Abstract
Autoimmune encephalitis is a rare spectrum of disease that can be a complication of chronic immunosuppression. Diagnosis often requires the presence of antineuronal antibodies, but many causative antibodies have not yet been identified. Antibody-negative autoimmune encephalitis (AbNAE) is especially difficult to diagnose and must rely largely on exclusion of other causes. In chronically immune-suppressed transplant recipients, the differential is broad, likely resulting in underdiagnosis and worse outcomes. Here, we present a 58-year-old liver transplant recipient taking tacrolimus for prevention of chronic rejection who presented with 5 days of confusion, lethargy and lightheadedness. He was diagnosed with AbNAE after an extensive workup and recovered fully after high-dose corticosteroids. Our case highlights the importance of recognising the association between chronic immunosuppression and autoimmune encephalitis. Autoimmune encephalitis, even in the absence of characterised antibodies, should be considered when transplant recipients present with central neurologic symptoms.
Keywords: immunology, neurooncology, unwanted effects / adverse reactions, gastroenterology
Background
Autoimmune encephalitis is a rare spectrum of disease usually associated with a viral central nervous system infection or underlying autoimmune disease. Associated symptoms include lethargy, focal deficits, seizures and changes in mentation and behaviour. Diagnosis often requires the presence of antineuronal antibodies, but many causative antibodies have not yet been identified. Antibody-negative autoimmune encephalitis (AbNAE) is especially difficult to diagnose and must rely largely on exclusion of other causes. In chronically immune-suppressed transplant recipients, the differential is broad, likely resulting in underdiagnosis and worse outcomes. Autoimmune encephalitis, even in the absence of characterised antibodies, should be considered when transplant recipients present with central neurologic symptoms, as early initiation of treatment results in better outcomes.
Case presentation
A 58-year-old man presented with intermittent confusion, lightheadedness, lethargy and dizziness. The patient’s wife reported episodes in which he forgot how to turn the lights off and forgot how to use a television remote, stating that episodes had occurred each of the last five nights. After sleeping, he returned to normal until the next evening. He was the recipient of a cytomegalovirus (CMV) positive, deceased donor liver transplant 14 years prior secondary to alcoholic cirrhosis and was maintained on tacrolimus for prevention of chronic rejection. The patient had recently made dietary and lifestyle changes due to hyperlipidaemia. He also had a sinus infection treated with amoxicillin 2 weeks prior. Other medical history included a single episode of autoimmune haemolytic anaemia (AIHA) triggered by vaccination, and a single episode of immune thrombocytopenic purpura (ITP) triggered by upper respiratory tract infection or antibiotic use. Both were refractory to steroids and required rituximab infusions but had completely resolved.
At presentation, he denied trauma, fevers, weakness or sensory changes. Physical examination revealed an alert and oriented, healthy appearing man with no focal neurological deficits. He had white, scaly patches on bilateral upper extremity extensor surfaces consistent with psoriasis. Following admission, he began having intermittent episodes of confusion which progressed to complete disorientation, inability to follow commands and agitation. He was noted to have an increased startle reflex and hyperreflexia but no nuchal rigidity or meningeal signs.
Investigations
Labs on admission were remarkable for a normal complete blood count, comprehensive metabolic panel with mildly low albumin (3.0) and bicarbonate level of 23.2. Tacrolimus level was 3.9 (goal 3–5) and erythrocyte sedimentation rate (ESR) was elevated (30).
Initial workup included normal thyroid stimulating hormone (TSH) and B12 levels and a negative urine drug screen. During an episode of confusion, blood glucose level was 96.
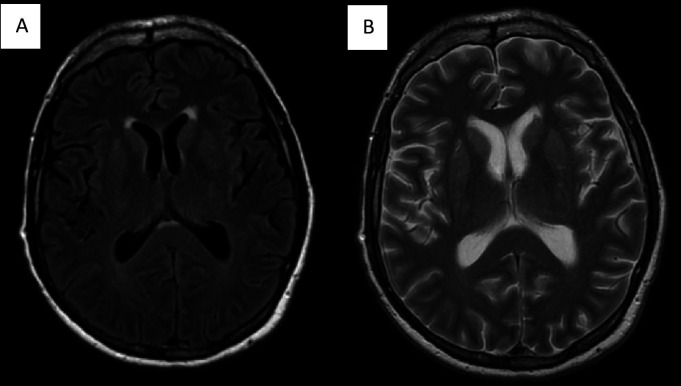
A normal CT of the brain was followed by an MRI of the brain under anaesthesia (figure 1), which revealed mild, hyperintense white matter lesions on T2 imaging of the anterior right putamen and periventricular white matter.
Figure 1.
(A) MRI of the brain T2 fluid-attenuated inversion recovery (FLAIR) image showing hyperintense signal in the anterior aspect of the right putamen and periventricular white matter. (B) T2 fast spin echo (FSE) image.
One-hour electroencephalogram (EEG) was performed showing mild, generalised, non-specific cerebral dysfunction without seizure tendencies and a slow, posterior dominant rhythm.
Lumbar puncture returned colourless cerebrospinal fluid with elevated protein, IgG, albumin quotient and glucose, but infectious testing of blood and cerebrospinal fluid (CSF) was negative (table 1).
Table 1.
Cerebrospinal fluid, metabolic and infectious laboratory findings
| Test | Type | Serum | CSF | Reference |
| Cerebrospinal fluid | Colourless | Colourless | ||
| Red blood cells | 0 | 0/HPF | ||
| White blood cells | 1 | 0–5/HPF | ||
| Neutrophils | 2% | 0%–5% | ||
| Lymphocytes | 0% | 40%–80% | ||
| Monocytes | 98% | 0%–5% | ||
| Glucose | 85 | 40–70 mg/dL | ||
| Lactic acid | 24 | 10–22 mg/dL | ||
| Protein | 133 | 15–45 mg/dL | ||
| Albumin | 92 | 11–48 mg/dL | ||
| Qalbumin | 23 | <9 | ||
| IgG | 10.9 | 0–8.6 mg/dL | ||
| IgG index | 0.5 | 0.00–0.70 | ||
| Malignant cells | 0 | 0/HPF | ||
| Oligoclonal bands | 0 | <4 | ||
| Metabolic | ||||
| Glucose | 93 | <100 mg/dL | ||
| Hemoglobin A1c | 4.70% | <5.7% | ||
| Insulin | 8.8 | Fasting <25 units/mL | ||
| Glucagon | 107 | 50–150 pg/mL | ||
| 8:00 cortisol | 13 | 10–20 µcg/dL | ||
| TSH | 1.85 | 0.50–5.0 µU/mL | ||
| Cholesterol | 277 | 120–200 mg/dL | ||
| Triglycerides | 260 | 10–190 mg/dL | ||
| Arterial ammonia | 45 | Fasting 9–55 | ||
| Infectious testing | ||||
| HSV1 | PCR | Negative | Negative | Negative |
| HSV2 | PCR | Negative | Negative | Negative |
| Streptococcus pneumoniae | Antigen | Negative | Negative | |
| CMV | IgM | Negative | Negative | |
| CMV | IgG | Negative | Negative | |
| CMV | PCR | Negative | Negative | |
| Borrelia burgdorferi | Antibody | 0.27 | <0.99 Lyme index value | |
| Cryptococcus | Antigen | Negative | Negative | Negative |
| Culture | Negative | Negative | Negative | |
| Hepatitis B virus DNA | PCR | Negative | Negative | |
| Human herpes virus 6 | PCR | Negative | Negative | Negative |
| Varicella zoster virus | PCR | Negative | Negative | |
| Eppestein-Barr virus | PCR | Negative | Negative | |
| WNV | IgM | Negative | Negative | |
| WNV | IgG | Positive | Negative | |
| Coxsackie B1 | Ab titre | <1:10 | <1:10 | |
| Coxsackie B2 | Ab titre | <1:10 | <1:10 | |
| Coxsackie B3 | Ab titre | <1:10 | <1:10 | |
| Coxsackie B4 | Ab titre | <1:10 | <1:10 | |
| Coxsackie B5 | Ab titre | <1:10 | <1:10 | |
| Coxsackie B6 | Ab titre | <1:10 | <1:10 | |
| Coxsackie A2 | Ab titre | <1:1 | <1:1 | |
| Coxsackie A4 | Ab titre | <1:1 | <1:1 | |
| Coxsackie A7 | Ab titre | <1:1 | <1:1 | |
| Coxsackie A9 | Ab titre | <1:1 | <1:1 | |
| Coxsackie A10 | Ab titre | <1:1 | <1:1 | |
| Coxsackie A16 | Ab titre | <1:1 | <1:1 |
HSV, herpes simplex virus; CMV, cytomegalovirus; WNV, west Nile virus; HPF, high powered field
MRI of the abdomen revealed a non-specific early-enhancing lesion of the right lobe of the liver suspicious for a portosystemic shunt, but fasting arterial ammonia was normal. Serum protein electrophoresis revealed no paraproteinaemia and IgG subgroups were within normal limits.
The Mayo Clinic Laboratories Autoimmune Encephalitis Panel (ENC2) did not reveal antineuronal antibodies, but negative results do not preclude diagnosis of autoimmune encephalitis (table 2).
Table 2.
Laboratory testing for autoimmune disease
| Autoimmune testing | Type | Blood | Cerebrospinal Fluid | Reference |
| Erythrocyte sed rate | Serum | 30 | <10 mm/hour | |
| Anti-Ro (anti–Sjögren's-syndrome-related antigen A) | Ab | <0.2 | 0–0.9 AI | |
| Anti-La (anti–Sjögren's-syndrome-related antigen B) | Ab | <0.2 | 0–0.9 AI | |
| Anti-thyroglobulin | Ab | <1.0 | <1.0 | |
| Anti-thyroperoxidase | Ab | 10 | 0–30 | |
| C3 | Serum | 108 | 80–150 mg/dL | |
| C4 | Serum | 25 | 18–55 mg/dL | |
| CH50 | Serum | 46 | 42–95 U/mL | |
| Total IgG | Serum | 985 | 10.9 | 700–1600 mg/dL (serum), 0.4–4.5 mg/dL (CSF) |
| IgG1 | Serum | 444 | 248–810 mg/dL | |
| IgG2 | Serum | 402 | 130–555 mg/dL | |
| IgG3 | Serum | 58 | 15–102 mg/dL | |
| IgG4 | Serum | 35 | 2–96 mg/dL | |
| Alpha 1 globulin | Electrophoresis | 0.18 | 0.11–0.34 g/dL | |
| Alpha 2 globulin | Electrophoresis | 1.02 | 0.37–1.11 g/dL | |
| Beta globulin | Electrophoresis | 0.96 | 0.69–1.55 g/dL | |
| Gamma globulin | Electrophoresis | 1.24 | 0.73–2.00 g/dL | |
| Aquaporin 4 | Ab titre | Negative | Negative | |
| MOG IgG1 | FACS | Negative | Negative | |
| Mayo Clinic Laboratories Autoimmune Encephalitis Panel (ENC2) | ||||
| AMPA-R | Ab CBA | Negative | Negative | |
| Amphiphysin | Ab titre | <1:2 | <1:2 | |
| AGNA-1 | Ab titre | <1:2 | <1:2 | |
| ANNA-1 | Ab titre | <1:2 | <1:2 | |
| ANNA-2 | Ab titre | <1:2 | <1:2 | |
| ANNA-3 | Ab titre | <1:2 | <1:2 | |
| CASPR2 | IgG CBA | Negative | Negative | |
| CRMP-5 | IgG titre | <1:2 | <1:2 | |
| DPPX | Ab IFA | Negative | Negative | |
| GABA-B-R | Ab CBA | Negative | Negative | |
| GAD65 | Ab | 0.00 | <0.02 nmol/L | |
| GFAP | IFA | Negative | Negative | |
| LGI1 | IgG CBA | Negative | Negative | |
| mGluR1 | Ab IFA | Negative | Negative | |
| NMDA-R | Ab CBA | Negative | Negative | |
| PCA-Tr | Ab titre | <1:2 | <1:2 | |
| PCA-1 | Ab titre | <1:2 | <1:2 | |
| PCA-2 | Ab titre | <1:2 | <1:2 | |
AGNA-1, antiglial nuclear antibody 1; AMPA-R, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antibody; ANNA, antineuronal nuclear antibody (ANNA-1 and 2 antibodies previously referred to as anti-Hu, anti-Ri, respectively); CASPR2, contactin-associated protein-like 2; CBA, antibody cell-binding assay; CRMP-5, collapsing response mediator protein-5; DPPX, dipeptidyl-peptidase-like protein 6; FACS, fluorescence activated cell sorting; GABA-B-R, gamma aminobutyric acid receptor type B; GAD65, glutamic acid decarboxylase 65-kd isoform; GFAP, glial fibrillar acid protein; IFA, immunofluorescence assay; LGI1, leucine-rich glioma-inactivated 1; mGluR, metabotropic glutamate receptor; MOG, myelin oligodendrocyte glycoprotein; NMDA-R, N-methyl-D-aspartate receptor; PCA, Purkinje cell cytoplasmic antibody types Tr, 1 and 2.
Differential diagnosis
Central nervous system infection was highest on the differential list and we treated our patient empirically for viral encephalitis while awaiting test results. Viral, Streptococcus, Cryptococcus and toxoplasmosis testing were negative in serum and CSF.
Due to history of intermittent confusion, lifestyle and dietary changes, hemoglobin A1c (HbA1c) of 4.7% and liver transplant status, episodes of hypoglycaemic were suspected. Since transplanted livers are denervated, glucose homeostasis relies on circulating hormones and can cause slower corrections from hypoglycaemic than in patients with native livers.1 During an episode of confusion, the patient’s blood glucose was normal, and insulin and glucagon levels were within the normal ranges.
Acquired hepatocerebellar degeneration was also suspected, due to either the presence of graft dysfunction or a portosystemic shunt, which was considered on MRI of the abdomen. When paramagnetic metals such as manganese bypass biliary excretion, they can deposit in susceptible areas of the brain with similar pathogenesis to Wilson’s hepatocerebral degeneration.2–4 Our patient had normal protein and only mildly reduced albumin indicating a functioning graft. Furthermore, a portosystemic shunt was ruled out with a normal fasting arterial ammonia.
Hashimoto’s encephalopathy was also on the differential since our patient’s history of autoimmune disease and the clinical presentation were suggestive.5 He had no antibodies against thyroperoxidase or thyroglobulin and had a normal thyroid stimulating hormone level.
Paraneoplastic limbic encephalitis due to onconeuronal antibodies from underlying malignancy is described similarly to our patients presentation.6 Diagnostic criteria proposed by Graus et al require a subacute onset, bilateral brain abnormalities highly restricted to the medial temporal lobes, at least one of the CSF pleocytosis or an EEG with epileptic or slow wave activity involving the temporal lobes, and reasonable exclusion of other causes, though the presence of specific antibodies is not required.6 Our patient did not meet these criteria and obvious malignancy was excluded with a normal serum protein electrophoresis and benign MRI of the abdomen.
Posterior reversible encephalopathy syndrome (PRES) due to calcineurin inhibitor toxicity was considered, as it is well described after organ transplantation and is associated with significant morbidity. PRES is caused by alterations in brain perfusion secondary to excess endothelin production causing inappropriate vasoconstriction.7 This causes disruption of the blood–brain barrier and increased leakage into the brain parenchyma, leading to hyperperfusion injury and free radical toxicity.8 9 MRI shows subcortical white matter changes in the posterior cerebrum, which generally appear in the first month after transplantation.7 Our patient had an MRI of the brain, which revealed white matter lesions inconsistent with PRES, and his tacrolimus level was within the desired range (3.9, goal 3.0–5.0).
Acute disseminated encephalomyelitis (ADEM) is a monophasic, multifocal demyelinating disorder precipitated by systemic infection. It is common in children and rare but reported in adults over the age of 40.6 10 The clinical course includes the presence of encephalopathy and other new neurologic signs such as cranial nerve palsies and motor deficits, commonly haemiparesis.6 10 Spinal cord involvement is common in adults and CSF typically shows mild pleocytosis, whereas our patient had normal CSF cell counts but elevated IgG.6 10 While our patient’s preceding history may have suggested ADEM, he did not meet MRI criteria with diffuse, large, cerebral white matter lesions or deep grey matter abnormalities, which are required for diagnosis.6 Therefore, we found another diagnosis to be most likely.
AbNAE was diagnosed using the criteria proposed by Graus et al (box 1). His clinical presentation was consistent with AbNAE, other causes were ruled out, and he met the required criteria with MRI findings of white matter lesions. Though his CSF IgG Index was within normal range at 0.50, (range 0.00–0.70) we believe the disease was diagnosed early in its course and his CSF IgG was elevated. Furthermore, his blood–brain barrier was disrupted as indicated by a high albumin quotient of 23 (normal <9). While his encephalitis antibody panel was negative, it is not all encompassing.6 11–14
Box 1. Diagnostic criteria for antibody-negative autoimmune encephalitis6.
Rapid progression (<3 months) of memory deficits, altered mental status or psychologic disorders.
Exclusion of limbic encephalitis, Birkerstaff's brainstem encephalitis and acute disseminated encephalomyelitis.
Absence of well-characterised antineuronal antibodies in serum and CSF.
Reasonable exclusion of other causes.
-
two or more of the following:
MRI findings suggestive of autoimmune encephalitis.
Brain biopsy with inflammatory infiltrates.
-
one or more of:
CSF pleocytosis.
CSF oligoclonal bands.
Increased CSF IgG Index.
Treatment
The patient was started on intravenous valacyclovir empirically while infectious studies were pending. After diagnosis of autoimmune encephalitis was made, he was started on intravenous methylprednisolone 1 g daily for 5 days followed by oral methylprednisolone taper from 60 mg daily for 6 weeks total duration. His tacrolimus was continued in hospital and at discharge.
Outcome and follow-up
By day 3 of high-dose steroids, the patient’s mental status began to improve. He was able to follow commands but continued to have poor concentration and short-term memory loss. By day 5, he was functioning at his baseline and was discharged home after a 10-day hospitalisation.
Post discharge, the patient was followed by transplant hepatology and neuroimmunology. He remained asymptomatic and independent over the next 4 months.
Discussion
To our knowledge, no other cases of AbNAE associated with chronic immune suppression for liver transplantation have been reported. In a literature search of autoimmune encephalitis related to immune suppression for liver transplant, two cases were identified. One involved a 69-year-old woman presenting with seizures. She was diagnosed with N-methyl-D-aspartate receptor (NMDAR) antibody-positive autoimmune encephalitis (AbPAE) refractory to treatment 10 years after transplantation and eventually died due to complications.15 The other case involved a 61-year-old man 1 month post transplant with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) AbPAE. He had complete recovery with plasmapheresis, rituximab, corticosteroids and transition from tacrolimus to cyclosporine.16
Rare cases have been reported of autoimmune encephalitis (AE) related to other solid organ or stem cell transplantation.16–19 Including the liver transplant cases above, five out of six patients presented more than 6 years post transplant. Five out of six cases had Eppstein-Barr virus (EBV) DNA isolated in CSF, five patients were positive for anti-NMDAR antibodies, and one was positive for AMPAR antibodies. All six patients were maintained on mycophenolate with the addition of tacrolimus in five and methylprednisolone in one patient.
Treatment followed standard guidelines with steroids, plasmapheresis or immunoadsorption therapy as first line, followed by rituximab, then cyclophosphamide as last line treatment, and tumour removal indicated if a causative malignancy was found.20
Outcomes varied, with two patients dying from complications, one patient who had a mild cognitive deficiency at 3 months of follow-up, and three patients with complete recovery. Since our patient had required rituximab for refractory AIHA and ITP in the past, we suspected that escalation of therapy would be required. His family was consented for rituximab but he recovered fully with corticosteroids only.
AE is due to complement-independent antibody autoreactivity.21 AE while on immune suppressants presents a clinical conundrum since the formation of autoantibodies should be limited.
Pathogenesis may result from long-term immune suppression causing an imbalance of B and T helper cell populations. The lack of proper immune regulation by T helper cells can predispose the development of B cells without immune tolerance.15 21 Supporting this, our patient was on immune suppression directed only at T cells and his symptoms resolved with corticosteroids. His previous AIHA and ITP required specific anti-B-cell therapy.
The development of autoantibodies may be precipitated by an infection.15 22 Most commonly identified are EBV and herpes simplex virus (HSV), though our patient had neither. His disease could have been precipitated by his sinus infection. Similarly, each of his previous events was precipitated by vaccination, infection or possibly his treatment with antibiotics.
This diagnosis of AbNAE is challenging for multiple reasons, and even more so in transplant patients. First, there is a range of non-specific presentations associated with autoimmune encephalitis that can lead to misdiagnosis and untimely treatment.14 In a multicenter retrospective study of 118 patients diagnosed with AE, symptoms at onset varied even among phenotypic subgroups. Due to this, infectious encephalitis and seizure disorders were suspected more frequently than AE prior to diagnosis.14 Subsequently, treatment was delayed for many patients, which results in worse outcomes.11 12 14
Many patients that are clinically diagnosed with autoimmune encephalitis lack identifiable antibodies. The same retrospective study determined that 31.4% of patients diagnosed with AE had no well-characterised antibodies identified.14 Hence, patients diagnosed with AbNAE received treatment even further in their disease course than those with antibodies detected.14
Finally, there is no gold standard for diagnosis, and diagnostic algorithms are largely based on exclusion.6 11 12 14 We used an algorithm first proposed in 2016 that included knowledge of most of the currently tested antibodies. However, even this algorithm relies on exclusion, which, in a transplant patient, requires extensive testing since they are more predisposed to a wide range of pathology.
Despite the challenges, recent developments are encouraging. While new antibodies continue to be characterised, specific biomarkers are also showing promise. In a cross-sectional study of patients with presumed AE and infectious, autoimmune and non-inflammatory controls, two potentially useful CSF biomarkers were identified.13 Interleukin 21, a cytokine that downregulates T regulatory cells thereby inducing autoimmunity, was found to be higher in both AbPAE and AbNAE groups. On the other hand, interferon-γ induced protein (IP10), also known as C-X-C motif chemokine 10 (CXCL10), a chemokine implicated in the helper T1 cells response to viral infection, was higher in patients with a viral infection than with AE.13 Since chronically immune-suppressed transplant patients are also at increased risk of infectious encephalitis, these biomarkers may be especially useful.
AE, especially AbNAE, is likely underdiagnosed in transplant recipients and patients on chronic immune suppression. Those patients that are diagnosed are treated later in their disease courses. Our case highlights the importance of including AE and AbNAE as a differential and demonstrated complete recovery with early treatment. Therefore, even in the absence of well-characterised antineuronal antibodies, AE should be considered when chronically immune-suppressed transplant patients present with central neurologic symptoms.
Learning points.
Although seemingly paradoxical, chronic immune suppression is a risk factor for the development of autoimmune encephalitis.
History of autoimmune disease, immune suppression and preceding infection are clues towards diagnosing autoimmune encephalitis.
Autoimmune encephalitis may be diagnosed in the absence of characterised antibodies.
There is no gold standard for diagnosis of antibody-negative autoimmune encephalitis and diagnostic algorithms are largely based on exclusion.
Outcomes favour early treatment, so autoimmune encephalitis should be considered in any transplant recipient presenting with central neurologic symptoms.
Acknowledgments
The authors thank Dr Fletcher for the support, knowledge and dedicated care of this patient.
Footnotes
Contributors: JS began the project and wrote the hospital course, discussion, data figures and contributed to the differential diagnoses and explanations. MH wrote the summary, treatment, teaching points and contributed to the differential diagnoses section. JS and LM edited and revised the manuscript. LM was the attending physician for this patient and oversaw all work on the project.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Perseghin G, Regalia E, Battezzati A, et al. Regulation of glucose homeostasis in humans with denervated livers. J Clin Invest 1997;100:931–41. 10.1172/JCI119609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renjen PN, Khanna L, Rastogi R, et al. Acquired hepatocerebral degeneration. BMJ Case Rep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pigoni A, Iuculano F, Saetti C, et al. Acquired hepatocerebral degeneration (AHD): a peculiar neurological impairment in advanced chronic liver disease. Metab Brain Dis 2018;33:347–52. 10.1007/s11011-017-0107-0 [DOI] [PubMed] [Google Scholar]
- 4.Maffeo E, Montuschi A, Stura G, et al. Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci 2014;35:523–30. 10.1007/s10072-013-1458-x [DOI] [PubMed] [Google Scholar]
- 5.Hori T, Oike F, Hata K, et al. Hashimoto's encephalopathy after interferon therapy for hepatitis C virus in adult liver transplant recipient accompanied by post-transplant lymphoproliferative disorder related to Epstein-Barr virus infection. Transpl Infect Dis 2010;12:347–52. 10.1111/j.1399-3062.2010.00508.x [DOI] [PubMed] [Google Scholar]
- 6.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Te HS. Altered mental status after liver transplant. Clin Liver Dis 2017;10:36–41. 10.1002/cld.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song T, Rao Z, Tan Q, et al. Calcineurin inhibitors associated posterior reversible encephalopathy syndrome in solid organ transplantation: report of 2 cases and literature review. Medicine 2016;95:e3173. 10.1097/MD.0000000000003173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anghel D, Tanasescu R, Campeanu A, et al. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica 2013;8:170–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Berzero G, Cortese A, Ravaglia S, et al. Diagnosis and therapy of acute disseminated encephalomyelitis and its variants. Expert Rev Neurother 2016;16:83–101. 10.1586/14737175.2015.1126510 [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan S, Mohammad SS, Brilot F, et al. Autoimmune encephalitis: recent updates and emerging challenges. J Clin Neurosci 2014;21:722–30. 10.1016/j.jocn.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 12.Dubey D, Sawhney A, Greenberg B, et al. The spectrum of autoimmune encephalopathies. J Neuroimmunol 2015;287:93–7. 10.1016/j.jneuroim.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 13.Jocelyn X, et al. Novel surrogate markers of CNS inflammation in CSF in the diagnosis of autoimmune encephalitis. Frontiers in Neurology 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gastaldi M, Mariotto S, Giannoccaro MP, et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol 2020;27:633–43. 10.1111/ene.14139 [DOI] [PubMed] [Google Scholar]
- 15.Konen FF, Schwenkenbecher P, Jendretzky KF, et al. Severe Anti-N-Methyl-D-Aspartate Receptor Encephalitis Under Immunosuppression After Liver Transplantation. Front Neurol 2019;10:987. 10.3389/fneur.2019.00987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen DA, Lopez-Chiriboga AS, Pittock SJ, et al. Posttransplant autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 2018;5:e497. 10.1212/NXI.0000000000000497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randall A, Huda S, Jacob A, et al. Autoimmune encephalitis (NMDAR antibody) in a patient receiving chronic post-transplant immunosuppression. Pract Neurol 2018;18:320–2. 10.1136/practneurol-2018-001923 [DOI] [PubMed] [Google Scholar]
- 18.Zhao CZ, Erickson J, Dalmau J. Clinical Reasoning: agitation and psychosis in a patient after renal transplantation. Neurology 2012;79:e41–4. 10.1212/WNL.0b013e3182616fad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garré J, Sprengers M, Van Melkebeke D, et al. EBV-NMDA double positive encephalitis in an immunocompromised patient. J Neurol Sci 2019;396:76–7. 10.1016/j.jns.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 20.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–65. 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saiz A, Graus F. Neurologic complications of hematopoietic cell transplantation. Semin Neurol 2010;30:287–95. 10.1055/s-0030-1255218 [DOI] [PubMed] [Google Scholar]
- 22.Gable MS, Sheriff H, Dalmau J, et al. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California encephalitis project. Clin Infect Dis 2012;54:899–904. 10.1093/cid/cir1038 [DOI] [PMC free article] [PubMed] [Google Scholar]