Abstract
Background
About 20% of individuals with autism spectrum disorders (ASD) showed tip-toe behavior (TTB). This behavior may be related to a decreased ankle joint range of motion (ROM) in dorsiflexion. Physiologically, gastrocnemius (GM) and soleus (SM) muscles influence ankle ROM independently. However, no studies investigated the relationship between the amount of time individuals with ASD spend in TTB and GM and SM muscle lengths.
Objective
To evaluate the relationship between three mutually exclusive clinical patterns of TTB i.e., during standing, walking and running (TTB Class 1), or during walking and running (TTB Class 2), or only when running (TTB Class 3), and GM and SM muscle lengths.
Methods
Sixty-nine individuals with ASD (average age: 14.1 ± 3.6 years, 56 males) were enrolled. In a clinical setting, SM and GM muscle lengths of both legs were assessed through a manual goniometer. Measurements were performed by two trained assessors blinded to TTB classifications.
Results
Individuals with ASD classified as TTB Class 1 demonstrated a shortening of both GM and SM compared with NO-TTB and TTB Class 3 individuals.
Conclusions
Our results support the relationship between TTB severity and GM and SM shortening assessed by a decreased ankle joint ROM in dorsiflexion. Further studies are needed to determine the factors associated with TTB and decreased ankle ROM.
Keywords: Toe walking, Autism spectrum disorder, Assessment, Ankle range of motion, Tip-toe behavior, Soleus muscle, Gastrocnemius muscle
1. Introduction
Motor deficits are not included in current autism spectrum disorders (ASD) diagnostic criteria1; however, they may be the first sign of atypical development in ASD.2 Recent evidence suggests that developmental motor delays and motor impairments during early childhood may also be significant predictors of ASD and may influence the cognitive development.2
In their meta-analysis, Fournier et al.3 found that individuals with ASD displayed motor impairments compared to healthy subjects, suggesting motor deficits as a salient feature of ASD. A wide range of motor impairments is reported among individuals with ASD as alterations in motor milestone development4 such as disturbances in reach-to-grasp movements, deficits in gross and fine motor movements and praxis, impaired postural capacity and gait impairment.5
Toe walking (TW) is a possible finding during the gait assessment of individuals with ASD. Clinically, TW refers to walking on the toes without heel strike upon initiation of the stance phase of gait.6 To be considered present, the duration of TW needs to be present for more than six months. While TW may be a manifestation of peripheral musculoskeletal motor impairments, it may also be considered as a sensory processing disorder employed by these individuals, as suggested in other research.7 TW prevalence has been estimated at about 20%.6 TW was found to be significantly more prevalent in children with ASD compared to individuals with developmental disorders,6 and typically developing peers.8
Earlier studies suggested that TW is not only present during walking but also in other functional activities such as standing or running.9 Recently, TW has been defined as tip-toe behavior (TTB), since TW was displayed across various motor activities in individuals with ASD, i.e., standing, walking, and running.9 In this study, the authors described the existence of three mutually exclusive clinical patterns of TTB: 1) TTB during standing, walking and running (TTB Class 1), 2) during walking and running (TTB Class 2), 3) only when running (TTB Class 3).9
Physiological gait studies have shown that safe gait requires 10–15° of ankle dorsiflexion, grating the tibia to advance over the foot. This range of motion (ROM) widely depends on the physiological length of the gastrocnemius muscle (GM), soleus muscles (SM), and Achilles tendon (AT).
Persistent TTB in individuals with ASD is thought to be related to the shortening of the AT,5 which may lead to a decrease of ankle ROM in dorsiflexion. However, it is not clearly understood why some individuals with ASD develop a decrease of ankle ROM in dorsiflexion while others do not.
Anatomically, the distal portion of GM and SM coalesce into the AT, while their proximal origin is different. GM originates from the lateral and medial condyle of the femur while SM just below the knee joint. Therefore, GM and SM length may affect ankle ROM independently.10 Clinically, GM length should be tested by placing the knee in extension and the ankle in dorsiflexion. In contrast, SM length should be tested by placing the knee in flexion and the ankle in dorsiflexion.10 Hence in clinical settings, it is crucial to consider the influence of GM and SM over the ankle joint when assessing ankle ROM on functional activities involving a complete knee extension (e.g., standing or walking).
Recently, Valagussa et al.9 suggested that a possible contributing factor leading to GM and/or SM shortening in children with ASD might be the amount of time they spend in TTB during the day. Clinically, individuals presenting TTB during standing, walking, and running may be more at risk to develop GM and/or SM shortening compared to subjects displaying TTB only during running or are NO-TTB. Muscle-skeletal adaptation to mechanical stimuli physiologically supports such hypothesis.11
While the use of reliable and valid measures of motor function is widely recognized to identify specific motor impairments, a recent systematic review on TTB assessment in individuals with ASD pointed out the lack of quantitative studies and the heterogeneity of evaluation methods.12 Moreover, to the best of our knowledge, an assessment of ankle ROM differentiating between SM and GM complex in individuals with ASD has not been reported. Therefore, the aims of this cross-sectional study were: 1) to evaluate ankle ROM discriminating between SM and GM lengths in a sample of individuals with ASD; 2) to compare muscle length values in NO-TTB and TTB ASD individuals and compare the muscle length values of each of the four subgroups (NO-TTB; TTB Class 1, TTB Class 2, and TTB Class 3).
2. Methods
2.1. Participants
The cross-sectional study included sixty-nine consecutive subjects attending a center for Autism Research and Treatment (Villa Santa Maria Institute, Italy). The inclusion criteria were: 1) an ASD diagnosis according to the DSM 5 criteria1; 2) a diagnosis confirmation based on the Autism Diagnostic Observation Scale - 2nd edition (ADOS–2; 13) assessed by physicians not involved in the study; 3) no previous surgical history affecting ankle ROM; 4) no history of congenital shortening of the Achilles tendon. The exclusion criteria were: 1) comorbidities that would impact gait, i.e., cerebral palsy, upper motor neuron syndromes, tethered spinal cord, spinal muscular atrophy, muscular dystrophy, trisomy 21, Rett Syndrome; 2) impaired of full knee extension ROM.
The severity of autism was established using the data from the ADOS Calibrated Severity Score (CSS).14 Intellectual disability (ID) was evaluated using the four categories proposed in DSM 5: mild, moderate, severe, and profound.1
Parents or guardians of all participants signed an informed consent form to participate in the study. Ethical approval was obtained from the local IRB Insubria's Ethics Committee.
2.2. Procedure
2.2.1. TTB assessment
Using a standardized methodology described in a previous study,9 a trained pediatric physical therapist assessed the presence/absence of TTB during standing, walking and running using direct observation and an interview of the primary caregiver living with the study participant.
2.2.2. Muscle lengths assessment
Clinically, GM and SM lengths can be measured indirectly by testing ankle ROM with the knee extended and knee flexed, respectively.10 The following procedure was performed. Two senior pediatric physical therapists (assessor 1 and assessor 2), blind to TTB assessment, tested GM and SM lengths.
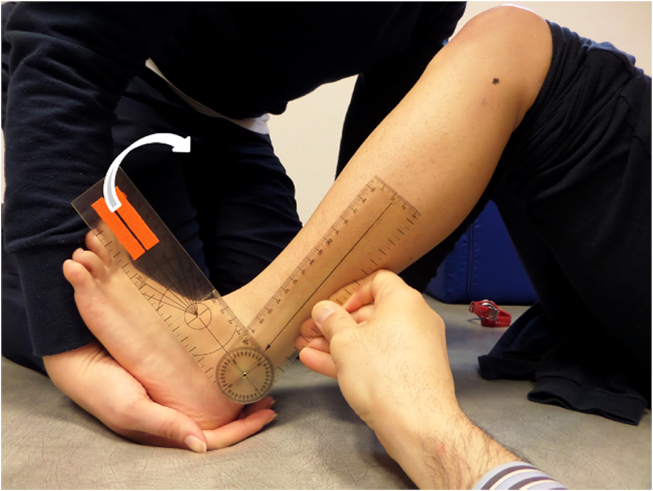
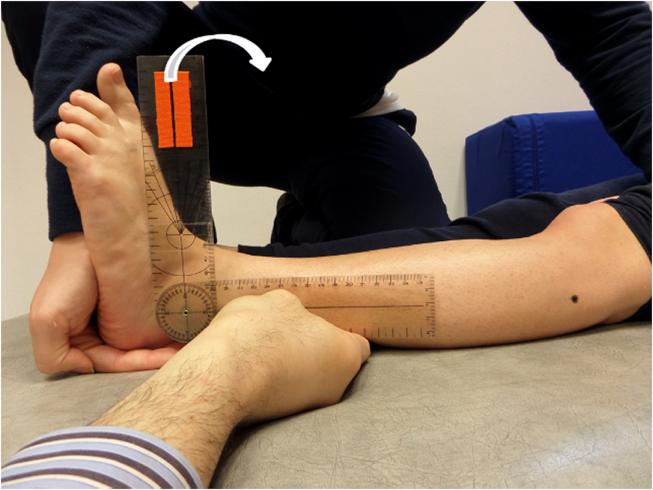
A universal manual goniometer 20 cm long with 2° increments10 was used. The goniometer fulcrum was placed over the lateral malleolus. The proximal arm was aligned with the lateral midline of the fibula using the head of the fibula as a reference. The distal arm was placed parallel to the distal profile of the hindfoot. Then, assessor 1 moved passively and slowly the ankle towards full dorsiflexion and assessor 2 recorded the angles. All measurements were performed with the participant in the supine position. The ankle dorsiflexion ROM was recorded at two knee positions i.e., knee flexed 90° to evaluate SM (Fig. 1) and fully extended to evaluate GM (Fig. 2). Both lower limbs were evaluated, and all assessments were randomized across participants. One measurement for each muscle length per subject was performed.
Fig. 1.
Assessment of soleus muscle length.
Fig. 2.
Assessment of gastrocnemius muscle length.
The same procedure was applied to all participants to reduce measurement error. Muscle lengths were expressed as degrees of ankle dorsiflexion starting from a zero position (i.e., 90° between the leg and rearfoot).
2.3. Statistical analysis
Data are presented as percentage and mean ± standard deviation (SD) for nominal and continuous variables, respectively. Data were checked for normal distribution (i.e., Shapiro-Wilk test p-value > 0.05 and by visual inspection of Q-Q plot). T-test and one-way ANOVA were used if assumptions for data normality and homogeneity were met. Mann–Whitney and Kruskal-Wallis were used for data non-normally distributed. In particular, chi-square (χ2) tests were used to test for differences between ID, TTB and NO-TTB individuals. Muscle lengths between TTB and NO-TTB individuals were examined using T-Test or Mann Whitney Test. To investigate differences in muscle length between NO-TTB and each TTB subgroup, a Kruskal-Wallis test was run for each muscle and each side separately. Dunn pairwise tests were performed for post hoc tests with Bonferroni corrections. The relationship between muscle lengths and lower limb side were analyzed with Spearman rank correlations. Significant level was set at p-value ≤0.05. Data analysis was performed using SPSS version 20.0 for Windows (IBM Corp., Armonk, NY).
3. Results
3.1. Demographic characteristics
Participants' characteristics are shown in Table 1. All individuals were compliant both during the TTB assessment and during the goniometric measurements.
Table 1.
Demographic characteristics and clinical features of ASD sample and subgroups.
| Total (N 69) | NO-TTB (n 46) | TTB (n 23) | TTB Class 1 (n 10) | TTB Class 2 (n 8) | TTB Class 3 (n 5) | |
|---|---|---|---|---|---|---|
| Age - mean (SD) | 14.10 (3.61) | 14.91 (3.69) | 12.42 (2.85) | 11.62 (3.29) | 12.77 (2.39) | 13.45 (2.70) |
| ADOS CSS - mean (SD) | 7.99 (1.71) | 7.74 (1.71) | 8.48 (1.65) | 9.00 (1.41) | 7.63 (1.69) | 8.8 (1.79) |
| Intellectual disability – mild (n° subjects) (%) | 2 (2.9%) | 1 (2.2%) | 1 (4.3%) | 1 (10%) | 0 | 0 |
| Intellectual disability – moderate (n° subjects) (%) | 4 (5.8%) | 3 (6.5%) | 1 (4.3%) | 0 | 0 | 1 (20%) |
| Intellectual disability – severe (n° subjects) (%) | 15 (21.7%) | 13 (28.3%) | 2 (8.7%) | 1 (10%) | 1 (12.5%) | 0 |
| Intellectual disability – profound (n° subjects) (%) | 48 (69.6%) | 29 (63%) | 19 (82.6%) | 8 (80%) | 7 (87.5%) | 4 (80%) |
Note. TTB = tip-toe behavior; SD = standard deviation; ADOS CSS = ADOS Calibrated Severity Score.
Twenty-three out of 69 (33.3%) individuals displayed TTB in three mutually exclusive modalities: (a) ten individuals (14.5%) exhibited it while standing, walking and running (TTB Class 1), (b) eight (11.6%) only during walking and running (TTB Class 2) and (c) five (7.3%) only during running (TTB Class 3).
The overall mean age of the study group was 14.1 ± 3.61 yr without a significant age difference between males and females (t = −0.317, p = 0.752). The mean age of NO-TTB individuals was slightly higher (14.91 ± 3.69 yr) than TTB individuals (12.41 ± 2.85 yr; t = −2.840, p = 0.006). There was a significant difference across the NO-TTB group and each of the three TTB subgroups (F (3,65) = 3,004, p = 0.037). A post hoc analysis revealed a significant difference between NO-TTB and TTB Class 1 subgroup (p = 0.049).
The overall mean ADOS CSS was 7.99 ± 1.71, with no significant differences between NO-TTB and TTB groups (Mann–Whitney U = 389.5, p = 0.069). No differences were found across NO-TTB and each TTB subgroups for ADOS CSS (H(3) = 6.256, p = 0.1).
Severe or profound ID was present in almost all the study sample, i.e., n = 63 (91.3%) subjects. In detail, the following ID classes were found: 28.3% NO-TTB and 8.7% TTB individuals showed severe ID, while 63% and 82.6% of NO-TTB and TTB individuals had a profound ID, respectively. ID displayed no significant difference between TTB and NO-TTB individuals (χ2 (3) = 3,919, p = 0.270).
3.2. Muscle lengths
Table 2 depicts the overall mean length of left GM, right GM, left SM, and right SM of the study group and the statistical analysis between NO-TTB and TTB groups. Mean values for each muscle and each subgroup are shown in Table 3 as well as Kruskal Wallis test values. Respect to left GM, pairwise comparisons using Dunn Test shown a significant difference between NO-TBB vs TTB class 1 (p = 0.002) and TTB class 1 vs TTB class 3 (p = 0.037). For the right GM, Dunn Test depicts a significant difference (p = 0.05) between TTB class 1 vs. TTB class 3. For SM, Dunn Test revealed significant differences between NO-TTB vs. TTB class 1 for both left and right side (p = 0.013 and p = 0.022, respectively) and between TTB class 1 vs. TTB class 3 for right one (p = 0.049). No other post hoc comparisons were significant.
Table 2.
Clinical features of ASD cohort muscle length, and NO-TTB and TTB subgroups.
| Total (N 69) | NO-TTB (n 46) | TTB (n 23) | Mann Whitney Test |
||
|---|---|---|---|---|---|
| Z Value | p | ||||
| Left GM length (degrees) (SD) | 7.54 (6.7) | 9.20 (5.16) | 4.22 (8.2) | −2819 | 0.005* |
| Right GM length (degrees) (SD) | 8.12 (6.78) | 9.02 (5.39) | 6.30 (8.8) | −0.804 | 0.421 |
| Left SM length (degrees) (SD) | 19.3 (8.91) | 21.07 (7.67) | 15.78 (10.29) | −1837 | 0.066 |
| Right SM length (degrees) (SD) | 18.1 (7.98) | 19.33 (6.87) | 15.65 (9.54) | −1371 | 0.170 |
Note. Data are mean (standard deviation) degrees; TTB = tip-toe behavior; SD = standard deviation; GM = gastrocnemius muscle; SM = soleus muscle. * significant p value.
Table 3.
Gastrocnemius and soleus muscle length values of NO-TTB and the three TTB subgroups.
| NO-TTB (n 46) | TTB Class 1 (n 10) | TTB Class 2 (n 8) | TTB Class 3 (n 5) | Kruskal Wallis test |
||||
|---|---|---|---|---|---|---|---|---|
| H Value | p | |||||||
| Left GM length (degrees) (SD) | 9.20 (5.16) | −0.2 (10.16) | 6 (2.73) | 10.2 (4.92) | 15.507 | 0.001* | ||
| Right GM length (degrees) (SD) | 9.02 (5.39) | 1.7 (10.91) | 8.75 (4.68) | 11.6 (4.39) | 8.005 | 0.046* | ||
| Left SM length (degrees) (SD) | 21.07 (7.67) | 10 (9.65) | 18.63 (9.9) | 22.8 (6.3) | 10.769 | 0.013* | ||
| Right SM length (degrees) (SD) | 19.33 (6.87) | 9.7 (8.85) | 19.25 (8.26) | 21.8 (6.61) | 10.315 | 0.016* | ||
Note. Data are mean (standard deviation) degrees; GM = gastrocnemius muscle; SM = soleus muscle. * significant p value. For pairwise comparison see results section.
Finally, significant correlations were found between GM and SM muscle lengths for the same side (left side: r = 0.663, p = 0.000; right side: r = 0.735, p = 0.000). A significant correlation was detected between right and left GM length values (r = 0.652, p = 0.000), and between right and left SM length values (r = 0.763, p = 0.000).
4. Discussion
Our study aimed to compare both left and right SM and GM length values of TTB and NO-TTB groups and each of the four subgroups (NO-TTB, TTB Class 1, TTB Class 2, and TTB Class 3). To our knowledge, this is the first study that clinically assessed both GM and SM muscle lengths in individuals with ASD exhibiting TTB.
4.1. Methodological consideration
A variety of positions are used to measure dorsiflexion ROM, including sitting with the knee flexed, supine with the knee either flexed or extended, prone with the knee either flexed or extended, and standing with the knee either flexed or extended.10 Dorsiflexion measurements taken in weight-bearing positions are usually higher than measurements taken in non-weight-bearing positions.10 While previous research assessed ankle dorsiflexion ROM in individuals with ASD, a detailed assessment position description was not clearly described.15 Shetreat-Klein et al.16 assessed ankle dorsiflexion ROM mobility in a seated position with the knee flexed: using this procedure, they assessed the SM length, but not the GM length. We decided to use the supine position since it is usually a comfortable position for the subjects. Moreover, in such position, the subject can see what is happening during all the tests, and the clinician can remain in visual contact with the subject and verify if he or she remains calm.
A wide range of instruments is used to measure muscle length and joint ROM (e.g., universal goniometers, inclinometers, and specialized measurement equipment and techniques). Of the studies aiming to assess ankle mobility in subjects with ASD, only Shetreat-Klein et al.16 used an instrumental approach (universal goniometer), whereas Ming et al.15 Barrow et al.6 and Accardo et al.17 used clinical judgment during physical examination (i.e., reduced/non-reduced degree of ankle dorsiflexion or heel cords that did not reduce past neutral or greater than 90 dorsiflexion). We used a universal goniometer since it is a standard instrument that can be applied in clinical settings. Reliability studies for measuring ROM and muscle length at the ankle using universal goniometer have been conducted on both healthy and impaired populations. Martin et al.18 reviewed the existing literature on the goniometric measurement of ankle dorsiflexion and plantarflexion ROM. For dorsiflexion, evidence suggests that ROM assessment displayed good to excellent intra-tester reliability and some for inter-tester reliability. Moreover, Martin et al.18 outlined that training sessions before measurement appeared to have a positive effect on intra-rater reliability. Notably, in a study of 27 healthy young adults, McPoil et al.19 examined the intra-tester reliability of goniometric measurements of ankle dorsiflexion ROM with the knee flexed and extended. Intra-tester reliability was excellent, with ICC values higher than 0.95 for all four of these motions. Kilgour et al.20 determined intra-tester reliability of passive ankle dorsiflexion with the knee flexed and extended in 25 children aged 6–17 years with spastic diplegic cerebral palsy and in 25 healthy age and sex-matched controls. Intra-tester reliability was excellent, with ICC values above 0.95. They also found that averaging the two measurements did not improve intersession reliability compared with just one measurement. Pandya et al.21 assessed the intra-tester and inter-tester reliability of goniometric measurements of ankle dorsiflexion carried out by five physiotherapists in 150 children (ages 1–20 years) with Duchenne muscular dystrophy. The intra-tester and inter-tester reliability had ICC values of 0.90 and 0.73, respectively. Macedo et al.22 investigated the standard error of measurement value for universal goniometer dorsiflexion assessment reporting 2.5°. To our knowledge, there are no studies that have investigated the validity of goniometric measurements of ankle and foot ROM values by comparing these measurements with a gold standard i.e., radiographs.
4.2. Muscle lengths in NO-TTB individuals
In the NO-TTB group, both GM and SM mean length values were in line with the normative data. Several studies have directly compared dorsiflexion ROM measurements taken with the knee flexed and with the knee extended in the same subject. McPoil et al.19 in a study of 27 healthy young adults, using a universal goniometer, found the mean dorsiflexion ROM (16.2°) with the knee flexed to be about 6° greater than the mean with the knee extended (10.1°). Baumbach et al.23 assessed ankle dorsiflexion values with the knee extended and flexed in non-weight bearing and weight-bearing in a sample of 64 young (i.e., 18–35 years) asymptomatic subjects. They reported mean ankle dorsiflexion values with the knee extended for the left/right limb to be 22.7° (95% CI 21.2°–24.3°] and 23.4° (95% CI 21.7°–25.1°) in non-weight bearing; ankle dorsiflexion with knee flexed resulted in an approximate increase of 10°. Alanen et al.,24 in a study of 7 to 14-year old, found that dorsiflexion measurements taken with the knee flexed to 90° were 10–19° greater than measurements taken with the knee extended.
Newborns and infants have greater dorsiflexion and smaller plantarflexion ROM than older children and adults. Soucie et al.25 reported a decrease in dorsiflexion for both males and females, with the most significant age-related differences being between children (aged 2–8 years) and all other groups. They found a mean ankle dorsiflexion range of 22.8° (5.6 SD) (male) and 24.8° (7.2 SD) (female) in subjects aged 2–8 years, and 16.3° (5.1 SD) (male) and 17.3° (6.4 SD) (female) in subjects aged 9–19 years.
4.3. Relation between muscle lengths in TTB and NO-TTB subgroups
When comparing the TTB and NO-TTB subgroups, both GM and SM mean length values were found to be not significantly different, except for the left GM length (p = 0.005). The non-significant differences might be due to the cohort of TTB group, including subjects that demonstrate TTB only when running.
We found a significant decrease of left GM, left SM, and right SM lengths in TTB Class1 subgroup (i.e., individuals that show TTB during standing, walking and running) when compared with the NO-TTB individuals. Another significant length decrease was found between TTB Class 1 vs. TTB Class 3 (i.e., subjects that show TTB only during running) in relation to left GM, right GM, and left SM lengths. Thus, the results of this study seem to suggest the clinical relevance of a TTB sub-grouping since not all the TTB individuals have decreased ankle ROM. A prospective cohort study would be useful to confirm these findings.
No differences were found between TTB Class 3 muscle length and NO-TTB group. This result supports the less severity of TTB Class 3, Indeed subjects in this subgroup exhibit TW only during running and not during standing and walking. All these findings are supported by studies about muscle-skeletal adaptation to mechanical stimuli (reviewed in Wisdom et al.11).
4.4. Limitations
The main limitation of our study is the generalizability of our findings: our sample can be considered representative of individuals with severe ASD and profound ID, limiting the applicability of the results to a broader spectrum severity. It would be interesting and to repeat the study with a less severe ASD sample. Further confirmation of our findings could be obtained by collecting and comparing quantitative data of TTB with the GM and SM length values using cross-sectional and prospective study designs.
5. Conclusion
In conclusion, this is the first study that systematically assessed clinically both GM and SM muscle lengths in NO-TTB and TTB ASD individuals comparing the results across different TTB clinical presentation patterns. Findings suggest a relationship between the presence and severity of TTB and the amount of GM and SM muscle lengths expressed as ankle ROM. Differences between the NO-TTB and the TTB Class 1 subgroup and also between the TTB Class 1 and TTB Class 3 subgroups were found. This support the clinical relevance of a TTB sub-grouping in individuals with ASD. Individuals showing TTB during standing, walking and running (TTB class 1) should be closely monitored using standardized assessment protocols. Moreover, our findings highlight the importance of assessing both SM and GM when aiming to evaluate their influence on ankle ROM in dorsiflexion in individuals with ASD in both clinical and research settings.
Authors contributions
G.V., V.B., and E.G. conceived and designed the study. L.T., V.B., and G.V. collected the data. E.G., M.Y., and D.P. designed and reviewed statistical analysis. G.V., V.B., and L.T. interpreted the results. L.T., V.B., G.V., E.G., M.Y., and D.P. revised it critically for important intellectual content. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data that support the findings of this study are available from the corresponding author [GV] upon reasonable request.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
The authors want to thank Mrs. Dianne Andreotti Jackson for her supervision of the English language.
References
- 1.American Psychiatric A. American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- 2.Harris S.R. Early motor delays as diagnostic clues in autism spectrum disorder. Eur J Pediatr. 2017;176:1259–1262. doi: 10.1007/s00431-017-2951-7. [DOI] [PubMed] [Google Scholar]
- 3.Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- 4.Provost B., Lopez B.R., Heimerl S. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. J Autism Dev Disord. 2007;37:321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- 5.Accardo P.J., Monasterio E., Oswald D. Toe walking in autism. In: Patel V.B., Preedy V.R., Martin C.R., editors. Comprehensive Guide to Autism. Springer Reference; New York: 2014. pp. 519–532. [Google Scholar]
- 6.Barrow W.J., Jaworski M., Accardo P.J. Persistent toe walking in autism. J Child Neurol. 2011;26:619–621. doi: 10.1177/0883073810385344. [DOI] [PubMed] [Google Scholar]
- 7.Eggleston J.D., Harry J.R., Dufek J.S. Lower extremity joint stiffness during walking distinguishes children with and without autism. Hum Mov Sci. 2018;62:25–33. doi: 10.1016/j.humov.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyden J., Fung L., Frick S. Autism and toe-walking: are they related? Trends and treatment patterns between 2005 and 2016. J Child Orthop. 2019;13:340–345. doi: 10.1302/1863-2548.13.180160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valagussa G., Trentin L., Balatti V., Grossi E. Assessment of presentation patterns, clinical severity, and sensorial mechanism of tip-toe behavior in severe ASD subjects with intellectual disability: a cohort observational study. Autism Res. 2017;10:1547–1557. doi: 10.1002/aur.1796. [DOI] [PubMed] [Google Scholar]
- 10.Norkin C.C., White D.J. F.A. Davis Company. fifth ed. 2016. Measurement of joint motion : a guide to goniometry. Philadelphia. [Google Scholar]
- 11.Wisdom K.M., Delp S.L., Kuhl E. Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. Biomech Model Mechanobiol. 2015;14:195–215. doi: 10.1007/s10237-014-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valagussa G., Trentin L., Signori A., Grossi E. Toe walking assessment in autism spectrum disorder subjects: a systematic review. Autism Res. 2018;11:1404–1415. doi: 10.1002/aur.2009. [DOI] [PubMed] [Google Scholar]
- 13.Lord C., Luyster R., Gotham K., Guthrie W. second ed. Western Psychological Services; Torrance, CA: 2012. Autism Diagnostic Observation Schedule. (ADOS-2) Manual (Part II): Toddler Module. [Google Scholar]
- 14.Gotham K., Pickles A., Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ming X., Brimacombe M., Wagner G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Shetreat-Klein M., Shinnar S., Rapin I. Abnormalities of joint mobility and gait in children with autism spectrum disorders. Brain Dev. 2014;36:91–96. doi: 10.1016/j.braindev.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Accardo P.J., Barrow W. Toe walking in autism: further observations. J Child Neurol. 2015;30:606–609. doi: 10.1177/0883073814521298. [DOI] [PubMed] [Google Scholar]
- 18.Martin R.L., McPoil T.G. Reliability of ankle goniometric measurements: a literature review. J Am Podiatr Med Assoc. 2005;95:564–572. doi: 10.7547/0950564. [DOI] [PubMed] [Google Scholar]
- 19.McPoil T.G., Cornwall M.W. The relationship between static lower extremity measurements and rearfoot motion during walking. J Orthop Sports Phys Ther. 1996;24:309–314. doi: 10.2519/jospt.1996.24.5.309. [DOI] [PubMed] [Google Scholar]
- 20.Kilgour G., McNair P., Stott N.S. Intrarater reliability of lower limb sagittal range-of-motion measures in children with spastic diplegia. Dev Med Child Neurol. 2003;45:391–399. doi: 10.1017/s0012162203000744. [DOI] [PubMed] [Google Scholar]
- 21.Pandya S., Florence J.M., King W.M., Robison J.D., Oxman M., Province M.A. Reliability of goniometric measurements in patients with Duchenne muscular dystrophy. Phys Ther. 1985;65:1339–1342. doi: 10.1093/ptj/65.9.1339. [DOI] [PubMed] [Google Scholar]
- 22.Macedo L.G., Magee D.J. Effects of age on passive range of motion of selected peripheral joints in healthy adult females. Physiother Theory Pract. 2009;25:145–164. doi: 10.1080/09593980802686870. [DOI] [PubMed] [Google Scholar]
- 23.Baumbach S.F., Braunstein M., Seeliger F., Borgmann L., Bocker W., Polzer H. Ankle dorsiflexion: what is normal? Development of a decision pathway for diagnosing impaired ankle dorsiflexion and M. gastrocnemius tightness. Arch Orthop Trauma Surg. 2016;136:1203–1211. doi: 10.1007/s00402-016-2513-x. [DOI] [PubMed] [Google Scholar]
- 24.Alanen J.T., Levola J.V., Helenius H.Y., Kvist M.H. Ankle joint complex mobility of children 7 to 14 years old. J Pediatr Orthop. 2001;21:731–737. [PubMed] [Google Scholar]
- 25.Soucie J.M., Wang C., Forsyth A. Range of motion measurements: reference values and a database for comparison studies. Haemophilia. 2011;17:500–507. doi: 10.1111/j.1365-2516.2010.02399.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [GV] upon reasonable request.