixed or temporary centres established near study health centres or hospitals. The families of children scheduled for vaccination on a particular day were notified the day before this was due to take place and asked to bring their child to the vaccination centre on that day. In the case of families who lived a long distance from a vaccination centre, project transport was provided to bring the children and caretakers to the vaccination centre and to take them home. Children were retained at the vaccination centre for at least 30 min after vaccination to ensure that there were no immediate adverse events. Home visits were made to the families of children who missed vaccination on the designated day and their caretakers were asked if they would still like their child to receive vaccination (or SMC). If they agreed, they were asked to bring their children to the vaccination/drug administration centre. Some children were unable to attend for vaccination at the time that this took place in their community because their family had travelled or due to an acute illness. A protocol amendment, approved by the data and safety monitoring board, allowed these children to receive the booster vaccination in 2018 and in 2019 if they presented for SMC administration later in the malaria transmission season.
SMC: SMC drugs were pre-packed by a pharmacist who took no further part in the trial, in re-sealable envelopes bearing the child’s unique number and containing tablets for the four cycles of treatment appropriate for the child’s age. Each dose of SMC or placebo was administered by trained project staff at a central point in the study village. Study children were given an identity card containing their photograph, study identity number and date of birth. At the time of vaccination and/or SMC administration, a child’s photo ID card was scanned to ensure that the child was given the allocated intervention. The Ministry of Health (MoH) introduced administration of SMC in the study areas in Mali and Burkina Faso in 2018. A member of the study team accompanied the MoH team to prevent the risk of study children receiving SMC again from the routine system.
ITNs: all study children were given an ITN at enrolment in 2017 before the malaria transmission season.
Study outcomes
The primary trial outcome is clinical malaria, defined as an episode of illness characterised by fever (temperature ≥37.5°C), or a history of fever within the previous 48 hours, that is severe enough to require treatment at a health centre or by a community health worker and which is accompanied by a positive blood film with a parasite density of 5000 /µL or more.
Secondary outcomes include the following:
Blood slide or rapid diagnostic test (RDT): positive malaria is defined as a clinical episode of an uncomplicated febrile illness (temperature ≥37.5°C), or a history of fever within the previous 48 hours, with a positive blood film (any level of asexual parasitemia) or a positive RDT.
Hospital admissions with malaria, including cases of severe malaria which meet WHO criteria for a diagnosis of severe malaria.
Malaria parasitaemia (symptomatic or asymptomatic) in a subset of randomly selected children seen during home visits.
Malaria parasitaemia (asymptomatic or symptomatic), moderate or severe anaemia, and malnutrition at the end of the malaria transmission season.
Serious adverse events (SAEs), including any deaths, with special reference to meningitis, cerebral malaria or immune deficiency illnesses.
Anti-circumsporozoite protein (CSP) concentrations obtained after priming and after each booster dose, determined in a sub-sample of children.
The presence of molecular markers of resistance to SP and AQ in parasite positive samples collected at the last cross-sectional survey.
The match of polymorphisms in the P. falciparum circumsporozite (csp) gene of parasite isolates obtained from children with clinical episodes of malaria to the genetic structure of the strain of parasite used to develop the RTS,S/AS01E vaccine.
Sample size
Based on the sample size calculations described below, the trial aimed to recruit approximately 3000 children in Burkina Faso and 3000 in Mali (total 6000) who would be followed for three years. A low dropout rate of around 5 % per year (15% overall) was anticipated based on findings from a previous trial conducted in the same study areas.12 Based on the results of a blinded interim analysis of an SMC+azithromycin study,12 it was assumed that the incidence of clinical malaria confirmed by blood slide would be 300 cases per 1000 children over a calendar year in the SMC alone group. Results obtained over a period of three years of observation in the two study sites will be combined for the comparison of the primary endpoint but secondary analyses will evaluate impact at each study site separately. The study is powered to (1) assess the statistical evidence against the null hypothesis of no difference between the combined group and either the SMC alone or RTS,S alone group and (2) estimate the efficacy of the combined group relative to the single intervention groups with a relatively high degree of precision. This latter aspect is important because if the combined intervention is to be used in practice, it is necessary to show that adding RTS,S/AS01E to SMC has a clinically significant benefit.
Superiority: the trial is designed to compare the two interventions combined with either used alone. The study is powered to (1) assess the statistical evidence against the null hypothesis of no difference between the combined group and either used alone, and (2) estimate the efficacy of the combined group with a relatively high degree of precision. This latter aspect is important because it is necessary to show that adding RTS,S/AS01E to SMC has clinically significant benefit.
Based on the incidence of events observed in the trial after two years of follow-up, with approximately 2000 individuals in each arm, if the efficacy is 30% or more, there is very high power over the three years of the study (close to 100%) to reject the null hypothesis of no difference between the treatment groups. There will be 90% power for the lower limit of the CI to exclude 15%, that is, to establish that the protection from the combined group is at least 15% better than SMC or vaccination alone.
Non-inferiority: SMC for 4 months of the year has an efficacy, assuming receipt of all 4 monthly cycles, of about 85%. If, without intervention, the peak 4 months would account for 60% of annual cases (with the other 40% falling during the other months), this equates to an efficacy over 12 months of at least 50%. The non-inferiority margin is the largest reduction in this efficacy that we would be willing to accept, or would consider unimportant, if RTS,S were to replace SMC. A reduction in efficacy from 50% to 40% translates to a 20% greater incidence in the RTS,S-alone arm compared with the SMC-alone arm. The analysis plan will specify a margin of 20%, this takes into account the potential advantages of RTS,S over SMC in terms of ease of delivery and hence the likelihood of being able to sustain high levels of coverage. The trial has 80% power to exclude, at the 2.5% significance level, a relative difference in the incidence of clinical episodes of malaria of 20% over the three year study period between the RTS,S/AS01E and SMC alone groups, if these two interventions were equally effective.
For the analysis of the serological response to RTS,S/AS01E, comparisons will be made between mean anti-CSP antibody titres before and after the primary series of vaccination and before and after each subsequent booster dose. Based on the SD in antibody titres observed in children enrolled in the RTS,S/AS01E phase 2 and phase 3 trials, inclusion of around 160 individuals in each group (prevaccination and postvaccination) will give approximately 80% power to detect a difference of 25%–30% in mean titre between children who receive RTS,S/AS01E with or without co-administration of SMC.
Follow-up and measurement of outcomes
Passive surveillance for cases of uncomplicated and severe malaria: project staff based in the study hospitals and health centres that serve the study communities identify and document all cases of malaria who present to these health facilities. Community health workers refer all suspected malaria cases to study health facilities. Blood films and filter paper strips are obtained from all these cases for subsequent confirmation of the diagnosis by microscopy.
Active surveillance for malaria: each week, 24 randomly selected children (8 from each arm of the study) in each country are visited at home, their temperature measured and a blood film collected. Any child who is febrile or who has other features suggestive of malaria is tested with an RDT and treated with a full course of an artemisinin combination therapy (ACT) if positive.
Prevalence of malaria parasitaemia and anaemia: a survey of all study children is undertaken at least one month after the last round of SMC administration at the end of each malaria transmission season. During these surveys, anthropometric measurements are taken and a finger prick blood sample is collected for preparing blood slides and blood spots. Children who are febrile are tested with an RDT and treated with an ACT if positive.
Malaria endemicity in the study area: a survey of schoolchildren aged 6–12 years resident in the same areas as the study children is conducted at the end of each malaria transmission season. Two hundred randomly selected schoolchildren per country (total 400) aged 6–12 years who are well and have not received SMC are tested for malaria by microscopy.
Measurement of the immune response to vaccination: blood samples (2 mL) have been collected from approximately 160 children in each of the groups (80 per country) who received RTS,S/AS01E prior to administering the first dose of vaccine and one month after the third dose of the primary series of vaccination had been given. In years 2 and 3, samples have been collected one month before and after administration of the fourth and fifth doses of vaccine for measurement of anti-CSP antibodies.
Measurement of resistance to the antimalarial drugs used for SMC: dried blood spots from a randomly group of children who have malaria parasitaemia detected by microscopy at the last cross-sectional survey will be used for analysis of molecular markers of resistance to SP and AQ.
SAEs: project staff based at the study hospitals have been provided with additional training on the recognition of meningitis, cerebral malaria and immune deficiency diseases, and standard operating procedures have been developed for management of children suspected of having one of these conditions. Definitions for meningitis and cerebral malaria developed by WHO for use in the pilot RTS,S/AS01E implementation trials are being applied. All deaths occurring outside the health facilities are assessed by verbal autopsy using the WHO 2016 Verbal Autopsy Questionnaire.13 Any SAEs that are (a) considered by the investigators likely to be linked to the administration of a study vaccine or study drug or (b) are suspected cases of meningitis or cerebral malaria or (c) are fatal or life threatening are thoroughly investigated and reported to GSK and to the Data Safety Monitoring Board (DSMB) within 72 hours of their detection. All SAEs, whether considered related to the study interventions or not, are tabulated in a blinded fashion and provided to the DSMB and to GSK quarterly.
Laboratory methods
Detection of malaria: a histidine-rich protein 2 (HRP2) based RDT is used for the initial diagnosis of malaria and to guide treatment. Blood films collected at the same time are read subsequently by two readers following the guidelines developed for the phase 3 RTS,S/AS01E trial.14 Slides which are judged to be discordant for either positivity or parasite density are read by a third reader, and the discrepancy was resolved following the algorithm used in the above study.14
Measurement of haemoglobin concentration: haemoglobin concentration is measured colourimetrically using a HemoCue colorimeter (HemoCue AB, Angelholm, Sweden).
Detection of markers of resistance to SP and AQ: parasite DNA will be extracted from dried blood spots and nested PCR reactions will be used to detect the presence of mutations in the dhfr and dhps genes associated with resistance to pyrimethamine and sulphadoxine, respectively, and the pfcrt and pfmdr mutations associated with resistance to AQ. PCR-Restriction Fragment Length Polymorphism (RFLP) will be used to detect the N511, C59R, S108N and I164L mutations in the dhfr gene, the A437G and K540E mutations in the dhps gene, the N86Y mutation in the pfmdr1 gene and the K76T mutation in the pfcrt gene.
Measurement of anti-CSP concentration: antibodies to CSP will be measured by a standardised ELISA at the University of Ghent laboratory using a method applied in previous trials of RTS,S/AS01E.
Detection of polymorphisms in the csp gene: sequencing of the C-terminal region of the CSP protein will be undertaken using methods described previously for detecting polymorphisms in this region of the csp gene to look for the selection effect of RTS,S/AS01E vaccine on malaria parasites.15
Investigation of suspected cases of meningitis: the aetiology of cases of meningitis is determined by microscopical examination of cerebrospinal fluid samples for bacteria and white blood cells at the district hospital and then by subsequent PCR testing at a reference laboratory.
Data management
Data are collected using electronic case record forms (eCRF) developed using Open Data Kit software. Tablet computers loaded with eCRFs are available at all study health centres that provide treatment. This system is based on electronic transfer of the CRFs from the research sites. Automatic checks are performed on clinical and laboratory forms to ensure that they are complete and contain valid responses prior to transferring data. eCRFs will be deposited with the study data on the LSHTM Data Compass System (http://datacompass.lshtm.ac.uk). All personal identifiers will be removed from the study database before archiving. The data will be assigned a digital object identifier. Twelve months after completing the analysis, the data will be made available in the London School of Hygiene and Tropical Medicine (LSHTM) data depository. A data access group will review requests to share archived data.
Analysis plan
The primary analysis will be by modified intention to treat (mITT). The mITT population will include all children who were screened and who received the first dose of RTS,S/AS01E or control vaccine, irrespective of the number of doses of subsequent vaccines or SMC/SMC placebo received. The primary endpoint of the trial is the incidence of all episodes of blood slide confirmed clinical malaria over the study period and this will be evaluated using Cox regression models with a robust SE to account for clustering of episodes within individuals (ie, the Andersen-Gill extension of the Cox model). Hypothesis testing will follow the closed testing procedure, whereby there is initially a test of the null hypothesis that the incidence in the three groups is the same. If this is rejected at the 5% level, pairwise comparisons will also be done using a 5% significance level. Pairwise comparisons can be considered statistically significant only if the overall null hypothesis is rejected. This preserves the overall type I error rate at 5%. There were no formal interim analyses planned or conducted.
The primary analysis will be based on data for both sites combined, with site as a stratification factor, but site-specific analyses will also be undertaken. A test of interaction will be used to determine if there is any evidence that efficacy of RTS,S varies between the two trial sites. An analysis will also be undertaken to assess efficacy of the booster dose of the vaccine each year.
The proportion of children in each group who experience at least one episode of malaria will be compared as a secondary outcome using Kaplan-Meier estimates of the risk. Evidence for provision of complete protection through the combination of SMC and vaccination each year will be explored using published methods.16 17 For the comparison of RTS,S/AS01E plus SMC versus the other two study groups, two-sided 95% CIs for the HR will be calculated. For the non-inferiority comparison of RTS,S/AS01E to SMC, two-sided 90%, 95% and 99% CIs for the rate ratio will be calculated, to indicate the degree of confidence with which the margin of 1.20 (a relative increase of 20%) can be excluded.
An analysis plan will be prepared, for approval by the DSMB before the study code is broken. Primary analyses will be by mITT. ATP analyses will also be undertaken. Efforts would be made during the trial to ensure high levels of adherence to minimise differences between ITT and ATP populations. All children who received the first dose of vaccine will be included in the intention to treat analysis. (Children who did not attend for the first vaccine dose were withdrawn from the study.) Children who received all scheduled doses of SMC, or treatment for a clinical episode of malaria at a time when SMC would have been given, and all scheduled doses of vaccine, will be included in the per protocol analysis for each year of the study. Additional subanalyses will include analysis by age, gender, bed net use during the transmission season and socioeconomic status.
Trial management
The LSHTM is the main sponsor for the trial. Delegated responsibilities are assigned locally. The LSHTM holds Public Liability (‘negligent harm’) and Clinical Trial (‘non-negligent harm’) insurance policies which apply to this trial. An independent trial steering committee provides scientific oversight and has approved the protocol. The steering committee holds teleconferencing or face-to-face meeting annually to monitor progress and advise on the scientific content of the study. The quality of data and the good clinical practice (GCP) standards of the trial are monitored by an independent, experienced GCP monitor.
Ethics and dissemination
Ethics
Inclusion in the trial of an RTS,S/AS01E alone group is justified, even though SMC is a recommended policy, on the grounds that RTS,S/AS01E could provide some added protection outside the main transmission season when SMC is not being given and that it may be easier to administer than SMC. Individual, written, informed consent has been obtained from the family or legally recognised guardian of each child entered into the trial. Conduct of the trial does not impose any additional costs on the local health services. The project contributes to the costs of routine clinical care of study subjects during the trial and to strengthening of clinical care at the district hospitals in the study areas.
Ethical approval has been obtained from the Ethics Committee of LSHTM, the Health Research Ethics Committee of Burkina Faso, the Institutional Ethics Committee of IRSS in Burkina Faso and the Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry, University of Bamako (protocol V.2; dated 9 December 2016). The trial has also been approved by the regulatory authorities in Burkina Faso and Mali. An independent DSMB monitors regularly the safety of children in the trial, especially those in the RTS,S/AS01E alone group who are not receiving SMC, which is a standard of care. In February 2018, the board reviewed unblinded data on the incidence of SAEs and SAEs due to malaria in each study group, and gave permission for the trial to proceed for a further year retaining all three study arms.
The trial adheres to the principles outlined in the International Conference on Harmonisation GCP guidelines, protocol and all applicable local regulations. The trial is registered on clinicaltrials.gov (https://www.clinicaltrials.gov/ct2/show/NCT03143218?term=NCT03143218&rank=1).
Dissemination plans
Results from the trial will be discussed with the study communities at the end of the study, presented at national and international conferences and in peer-reviewed, open access journals. Trial results will be shared with the WHO’s technical expert groups and Malaria Policy Advisory Group. Strong links have been established already with the MoHs, NMCPs and EPI programmes in Burkina Faso and Mali. The trial team has also established good links with many other organisations involved in the delivery of SMC trials, including the SMC ACCESS programme and with the WHO staff responsible for conducting the RTS,S/AS01E implementation studies. Thus, if it is found that RTS,S/AS01E vaccine is a useful replacement or an addition to SMC regimens, routes have already been established through which this knowledge could be disseminated rapidly.
Supplementary Material
Footnotes
Contributors: DC and BG wrote the first draft of the manuscript. AD, IZ, IS, HT, OO-A and J-BO reviewed the manuscript and made significant contributions to the protocol. MC, IK, IS and PM wrote sections on sample size, data management and analysis plan. MD, IT, AT, DI, KS, AM, FS, and SY reviewed the paper and are involved in the field operations of the study.
Funding: The trial is being funded by the UK Joint Global Health Trials (the Department of Health and Social Care, the Department for International Development, the Global Challenges Research Fund, the Medical Research Council and the Wellcome Trust) grant no. MR/P006876/1, with supplementary funding from PATH MVI, grant no. 18269. The trial is sponsored by the London School of Hygiene and Tropical Medicine (LSHTM), Keppel Street, London WC1E 7HT. The investigators from the LSHTM are responsible for the design, data collection, analysis, interpretation and writing the report. The funding organisations have no role in these aspects.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cohen J, Nussenzweig V, Nussenzweig R, et al. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin 2010;6:90–6. 10.4161/hv.6.1.9677 [DOI] [PubMed] [Google Scholar]
- 2.RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386:31–45. 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 2001;358:1927–34. 10.1016/S0140-6736(01)06957-4 [DOI] [PubMed] [Google Scholar]
- 4.BACKGROUND PAPER ON THERTS,S/AS01 MALARIA VACCINE, 2015. Available: https://www.who.int/immunization/sage/meetings/2015/october/1_Final_malaria_vaccine_background_paper_v2015_09_30.pdf
- 5.Klein SL, Shann F, Moss WJ, et al. RTS,S Malaria Vaccine and Increased Mortality in Girls. mBio 2016;7:e00514–6. 10.1128/mBio.00514-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson AL, IPTc Taskforce . A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS One 2011;6:e16976. 10.1371/journal.pone.0016976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicko A, Diallo AI, Tembine I, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med 2011;8:1000407. 10.1371/journal.pmed.1000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konaté AT, Yaro JB, Ouédraogo AZ, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med 2011;8:e1000408. 10.1371/journal.pmed.1000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndiaye JL, Cissé B, EH B, et al. Safety of seasonal intermittent preventive treatment against malaria with sulfadoxine pyrimethamine + amodiaquine when delivered to children under 10 years of age by district health staff in Senegal. PLoS One. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaria Consortium Seasonal malaria chemoprevention, 2020. Available: http://www.malariaconsortium.org/pages/access-smc.htm
- 11.Chandramohan D, Dicko A, Zongo I, et al. Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med 2019;380:2197–206. 10.1056/NEJMoa1811400 [DOI] [PubMed] [Google Scholar]
- 12.Piaggio G, Elbourne DR, Altman DG, et al. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152–60. 10.1001/jama.295.10.1152 [DOI] [PubMed] [Google Scholar]
- 13.Leitao J, Chandramohan D, Byass P, et al. Revising the who verbal autopsy instrument to facilitate routine cause-of-death monitoring. Glob Health Action 2013;6:21518. 10.3402/gha.v6i0.21518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swysen C, Vekemans J, Bruls M, et al. Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J 2011;10:e223. 10.1186/1475-2875-10-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med 2015;373:2025–37. 10.1056/NEJMoa1505819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Lam KF, Cheung YB. Estimation of intervention effects using recurrent event time data in the presence of event dependence and a cured fraction. Stat Med 2014. [DOI] [PubMed] [Google Scholar]
- 17.Cairns M, Cheung YB, Xu Y, et al. Analysis of preventive interventions for malaria: exploring partial and complete protection and total and primary intervention effects. Am J Epidemiol 2015;181:1008–17. 10.1093/aje/kwv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035433supp001.pdf (115.4KB, pdf)


