Abstract
STUDY QUESTION
What is the standpoint of an international expert panel on ovarian tissue cryopreservation (OTC) in young females with Turner syndrome (TS)?
SUMMARY ANSWER
The expert panel states that OTC should be offered to young females with TS, but under strict conditions only.
WHAT IS KNOWN ALREADY
OTC is already an option for preserving the fertility of young females at risk of iatrogenic primary ovarian insufficiency (POI). Offering OTC to females with a genetic cause of POI could be the next step. One of the most common genetic disorders related to POI is TS. Due to an early depletion of the ovarian reserve, most females with TS are confronted with infertility before reaching adulthood. However, before offering OTC as an experimental fertility preservation option to young females with TS, medical and ethical concerns need to be addressed.
STUDY DESIGN, SIZE, DURATION
A three-round ethical Delphi study was conducted to systematically discuss whether the expected benefits exceed the expected negative consequences of OTC in young females with TS. The aim was to reach group consensus and form an international standpoint based on selected key statements. The study took place between February and December 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Anonymous panel selection was based on expertise in TS, fertility preservation or medical ethics. A mixed panel of 12 gynaecologists, 13 (paediatric) endocrinologists, 10 medical ethicists and 20 patient representatives from 16 different countries gave consent to participate in this international Delphi study. In the first two rounds, experts were asked to rate and rank 38 statements regarding OTC in females with TS. Participants were offered the possibility to adjust their opinions after repetitive feedback. The selection of key statements was based on strict inclusion criteria.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 46 participants completed the first Delphi round (response rate 84%). Based on strict selection criteria, six key statements were selected, and 13 statements were discarded. The remaining 19 statements and two additional statements submitted by the expert panel were re-evaluated in the second round by 41 participants (response rate 75%). The analysis of the second survey resulted in the inclusion of two additional key statements. After the approval of these eight key statements, the majority of the expert panel (96%) believed that OTC should be offered to young females with TS, but in a safe and controlled research setting first, with proper counselling and informed consent procedures, before offering this procedure in routine care. The remaining participants (4%) did not object but did not respond despite several reminders.
LIMITATIONS, REASONS FOR CAUTION
The anonymous nature of this study may have led to lack of accountability. The selection of experts was based on their willingness to participate. The fact that not all panellists took part in all rounds may have resulted in selection bias.
WIDER IMPLICATIONS OF THE FINDINGS
This international standpoint is the first step in the global acceptance of OTC in females with TS. Future collaborative research with a focus on efficacy and safety and long-term follow-up is urgently needed. Furthermore, we recommend an international register for fertility preservation procedures in females with TS.
STUDY FUNDING/COMPETING INTEREST(S)
Unconditional funding (A16-1395) was received from Merck B.V., The Netherlands. The authors declare that they have no conflict of interest.
Keywords: Turner syndrome, fertility preservation, ovarian tissue cryopreservation, Delphi study, ethics, primary ovarian insufficiency, international statement, paediatrics, endocrinology—disorders of sex development
Introduction
Removal of one of the two ovaries for cryopreservation followed by autotransplantation of ovarian cortex fragments in the future (i.e. ovarian tissue cryopreservation: OTC) is already an option for preserving the fertility of young females at risk of iatrogenic primary ovarian insufficiency (POI) (Jensen et al., 2015; ‘Netherlands Network of Fertility Preservation (NNF),’ 2016; ‘Oncoline,’ 2016; Van der Ven et al., 2016). Offering OTC to females with a genetic cause of POI could be the next step. One of the most common genetic conditions related to POI is Turner syndrome (TS), affecting 25–50 per 100 000 live-born girls (Gravholt et al., 2017).
TS is caused by the partial or complete absence of one of the sex chromosomes. Missing an X or Y chromosome affects foetal development. Signs and symptoms vary greatly among females with TS but are mostly related to the patient’s karyotype. Monosomy 45,X is associated with a more severe lymphatic and skeletal phenotype, while the dysmorphic features of patients with a mosaic karyotype can be very mild, depending on the level of mosaicism. No association was found between karyotype and cardio-aortic malformations (Noordman et al., 2018). The majority of females with TS are diagnosed before the age of 12 years because of growth retardation (Massa et al., 2005). TS may be diagnosed prenatally by chorionic villus sampling or amniocentesis in cases of foetal cystic hygroma, intra-uterine growth retardation, cardiovascular malformations or advanced maternal age, or shortly after birth because of dysmorphic signs such as neck webbing, cubitus valgus and lymphoedema. In a few cases, TS is diagnosed in adolescence because of delayed puberty or primary or secondary amenorrhea.
Females with TS are known to have a shorter reproductive lifespan due to an accelerated loss of germ cells. This process starts during meiosis I of the foetal oocyte and continues until the point when the ovarian reserve is completely exhausted (Weiss, 1971; Reynaud et al., 2004). In most females with TS, this point is reached during childhood or early adolescence (Reynaud et al., 2004). Up to 33% have some pubertal development and 10–15% experience one or more spontaneous menstruation cycles (Pasquino et al., 1997; da Silva Negreiros et al., 2014; Tanaka et al., 2015). Hence, spontaneous pregnancies in adult females with TS are rare, occurring in ~2.0–7.6% of cases (Hovatta, 1999; Birkebaek et al., 2002; Bryman et al., 2011; Hadnott et al., 2011; Bernard et al., 2016).
As the majority of females with TS are unable to conceive a child naturally, most of them depend on alternative parenting options such as adoption, foster care or oocyte donation. However, like most women, females with TS prefer a genetically related child above other forms of parenting, as adoption, foster care or oocyte donation have their own limitations (Hallebone, 1991; Bracewell-Milnes et al., 2016). The inability to bear biological children is the most prevalent and painful challenge experienced by most females with TS, especially at the moment that their family and friends begin to procreate (Sylven et al., 1993; Sutton et al., 2005). In an interview study with 97 females with TS aged 7–59 years and 21 parents, uncertainty about their fertility started at a young age and was a major concern for both groups (Sutton et al., 2005). In females with other causes of POI, uncertainty about fertility and the inability to have biological offspring was associated with a reduction of quality of life and serious psychosocial disorders (Nilsson, 2014).
It is, therefore, unsurprising that physicians are increasingly being asked about fertility preservation (FP) options for females with TS (Grynberg et al., 2016), especially as research shows that oocytes can still be found in the ovaries of some girls with TS (Hreinsson et al., 2002; Huang et al., 2008; Kavoussi et al., 2008; Borgstrom et al., 2009; Lau et al., 2009; Balen et al., 2010; El-Shawarby et al., 2010; Oktay et al., 2010; Oktay & Bedoschi, 2014; Balkenende et al., 2015; von Wolff et al., 2015; Finlayson et al., 2017; Jensen et al., 2017; Mamsen et al., 2019; Talaulikar et al., 2019).
FP is the process of safeguarding the patient’s own gametes so that these can be used to have biological children in the future. In single females, FP can be performed by either the vitrification of mature oocytes or by cryopreserving ovarian tissue containing primordial follicles. Vitrification of mature oocytes (oocyte cryopreservation, OC) is the most established FP approach but is limited to a small percentage of females with TS only, i.e. those with a spontaneous menstrual cycle during adolescence or adulthood (Oktay et al., 2015). Furthermore, females interested in OC have to be emotionally mature enough to undergo the procedure, which involves a period of at least 2 weeks with daily hormone injections, frequent ultrasonographic monitoring, and transvaginal oocyte retrieval (Oktay et al., 2015). In view of these limitations, OTC appears to be a more promising technique to preserve the fertility of females with TS, as it can be performed regardless of the patient’s age or ovarian activity. This procedure may offer more females with TS the possibility to store a number of primordial follicles before their disappearance (Borgstrom et al., 2009).
Since 2002, OTC procedures have been performed experimentally in more than 100 young females with TS (Hreinsson et al., 2002; Huang et al., 2008; Borgstrom et al., 2009; Balen et al., 2010; von Wolff et al., 2015; Finlayson et al., 2017; Jensen et al., 2017; Mamsen et al., 2019) [ClinicalTrials.gov: NCT01410045]. Unfortunately, optimal discriminative markers for the presence or absence of follicles are lacking, but there is a general agreement that the mosaic karyotype is the most likely group to have ovarian follicles and to benefit from FP (Borgstrom et al., 2009; Oktay et al., 2015; Grynberg et al., 2016; Mamsen et al., 2019).
Thus far, there are no published records of girls with TS who have returned for autotransplantation of cryopreserved ovarian tissue. Hence, the efficacy of OTC in females with TS remains unknown. In other patient groups, the occurrence of pregnancy and live birth after autotransplantation of cryopreserved ovarian tissue is highly correlated with the number of functional primordial follicles found in the ovarian tissue (Donnez, 2011). However, even in these patient groups there is limited data regarding the efficacy of the procedure when OTC is performed at a very young age (Demeestere et al., 2015). Autotransplantation of ovarian cortex fragments with a decreased follicular density combined with the risk of re-initiation of accelerated follicle apoptosis might be less effective. Possibly, in vitro activation (IVA) of residual dormant follicles (Kawamura et al., 2013; Suzuki et al., 2015; Kawamura et al., 2016; Zhai et al., 2016) prior to autotransplantation might be helpful in females with TS to optimize their fertility chances. However, no cases of IVA in patients with TS have been reported thus far. The isolation and IVM of primordial follicles from cryopreserved ovarian tissue could become an effective alternative to autotransplantation in the future (McLaughlin et al., 2018). However, this method is still experimental and not yet available in the clinic. Hence, females with TS who are currently undergoing OTC are still depending on autotransplantation.
Even if the follicular density is normal or slightly decreased, it remains questionable if autotransplantation of cryopreserved ovarian tissue in females with TS will lead to healthy offspring, as females with TS females who conceived spontaneously are known to have an increased risk of miscarriages and chromosomal abnormalities among their offspring (Bernard et al., 2016). Whether these increased risks are related to the quality and functional integrity, or the chromosome profile, of the follicular cells remains unclear.
Hence, offering OTC in routine clinical care could give false hope and psychological harm in the future. Young patients might not be able to fully understand the possible risks and benefits of the procedure (Di Pietro et al., 2012; McDougall, 2015; Wallace et al., 2016), and thus, parents will be burdened with this decision (Hreinsson & Fridstrom, 2004).
Another concern that should be taken into account is that OTC requires laparoscopic surgery under general anaesthesia with a possible risk of complications (Jansen et al., 1997). Furthermore, removing half of the ovarian reserve in females with TS might impact their chances for spontaneous puberty, menstruation and pregnancy. The short-term and long-term effects of the surgical removal of one ovary in females with TS are currently unknown, but recent studies (Bellati, 2014; Geomini, 2014; Khan, 2014; Lass, 1999) have shown that the surgical removal of one ovary in females with a normal ovarian reserve does not affect the patient’s menstrual cycle, or their chance for spontaneous pregnancies in the future (Coccia et al., 2011). However, the procedure could lead to early menopause, of up to 3 years earlier in comparison to a woman who still has both ovaries (Bjelland et al., 2014).
Lastly, one should consider that pregnancies in females with TS show more foetal and maternal complications compared to pregnancies in healthy females. Pregnant females with TS have an increased risk of intra-uterine growth restriction and preterm labour (Hewitt et al., 2013). Thyroid dysfunction, diabetes, obesity, hypertension and pre-eclampsia occur in ~40% of pregnant women with TS (Hewitt et al., 2013). In the past, women with TS were advised to avoid pregnancy due to the risk of mortality. Recent studies have shown that the risk for aorta dissection and maternal mortality associated with pregnancy have decreased from 2.0 to 0.5% due to increased awareness of cardiovascular complications, stringent preconception screening and cardiovascular follow up during pregnancy (Gravholt et al., 2017; van Hagen et al., 2017). Pregnancies in females with TS should be strictly monitored by a multidisciplinary team including high-care obstetricians, and cardiologists and anaesthesiologists with expertise in maternal heart disease and/or disease of the aorta. If this care is unavailable, pregnancies in females with TS might be contraindicated because of an increased risk of complications.
For the above mentioned reasons, FP in females with TS remains a controversial topic for clinicians (Borini & Coticchio, 2019). However, patient organizations are optimistic and demand equal access to FP options worldwide (Borgstrom et al., 2009; Di Pietro et al., 2012). To further explore the opinion of international professionals and patient representatives, we conducted a three-stage ethical Delphi study to systematically discuss the advantages and disadvantages of OTC in females with TS. The aim of this study was to reach group consensus and to form an internationally accepted standpoint as to whether OTC should be offered to females with TS, or not.
Materials and Methods
The RAND/UCLA Delphi procedure (Dalkey, 1969; Fitch et al., 2001; Boulkedid et al., 2011) was used to combine scientific evidence with the expertise and opinion of different international experts within the field of TS, OTC or medical ethics. The Delphi procedure is a well-accepted method for attaining group consensus. It is a structured process that uses a series of questionnaires or rounds to gather information from different experts anonymously. Rounds are held until group consensus is reached according to predetermined defined consensus rules. In medical research, the Delphi procedure is commonly used to reach consensus on key recommendations (Schleedoorn et al., 2016), quality indicators (Campbell et al., 2003) or key statements (Carley et al., 1999).
In this study, the Delphi procedure was used to determine whether the expected benefits exceed the expected negative consequences of OTC in TS (Brook et al., 1986; Boulkedid et al., 2011). The outcome of this study was an international standpoint for or against OTC in females with TS, supported by a set of key statements.
Two questionnaire rounds and one agreement round were performed. Panel members were polled individually and anonymously. Participants were offered the possibility to adjust their opinions after repetitive feedback after each round, thus avoiding the negative social influences associated with face-to-face discussion (Fitch et al., 2001). Questionnaires were conducted by electronic data capture, using CastorEDC® (Castor, George Westinghousestraat 2, 1097 BA, Amsterdam, The Netherlands). Possibilities to add new statements or comments were provided in each questionnaire. Invitations and reminders were sent via CastorEDC®. All scores were listed in a database created with IBM SPSS Statistics version 25.0 (IBM Netherlands, Johan Huizingalaan 765, P.O. Box 9999, 1066 VH Amsterdam, Netherlands). The consensus procedure took place between February and December 2018.
Identifying ethical issues and formulating statements
A total number of 65 articles were screened for arguments both for and against OTC in TS after a comprehensive literature search (Fig. 1). Each article was screened independently by two researchers from a multidisciplinary research group (i.e. two gynaecologists (n = K.F., C.B.), one paediatric endocrinologist (n = A.v.d.V.), one medical ethicist (E.v.L.), one senior scientist in reproductive biology (n = R.P.) and one physician in reproductive medicine (M.S.) for arguments regarding OTC in females with TS. None of these researchers participated in the Delphi selection procedure. Arguments were extracted if both independent researchers agreed.
Figure 1.
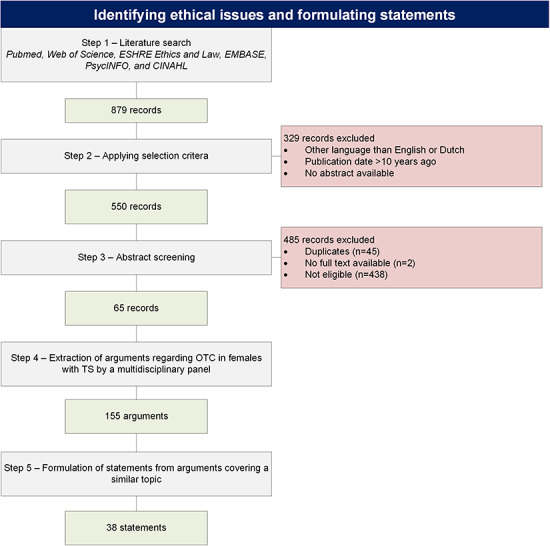
Identifying ethical issues and formulating statements concerning potential use of ovarian tissue cryopreservation in young females with Turner syndrome. The figure provides a detailed description of the comprehensive literature search, extraction of arguments and formulation of statements. OTC: ovarian tissue cryopreservation, TS: Turner syndrome.
This selection procedure resulted in a total number of 155 arguments regarding OTC in TS. Arguments focusing on a similar topic were grouped and brought together into a framework of 38 statements (Supplementary Table SI). These statements were divided over the four basic ethical domains (Gillon, 1994), i.e. beneficence (n = 18), autonomy (n = 5), non-maleficence (n = 8) and justice (n = 7). Each statement highlighted a specific ethical concern regarding OTC in females with TS. The original 155 arguments are presented as additional information below each statement.
Selection of key statements and formulation of a common stand
Step 1: Composition of the expert panel
To enhance the acceptance of this international standpoint in clinical practice, the expert panel consisted of a representative diversity of international professionals and patient representatives. Sufficient English language proficiency was an admission requirement for all experts.
Invitations for the Delphi study were sent out by e-mail to 59 international professionals (female professionals n = 37, male professionals n = 22) with expertise in the field of FP, TS and/or medical ethics. Eligible experts were gynaecologists (n = 18) and (paediatric-) endocrinologists (n = 17) with either a prominent role in one or more international expert groups (i.e. ESHRE Special Interest Group for Fertility Preservation, DSD-Life, Oncofertility, FertiPROTEKT or Turner Syndrome Guideline Group) and/or an author of one or more key publications regarding FP in females with TS. Medical ethicists (n = 24) were recruited by the ESHRE Task Force Ethics & Law and the personal network of the senior research team members (K.F., A.v.d.V., E.v.L., W.N., C.B., R.P., D.B.). Patient representatives should have had a prominent role in one of the international TS patient organizations. Therefore, a request for patient representatives (i.e. patients and/or parents of patients) was sent out to 17 patient organizations in 15 different countries. Contact data from 38 patient representatives were obtained, and invitations were sent out to them by e-mail.
Step 2: First Delphi round
In the first Delphi round, the panel was asked to rate the 38 statements on a 9-point Likert scale ranging from 1 (extremely irrelevant) to 9 (extremely relevant). Relevance was graded by the experts in response to the following question: ‘To what extent is the following statement an important determinant to offer/discourage OTC in young females with TS?’. Participants were encouraged to read the original arguments and were provided with supporting evidence and background information. By the end of the questionnaire, participants were asked to create a top 5 of their most relevant statements to promote discrimination between recommendations with a high Likert score. In addition, they were given the option to add comments and new statements. Participants were given 4 weeks to complete the first round. Reminders were sent after 2 and 3 weeks.
The results of the first round were analysed using predefined consensus criteria based on Campbell’s criteria (Campbell et al., 2000). These criteria include a median score of 8 or higher without panel disagreement. Panel disagreement was defined as the case in which 25% or more of the individual scores was in the lowest tertile of the scale (Likert score 1–3). Previous studies (Mourad et al., 2007; van den Boogaard et al., 2010; Stienen et al., 2011; Uphoff et al., 2012; Dancet et al., 2013; den Breejen, 2013; Luitjes et al., 2013; Woiski et al., 2015) have shown that Campbell’s criteria alone are often not discriminative enough. Therefore, a third criterion was added, which is commonly used in Delphi studies, namely a top 5 score (Mourad et al., 2007; van den Boogaard et al., 2010; Stienen et al., 2011; Uphoff et al., 2012; Dancet et al., 2013; Luitjes et al., 2013; Woiski et al., 2015). Recommendations should have at least a top 5 score of 35 points or higher. Points were awarded to each top-five ranking position, with number 1 position = 5 points, number 2 position = 4 points, number 3 position = 3 points, number 4 position = 2 points and number 5 position = 1 point. The authors combined the three criteria as described above and converted them into three possible outcomes ‘selected’, ‘rejected’, or ‘no consensus’. Recommendations that met all three criteria were classified as ‘selected’, those who met none of the criteria as ‘rejected’ and the remaining recommendations as ‘no consensus’. The ‘no consensus’ recommendations were again discussed in the second questionnaire round.
Step 3: Second Delphi round
The second round started with an overview of the selected, rejected and ‘no consensus’ recommendations. First, the experts were asked for their approval of the key statements that have been selected in the first round. Second, the expert panel was asked to revise their opinion for the ‘no consensus’ recommendations in light of the replies of the other panel members. The overall median score, the median score of each subgroup, the total top 5 score and their own previous rating were shown for each statement. When experts were not able to participate in the first Delphi round (n = 9), but wanted to participate in the second Delphi round, the overall median score, the median score of each subgroup and the total top 5 score were shown for each statement. Participants were asked once again to score the ‘no consensus’ recommendations on the same 9-point Likert scale as used in the first Delphi round (ranging from 1 (extremely irrelevant) to 9 (extremely relevant)). By the end of the questionnaire, the recommendations that they scored with 8 or higher were shown, and participants were asked to select up to three additional statements.
Participants were given 3 weeks to complete the second round. Reminders were sent after 1 week and after 2 weeks.
The selection of additional key statements during the second round was based on two predefined criteria that were used during the first round (i.e. a median score of 8 or higher and panel disagreement below 25%). Furthermore, additional key statements should be selected by at least 30% of the experts. These three criteria were combined and converted into two possible outcomes: ‘selected’ or ‘rejected’. Recommendations that met all criteria were classified as ‘selected’ and the remaining recommendations as ‘rejected’.
After having discussed the medical and ethical aspects of OTC in females with TS, the expert panel was asked to form a current standpoint if OTC should be offered to young females with TS or not.
Step 4: Final approval
An overview of the selected key statements and the current expert panel’s standpoint was sent out by e-mail to all 55 experts who initially gave consent for study participation, as 14 out of the 55 experts (25%) did not participate in the second Delphi round. They were provided with a last opportunity to make remarks and asked for their approval of the final set of key statements and the expert panel’s standpoint in order to reach international consensus.
Results
Selection of key statements and formulation of a common stand
Step 1: Composition of the expert panel
A total number of 12 gynaecologists, 13 (paediatric) endocrinologists, 10 medical ethicists and 20 patient representatives (patients n = 7, parents n = 13) from 16 different countries gave consent to participate in this study, forming an international expert panel of 55 members (Fig. 2). The composition of the expert panel for each Delphi round has been visualized in Supplementary Figure S1.
Figure 2.
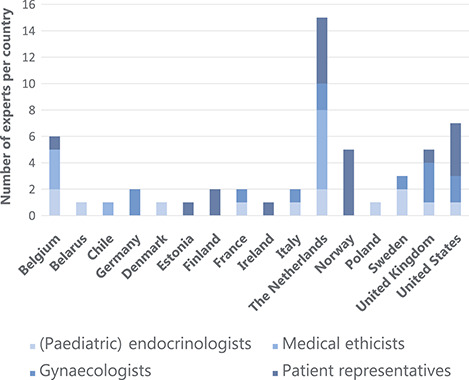
Members of the TurnerFertility expert panel (n = 55) divided by country. (Paediatric) endocrinologists: (n = 13) representing 10 countries: Belgium (n = 2), Belarus (n = 1), Denmark (n = 1), France (n = 1), Italy (n = 1), The Netherlands (n = 2), Poland (n = 1), Sweden (n = 2), UK (n = 1), USA (n = 1). Medical ethicists (n = 10) representing three countries: Belgium (n = 3), The Netherlands (n = 6), Chile (n = 1). Gynaecologists: (n = 12) representing seven countries: Germany (n = 2), France (n = 1), Italy (n = 1), The Netherlands (n = 2), Sweden (n = 1), UK (n = 3), USA (n = 2). Patient representatives: (n = 20) (7 patients with TS and 13 parents) representing eight countries: Belgium (n = 1), Estonia (n = 1), Finland (n = 2), Ireland (n = 1), The Netherlands (n = 5), Norway (n = 5), UK (n = 1), USA (n = 4).
All professionals, except one of the (paediatric) endocrinologists, were employed in academic hospitals or were working in an academic setting. Most of them were females (n = 23), and about one-third of them were male professionals (n = 12).
Gynaecologists had an average work experience of 23 years (range 9–40 years), (paediatric) endocrinologists had an average work experience of 21.4 years (range 7–40 years) and medical ethicists had an average work experience of 22 years (range 9–38 years).
Most of the professionals (77%) had children themselves, and most of them conceived spontaneously. Two professionals conceived using ART, and one professional adopted a child.
The remaining panel consisted of 20 patient representatives, 7 females with TS and 13 parents of patients with TS. The average age for patients was 32 years (range 18–50) and for parents 45 years (range 36–55).
Most patient representatives were highly educated, with at least an associate degree (n = 15), while the remaining five patient representatives finished at least high school. None of the women with TS had children themselves, but all except one expressed a desire to have children in the near future. None of them had tried ART. One woman with TS tried to adopt a child but was rejected for unknown reasons. One of the parents adopted a child, and the others conceived spontaneously.
Step 2: First Delphi round
A total number of 46 participants completed the first Delphi round (response rate 84%). Reasons for not participating were concerns about privacy (n = 1), not enough time (n = 4), not feeling sufficiently involved with the subject (n = 2), a conflict of interest (n = 1) and software problems (n = 1). The average time for completing the first survey was 30 min.
Based on the predefined selection criteria, 6 of the 38 statements were selected as key statements (Fig. 3). These six key statements were divided over the four ethical domains (beneficence (n = 2), autonomy (n = 1), non-maleficence (n = 2) and justice (n = 1)). In the first round, 13 statements could be discarded, and 19 statements remained undetermined. In addition, two new statements submitted by the expert panel, were added to the second Delphi round (Supplementary Table SI).
Figure 3.
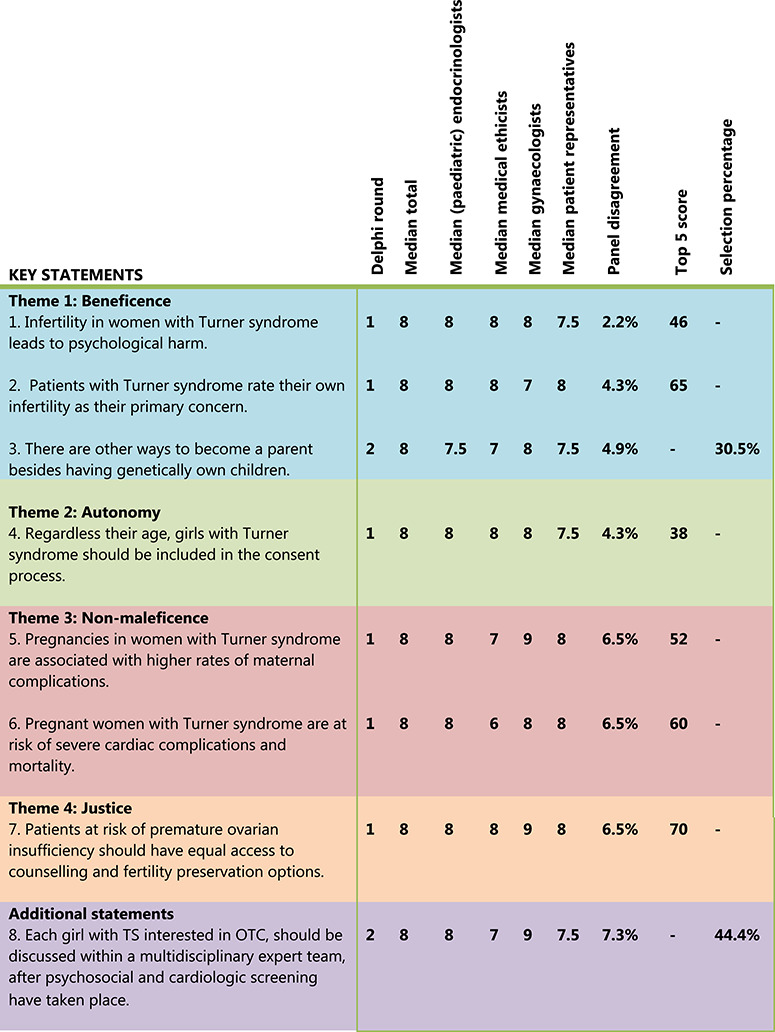
The final set of eight key statements divided among the four basic ethical themes (beneficence, autonomy, non-maleficence and justice). Inclusion was based on predetermined selection criteria (i.e. a median score of 8 or higher AND panel disagreement below 25%) AND a top 5 score of 35 points or higher (first Delphi round) OR a selection percentage of 30% or more (second Delphi round).
Step 3: Second Delphi round
The remaining 19 statements and the two additional statements submitted by participants were re-evaluated in the second round by 41 participants (response rate 75%). Time investment was reported as the main reason for not participating. The average time for completing the second survey was 10 min.
The analysis of the second survey resulted in the inclusion of two additional key statements (Fig. 3). The other 19 statements could be discarded.
By the end of the second Delphi round, 30 experts (75%) believed that OTC should be offered to young females with TS in a safe and controlled research setting, one participant voted against (2%) and 10 experts chose to remain neutral (24%).
Step 4: Final approval
The final set of eight key statements (Fig. 3) and the current expert panel’s standpoint were approved by 53 out of 55 participants (response rate 96%). All experts, including those who voted against, remained neutral or did not respond in the second Delphi round, now agreed that OTC should be offered to young females with TS, but under strict conditions only. The expert panel suggested a research setting, with proper counselling and informed consent procedures, and a long-term follow-up to study the efficacy of OTC in young females with TS first, before offering this procedure in routine care.
Two experts (4%), one gynaecologist and one medical ethicist, did not respond despite several reminders.
The detailed process of study enrolment and response rates by Delphi round is shown in Figure 4. A process description of the key statement selection by Delphi round can be found in Supplementary Figure S2.
Figure 4.
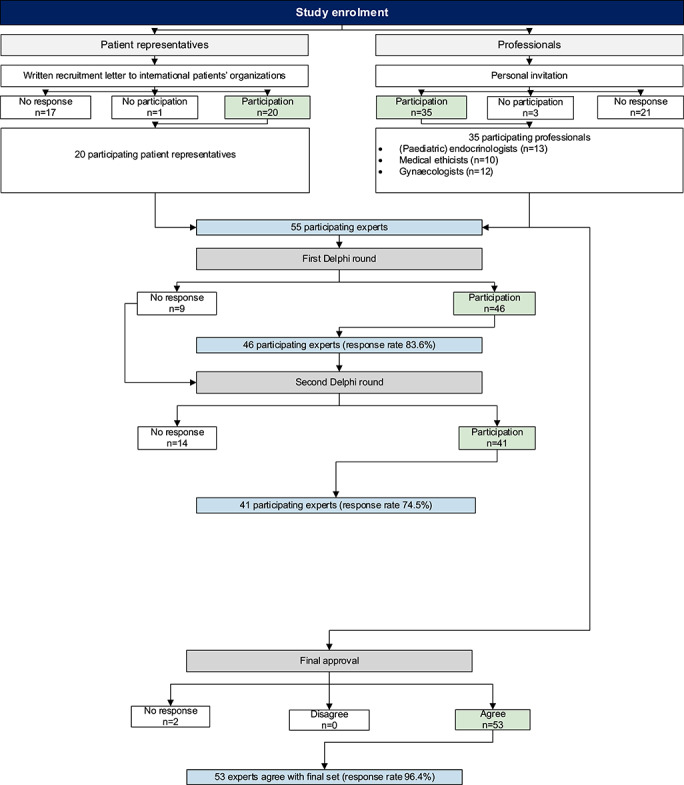
Detailed process of study enrolment and response rates by Delphi round.
Differences in scoring behaviour between experts
In the first Delphi round, patient representatives mainly highlighted arguments focusing on the psychological harm of infertility, and one of the key statements was selected by the patient representatives despite a moderate popularity among the professionals. This statement focused on the impact of infertility on the quality of life. Medical ethicists were more concerned about creating false hope resulting in psychological harm in the future. Gynaecologists and (paediatric-) endocrinologists underlined statements regarding non-maleficence. The fact that young TS patients might be too immature to give informed consent was considered a major issue for both patient representatives and (paediatric) endocrinologists but was not recognized by the gynaecologists and medical ethicists. Younger participants (<25 years old) expressed the importance of including all patients in the consent process, regardless of their age. The statement that ‘infertility leads to psychological harm’ was selected twice as frequently by participants without children compared to participants with children. Male participants were more concerned about pregnancy-related complications, whereas the female participants highlighted the psychological harm of infertility. Religion, age and the availability of OTC in the participant’s country did not influence the scoring behaviour between participants in the first Delphi round.
Differences in scoring behaviour between the various experts were only seen in the first Delphi round and disappeared in the following two rounds when opinions were exchanged.
Discussion
In this Delphi study, we demonstrated that an international expert panel of both professionals and patient representatives agreed that OTC in patients with TS should be offered, but in a safe and controlled research setting only. The expert panel’s standpoint was supported by eight key statements (Fig. 3). The first two statements highlighted that infertility leads to psychological harm, and that patients with TS consider this their main concern (key statements 1 and 2). In addition, patients with TS should have equal access to FP options, in line with other patient groups (e.g. patients awaiting cancer treatments) (key statement 7). However, there were concerns about the increased risk of maternal morbidity and mortality associated with TS during a future pregnancy (key statements 5 and 6). Patients with TS should be counselled about the alternative options for future parenthood (i.e. adoption, fostering and oocyte donation) (key statement 3). Furthermore, the option of voluntary childlessness should be discussed. All patients with TS interested in OTC should undergo psychosocial and cardiac screening and should be discussed by a multidisciplinary expert team (key statement 8). Great caution and restrictive (i.e. more negative, or even discouraging) counselling are recommended if laparoscopic surgery or pregnancy is contraindicated (i.e. in patients with severe cardiac comorbidity).
In addition, patients with TS should always be included in the consent process, regardless of their age (key statement 4).
To our knowledge, this is the first international standpoint regarding OTC in females with TS. To reach international group consensus we used the RAND/UCLA Delphi procedure, which is a well-accepted method to perform Delphi studies. A key strength of this study was the combination of evidence and expert opinion, involving both professionals and patient representatives from 16 different countries. Furthermore, our expert panel represented a robust sample of the most important stakeholders to ensure that all the medical and ethical aspects of OTC in females with TS were discussed. The literature shows that a diversity of expert panel members leads to the inclusion of different perspectives, in turn leading to better overall performance (Murphy et al., 1998). This diversity provided a suitable set of key statements and consensus on a final standpoint, which should support broad acceptance in daily practice internationally. Remarkably, ours was one of the few studies where a combined panel of medical professionals and patient representatives was involved in defining a standpoint regarding the indication for medical treatment for a specific patient group. It is well known that patient input is invaluable when it comes to clinical practice guideline development (Dancet et al., 2013; den Breejen, 2013; Pohontsch et al., 2015). Essentially, patients are the ultimate experts in patient-centeredness of care (Grol, 2001; Epstein & Street, 2011), which is possibly the dominant paradigm in modern healthcare systems. The final set of key statements, and thus the outcome of this Delphi study, could have been different if only professionals had been involved in the selection procedure (Krahn & Naglie, 2008; Aarts et al., 2011; van Empel et al., 2011; Uphoff et al., 2012; den Breejen, 2013; Kotter et al., 2013). In the first Delphi round, patient representatives mainly highlighted arguments focusing on the psychological harm of infertility, whereas medical ethicists were more concerned about creating false hope resulting in psychological harm in the future. Gynaecologists and (paediatric-) endocrinologists underlined statements regarding non-maleficence. This is in line with previous studies reporting that professionals underestimate ‘softer’ dimensions of healthcare (e.g. quality of life) and overestimate the importance of biomedical outcomes compared to patients (Laine et al., 1996; Rothwell et al., 1997; Mack et al., 2005; Wessels et al., 2010; van Empel et al., 2011).
However, differences in scoring behaviour between the various experts were only seen in the first Delphi round and disappeared in the following two rounds when opinions were exchanged. Only one of the key statements (Infertility in women with TS leads to psychological harm) was selected by the patient representatives, despite having a moderate popularity among the professionals.
Although we considered the expert panel to be representative because of their diverse backgrounds (Hermens et al., 2006; Ouwens et al., 2010; Kesmodel & Jolving, 2011; Kotter et al., 2013), the recruitment of professionals and patient representatives based on their expertise, having a leading role in one of the Turner patient’s organizations, and willingness to participate may have led to selection bias. As a result, panel members with a prominent opinion regarding OTC in females with TS might have been preferentially motivated to participate in this Delphi study. Furthermore, as not all panel members took part in all three rounds, there might be some response bias (Sica, 2006) because of time constraints or technical problems. Therefore, the final set of key statements and the expert panel’s standpoint might reflect the opinion of the most motivated panel members (Sica, 2006). However, we tried to overcome this by inviting all 55 experts who initially agreed to participate in this Delphi study to participate in the final approval round.
The anonymous nature of this study was both a strength and a weakness. The purpose of anonymity in a Delphi study is to allow a safe exchange of opinions, without the bias of the more influential responders dominating the discussion. External influences are eliminated, as participants do not have to worry about their reputation. However, it might encourage hasty decision-making and a lack of accountability for their answers.
This study described the systematic selection of key statements and the formulation of a final standpoint regarding use of OTC in females with TS by an international panel of patient representatives and professionals. International group consensus was reached after three rounds. The approval to perform OTC in a safe and controlled research setting is the first step in the global acceptance of this FP option in females with TS. The eight supporting key statements will contribute to the implementation of and patients’ access to this new treatment method. Our results reinforce the importance of involving patient representatives in decision-making and guideline development.
Future collaborative research with a focus on the efficacy and safety of OTC in females with TS is urgently needed before OTC is performed in females with TS in routine care. Therefore, we recommend an international prospective cohort study with long-term follow-up with a research protocol based on the eight selected key statements. Furthermore, we recommend an international register for FP procedures in females with TS.
Supplementary Material
Acknowledgements
We would like to acknowledge all members of the TurnerFertility expert panel for their participation in this Delphi study: Prof. Dr Richard Anderson, Dr Natallia Akulevich, Prof. Dr Carmen Astete, Prof. Dr Philippe F. Backeljauw, Prof. Dr Adam Balen, Prof. Dr Inez D. de Beaufort, Prof. Dr Theo A. Boer, Dr Birgit Borgstrom, Prof. Dr Pascal Borry, Prof. Dr Annelien L. Bredenoord, Dr Eline M. Bunnik, Prof. Dr Ralf Dittrich, Dr Marie-Madeleine Dolmans, Dr Lise Duranteau, Dr Courtney Finlayson, Dr Elisa B. Garcia, Dr Aneta Gawlik, Prof. Dr Claus H. Gravholt, Prof. Dr John W. Gregory, Dr Solange Grunenwald, Dr Michelle Habets, Dr Sabine Hannema, Prof. Dr Outi Hovatta, Prof. Dr Nils B. Lambalk, Drs Leoni Louwé, Prof. Dr Laura Mazzanti, Dr Anna Nordenström, Prof. Dr Kutluk Oktay, Prof. Dr Guido Pennings, Prof. Dr Eleonora Porcu, Prof. Dr Veerle Provoost, Dr Katharina Rall, Dr Theo C.J. Sas, Dr Francoise Shenfield, Dr Teresa K. Woodruf and all 20 patient representatives from Belgium, Estonia, Finland, Ireland, the Netherlands, Norway, UK and the USA.
Authors’ roles
M.S., W.N., E.v.L., A.v.d.V. and K.F. designed the research project. R.P., C.B., M.S., W.N., E.v.L., A.v.d.V. and K.F. obtained all arguments from the literature and brought them together into a framework. M.S. and B.M. composed the expert panel, conducted the surveys, led data collection, performed data analysis and wrote this manuscript. R.P., C.B., D.B., M.S., W.N., E.v.L., A.v.d.V. and K.F. contributed substantially to data interpretation and manuscript revisions. All authors read and approved the final manuscript.
Funding
Unconditional funding (A16-1395) was received from Merck B.V., The Netherlands. Members of the expert panel did not receive payment for study participation.
Conflict of interest
The authors and members of the expert panel declare that they have no conflict of interest in this procedure.
References
- Aarts JW, Faber MJ, van Empel IW, Scheenjes E, Nelen WL, Kremer JA. Professionals' perceptions of ScienceDirect’s AI-generated care. Hum Reprod 2011;26:1119–1127. [DOI] [PubMed] [Google Scholar]
- Balen AH, Harris SE, Chambers EL, Picton HM. Conservation of fertility and oocyte genetics in a young woman with mosaic Turner syndrome. BJOG 2010;117:238–242. [DOI] [PubMed] [Google Scholar]
- Balkenende E., Dahhan T., Repping S., de Melker A. A., van der Veen F., & Goddijn M. (2015). [Oocyte vitrification: for whom?]. Ned Tijdschr Geneeskd ,159, A9361. [PubMed] [Google Scholar]
- Bellati F, I R, Gasparri ML, Antonilli M, Pernice M, Vallone C et al. Effects of unilateral ovariectomy on female fertility outcome. Arch Gynecol Obstet 2014;290:349–353. [DOI] [PubMed] [Google Scholar]
- Bernard V, Donadille B, Zenaty D, Courtillot C, Salenave S, Brac de la Perrière A, Christin-Maitre S. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum Reprod 2016;31:782–788. [DOI] [PubMed] [Google Scholar]
- Birkebaek NH, Crüger D, Hansen J, Nielsen J, Bruun-Petersen G. Fertility and pregnancy outcome in Danish women with Turner syndrome. Clin Genet 2002;61:35–39. [DOI] [PubMed] [Google Scholar]
- Bjelland EK, Wilkosz P, Tanbo TG, Eskild A. Is unilateral oophorectomy associated with age at menopause? A population study (the HUNT2 Survey). Hum Reprod 2014;29:835–841. [DOI] [PubMed] [Google Scholar]
- Borgstrom B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, Hovatta O. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab 2009;94:74–80. [DOI] [PubMed] [Google Scholar]
- Borini A, Coticchio G. Oocyte quantity and quality are crucial for a perspective of fertility preservation in women with Turner syndrome. Fertil Steril 2019;111:461–462. [DOI] [PubMed] [Google Scholar]
- Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011;6:e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracewell-Milnes T, Saso S, Bora S, Ismail AM, Al-Memar M, Hamed AH, Thum M-Y. Investigating psychosocial attitudes, motivations and experiences of oocyte donors, recipients and egg sharers: a systematic review. Hum Reprod Update 2016;22:450–465. [DOI] [PubMed] [Google Scholar]
- Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care 1986;2:53–63. [DOI] [PubMed] [Google Scholar]
- Bryman I, Sylven L, Berntorp K, Innala E, Bergstrom I, Hanson C, Landin-Wilhelmsen K. Pregnancy rate and outcome in Swedish women with Turner syndrome. Fertil Steril 2011;95:2507–2510. [DOI] [PubMed] [Google Scholar]
- Campbell SM, Braspenning J, Hutchinson A, Marshall MN. Research methods used in developing and applying quality indicators in primary care. BMJ 2003;326:816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SM, Cantrill JA, Roberts D. Prescribing indicators for UK general practice: Delphi consultation study. BMJ 2000;321:425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley SD, Mackway-Jones K, Donnan S. Delphi study into planning for care of children in major incidents. Arch Dis Child 1999;80:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod 2011;26:3000–3007. [DOI] [PubMed] [Google Scholar]
- da Silva Negreiros Liza P, Bolina Eduardo R, Guimarães Marilia M. Pubertal development profile in patients with Turner syndrome. J Pediatr Endocrinol Metab 2014;27:845. [DOI] [PubMed] [Google Scholar]
- Dalkey NCBB, Cochran S. The Delphi method III: use of self ratings to improve group estimates. Santa Monica, CA: Rand, 1969 [Google Scholar]
- Dancet EA, D'Hooghe TM, Spiessens C, Sermeus W, De Neubourg D, Karel N, Nelen WL. Quality indicators for all dimensions of infertility care quality: consensus between professionals and patients. Hum Reprod 2013;28:1584–1597. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 2015;30:2107–2109. [DOI] [PubMed] [Google Scholar]
- den Breejen EM, W N, Schol SF et al. Development of guideline-based indicators for patient-centredness in fertility care: what patients add. Hum Reprod 2013;28:987–996. [DOI] [PubMed] [Google Scholar]
- Di Pietro ML, Virdis A, Gonzalez-Melado FJ, De Luca D. Cryopreservation of ovarian tissue in pediatrics: what is the child's best interest? J Matern Fetal Neonatal Med 2012;25:2145–2148. [DOI] [PubMed] [Google Scholar]
- Donnez JSK. S.S. Medical: Principles and Practice of Fertility Preservation., 2011
- El-Shawarby SA, Sharif F, Conway G, Serhal P, Davies M. Oocyte cryopreservation after controlled ovarian hyperstimulation in mosaic Turner syndrome: another fertility preservation option in a dedicated UK clinic. BJOG 2010;117:234–237. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Street RLJ. The values and value of patient-centered care. Ann Fam Med 2011;9:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson C, Fritsch MK, Johnson EK, Rosoklija I, Gosiengfiao Y, Yerkes E, Cheng E. Presence of germ cells in disorders of sex development: implications for fertility potential and preservation. J Urol 2017;197:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch K, Bernstein SJ, Aguilar MD. The RAND/UCLA Appropriateness Method User’s Manual, 2001.
- Geomini PM, R. Z., Vlemminx M, Mol BW, Coppus SF. (2014). Unilateral ovariectomy, is there a risk for early menopause?[oral presentation]. Paper presented at the European Society for Gynaecological Endoscopy 23rd Annual Congress Brussels, Belgium.
- Gillon R. Medical ethics: four principles plus attention to scope. BMJ 1994;309:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Backeljauw PF. On behalf of the International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017;177:G1–G70. [DOI] [PubMed] [Google Scholar]
- Grol R. Improving the quality of medical care: building bridges among professional pride, payer profit, and patient satisfaction. JAMA 2001;286:2578–2585. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Bidet M, Benard J, Poulain M, Sonigo C, Cedrin-Durnerin I, Polak M. Fertility preservation in Turner syndrome. Fertil Steril 2016;105:13–19. [DOI] [PubMed] [Google Scholar]
- Hadnott TN, Gould HN, Gharib AM, Bondy CA. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the U.S. National Institutes of Health experience. Fertil Steril 2011;95:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallebone EL. Non-genetic mothers and their own children: infertility and IVF donor birth. Aust J Soc Issues 1991;25:122–136. [DOI] [PubMed] [Google Scholar]
- Hermens RP, Ouwens MM, Vonk-Okhuijsen SY, van der Wel Y, Tjan-Heijnen VC, van den Broek LD, Wollersheim HC. Development of quality indicators for diagnosis and treatment of patients with non-small cell lung cancer: a first step toward implementing a multidisciplinary, evidence-based guideline. Lung Cancer 2006;54:117–124. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Jayasinghe Y, Amor DJ, Gillam LH, Warne GL, Grover S, Zacharin MR. Fertility in Turner syndrome. Clin Endocrinol (Oxf) 2013;79:606–614. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Pregnancies in women with Turner’s syndrome. Ann Med 1999;31:106–110. [PubMed] [Google Scholar]
- Hreinsson J, Fridstrom M. In vitro oocyte maturation for safer treatment of infertility. The risk of ovarian overstimulation syndrome is minimized. Lakartidningen 2004;101:3665–3668, 3671. [PubMed] [Google Scholar]
- Hreinsson JG, Otala M, Fridstrom M, Borgstrom B, Rasmussen C, Lundqvist M, Hovatta O. Follicles are found in the ovaries of adolescent girls with Turner’s syndrome. J Clin Endocrinol Metab 2002;87:3618–3623. [DOI] [PubMed] [Google Scholar]
- Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, Chian RC. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: case report. Hum Reprod 2008;23:336–339. [DOI] [PubMed] [Google Scholar]
- Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicentre observational study. Br J Obstet Gynaecol 1997;104:595–600. [DOI] [PubMed] [Google Scholar]
- Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, Andersen CY. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod 2015. [DOI] [PubMed] [Google Scholar]
- Jensen AK, Rechnitzer C, Macklon KT, Ifversen MR, Birkebaek N, Clausen N, Andersen CY. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod 2017;32:154–164. [DOI] [PubMed] [Google Scholar]
- Kavoussi SK, Fisseha S, Smith YR, Smith GD, Christman GM, Gago LA. Oocyte cryopreservation in a woman with mosaic Turner syndrome: a case report. J Reprod Med 2008;53:223–226. [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol 2016;28:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesmodel US, Jolving LR. Measuring and improving quality in obstetrics--the implementation of national indicators in Denmark. Acta Obstet Gynecol Scand 2011;90:295–304. [DOI] [PubMed] [Google Scholar]
- Khan Z, R G, Tabbaa ZM, Laughlin-Tommaso SK, Jensen JR, Coddington CC 3rd et al. Unilateral oophorectomy results in compensatory follicular recruitment in the remaining ovary at time of ovarian stimulation for in vitro fertilization. Fertil Steril 2014;101:722–727. [DOI] [PubMed] [Google Scholar]
- Kotter T, Schaefer FA, Scherer M, Blozik E. Involving patients in quality indicator development - a systematic review. Patient Prefer Adherence 2013;7:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA 2008;300:436–438. [DOI] [PubMed] [Google Scholar]
- Laine C, Davidoff F, Lewis CE, Nelson EC, Nelson E, Kessler RC, Delbanco TL. Important elements of outpatient care: a comparison of patients’ and physicians’ opinions. Ann Intern Med 1996;125:640–645. [DOI] [PubMed] [Google Scholar]
- Lass A. The fertility potential of women with a single ovary. Hum Reprod Update 1999;5:546–550. [DOI] [PubMed] [Google Scholar]
- Lau NM, Huang JY, MacDonald S, Elizur S, Gidoni Y, Holzer H, Tan SL. Feasibility of fertility preservation in young females with Turner syndrome. Reprod Biomed Online 2009;18:290–295. [DOI] [PubMed] [Google Scholar]
- Luitjes SH, Wouters MG, Franx A, Bolte AC, de Groot CJ, van Tulder MW, Hermens RP. Guideline-based development of quality indicators for hypertensive diseases in pregnancy. Hypertens Pregnancy 2013;32:20–31. [DOI] [PubMed] [Google Scholar]
- Mack JW, Hilden JM, Watterson J, Moore C, Turner B, Grier HE, Wolfe J. Parent and physician perspectives on quality of care at the end of life in children with cancer. J Clin Oncol 2005;23:9155–9161. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Charkiewicz K, Anderson RA, Telfer EE, McLaughlin M, Kelsey TW, Andersen CY. Characterization of follicles in girls and young women with Turner syndrome who underwent ovarian tissue cryopreservation. Fertil Steril 2019;111:1217–1225e1213. [DOI] [PubMed] [Google Scholar]
- Massa G, Verlinde F, De Schepper J, Thomas M, Bourguignon JP, Craen M, Heinrichs C. Trends in age at diagnosis of Turner syndrome. Arch Dis Child 2005;90:267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall R. The ethics of fertility preservation for paediatric cancer patients: from offer to rebuttable presumption. Bioethics 2015;29:639–645. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- Mourad SM, Hermens RP, Nelen WL, Braat DD, Grol RP, Kremer JA. Guideline-based development of quality indicators for subfertility care. Hum Reprod 2007;22:2665–2672. [DOI] [PubMed] [Google Scholar]
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, Marteau T. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2i-iv:1–88. [PubMed] [Google Scholar]
- Netherlands Network of Fertility Preservation (NNF) (2016).
- Nilsson J, A J, Lampic C, Eriksson LE, Widmark C, Armuand GM, Malmros J, Marshall Heyman M, Wettergren L. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod, Dec 2014;29:2704–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordman I, Duijnhouwer A, Kapusta L, Kempers M, Roeleveld N, Schokking M, van Alfen-van der Velden J. Phenotype in girls and women with Turner syndrome: association between dysmorphic features, karyotype and cardio-aortic malformations. Eur J Med Genet 2018;61:301–306. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G. Oocyte cryopreservation for fertility preservation in post-pubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J Pediatr Adolesc Gynecol 2014;27:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G, Berkowitz K, Bronson R, Kashani B, McGovern P, Rubin K. Fertility preservation in females with Turner syndrome: A comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Rodriguez-Wallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril 2010;94, 753:e715–e759. [DOI] [PubMed] [Google Scholar]
- Oncoline (2016). Retrieved from https://www.oncoline.nl
- Ouwens M, Hermens R, Hulscher M, Vonk-Okhuijsen S, Tjan-Heijnen V, Termeer R, Grol R. Development of indicators for patient-centred cancer care. Support Care Cancer 2010;18:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner's syndrome. Italian study Group for Turner’s syndrome. J Clin Endocrinol Metab 1997;82:1810–1813. [DOI] [PubMed] [Google Scholar]
- Pohontsch NJ, Herzberg H, Joos S, Welti F, Scherer M, Blozik E. The professional perspective on patient involvement in the development of quality indicators: a qualitative analysis using the example of chronic heart failure in the German health care setting. Patient Prefer Adherence 2015;9:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J. Number of ovarian follicles in human fetuses with the 45,x karyotype. Fertil Steril 2004;81:1112–1119. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, McDowell Z, Wong CK, Dorman PJ. Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ 1997;314:1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleedoorn MJ, Nelen WL, Dunselman GA, Vermeulen N. Selection of key recommendations for the management of women with endometriosis by an international panel of patients and professionals. Hum Reprod 2016;31:1208–1218. [DOI] [PubMed] [Google Scholar]
- Sica GT. Bias in research studies. Radiology 2006;238:780–789. [DOI] [PubMed] [Google Scholar]
- Stienen JJ, Tabbers MM, Benninga MA, Harmsen M, Ouwens MM. Development of quality indicators based on a multidisciplinary, evidence-based guideline on pediatric constipation. Eur J Pediatr 2011;170:1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EJ, McInerney-Leo A, Bondy CA, Gollust SE, King D, Biesecker B. Turner syndrome: four challenges across the lifespan. Am J Med Genet A 2005;139A:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod 2015;30:608–615. [DOI] [PubMed] [Google Scholar]
- Sylven L, Magnusson C, Hagenfeldt K, von Schoultz B. Life with Turner’s syndrome--a psychosocial report from 22 middle-aged women. Acta Endocrinol 1993;129:188–194. [DOI] [PubMed] [Google Scholar]
- Talaulikar VS, Conway GS, Pimblett A, Davies MC. Outcome of ovarian stimulation for oocyte cryopreservation in women with Turner syndrome. Fertil Steril 2019;111:505–509. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Igarashi Y, Ozono K, Ohyama K, Ogawa M, Osada H, Yokoya S. Frequencies of spontaneous breast development and spontaneous menarche in Turner syndrome in Japan. Clin Pediatr Endocrinol 2015;24:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff EP, Wennekes L, Punt CJ, Grol RP, Wollersheim HC, Hermens RP, Ottevanger PB. Development of generic quality indicators for patient-centered cancer care by using a RAND modified Delphi method. Cancer Nurs 2012;35:29–37. [DOI] [PubMed] [Google Scholar]
- van den Boogaard E, Goddijn M, Leschot NJ, Veen F, Kremer JA, Hermens RP. Development of guideline-based quality indicators for recurrent miscarriage. Reprod Biomed Online 2010;20:267–273. [DOI] [PubMed] [Google Scholar]
- Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, Dittrich R. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod 2016;31:2031–2041. [DOI] [PubMed] [Google Scholar]
- van Empel IW, Dancet EA, Koolman XH, Nelen WL, Stolk EA, Sermeus W, Kremer JA. Physicians underestimate the importance of patient-centredness to patients: a discrete choice experiment in fertility care. Hum Reprod 2011;26:584–593. [DOI] [PubMed] [Google Scholar]
- van Hagen IM, Duijnhouwer AL, Ten Kate-Booij MJ, Dykgraaf RH, Duvekot JJ, Utens EM, Roos-Hesselink JW. Wish to conceive and concerns to develop cardiovascular complications during pregnancy in patients with Turner syndrome. J Psychosom Obstet Gynaecol 2017;38:45–52. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Dittrich R, Liebenthron J, Nawroth F, Schüring AN, Bruckner T, Germeyer A. Fertility-preservation counselling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online 2015;31:605–612. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril 2016;105:6–12. [DOI] [PubMed] [Google Scholar]
- Weiss L. Additional evidence of gradual loss of germ cells in the pathogenesis of streak ovaries in Turner’s syndrome. J Med Genet 1971;8:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels H, de Graeff A, Wynia K, de Heus M, Kruitwagen CL, Teunissen SC, Voest EE. Are health care professionals able to judge cancer patients’ health care preferences correctly? A cross-sectional study. BMC Health Serv Res 2010;10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiski MD, Scheepers HC, Liefers J, Lance M, Middeldorp JM, Lotgering FK, Hermens RP. Guideline-based development of quality indicators for prevention and management of postpartum hemorrhage. Acta Obstet Gynecol Scand 2015;94:1118–1127. [DOI] [PubMed] [Google Scholar]
- Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, Sun Y. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab 2016;101:4405–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


