Abstract
Cocaine abuse continues to be a serious health problem worldwide. Despite intense research there is currently no FDA-approved medication to treat cocaine use disorder. The recent search has been focused on agents targeting primarily the dopamine system, while limited success has been achieved at the clinical level. Cannabidiol (CBD) is a U.S. FDA-approved cannabinoid for the treatment of epilepsy and recently was reported to have therapeutic potential for other disorders. Here we systemically evaluated its potential utility for the treatment of cocaine use disorder and explored the underlying receptor mechanisms in experimental animals. Systemic administration (10–40 mg/kg) of CBD dose-dependently inhibited cocaine self-administration, shifted a cocaine dose-response curve downward, and lowered break-points for cocaine self-administration under a progressive-ratio schedule of reinforcement. CBD inhibited cocaine self-administration maintained by low, but not high, doses of cocaine. In addition, CBD (3–20 mg/kg) dose-dependently attenuated cocaine-enhanced brain-stimulation reward (BSR) in rats. Strikingly, this reduction in both cocaine self-administration and BSR was blocked by AM630 (a cannabinoid CB2 receptor antagonist), WAY100135 (a 5-HT1A receptor antagonist), or capsazepine (a TRPV1 channel blocker), but not by AM251 (a CB1 receptor antagonist), CID16020046 (a GPR55 antagonist), or naloxone (an opioid receptor antagonist), suggesting the involvement of CB2, 5-HT1A, and TRPV1 receptors in CBD action. In vivo microdialysis indicated that pretreatment with CBD (10–20 mg/kg) attenuated cocaine-induced increases in extracellular dopamine (DA) in the nucleus accumbens, while CBD alone failed to alter extracellular DA. These findings suggest that CBD may have certain therapeutic utility by blunting the acute rewarding effects of cocaine via a DA-dependent mechanism.
Keywords: Cannabidiol, Cocaine, Dopamine, Self-administration, Brain-stimulation reward, CB1, CB2, 5-HT1A, TRPV1
1. Introduction
Cocaine is a commonly used psychostimulant worldwide. Despite several decades of intense research, there is currently no U.S. Food and Drug Administration (FDA)-approved medication to treat cocaine use disorder. Given that the rewarding effects of cocaine are mediated mainly by blockade of dopamine (DA) transporters (DAT), most medication development research has focused on DAT or DA receptors (Galaj et al., 2018; Newman et al., 2012). However, limited success has been achieved at the clinical level.
Recent studies have shown that endocannabinoids modulate the mesolimbic DA system and cocaine-related behaviors (Arnold, 2005; Manzanares et al., 2018; Wiskerke et al., 2008; Xi et al., 2011), and therefore, the endocannabinoid system, particularly CB1 receptors, have become new targets for the treatment of cocaine use disorder (Le Foll et al., 2009; Sloan et al., 2017). CB1 receptor (CB1R) antagonists were reported to be promising therapeutics since such compounds can attenuate cocaine reward, as assessed in conditioned place preference (CPP), intracranial self-stimulation and self-administration paradigms (Xi et al., 2008; Yu et al., 2011). Further, these compounds can inhibit reinstatement of cocaine-seeking behavior (De Vries et al., 2001; Ward et al., 2009; Xi et al., 2006a). However, clinical studies with the selective CB1R antagonist, rimonabant, have been terminated worldwide due to significant adverse effects such as depression and suicidal tendencies (Gaal et al., 2005; Le Foll et al., 2009). Therefore, many scientists have shifted their research interests to other targets of the endocannabinoid system such as CB2 receptor (Jordan and Xi, 2019; Manzanares et al., 2018), fatty acid amide hydroxylase (FAAH, the anandamide degradation enzyme) (Chauvet et al., 2014; Deutsch, 2016), monoacylglycerol lipase (MAGL, the 2-arachydonoylglycerol degradation enzyme) (Gil-Ordóñez et al., 2018), or non-psychoactive phytocannabinoids (Mandolini et al., 2018; Sloan et al., 2017).
Cannabidiol (CBD) is a major constituent in cannabis devoid of psychotomimetic effects. Recent research indicates that CBD has nanomolar allosteric binding affinity to CB1R and CB2R (acting as negative allosteric modulator) (Martínez-Pinilla et al., 2017; Navarro et al., 2018; Pertwee, 2008; Tham et al., 2019; Thomas et al., 2007). In addition, in vitro binding assays suggest that CBD can act as a GPR55 antagonist (Ryberg et al., 2007), transient receptor potential vallinoid 1 (TRPV1) agonist (Bisogno et al., 2001), partial agonist to serotonin 5-HT1A receptors (Russo et al., 2005), and allosteric modulator of mu opioid receptors (MORs) (Kathmann et al., 2006). CBD is the first cannabinoid approved by the U.S. FDA for the treatment of epilepsy. In addition, CBD has been shown to have therapeutic utility for many other medical conditions such as neuropathic pain, anxiety, schizophrenia, and substance use disorders (Crippa et al., 2018; Mandolini et al., 2018; Russo, 2018; Sanmartin and Detyniecki, 2018). Unlike Δ9-tetrahydrocannabinol, the major psychoactive component in cannabis, CBD does not produce psychotomimetic effects (Martin-Santos et al., 2012) nor does it have rewarding or abuse potential (Schoedel et al., 2018), making it an attractive candidate for future clinical use. Preclinical research has suggested that a single injection or repeated administration of CBD can 1) reduce the rewarding effects of cocaine, methamphetamine or alcohol, 2) decrease drug intake (Hay et al., 2018; Luján et al., 2018; Viudez-Martínez et al., 2018a) and 3) attenuate relapse to drug-seeking (Gonzalez-Cuevas et al., 2018; Hay et al., 2018; Ren et al., 2009; Viudez-Martínez et al., 2018a). However, it was also reported that CBD has no effect on cocaine or heroin self-administration and cue-induced reinstatement of cocaine-seeking behavior (Mahmud et al., 2017; Ren et al., 2009).
In the present study, we systemically evaluated pharmacological effects of CBD on cocaine reward as assessed by multiple models of cocaine self-administration with a wide range of cocaine doses and CBD, by highly sensitive electrical brain-stimulation reward (BSR), and in vivo microdialysis with HPLC to detect brain DA in response to cocaine or CBD. We also explored the possible receptor mechanisms underlying CBD action in the intravenous self-administration and BSR paradigms by targeting CB1R, CB2R, GPR55, TRPV1, 5-HT1A, and MOR based on their binding profiles in vitro cell lines.
2. Materials and methods
2.1. Animals
A total of 75 male Long–Evans rats (Charles River Laboratories, Frederick, MD) (initially weighing 250–300 g) were used in our experiments. During experimentation animals were housed individually in a climate-controlled animal colony room on a reversed 12-h light–-dark cycle (lights on at 7 a.m.) with free access to food and water. All experiments were conducted during the animals’ active period (dark cycle). The housing conditions and care of the animals were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). The protocols (16-BNRB-48) used in the present experiments were approved by the National Institute on Drug Abuse Animal Care and Use Committee.
2.2. Drugs
Cocaine hydrochloride (provided by NIDA Pharmacy) was dissolved in 0.9% saline. Cannabidiol (provided by NIDA Research Resources Drug Supply Program) was dissolved in 5% cremophor. AM251 (CB1R antagonist; 3 mg/kg), AM630 (CB2R antagonist; 3 mg/kg), CID16020046 (GPR55 antagonist; 3 and 5 mg/kg), capsazepine (TRPV1 antagonist, 3 and 5 mg/kg) were purchased from Tocris and dissolved in 5% cremophor. Naloxone (MOR antagonist; 3 mg/kg) and WAY100135 (5-HT1A antagonist; 3 mg/kg) were dissolved in 0.9% saline. All compounds were administered intraperitoneally (i.p.) in the volume of 1 ml/kg. The doses of CBD and antagonists and the intraperitoneal route of administration are based on previous reports (Bi et al., 2019; Hay et al., 2018; Ren et al., 2009; Spiller et al., 2019), our pilot studies, and the pharmacokinetics of CBD, assessed after i.p. administration.
2.3. Surgery
Briefly, for the brain-stimulation reward experiment, under sodium pentobarbital anesthesia (65 mg/kg i.p.) bilateral monopolar stainless-steel stimulation electrodes were implanted so as their tip would be situated in the medial forebrain bundle at the level of the lateral hypothalamus (AP −2.56, ML ±1.9, and DV −8.6) as described in (Xi et al., 2008). For self-administration experiments, as previously described in (Xi et al., 2008), a microrenathane catheter was implanted in the right jugular vein and its free end fed subcutaneously along the scapula was connected to a 22-gauge stainless connector that was mounted to the rat’s skull using stainless steel screws and dental acrylic. For the microdialysis experiment, as described previously (Xi et al., 2006a), two guide cannulae were surgically implanted into the rat NAc (AP +1.6, ML ±2.0, DV −5.0 mm, at 6° angle away from the midline) to collect local extracellular fluid samples.
2.4. Apparatus
Cocaine self-administration sessions were conducted in standard operant conditioning chambers (Med-Associates, VT, US), each housed in a sound-attenuating box. Each chamber was equipped with two retractable levers, a white light above the active lever and a drug line connected to a syringe pump (Razel, 3.33 rpm). Operant chambers for brain-stimulation reward were equipped with one retractable lever and a stimulation line composed of electrodes connected through a swivel commutator (PlasticsOne), and insulated wire to a constant current stimulator (Med-Associates, VT, US).
2.5. Cocaine self-administration
2.5.1. Cocaine self-administration under a FR1 schedule of reinforcement
After 5–7 days of recovery from surgery, we first assessed whether CBD can reduce cocaine reward in rats self-administering cocaine under a fixed ratio 1 (FR1) schedule of reinforcement. To facilitate the acquisition of self-administration, animals (n = 24) were initially trained to self-administer cocaine (1.0 mg/kg/infusion) under a FR1 reinforcement schedule for one week, and then they were switched to self-administer a lower dose of cocaine (0.5 mg/kg/infusion). The 0.5 mg/kg/infusion is a routine dose that we use in our laboratory since it maintains reliable and evenly-distributed cocaine self-administration during daily 3 h sessions (Xi et al., 2006b). Responding on the active lever activated the syringe pump for 4.65 s causing the delivery of cocaine and the illumination of the light above the active lever. Responses on the inactive lever were counted but had no consequences. During the infusion period, additional responses on the active lever were recorded, but did not lead to additional infusions. Once animals demonstrated a pattern of stable responding, defined as less than 20% variability in daily cocaine intake across three consecutive sessions, and an active/inactive lever pressing ratio exceeding 2:1, they were tested with different doses of CBD (Vehicle, n = 8; 20 mg/kg, n = 8; 40 mg/kg, n = 10).
2.5.2. Multiple-dose cocaine self-administration
A different group of rats (n = 12) was used to evaluate the effects of CBD on self-administration maintained by a full dose range of cocaine (0.031, 0.0625, 0.125, 0.25, 0.5 and 1 mg/kg/infusion) under a FR2 schedule of reinforcement. A FR2 reinforcement schedule was chosen since self-administration for each cocaine dose lasted only 20 min, and the total numbers of infusions and active lever responses for each dose cocaine could be too low to detect drug-induced changes in cocaine self-administration. Elevation of the FR level from FR1 to FR2 would increase the number of active lever responding for cocaine reward, and therefore, increases sensitivity to measure drug-induced changes in self-administration behavior. Within each session each rat self-administered multiple doses of cocaine in a daily 3-h session in an ascending dose sequence (20 min at each dose with 10 min time intervals between different doses of cocaine) (Keck et al., 2013). Cocaine concentration was adjusted by changes in the infusion volumes and durations of pump activation. After stable cocaine self-administration was achieved for at least 2–3 days, animals received a systemic injection of CBD (0, 10 or 20 mg/kg, i.p.) 30 min prior to the test session. After each drug testing, the animals were allowed to self-administer cocaine under the same conditions until the behavior was re-stabilized for at least 2–3 days. Each animal was tested 3 times with time intervals of 5–7 days.
2.5.3. Cocaine self-administration under a PR schedule of reinforcement
We then evaluated the effects of CBD on the rewarding effects of cocaine and/or motivation for cocaine in additional groups of rats. Rats (n = 24) were initially trained under a FR1 schedule of reinforcement as outlined above. Rats with stable responding were then allowed to self-administer cocaine (0.5 mg/kg/infusion) under a progressive ratio (PR) schedule of reinforcement (Richardson and Roberts, 1996). Given that CBD failed to significantly alter PR cocaine self-administration maintained by 0.5 mg/kg/infusion, we then observed the effects of CBD on PR responding maintained by lower doses of cocaine (0.25 mg/kg/infusion). The number of lever presses required to receive a single infusion of cocaine was increased according to the following schedule: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50 and so on. Under this PR schedule the animals eventually stopped responding and reached a break-point (BP) with final ratio. BP was operationally defined as a number of lever presses for the last cocaine infusion before a 1-h period during which no cocaine reward was obtained. The sessions lasted between 1 and 4 h and ended once the BP was reached. Once responding stabilized with day-to-day variability in total infusions within 1 or 2 ratio increments for 3 consecutive days, animals were divided into 3 CBD dose groups (n = 6–10 per group). Thirty min prior to a test session, rats were injected with one of the CBD doses (Vehicle, n = 8; 20 mg/kg, n = 7; 40 mg/kg, n = 6) and later allowed to press the lever for cocaine under the PR schedule of reinforcement. The effects of CBD on BP for cocaine self-administration were evaluated. We then repeated the PR experiment with a lower dose of cocaine (0.25 mg/kg/inf) where the animals were re-stabilized and tested with CBD (Vehicle, n = 7; 20 mg/kg, n = 6; 40 mg/kg, n = 10).
2.5.4. Cocaine self-administration in the presence of CBD and a receptor antagonist
In this experiment, we explored the receptor mechanisms by which CBD inhibits cocaine self-administration. Here rats (n = 36) self-administered multiple doses of cocaine under the CBD treatment in the presence or absence of CB1, CB2R, GPR55, TRPV1, 5-HT1A or MOR antagonism. Different groups of rats were tested with different compounds, but each rat was exposed to all doses of cocaine within a session. Prior to the test session rats were pre-treated (i.p) with one of the compounds: AM251 (a CB1R antagonist; 3 mg/kg), AM630 (a CB2R antagonist; 3 mg/kg), CID16020046 (GPR55 antagonist; 3 or 5 mg/kg), capsazepine (a TRPV1 antagonist; 3 or 5 mg/kg), naloxone (MOR antagonist; 3 mg/kg) or WAY100135 (a 5-HT1A antagonist; 3 mg/kg). Thirty minutes later rats were injected with 20 mg/kg of CBD and later allowed to self-administer different doses of cocaine. Six groups of animals (n = 6–9 per group; see more details in Figure legend 2) were used to study the receptor mechanisms stated above, and each animal was tested for 3–5 times in the presence or absence of CBD and/or a receptor antagonist.
Fig. 2.
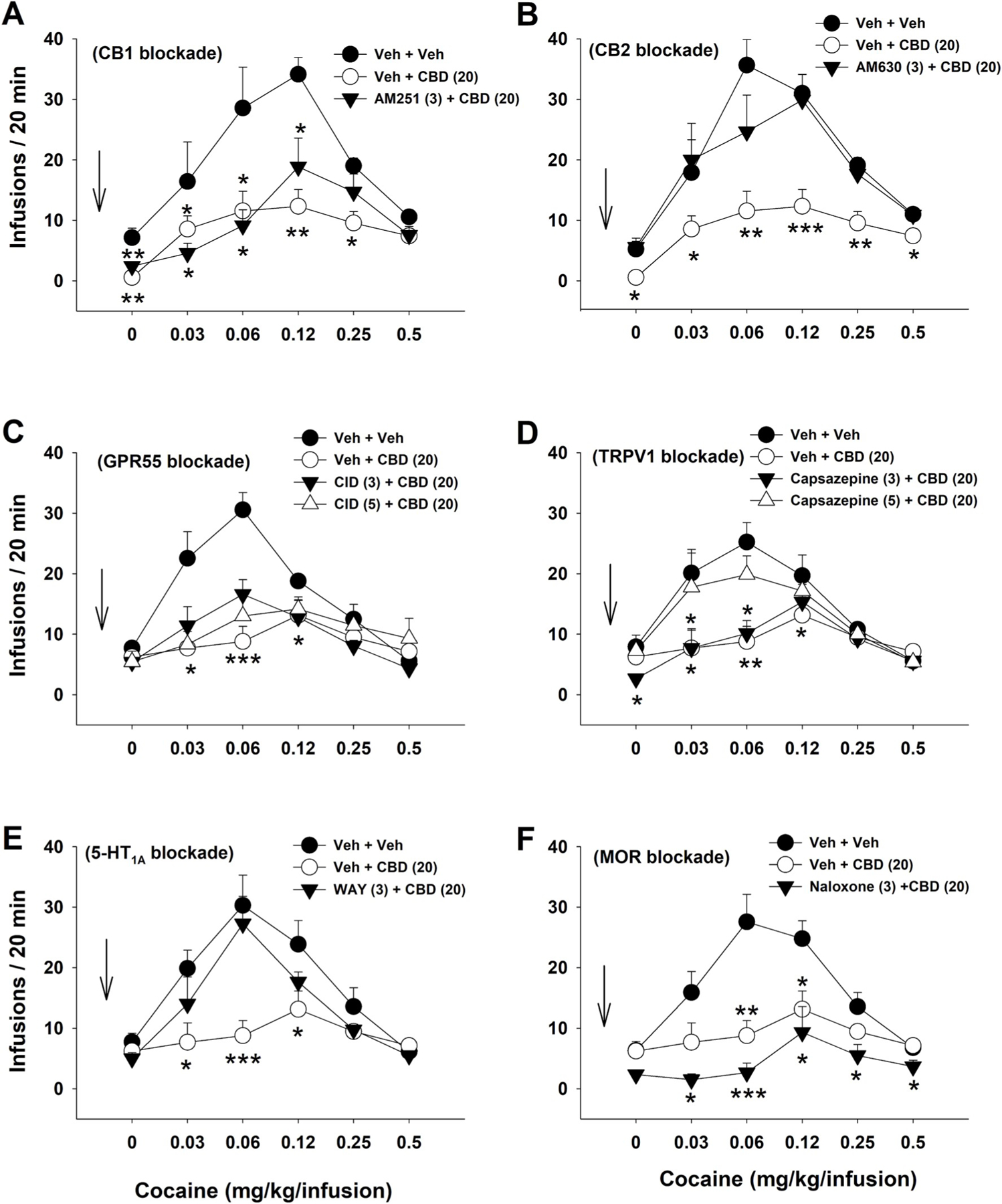
The effects of CBD (20 mg/kg, i.p.) on multiple doses of cocaine self-administration in the presence or absence of a receptor antagonist. A: Pretreatment with AM251 (a CB1 antagonist, 3 mg/kg) failed to block CBD-induced reductions in cocaine self-administration. Group sizes: (Veh + Veh, n = 7; Veh + CBD, n = 9; AM251 + CBD, n = 7). B: Pretreatment with AM630 (a CB2 antagonist, 3 mg/kg) blocked CBD-induced reductions in cocaine self-administration (Veh + Veh, n = 9; Veh + CBD, n = 9; AM630 + CBD, n = 9). C: Pretreatment with CID16020046 (a GPR55 antagonist, 3 or 5 mg/kg) failed to block CBD-induced reduction in cocaine self-administration. n = 9 in each dose group. D: Pretreatment with capsezapine (a TRPV1 antagonist, 3 or 5 mg/kg) dose-dependently blocked CBD-induced reductions in cocaine self-administration. n = 9 in each dose group. E: Pretreatment with WAY100135 (a 5-HT1A antagonist, 3 mg/kg) blocked CBD-induced reductions in cocaine self-administration (Veh + Veh, n = 7; Veh + CBD, n = 9; WAY100135 + CBD, n = 8). F: Pretreatment with naloxone (a MOR antagonist, 3 mg/kg) did not block CBD-induced reductions in cocaine self-administration (Veh + Veh, n = 9; Veh + CBD, n = 9; Naloxone + CBD, n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 as compared to (Veh + Veh) control group.
2.6. Brain-stimulation reward
To confirm the findings observed in the above self-administration experiments, we also assessed the effects of CBD on intracranial electrical brain-stimulation reward (BSR) itself or on cocaine-enhanced BSR. The BSR procedures are the same as reported previously (Spiller et al., 2019). Briefly, active lever pressing for electrical brain stimulation was shaped by the delivery of a 500-ms train of 0.1-ms rectangular cathodal pulses (Hz) and the illumination of a light above the lever. Responses made during the 500-ms stimulation period were recorded but earned no additional stimulation. Current was initially adjusted for each individual rat and held constant throughout the experiment at the lowest intensity that maintained at least 45–60 responses/30 s. After optimizing current intensity, the stimulation frequency, ranging from 141 to 25 Hz, was decreased in a series of 16 discrete 0.05 log steps. At each pulse frequency, there were two 30-s trials, each followed by lever retraction for 5 s. Response rate for each frequency was defined as the mean number of lever responses during two 30-s trials. The BSR threshold (θ0) was defined as the minimum frequency at which an animal responded for stimulation, calculated using the Gompertz sigmoidal model (Coulombe and Miliaressis, 1987). M50 was defined as stimulation frequency for half maximal lever responding (i.e., 50% Ymax). Ymax was defined as the maximal rate of lever responding. The testing phase began once stable BSR responding was achieved (<10% variation in θ0 over 5 consecutive days). On the test day animals received an intraperitoneal (i.p.) injection of CBD alone (0, 3, 10 or 20 mg/kg) 30 min prior to testing or 30 min prior to cocaine (2 mg/kg, i.p.). Fifteen min after cocaine injection animals were allowed to lever-press for brain stimulation reward. After each test, animals received additional 5–7 days of self-stimulation sessions until a new baseline θ0 was established, followed by the next drug treatment.
To study the possible receptor mechanisms underlying CBD action in BSR, an additional group (n = 12) was used to determine whether pretreatment with any of the receptor antagonists stated above blocks CBD action in cocaine-enhanced BSR. The dose(s) of each receptor antagonist (AM251, n = 7; AM630, n = 9; capsazepine, n = 7; naloxone, n = 7; CID16020046, n = 7; WAY100135, n = 7) were chosen based on our pilot studies or literature reports. The doses of CBD (3, 10 and 20 mg/kg) and cocaine (2 mg/kg) were chosen based on their pharmacological actions observed in this experiment. After each test, rats were later allowed to press the lever for brain stimulation until the baseline was re-established for the next test. The time intervals between different drug combination tests were 3–5 days. The mean θ0 and Ymax values (% baseline) were used to evaluate drug effects.
2.7. In vivo microdialysis with HPLC assays
We then further observed the effects of CBD alone on extracellular DA levels or on cocaine-enhanced extracellular DA in the NAc in rats (n = 27). The procedures for brain microdialysis were the same as we reported previously (Xi et al., 2006a). Briefly, unilateral microdialysis probes were inserted into the left or right NAc 12 h before the start of microdialysis. Microdialysate samples were collected every 20 min during baseline (total: 120 min) and after each rat was injected with CBD alone (Veh, n = 12) or CBD pretreatment (10 mg/kg, n = 8; 20 mg/kg, n = 10, i.p.) 30 min prior to cocaine (10 mg/kg (Veh + Cocaine, n = 6; 10 mg CBD + Cocaine, n = 11; 20 mg CBD + Cocaine, n = 7). Three days later microdialysis was performed again by collecting samples from the controlateral side of the NAc in rats treated with different doses of CBD or (CBD + cocaine). After collection, samples were frozen at −80 °C. Dialysate DA was measured with the ESA electrochemical detection system (ESA, Chelmsford, MA), as described previously (Xi et al. 2006a,b). After completion of the BSR and microdialysis experiments, placements of guide cannula were histologically verified.
2.8. Data analysis
All data were expressed as means ± S.E.M. One-way or two-way Analyses of Variance (ANOVA) were used for data analysis based on different experimental designs or data sets. Significant interactions and significant effects were followed by post-hoc Dunnett’s test or in cases of group comparisons involving more than 3 groups we used Bonferroni tests with correction for multiple group comparisons.
3. Results
3.1. CBD reduced self-administration maintained by low doses of cocaine
A previous study indicated that acute administration of lower doses of CBD (5, 10 mg/kg, i.p.) failed to alter cocaine self-administration in rats (Mahmud et al., 2017). Therefore, in this study, we examined whether higher doses of CBD are required to inhibit cocaine self-administration. Fig. 1A shows that systemic administration of CBD (20, 40 mg/kg, i.p.) also failed to alter cocaine self-administration under a FR1 schedule of reinforcement. A one-way ANOVA revealed no significant CBD effect (F2,24 = 1.104; p = 0.349). CBD treatment had no significant effect on active and inactive lever presses (see Fig. S1 in Supplementary Materials). To determine whether such ineffectiveness of CBD could be due to a higher unit dose of cocaine (0.5 mg/kg/infusion) used in self-administration, we then examined the effects of CBD on a full dose range (1, 0.5, 0.25, 0.125, 0.06, 0.03 mg/kg/infusion) of cocaine self-administration under a FR2 reinforcement schedule. We observed a typical inverted U-shaped curve of cocaine self-administration across cocaine doses after the vehicle treatment (Fig. 1B). Strikingly, treatment with CBD (10, 20 mg/kg) dose-dependently decreased cocaine self-administration and shifted the cocaine self-administration dose-response curve downward. A two-way ANOVA revealed a significant cocaine dose × CBD treatment interaction (F10,120 = 2.33, p < 0.05). Bonferroni tests for multiple group comparisons revealed that the 20 mg/kg dose of CBD significantly reduced self-administration maintained by lower doses of cocaine (0.03, 0.06 and 0.12 mg/kg/infusion), but not by higher doses of cocaine (0.25, 0.5 and 1 mg/kg/infusion).
Fig. 1.
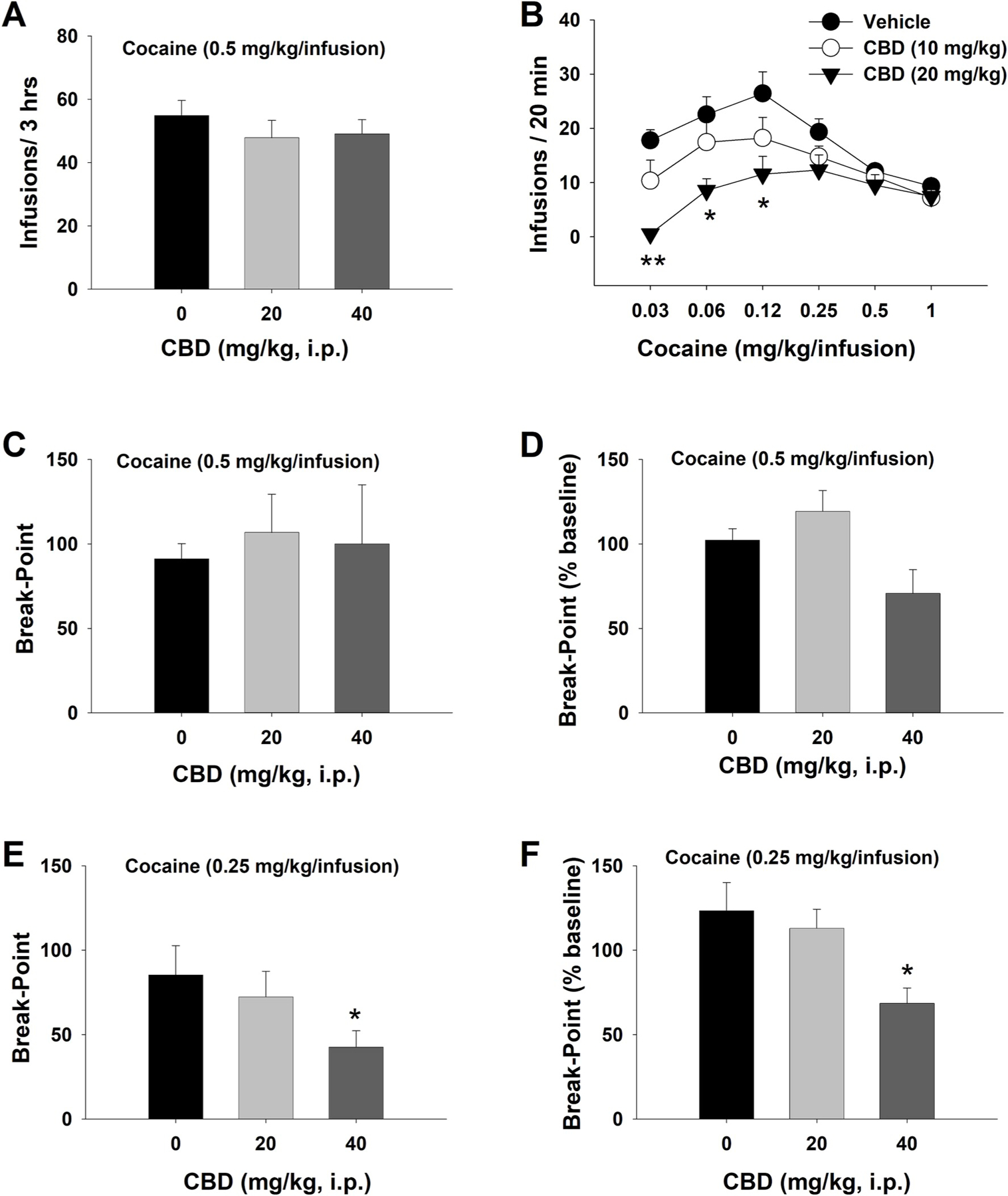
The effects of CBD on cocaine self-administration under different schedules of reinforcement in rats. A. Mean numbers of cocaine infusions, indicating that CBD failed to alter cocaine (0.5 mg/kg/infusion) self-administration under a FR1 schedule of reinforcement (Vehicle, n = 8; 20 mg/kg, n = 8; 40 mg/kg, n = 10). B. Mean numbers of cocaine infusions under a FR2 schedule of reinforcement for different doses of cocaine. CBD dose-dependently inhibited self-administration maintained by lower doses of cocaine and shifted the cocaine dose-response curve downward. N = 6 in each CBD dose group. C/D: Mean numbers of break-point and % changes in break-point (over baseline) for cocaine self-administration maintained by a high dose of cocaine (0.5 mg/kg/infusion) under a PR schedule of reinforcement, indicating that CBD failed to alter PR cocaine self-administration (Vehicle, n = 8; 20 mg/kg, n = 7; 40 mg/kg, n = 6). E/F: Mean numbers of break-point and % changes in break-point (over baseline) for cocaine self-administration maintained by a low dose of cocaine (0.25 mg/kg/infusion), indicating that CBD dose-dependently inhibited PR cocaine self-administration (Vehicle, n = 7; 20 mg/kg, n = 6; 40 mg/kg, n = 10). *p < 0.05, compared to the vehicle (0 mg/kg) control group.
We then investigated whether CBD administration can lower the rewarding effects of cocaine and/or motivation for cocaine as assessed by cocaine self-administration under a PR schedule of reinforcement. Fig. 2C shows that CBD, at 20 or 40 mg/kg, failed to alter the PR break-point for cocaine self-administration maintained by 0.5 mg/kg/infusion (one-way ANOVA, F2,18 = 3.42; p = 0.07). However, when the data were normalized to percent change over baseline immediately before the testing session, CBD caused a reduction in percent baseline of break-point for cocaine self-administration (F2,18 = 4.69; p = 0.02, where 20 mg/kg dose was significantly different from 40 mg/kg dose but not from the vehicle). Next, we lowered a cocaine dose from 0.5 mg/kg/infusion to 0.25 mg/kg/infusion (Fig. 1–E, F). We observed a dose-dependent reduction in either PR break-point (Fig. 1E, F2,20 = 6.25; p < 0.01) or % changes in PR break-point (Fig. 1F, F2,20 = 6.25; p < 0.01) after the same doses of CBD administration. Post-hoc individual group comparisons revealed a significant reduction in PR break-point after 40 mg/kg CBD administration (Fig. 1–E, F, p < 0.05). Although CBD reduced break points for a lower dose of cocaine, it did not significantly affect the total numbers of active or inactive lever presses throughout the session (see Fig. S1).
3.2. CB2, 5-HT1A and TRPV1 receptors are responsible for CBD action in cocaine self-administration
As stated above, in vitro binding assays suggest that CBD has binding affinities to multiple receptors and functional proteins such as CB1, CB2, GPR55, TRPV1, 5-HT1A and MORs. Here we determined whether blockade of each receptor attenuates CBD action in cocaine self-administration in vivo. Fig. 2A shows that CBD (20 mg/kg) caused a significant downward shift of the cocaine dose–response curve, whereas a pretreatment with AM251 (a CB1R antagonist, 3 mg/kg, 30 min prior to CBD) failed to alter CBD-induced reductions in cocaine self-administration. A two-way ANOVA revealed a significant cocaine dose × treatment interaction (F10,100 = 3.09; p < 0.01). Post-hoc Bonferroni tests revealed that (vehicle + CBD) and (AM251 + CBD) groups of rats self-administered similar amounts of cocaine at each dose (p > 0.05), but significantly less cocaine than the (vehicle + vehicle) control group at the 0 mg/kg/infusion (p < 0.01), 0.03 mg/kg/infusion (p < 0.05), 0.06 mg/kg/infusion (p < 0.01), and 0.12 mg/kg/infusion cocaine doses (p < 0.01).
In contrast to AM251, pretreatment with AM630 (a CB2R antagonist, 3 mg/kg, i.p., 30 min prior to CBD) prevented 20 mg/kg CBD-induced reductions in cocaine self-administration (Fig. 2B). A two-way ANOVA revealed a significant cocaine dose × treatment interaction (F10,120 = 2.75, p < 0.01). Post-hoc Bonferroni tests for multiple comparisons revealed that the (Veh + CBD) group of rats self-administered significantly less cocaine at each cocaine dose than the (vehicle + vehicle) control group or the (AM630 + CBD) group (p < 0.01). There were no significant differences in cocaine self-administration at each cocaine dose between the (vehicle + CBD) and (AM630 + CBD) groups. These findings suggest that the CBD-induced reduction in cocaine self-administration is at least in part mediated by CB2Rs.
Fig. 2C shows that pretreatment with CID16020046 (a GPR55 antagonist, 3 or 5 mg/kg, i.p. 30 min prior to CBD) failed to alter CBD-induced reduction in cocaine self-administration. A two-way ANOVA revealed a significant treatment × cocaine dose interaction (F15,150 = 3.72, p < 0.001). Post-hoc Bonferroni tests revealed that, although the (Veh + CBD) and (CID + CBD) groups were significantly different from the (Veh + Veh) control group at 0.03 and 0.06 mg/kg/infusion doses of cocaine, they did not differ from each other, suggesting the CBD action is not mediated by activation of GPR55.
Fig. 2D shows that pretreatment with capsazepine (a TRPV1 channel blocker, 3 or 5 mg/kg, 30 min prior to CBD) dose-dependently blocked CBD attenuating effects on cocaine self-administration. A two-way ANOVA revealed a significant treatment × cocaine dose interaction (F15,155 = 2.59, p < 0.01). Post-hoc Bonferroni tests revealed that (Veh + CBD) and (3 mg/kg capsazepine + CBD) groups self-administered similar amounts of cocaine, but were significantly different from the (Veh + Veh) group at 0.03 and 0.06 mg/kg/infusion doses of cocaine. However, there was no difference between the (5 mg/kg capsazepine + CBD) group and the (Veh + Veh) group, suggesting that the CBD action in cocaine self-administration may require TRPV1 activation.
Similar to AM630 or capsazepine, pretreatment with WAY100135 (a 5-HT1A receptor antagonist, 3 mg/kg, i.p., 30 min prior to CBD) also prevented the 20 mg/kg CBD-induced reduction in cocaine self-administration (Fig. 2E). A two-way ANOVA revealed a significant cocaine dose × treatment interaction F10,105 = 4.10, p < 0.001. Post-hoc Bonferroni tests revealed that the (Veh + CBD) group self-administered significantly less cocaine than the (Veh + Veh) or (WAY + CBD) group at 0.03 (p < 0.05), 0.06 (p < 0.01) and 0.12 mg/kg cocaine doses (p < 0.01), suggesting an involvement of 5-HT1A in CBD action.
Fig. 2E shows that pretreatment with naloxone (an opioid receptor antagonist, 3 mg/kg, i.p., 30 min prior to CBD) failed to prevent 20 mg/kg CBD-induced reduction in cocaine self-administration. In contrast, naloxone treatment appeared to potentiate CBD-induced reduction in cocaine self-administration. A two-way ANOVA revealed a significant cocaine dose × treatment interaction (F10,104 = 4.32, p < 0.001). Post-hoc Bonferroni tests revealed that the (Veh + CBD) and (naloxone + CBD) groups self-administered similar amounts of cocaine at each dose (p > 0.05) but significantly less cocaine than the (Veh + Veh) control group at 0.03 (p < 0.05), 0.06 (p < 0.01) and 0.12 mg/kg cocaine doses (p < 0.01). These findings suggest that mu opioid receptors are unlikely involved in the CBD action in cocaine self-administration. None of the combined treatments affected responding on the inactive lever (see Fig. S3 in Supplementary Materials).
3.3. Blockade of CB1R, but not other tested receptors/channel, attenuated cocaine self-administration
We then evaluated whether each antagonist alone used above also alters cocaine self-administration. Fig. 3 shows that pretreatment with the same dose of AM251 (3 mg/kg) alone also significantly reduced cocaine self-administration (Fig. 3A). A two-way ANOVA revealed a significant AM251 treatment effect (F5,80 = 7.12, p < 0.001). Post-hoc Bonferroni tests for multiple comparisons revealed that the AM251-treated group was significantly different from the vehicle-treated group at the 0.03, 0.06 and 0.12 mg/kg/infusion doses of cocaine (p < 0.05, p < 0.001, p < 0.05, respectively), suggesting that CB1R mechanism is also involved in cocaine reward as we reported previously (Xi et al., 2006a, 2008). In contrast, treatment with AM630, CID16020046, capsazepine, WAY100135, or naloxone alone, at the same doses as used above in Fig. 2, did not alter cocaine self-administration (p > 0.05) (Fig. 3B–E).
Fig. 3.
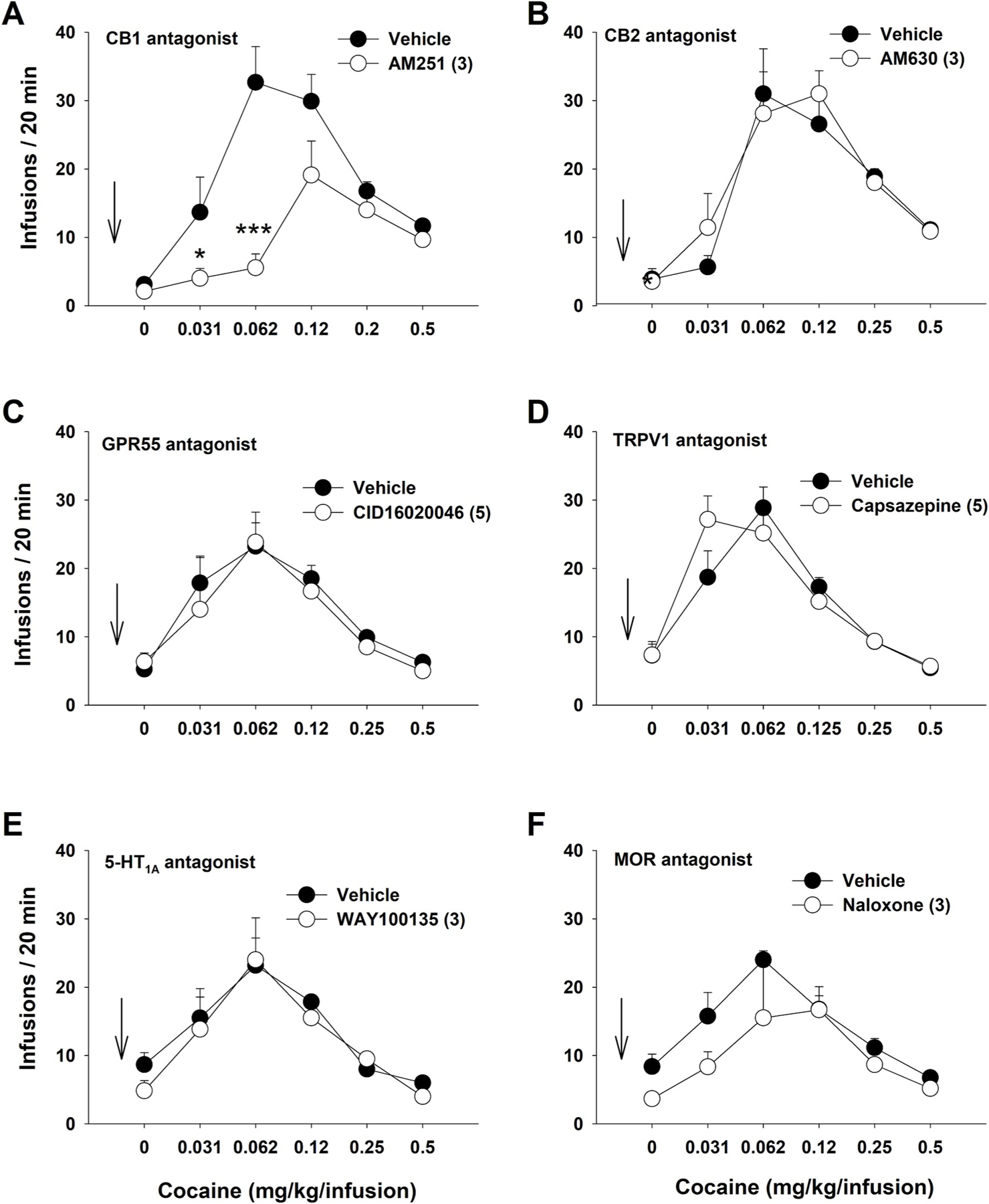
The effects of each receptor antagonist (tested above in Fig. 2) on multiple-dose cocaine self-administration. A: Prereatment with AM251 (3 mg/kg) inhibited cocaine self-administration. n = 9 in each dose group. B ~ F: Pretreatment with AM630 (3 mg/kg, n = 9) (B), CID16020046 (5 mg/kg, n = 6) (C), capsazepine (Veh, n = 8; 5 mg/kg, n = 6) (D), WAY100135 treatment (3 mg/kg, n = 6) (E), or naloxone (Veh, n = 8; 3 mg/kg naloxone, n = 6) (F) failed to alter cocaine self-administration. *p < 0.05, ***p < 0.001, compared to the vehicle control group.
3.4. CBD reduces cocaine-enhanced brain-stimulation reward (BSR)
To confirm the findings from the above cocaine self-administration experiment, we then used another highly sensitive BSR paradigm to evaluate the interactions between cocaine and CBD and the receptor mechanisms underlying CBD action. Fig. 4A shows representative rate-frequency functions for BSR, indicating the BSR threshold θ0, Ymax, and the effects of cocaine in the presence or absence of CBD. Cocaine (2 mg/kg, i.p.) significantly decreased the BSR threshold θ0 value (i.e., shifted the curve to the left) without affecting asymptotic rates of responding (aka no change in Ymax level), indicating potentiation of the rewarding effects of brain stimulation after cocaine administration. While CBD (20 mg/kg, i.p.) alone failed to alter BSR, pretreatment with the same dose of CBD blocked cocaine-enhanced BSR. Fig. 4B shows that pretreatment with CBD significantly and dose-dependently decreased cocaine-enhanced BSR. A one-way ANOVA revealed a significant dose effect (F3,30 = 3.17; p < 0.05). Post-hoc individual group comparisons showed that the 20 mg/kg CBD dose produced a significant reduction in cocaine-enhanced BSR compared to the vehicle treatment group (p < 0.05). Fig. 4C shows CBD alone, at the same doses as used above in Fig. 4B, failed to alter BSR (F3,31 = 0.062; p = 0.972).
Fig. 4.
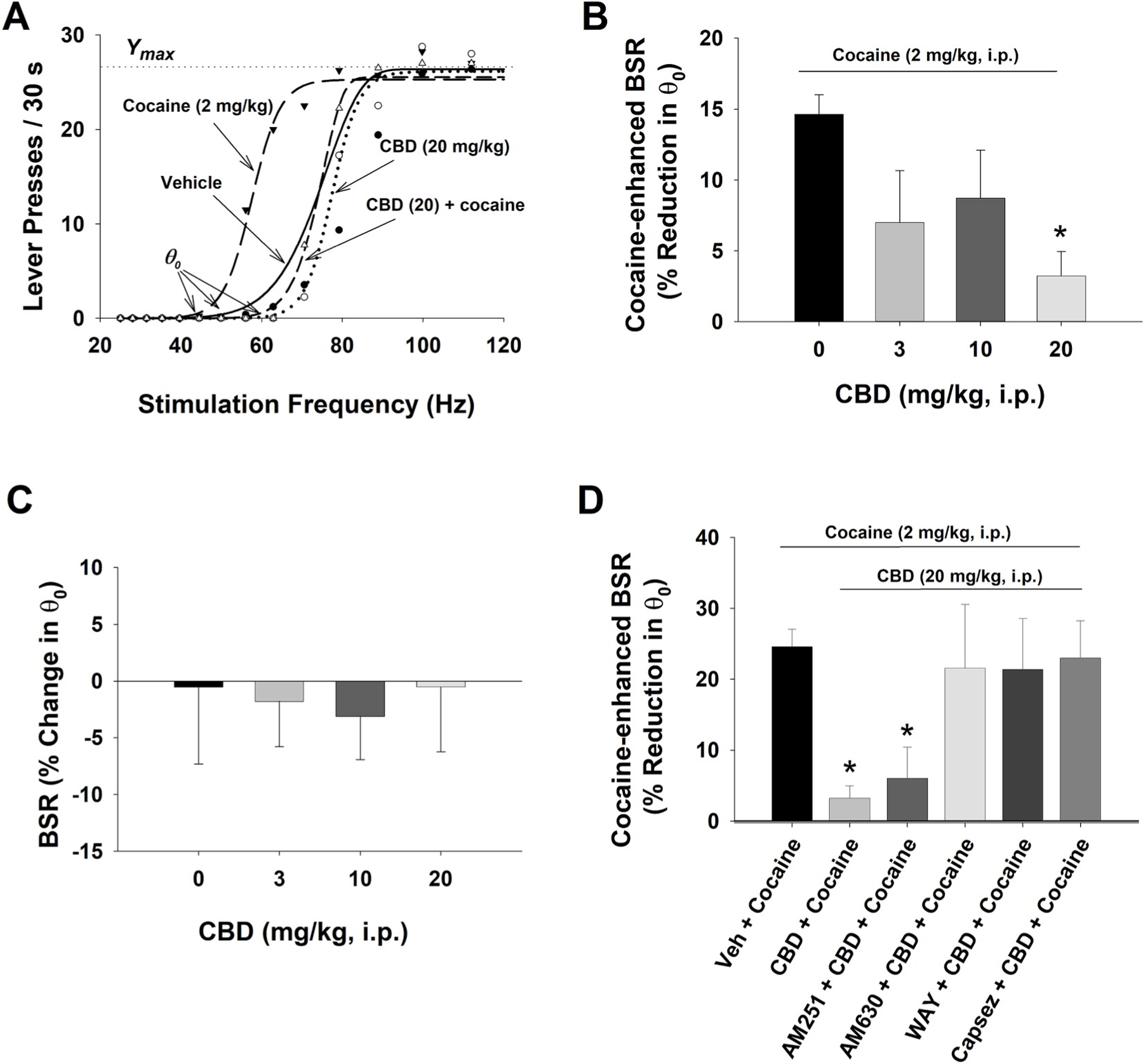
The effects of CBD on electrical brain-stimulation reward (BSR). A. Representative rate-frequency function curves for BSR, illustrating the BSR threshold θ0, Ymax, and the effects of cocaine and/or CBD on BSR. Cocaine, at 2 mg/kg (i.p.) shifted the rate-frequency function curve to the left, lowering the θ0 value (i.e., enhancing BSR), without a change in the Ymax level. Pretreatment with CBD (20 mg/kg) blocked cocaine-enhanced effect. B: Percent changes in the θ0 value, indicating that cocaine significantly enhanced BSR, which was dose-dependently attenuated by CBD treatment. n = 11 in each dose group. C: CBD alone, at the doses of 3, 10, 20 mg/kg, failed to alter BSR. n = 8 in each dose group. D: Pretreatment with AM630 (n = 9), WAY100135 (n = 7) or capsazepine (n = 7), but not AM251 (n = 7), blocked CBD-induced reduction in cocaine-enhanced BSR. *p < 0.05, compared to the (Veh + Cocaine) group.
To further confirm the above findings that CBD attenuating effects on cocaine self-administration could be mediated by stimulation of CB2, TRPV1 and 5-HT1A receptors, we pretreated animals with antagonists of these receptors before treating them with CBD (20 mg/kg) and cocaine (2 mg/kg). As shown in Fig. 4D, CBD treatment reduced cocaine-enhanced BSR. Pretreatment with AM251 failed to block the CBD-induced reduction in cocaine-enhanced BSR whereas pretreatments with AM630, capsazepine or WAY100135 blocked the CBD action. A one-way ANOVA showed a significant treatment effect (F5,45 = 2.43, p < 0.05). Post-hoc Bonferroni tests for multiple group comparisons revealed a significant reduction in BSR (p < 0.05) only in the (CBD + cocaine) and (AM251 + CBD + cocaine) groups, but not in other treatment groups. Neither AM251 nor AM630 alone altered BSR (Spiller et al., 2019). WAY100135 or capsazepine alone, at 3 mg/kg, also failed to alter BSR by themselves (Fig. S4).
3.5. CBD attenuates cocaine-induced dopamine in the nucleus accumbens
Finally, to determine whether CBD affects cocaine-enhanced DA in the NAc, we used in vivo microdialysis to measure cocaine- and/or CBD-induced changes in extracellular DA. Fig. 5A shows that systemic injections of CBD alone (10, 20 mg/kg, i.p.) failed to significantly alter extracellular DA level. A two-way ANOVA revealed a non-significant CBD treatment main effect (F2,29 = 0.45; p = 0.64), time main effect (F11,319 = 2.00, p = 0.12), or treatment × time interaction (F22,319 = 0.93; p = 0.55).
Fig. 5.
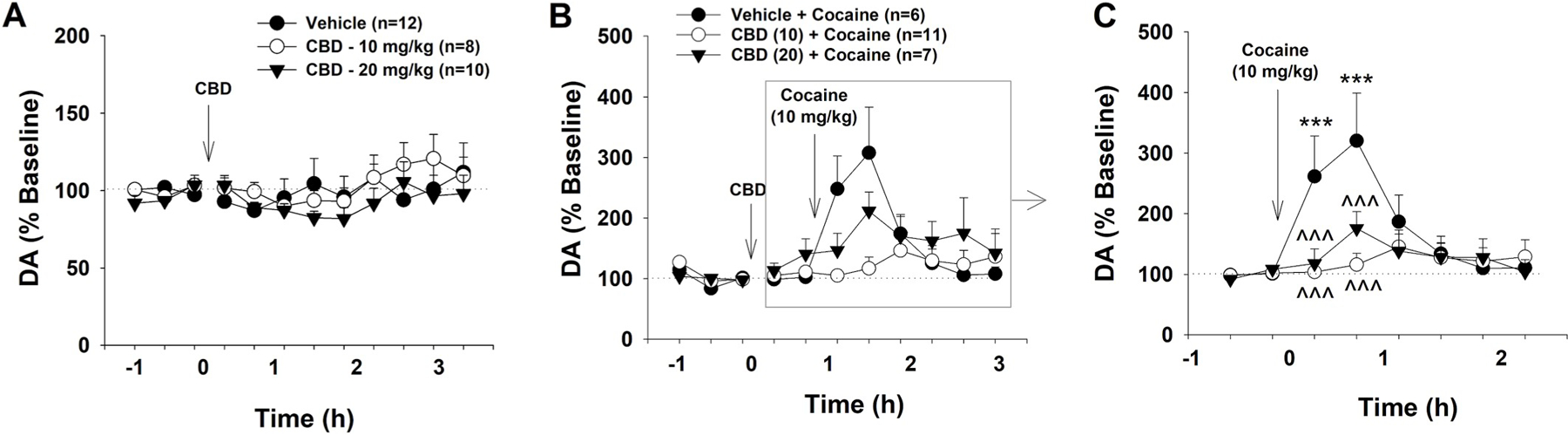
The effects of cocaine and CBD on extracellular DA in the NAc. A: Systemic administration of CBD (0, 10, 20 mg/kg) did not significantly alter extracellular DA level in the NAc. B: Effects of cocaine (10 mg/kg) and CBD on extracellular DA in the NAc. C: Renormalized data (% change over new baselines immediately before cocaine injection, gray box), illustrating that systemic administration of cocaine produced a significant increase in extracellular DA in the NAc, which was dose-dependently attenuated by CBD pretreatment. ***p < 0.001, compared to baselines before cocaine injection; ^^^ p < 0.001, compared to the (Veh + Cocaine) group.
Fig. 5B shows that cocaine (10 mg/kg) caused a rapid and significant increase in extracellular NAc DA level in naive rats that lasted 2–3 h with a peak effect at 1 h after cocaine injection. Pretreatment with CBD appeared to significantly attenuate cocaine-induced increases in extracellular DA in the NAc. Given that CBD, at 20 mg/kg, slightly-to moderately elevated extracellular DA levels before cocaine administration, we renormalized cocaine-induced changes in extracellular DA over the new baseline (mean values of two samples) immediately before cocaine injection (Fig. 5C). A two-way ANOVA for repeated measures over time revealed a significant time main effect (F7,147 = 8.37, p < 0.001) and CBD treatment × time interaction (F14,147 = 4.28, p < 0.001), although the CBD treatment main effect is not significant (F2,21 = 1.87, p > 0.05). Post-hoc multiple group comparisons indicated that the cocaine-induced increase in extracellular DA was statistically significant only in the (Veh + Cocaine) group, but not in the other two CBD treatment groups (Fig. 5C, ***p < 0.001). The differences between the vehicle and either dose of CBD pretreatment groups were also statistically significant (Fig. 5C, ^^^ p < 0.001).
4. Discussion
In the present study we demonstrated that systemic administration of CBD dose-dependently shifted the cocaine self-administration dose-response curve downward, lowed PR break-points in responding for a low dose of cocaine, and attenuated cocaine-enhanced BSR. In addition, CBD attenuated the rapid increase in extracellular NAc DA levels caused by a cocaine injection. The observed reductions in cocaine-taking and BSR are not likely caused by CBD-induced locomotor impairment since CBD pretreatment neither altered inactive lever responding in the self-administration experiments nor altered Ymax in the BSR experiments. CBD, at the same doses as used in the present study, significantly inhibited oral sucrose self-administration only in WT or CB1–KO mice, but not in CB2–KO mice (Bi et al., 2019). Together, these findings suggest that CBD has the ability to reduce the rewarding effects of cocaine possibly via a DA-dependent mechanism.
4.1. CBD inhibits cocaine reward
Our above findings are consistent with previous reports that CBD also attenuates the rewarding effects of alcohol (Viudez-Martínez et al., 2018a; 2018b), methamphetamine (Hay et al., 2018), morphine (Katsidoni et al., 2013; Markos et al., 2018) and cocaine (Luján et al., 2018). We note another report that low doses of CBD (5, 10 mg/kg, i.p.) failed to alter cocaine self-administration (Mahmud et al., 2017). We report here that higher doses of CBD (10–40 mg/kg) are required to inhibit cocaine self-administration maintained by low, but not high, doses of cocaine. Our findings are also consistent with previous reports that CBD dose-dependently attenuates cue or stress-induced drug seeking during extinction (i.e. in the absence of heroin or alcohol) (Gonzalez-Cuevas et al., 2018; Ren et al., 2009) and reinstatement of methamphetamine-induced CPP (Karimi-Haghighi and Haghparast, 2018). In addition, CBD also facilitates the extinction of cocaine- or amphetamine-induced CPP (Parker et al., 2004), disrupts reconsolidation of context-associated drug memories (de Carvalho and Takahashi, 2017) and attenuates impulsive discounting of delayed rewards (Gonzalez-Cuevas et al., 2018). Furthermore, chronic treatment with CBD not only reduces cocaine intake but also leads to long-term neuroplasticity of the mesolimbic system by increasing the expression of hippocampal CB1Rs and brain derived neurotrophic factor (Luján et al., 2018). Clinical studies showed that CBD was well tolerated (Taylor et al., 2018); it reduced heroin-induced drug cravings (Hurd et al., 2015), as well as the euphoric effects of Δ9-THC (Dalton et al., 1976). Importantly, CBD itself lacks rewarding effects as it does not induce CPP on its own (Parker et al., 2004; Viudez-Martínez et al., 2019) nor does it affect BSR in rodents (Katsidoni et al., 2013; present study). In healthy individuals, CBD fails to produce Δ9-THC-like psychoactive effects (Martin-Santos et al., 2012), and in poly-drug users it exhibits low abuse potential (Schoedel et al., 2018). CBD is also protective against acute cocaine toxicity, particularly liver damage and seizures (Vilela et al., 2015). Taken together, all these findings suggest that CBD is promising as a therapeutic for the treatment of cocaine abuse and addiction.
4.2. Receptor mechanisms underlying CBD action in cocaine reward
Another important finding in this study is the multiple receptor mechanisms including CB2, TRPV1 and 5-HT1A, involved in CBD action. This is based on the findings that pretreatment with AM630, capsazepine or WAY100135 blocked CBD-attenuating effects on cocaine reward, while pretreatments with AM251, CID16020046 or naloxone did not block CBD action. Furthermore, systemic administrations of AM630, CID16020046, capsazepine, naloxone or WAY100135 alone failed to alter cocaine self-administration, while AM251 alone significantly inhibited cocaine self-administration, suggesting an important role of CB1Rs in cocaine reward. This is consistent with our previous reports (Xi et al., 2006a, 2008).
4.2.1. CB1R involvement in CBD action
As stated above, although AM251 failed to alter CBD-induced reduction in cocaine self-administration, we cannot completely exclude the possible involvement of CB1Rs in CBD action. This is based on the following reasons. First, CBD displays nM high affinity for the CB1R allosteric binding site and behaves as a negative allosteric modulator (NAM) (or antagonist) at CB1Rs (Laprairie et al., 2015; Straiker et al., 2015; Tham et al., 2019; Thomas et al., 2007), although it has low affinity for the CB1R orthosteric binding site (Bisogno et al., 2001). Second, CBD or AM251 alone inhibits cocaine self-administration, suggesting that CBD may inhibit cocaine action by negative modulation of CB1Rs via its allosteric binding site. This is supported by the finding that pretreatment with AM251 augmented CBD-induced reduction in sucrose self-administration in WT mice (Bi et al., 2019) and genetic deletion of CB1Rs blocked the pro-neurogenic effects of CBD in hippocampus (Wolf et al., 2010). However, the combination of AM251 and CBD did not produce synergistic attenuating effects on cocaine self-administration in the present study. This may be related to the use of high doses of AM251 and CBD that produces maximal inhibitory effects. Thus, it is likely that subthreshold doses of AM251 and CBD might be able to produce their additive or synergistic effects. In addition, there are also conflicting reports that CB1R antagonism either prevented (Casarotto et al., 2010; Stern et al., 2017) or failed to block pharmacological effects of CBD (De Gregorio et al., 2019; Thapa et al., 2017). More studies are required to further address this issue.
4.2.2. CB2R involvement in CBD action
In addition, CBD acts as a CB2R antagonist or inverse agonist (Thomas et al., 2007; Pertwee, 2008), CB2R agonist or partial agonist (Navarro et al., 2018; Tham et al., 2019), or a negative allosteric modulator of CB2Rs with nM binding affinity (Martínez-Pinilla et al., 2017). In the present study, we found that pretreatment with AM630 prevented CBD attenuating effects on cocaine self-administration and BSR, suggesting CB2R involvement in CBD action and CBD itself may act as a functional CB2R agonist. This is consistent with our recent report indicating that pharmacological blockade or genetic deletion of CB2R blocked CBD-induced reduction in oral sucrose self-administration (Bi et al., 2019). Blockade of CB2R also attenuated CBD-induced reduction in food intake, body weight and obesity (Ignatowska-Jankowska et al., 2011; Ishiguro et al., 2010) and attenuated CBD-produced neuroprotection (Castillo et al., 2010). Our findings also correspond with previous reports that stimulation of CB2Rs by JWH133 inhibited cocaine self-administration and cocaine-induced CPP and locomotor sensitization (Delis et al., 2017; Xi et al., 2011).
Growing evidence indicates that CB2Rs are expressed in the brain and are functionally involved in drug reward and addiction (Jordan and Xi, 2019; Manzanares et al., 2018). CB2Rs have been identified on the cell bodies of dopaminergic (DA) neurons in the ventral tegmental area (VTA) (Zhang et al., 2014, 2017) as well as on the terminals of these neurons in the nucleus accumbens (NAc) (Aracil-Fernández et al., 2012; Foster et al., 2016), two brain regions critical for reward and addiction. We therefore hypothesized that a DA-dependent mechanism may underlie CBD-induced reduction in cocaine reward via a CB2R mechanism. This is supported by the finding that pretreatment with CBD significantly attenuates cocaine-induced increases in extracellular DA in the NAc (present study) and that intra-NAc microinjections of CBD inhibits VTA DA neuronal activity (Renard et al., 2016) and attenuates amphetamine-induced hyperlocomotion (Renard et al., 2016). However, systemic administration of the same doses of CBD alone failed to significantly alter extracellular DA in the NAc in the present study. This may explain why CBD itself is not rewarding and has no significant abuse potential. The ineffectiveness or a mild reduction (~20%) in NAc DA after CBD administration could be an outcome of multiple receptor mechanisms (see more discussion below) in addition to allosterically modulating CB2Rs.
It is worth noting that the VTA-NAc DA system is not the only one involved in CBD action. As mentioned above, CBD has multiple binding sites, and therefore, it may act at many other brain regions or neurotransmitter systems (see more discussion below).
4.2.3. 5-HT1A involvement in CBD action
In addition to DA, serotonin also plays an important role in cocaine reward and addiction. Cocaine is well known to block monoamine transporters, causing an increase in extracellular DA and serotonin (Andrews et al., 2005; Andrews and Lucki, 2001; Müller et al., 2002). A number of serotonin receptors have been implicated in cocaine-related behaviors (Cunningham et al., 2013; Fletcher et al., 2008). Particularly, the 5-HT1A receptor subtype has become a target in medication development for the treatments of cocaine addiction since activation of 5-HT1A receptors by buspirone, 8-OH-DPAT, or osemozotan attenuates cocaine self-administration in rats and monkeys (Collins and France, 2018; Gold and Balster, 1992; Peltier and Schenk, 1993) and cocaine-induced hyperlocomotion (Nakamura et al., 2006), whereas optogenetic activation of 5-HT1A autoreceptors on the dorsal raphe neurons abolishes cocaine CPP in mice (You et al., 2016).
Russo et al. (2005) were the first to suggest that CBD could act as a 5HT1A receptor agonist. Since then, a number of studies indicated the involvement of 5-HT1A receptors in CBD action in vivo, primarily its anxiolytic effects (You et al., 2016). In the present study, we found that pretreatment with WAY-100135, a selective 5-HT1A receptor antagonist, blocked CBD-induced reductions in cocaine self-administration and in cocaine-enhanced BSR, suggesting an important role of 5-HT1A in the CBD action. We note that an early study using the BSR paradigm indicated that CBD inhibited the rewarding effects of morphine, but not cocaine, and this inhibitory effect was also blocked by 5-HT1A antagonism (Katsidoni et al., 2013). This striking discrepancy in both the studies most likely derives from differences in drug doses (10–20 mg/kg CBD in the present study versus 5 mg/kg CBD in Katsidoni’s study). In our hands, doses lower than 20 mg/kg failed to produce significant effects in the BSR and self-administration paradigms. Our findings correspond with work of Viudez-Martínez and colleagues who demonstrated that CBD can reduce the rewarding effects of alcohol via stimulation of 5-HT1A receptors (Viudez-Martínez et al., 2018a, 2018b). It is conceivable that activation of 5-HT1A receptors by CBD attenuates reward-related processing by reducing serotonin release and modulating the mesolimbic DA system. This hypothesis is supported by the findings that intra-NAc CBD significantly inhibits spontaneous mesolimbic DA neuronal activity and burst firing, which can be reversed by 5-HT1A antagonism (Norris et al., 2016). Future studies should uncover the 5-HT1A-dependent neuronal pathways through which CBD inhibits cocaine self-administration.
4.2.4. TRPV1 involvement in CBD
Our data also suggest that the CBD action might be related to transient receptor potential channels of subfamily V1 (TRPV1) channel activation. TRPV1 are nonselective catanion membrane channels primarily involved in the transduction of somatosensory information such as taste, temperature, and pain (Tominaga et al., 1998). TRPV1 can be activated by capsaicin or phytocannabinoids (e.g. CBD and cannabidivarin) (Iannotti et al., 2014).
Previous studies have shown that blockade of TRPV1 inhibits opioid reward as assessed by morphine-induced conditioned place preference (Nguyen et al., 2014), but failed to alter cocaine self-administration (Adamczyk et al., 2012). In the present study, we found that CBD inhibited cocaine self-administration and brain-stimulation reward, an effect that was blocked by a TRPV1 antagonist, suggesting that TRPV1 activation may contribute to the therapeutic effects of CBD. The precise mechanisms through which TRPV1 underlies the CBD action in cocaine self-administration and BSR are unclear. Activation of presynaptic TRPV1 channels facilitates neurotransmitter release, while activation of postsynaptic TRPV1 leads to depolarization of neurons (Edwards, 2014). Given that TRPV1 channels are located on glutamatergic neurons in the frontal cortex and on striatal GABAergic neurons (Edwards, 2014), one possibility is that activation and then desensitization of TRPV1 by CBD may disrupt these processes, leading to a reduction in cocaine reward. Another possibility is that CBD may act as a fatty acid amide hydrolase (FAAH) inhibitor or fatty acid binding protein (FABPS) inhibitor, leading to an increase in the anandamide level (Bisogno et al., 2001), which subsequently affects cocaine-taking and/or cocaine-seeking (Adamczyk et al., 2009) by activation of both CB1Rs and TRPV1 channels (Fenwick et al., 2017).
4.2.5. Other mechanisms in CBD action
A body of literature suggests that pharmacological effects of CBD might be related to many other receptor mechanisms such as activation of GPR55 (Ryberg et al., 2007) or mu and delta opioid receptors (Kathmann et al., 2006). GPR55 activation has been attributed to intracellular Ca2+ stores and facilitation of glutamate release, which can be suppressed by CBD (Bouron, 2018; Sylantyev et al., 2013). Beneficial effects of CBD mediated by GPR55 appear to be mostly antiepileptic (Kaplan et al., 2017) and anti-inflammatory (Li et al., 2013), with very limited evidence for its therapeutic utility in treatment of addiction. Our data provide no direct evidence for the involvement of GPR55 in CBD-attenuating effects on cocaine reward. Correspondingly, we also failed to find strong supporting evidence for the role of MORs in CBD action. Although the combined treatment of CBD and naloxone reduced cocaine self-administration to a greater degree than CDB alone, differences between CBD and CBD + naloxone treatment groups for any cocaine doses were not statistically significant. In line with our finding is a previous report that CBD plus naltrexone also reduced motivation and alcohol self-administration to a greater degree than CBD or naltrexone alone (Viudez-Martínez et al., 2018b). In addition, CBD may indirectly activate CB1 and CB2 receptors through inhibition of either FAAH, the enzyme that breaks down anandamide and/or fatty-acid-binding proteins (FABPs), the intracellular “carries” that transport anandamide to FAAH (Deutsch, 2016). Although both mechanisms have been implicated in a number of drug-related behaviors (Scherma et al., 2019), currently there is a lack of evidence for dysregulation of these mechanisms by CBD as the primary means by which CBD attenuates the rewarding effects of cocaine.
It is unknown exactly how such multiple receptor mechanisms mediate CBD-attenuated effects on cocaine reward. One possibility is that CBD, at different doses, may produce its therapeutic effects through different receptor mechanisms. At low doses, CBD may act mainly through CB2 and TRPV1 receptors since it has higher binding affinity to these two receptors (Thomas et al., 2007; Pertwee, 2008; Martínez-Pinilla et al., 2017; Iannotti et al., 2014), while at high doses CBD action might require the activation of other receptors such as 5-HT1A receptors (Russo et al., 2005). Another possibility is that different receptors may form functional heterodimers or interact at intracellular signal molecule levels. Thus, pharmacological blockade of one receptor affects CBD action on other receptors. More studies are needed to address this issue.
In summary, the present findings suggest that CBD may have certain therapeutic potential in treatment of cocaine addiction, but some limiting factors should be taken into consideration. As demonstrated here, CBD has the ability to reduce cocaine self-administration maintained by low doses of cocaine and motivation for cocaine seeking, but it is not effective for high doses of cocaine. Such therapeutic effects may be related to multiple receptor mechanisms including inhibition of CB1R and activation of CB2R, TRPV1 and 5-HT1A. Although CBD is not so potent in attenuation of high dose cocaine action, CBD itself has no abuse liability or significant other side effects. Therefore, CBD deserves further study as a valuable supplementary pharmacotherapy for cocaine abuse and addiction.
Supplementary Material
HIGHLIGHTS.
CBD inhibited cocaine self-administration and shifted cocaine dose-response curve downward under FR schedule of reinforcement.
CBD lowered break-points cocaine self-administration under a PR schedule of reinforcement.
CBD dose-dependently attenuated cocaine-enhanced electrical brain-stimulation reward (BSR).
Blockade of CB2, 5-HT1A or TRPV1 receptors attenuated CBD action in cocaine self-administration and BSR.
CBD dose-dependently attenuated cocaine-induced increases in extracellular dopamine in the nucleus accumbens.
Acknowledgement
This work was supported by the Intramural Research Program (IRP) at the National Institute on Drug Abuse (NIDA) (DA000620-02), National Institutes of Health, U.S. Public Health Service, USA
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2019.107740.
References
- Viudez-Martínez A, García-Gutiérrez MS, Navarrón CM, Morales-Calero MI, Navarrete F, Torres-Suárez AI, Manzanares J, 2018Ea Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict. Biol 23, 154–164. [DOI] [PubMed] [Google Scholar]
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M, 2009. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J. Physiol. Pharmacol 60, 119–125. [PubMed] [Google Scholar]
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegaliński E, 2012. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res 1444, 45–54. [DOI] [PubMed] [Google Scholar]
- Andrews CM, Lucki I, 2001. Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology (Berl.) 155, 221–229. [DOI] [PubMed] [Google Scholar]
- Andrews CM, Kung HF, Lucki I, 2005. The 5-HT1A receptor modulates the effects of cocaine on extracellular serotonin and dopamine levels in the nucleus accumbens. Eur. J. Pharmacol 508, 123–130. [DOI] [PubMed] [Google Scholar]
- Aracil-Fernández A, Trigo JM, García-Gutiérrez MS, Ortega-Álvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J, 2012. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB₂ receptors. Neuropsychopharmacology 37, 1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JC, 2005. The role of endocannabinoid transmission in cocaine addiction. Pharmacol. Biochem. Behav 81, 396–406. [DOI] [PubMed] [Google Scholar]
- Bi GH, Galaj E, He Y, Xi ZH, 2019. Cannabidiol inhibits sucrose self-administration by Cb1 and CB2 receptor mechanism in rodents. 2019 Jun 19:e12783. Addict. Biol 10.1111/adb.12783. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanuš L, Petrocellis LD, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Marzo VD, 2001. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol 134, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, 2018. Phyto and endocannabinoids exert complex actions on calcium and zinc signaling in mouse cortical neurons. Biochem. Pharmacol 152, 244–251. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Gomes FV, Resstel LBM, Guimarães FS, 2010. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav. Pharmacol 21, 353–358. [DOI] [PubMed] [Google Scholar]
- Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J, 2010. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-is-chemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis 37, 434–440. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Nicolas C, Thiriet N, Lardeux MV, Duranti A, Solinas M, 2014. Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int. J. Neuropsychopharmacol 18, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, France CP, 2018. Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self-administration in male and female rhesus monkeys. Exp. Clin. Psychopharmacol 26, 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E, 1987. Fitting intracranial self-stimulation data with growth models. Behav. Neurosci 2, 209–214. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Guimarães FS, Campos AC, Zuardi AW, 2018. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front. Immunol 9 2009 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG, 2013. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem. Neurosci 4, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB, 1976. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin. Pharmacol. Ther 19, 300–309. [DOI] [PubMed] [Google Scholar]
- de Carvalho CR, Takahashi RN, 2017. Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict. Biol 22, 742–751. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, Gobbi G, 2019. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 160, 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJMJ, Schoffelmeer ANM, 2001. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med 7, 1151–1154. [DOI] [PubMed] [Google Scholar]
- Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K, 2017. Attenuation of cocaine-induced conditioned place preference and motor activity via cannabinoid CB2 receptor agonism and CB1 receptor antagonism in rats. Int. J. Neuropsychopharmacol 20, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, 2016. A personal retrospective: elevating anandamide (AEA) by targeting fatty acid amide hydrolase (FAAH) and the fatty acid binding proteins (FABPs). Front. Pharmacol 7 10.3389/fphar.2016.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JG, 2014. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog. Drug Res 68, 77–104. [DOI] [PubMed] [Google Scholar]
- Fenwick AJ, Fowler DK, Wu SW, Shaffer FJ, Lindberg JEM, Kinch DC, Peters JH, 2017. Direct anandamide activation of TRPV1 produces divergent calcium and current responses. Front. Mol. Neurosci 10, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA, 2008. The 5-HT2C receptor agonist Ro60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology 33, 1402–1412. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2016. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal LFV, Rissanen AM, Scheen AJ, Ziegler O, Rössner S, 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. The Lancet 365, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Galaj E, Ewing S, Ranaldi R, 2018. Dopamine D1 and D3 receptor polypharmacology as a potential treatment approach for substance use disorder. Neurosci. Biobehav. Rev 89, 13–28. [DOI] [PubMed] [Google Scholar]
- Gil-Ordóñez A, Martín-Fontecha M, Ortega-Gutiérrez S, López-Rodríguez ML, 2018. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem. Pharmacol 157, 18–32. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL, 1992. Effects of buspirone and gepirone on i.v. cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl.) 108, 289–294. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, Banks SL, Stinchcomb AL, Weiss F, 2018. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology 43, 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay GL, Baracz SJ, Everett NA, Roberts J, Costa PA, Arnold JC, McGregor IS, Cornish JL, 2018. Cannabidiol treatment reduces the motivation to self-administer methamphetamine and methamphetamine-primed relapse in rats. J. Psychopharmacol 32, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M, Jutras-Aswad D, 2015. Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics 12, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, Russo E, Whalley BJ, Di Marzo V, Stephens GJ, 2014. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci 5, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Jankowski MM, Swiergiel AH, 2011. Cannabidiol decreases body weight gain in rats: involvement of CB2 receptors. Neurosci. Lett 490, 82–84. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, Benno R, Arinami T, Onaivi ES, 2010. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 64, 92–96. [DOI] [PubMed] [Google Scholar]
- Jordan C, Xi ZX, 2019. Progress in brain cannabinoid CB2 receptors: from gene to behavior. 2019 Apr 19:107609. Neurosci. Biobehav. Rev 10.1016/j.neuropharm.2019.04.015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Stella N, Catterall WA, Westenbroek RE, 2017. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. U.S.A 114, 11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Haghighi S, Haghparast A, 2018. Cannabidiol inhibits priming-induced reinstatement of methamphetamine in REM sleep deprived rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 82, 307–313. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E, 2006. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmied Arch. Pharmacol 372, 354–361. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Anagnostou I, Panagis G, 2013. Cannabidiol inhibits the reward-facilitating effect of morphine: involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addict. Biol 18, 286–296. [DOI] [PubMed] [Google Scholar]
- Keck TM, Yang H-J, Bi G-H, Huang Y, Zhang H-Y, Srivastava R, Gardner EL, Newman AH, Xi Z-X, 2013. Fenobam sulfate inhibits cocaine-taking and cocaine-seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology (Berl.) 229, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM, 2015. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol 172, 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR, 2009. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology 205, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Feng J, Li Yong-yu, Yuece B, Lin X, Yu L, Li Yan-na, Feng Y, Storr M, 2013. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas 42, 123–129. [DOI] [PubMed] [Google Scholar]
- Luján MÁ, Castro-Zavala A, Alegre-Zurano L, Valverde O, 2018. Repeated Cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology 143, 163–175. [DOI] [PubMed] [Google Scholar]
- Mahmud A, Gallant S, Sedki F, D’Cunha T, Shalev U, 2017. Effects of an acute cannabidiol treatment on cocaine self-administration and cue-induced cocaine seeking in male rats. J. Psychopharmacol 31, 96–104. [DOI] [PubMed] [Google Scholar]
- Mandolini GM, Lazzaretti M, Pigoni A, Oldani L, Delvecchio G, Brambilla P, 2018. Pharmacological properties of cannabidiol in the treatment of psychiatric disorders: a critical overview. Epidemiol. Psychiatr. Sci 27, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Cabañero D, Puente N, García-Gutiérrez MS, Grandes P, Maldonado R, 2018. Role of the endocannabinoid system in drug addiction. Biochem. Pharmacol 157, 108–121. [DOI] [PubMed] [Google Scholar]
- Markos JR, Harris HM, Gul W, ElSohly MA, Sufka KJ, 2018. Effects of cannabidiol on morphine conditioned place preference in mice. Planta Med 84, 221–224. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi AW, McGuire PK, 2012. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharmaceut. Des 18, 4966–4979. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, Oyarzabal J, Canela EI, Lanciego JL, Nadal X, Navarro G, Borea PA, Franco R, 2017. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol 8, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, De Souza Silva MA, Jocham G, Huston JP, 2002. Cocaine increases serotonergic activity in the hippocampus and nucleus accumbens in vivo: 5-HT1a-receptor antagonism blocks behavioral but potentiates serotonergic activation. Synapse 45, 67–77. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Ago Y, Hayashi A, Itoh S, Kakuda M, Hashimoto H, Baba A, Matsuda T, 2006. Modification of cocaine-induced behavioral and neurochemical effects by serotonin1A receptor agonist/antagonist in mice. Synapse 60, 479–484. [DOI] [PubMed] [Google Scholar]
- Navarro G, Reyes-Resina I, Rivas-Santisteban R, Sánchez de Medina V, Morales P, Casano S, Ferreiro-Vera C, Lillo A, Aguinaga D, Jagerovic N, Nadal X, Franco R, 2018. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol 157, 148–158. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P, 2012. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem. Pharmacol 84, 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TL, Kwon SH, Hong SI, Ma SX, Jung YH, Hwang JY, Kim HC, Lee SY, Jang CG, 2014. Transient receptor potential vanilloid type 1 channel may modulate opioid reward. Neuropsychopharmacology 39, 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C, Loureiro M, Kramar C, Zunder J, Renard J, Rushlow W, Laviolette SR, 2016. Cannabidiol modulates fear memory formation through interactions with serotonergic transmission in the mesolimbic system. Neuropsychopharmacology 41, 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R, 2004. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl.) 175, 360–366. [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S, 1993. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl.) 110, 390–394. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol 153, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL, 2009. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J. Neurosci 29, 14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Loureiro M, Rosen LG, Zunder J, de Oliveira C, Schmid S, Rushlow WJ, Laviolette SR, 2016. Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J. Neurosci 36, 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Russo EB, 2018. Cannabis therapeutics and the future of neurology. Front. Integr. Neurosci 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK, 2005. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res 30, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N-O, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ, 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin PE, Detyniecki K, 2018. Cannabidiol for epilepsy: new hope on the horizon? Clin. Ther 40, 1438–1441. [DOI] [PubMed] [Google Scholar]
- Scherma M, Masia P, Satta V, Fratta W, Fadda P, Tanda G, 2019. Brain activity of anandamide: a rewarding bliss? Acta Pharmacol. Sin 40, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, Etges T, Sommerville K, 2018. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav 88, 162–171. [DOI] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Ramchandani VA, Hurd YL, Le Foll B, 2017. The endocannabinoid system as a target for addiction treatment: trials and tribulations. Neuropharmacology 124, 73–83. [DOI] [PubMed] [Google Scholar]
- Spiller K, Bi GH, Galaj E, Garder EL, Xi ZX, 2019. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br. J. Pharmacol 176 (9), 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CAJ, da Silva TR, Raymundi AM, de Souza CP, Hiroaki-Sato VA, Kato L, Guimarães FS, Andreatini R, Takahashi RN, Bertoglio LJ, 2017. Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology 125, 220–230. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K, 2015. Aiming for allosterism: evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol. Res 99, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylantyev S, Jensen TP, Ross RA, Rusakov DA, 2013. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. U. S. A 110, 5193–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Gidal B, Blakey G, Tayo B, Morrison G, 2018. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32, 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB, 2019. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol 176, 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa D, Toguri JT, Szczesniak AM, Kelly ME, 2017. The non-psychoactive phytocannabinoid, cannabidiol (CBD), and the synthetic derivatives, HU308 and CBD-DMH, reduces hyperalgesia and inflammation in a mouse model of corneal injury. FASEB J 31 811.817–811.7. [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG, 2007. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol 150, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D, 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Vilela LR, Gomides LF, David BA, Antunes MM, Diniz AB, Moreira F. de A., Menezes GB, 2015. Cannabidiol rescues acute hepatic toxicity and seizure induced by cocaine. Mediat. Inflamm 2015, 523418 10.1155/2015/523418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez-Martínez A, García-Gutiérrez MS, Fraguas-Sánchez AIJ, Torres-Suárez AI, Manzanares J, 2018Eb Effects of cannabidiol plus naloxone on motivation and ethanol consumption. Br. J. Pharmacol 175, 3369–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez-Martínez A, García-Gutiérrez MS, Medrano-Relinque J, Navarrón CM, Navarrete F, Manzanares J, 2019. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacol. Sin 40, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA, 2009. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend 105, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer ANM, De Vries TJ, 2008. The role of CB1 receptors in psychostimulant addiction. Addict. Biol 13, 225–238. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Müller A, Melnik A, Waltinger TP, Ullrich O, Kempermann G, 2010. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL, 2006a. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J. Neurosci 26, 8531–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Newman AH, Gilbert JG, Pak A-C, Peng X-Q, Ashby CR Jr., Gitajn L, Gardner EL, 2006b. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 31, 1393–13405. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng X-Q, Gardner EL, 2008. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology 33, 1735–1745. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Peng X-Q, Li X, Song R, Zhang H-Y, Liu Q-R, Yang H-J, Bi G-H, Li J, Gardner EL, 2011. Brain cannabinoid CB₂ receptors modulate cocaine’s actions in mice. Nat. Neurosci 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You I-J, Wright SR, Garcia-Garcia AL, Tapper AR, Gardner PD, Koob GF, David Leonardo E, Bohn LM, Wee S, 2016. 5-HT1A autoreceptors in the dorsal raphe nucleus convey vulnerability to compulsive cocaine seeking. Neuropsychopharmacology 41, 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L-L, Zhou S-J, Wang X-Y, Liu J-F, Xue Y-X, Jiang W, Lu L, 2011. Effects of cannabinoid CB₁ receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav. Brain Res 217, 111–116. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, Gardner EL, Wu J, Xi Z-X, 2014. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. U.S.A 111, E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Shen H, Bi G-H, Yang H-J, Liu Q-R, Wu J, Gardner EL, Bonci A, Xi Z-X, 2017. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict. Biol 22, 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


