Abstract
Split thickness skin graft (STSG) is a versatile procedure performed for the treatment of wounds resulting from varying pathology. This remains very useful because of its ability for quick healing and low complication rate. The surface of the foot and ankle is an area frequently affected by severe skin and soft tissue structure infections (SSTIs) whose treatment results in wounds. These infections and resultant surgical wounds are commonly seen patients with diabetes. The objective of the present study was to retrospectively evaluate initial healing and immediate post-operative outcomes following STSG application in a diabetic population when negative pressure wound therapy (NPWT) was used as a bolster. Ten patients were identified, including 11 surgical wounds, who underwent STSG bolstered with NPWT from January 2016 to October 2018. Mean follow-up was 13 months (range 1-33 months) with an average time to heal of 17 days (range 14-30 days) for 11 surgical wounds averaging 57 cm2 (range 6.3 - 91 cm2). Consistent improved outcomes have been demonstrated when compared to alternative bolstering techniques available in the literature making a STSG bolstered with NPWT a powerful tool in the reconstruction of diabetic foot wounds resulting from the treatment of infection.
Levels of Evidence: Level IV
Keywords: foot, reconstruction, wound, diabetes, split thickness skin graft, infection, negative pressure wound therapy
“. . . the role of STSG [split thickness skin graft] in reconstruction of the diabetic foot and ankle wounds has recently been exemplified.”
The use of split thickness skin graft (STSG) has withstood the test of time for coverage of wounds of the entire body. Documentation of its use dates to 3000 bc for the treatment of traumatic facial reconstruction.1 Monumental steps leading to the modern techniques were made by Padgett and Hood in 1939 with the use of electrodermatome and meshing of the graft originally described by Tanner et al.2,3
Lower extremity studies have confirmed its utility for coverage of burns, but the role of STSG in reconstruction of the diabetic foot and ankle wounds has recently been exemplified.4-10 Deep abscess of the foot and ankle necessitates timely surgical intervention to control infection and to prevent sepsis and septic shock. Often the intervention results in large wounds that primary closure is not an option. Timely healing in the diabetic population is of paramount importance to be able to avoid recurrent infection and amputation. Pathophysiological factors such as endothelial dysfunction, impaired microcirculation, neuropathy, and hyperglycemia all contribute in longer healing times, which increase susceptibility to infection and ultimately propagation to further tissue loss.11-13
We hypothesized that use of negative pressure wound therapy (NPWT) as a bolster will improve overall recovery time and success at the graft site as measured by time to heal in a diabetic population. This retrospective review aims to further validate the use of STSG for reconstructive efforts in the diabetic population.
Patients and Methods
With institutional review board approval (Baylor College of Medicine, protocol H-44423), after an expedited review, a retrospective medical record review was performed. Current Procedural Terminology codes were used to identify a total of 15 patients who received STSG bolstered with NPWT as delivered by Vacuum Assisted Closure (VAC) Therapy System (KCI USA, San Antonio, TX, USA) from January 2016 to October 2018. Of those, 10 patients (66%) with 11 wounds met the inclusion criteria. These patients were evaluated by the author at the clinic or hospital setting. The inclusion criteria were previous diagnosis of diabetes mellitus, lower extremity infection that required surgical debridement in the operating room, and application of STSG using NPWT Vacuum Assisted Closure (VAC) Therapy System as a bolster during the reconstructive process. The patients were required to be older than 18 years and have not undergone previous surgical intervention for the defect such as skin graft or flap coverage.
Healed wound was defined based on US Food and Drug Administration as reepithelialized skin without drainage or dressing requirements confirmed in 2 consecutive visits 2 weeks apart.14
Demographic data (Table 1), including age, sex, tobacco use, and comorbidities were obtained from the electronic medical record for analysis in addition to lab values (HbA1c, albumin), vascular status, and wound characteristics (Table 2), including size, time to heal, and clinical progress based on clinical notes and pictures. Time to heal and percent take of the STSG was determined based on clinical notes and clinical pictures. Pictures were taken at each visit and findings were recorded (Figures 1A to 3K). Epithelization was visually estimated as a percent of incorporation by the surgeon at 2 weeks (Figures 2A-K) during removal of the bolster and confirmed at consequent weekly visits. Outcome assessors were participants in this intervention.
Table 1.
Patients’ Demographic Characteristics, Systemic Comorbidities, and Lab and Vascular Data Characteristics (N = 11 Feet in 10 Patients).
| Wound No. | Sex | Age (y) | HbA1c (%) | Albumin | Vascular Status | Comorbidities |
|---|---|---|---|---|---|---|
| 1 | M | 65 | 7.2 | 2.4 | N/A | DM 2 |
| 2 | F | 76 | 6.6 | 2.4 | N/A | DM 2 |
| 3 | F | 76 | 6.6 | 2.4 | N/A | DM 2 |
| 4 | M | 70 | 8 | 3.6 | Revascularized | DM 2, Charcot |
| 5 | M | 67 | 7 | 2.5 | N/A | DM 2, Tobacco |
| 6 | M | 87 | 8.1 | 2.7 | Revascularized | DM 2, Charcot |
| 7 | F | 57 | 7.4 | 3.7 | Revascularized | DM 2, Charcot |
| 8 | F | 41 | 6 | 2.9 | N/A | DM 2, CKD IV |
| 9 | M | 41 | 8 | 2.7 | N/A | DM 2 |
| 10 | M | 37 | 14 | 3.5 | N/A | DM 2, Charcot |
| 11 | M | 49 | 9.6 | 3.6 | N/A | DM 2, Charcot |
| Avg 60.5 | Avg 8.05 | Avg 2.95 |
Abbreviations: M, male; F, female; Avg, average; DM 2, diabetes mellitus type 2; CKD, chronic kidney disease; N/A, not applicable.
Table 2.
Wound Characteristics of 10 Patients 11 Wounds With Diabetes Mellitus Type 2 Who Received Split Thickness Graft Bolstered With Negative Pressure Wound Therapy (N = 11 Feet in 10 Patients).
| Wound No. | Age (y) | Follow-up (mo) | Time to Heal (d) | Wound Area (cm2) | Location |
|---|---|---|---|---|---|
| 1 | 65 | 33 | 30 | 91 | Dorsal |
| 2 | 76 | 24 | 14 | 16.5 | Posterior leg |
| 3 | 76 | 24 | 14 | 6.3 | Posterior leg |
| 4 | 70 | 21 | 14 | 41.2 | Plantar |
| 5 | 67 | 15 | 14 | 42 | Dorsal |
| 6 | 87 | 11 | 14 | 80.5 | Plantar |
| 7 | 57 | 7 | 14 | 21.6 | Plantar |
| 8 | 41 | 5 | 14 | 50 | Plantar |
| 9 | 41 | 4 | 14 | 67.5 | Dorsal |
| 10 | 37 | 2 | 21 | 45 | Plantar |
| 11 | 49 | 1 | 21 | 22.5 | Plantar |
| Avg 60.5 | Avg 13.3 mo | Avg 16.7 d | 57 cm2 |
Abbreviation: Avg, average; d, Days; sq cm, square centimeters; post, posterior.
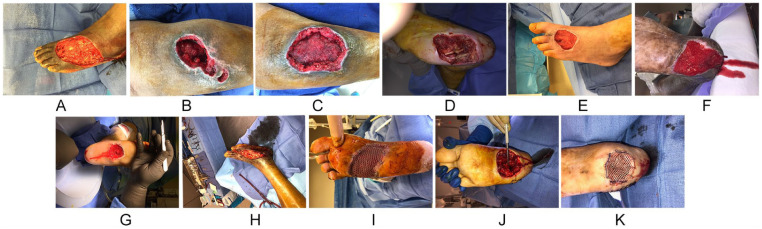
Figure 1.
(A) Intraoperative picture of a dorsal foot wound resulting from the surgical treatment of necrotizing fasciitis. (B) Intraoperative picture of a posterior leg wound resulting from the surgical treatment of an infected hematoma following a motor vehicle accident. (C) Intraoperative picture of a posterior leg wound resulting from the surgical treatment of an infected hematoma following a motor vehicle accident. (D) Intraoperative picture of a plantar foot wound resulting from surgical treatment of infection associated with Charcot neuroarthopathy. (E) Intraoperative picture of a dorsal foot wound resulting from surgical treatment of a dorsal foot abscess with concomitant osteomyelitis of the second metatarsal. (F). Clinical picture of plantar wound resulting from surgical treatment of calcaneal osteomyelitis after failed previous surgical intervention. (G) Intraoperative picture of a plantar wound resulting from surgical treatment of infection associated with Charcot neuroarthopathy. (H) Intraoperative picture of a dorsal foot wound resulting from surgical treatment of a dorsal foot abscess extending to the ankle with concomitant osteomyelitis of the fourth metatarsal. (I) Intraoperative picture of a plantar foot wound with split thickness skin graft secured with staples. (J) Intraoperative picture of a plantar foot wound associated with Charcot neuroarthopathy with visible sutures from a lateral-based approach to plantar ostectomy. (K) Intraoperative picture of a plantar foot wound associated with Charcot neuroarthropathy covered with split thickness skin graft secured with staples with visible sutures from a lateral based approach to plantar ostectomy.
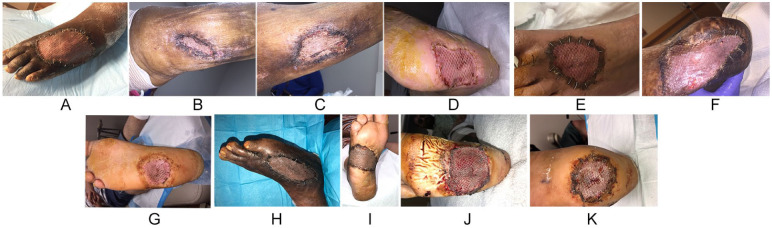
Figure 3.
(A-K) Clinical pictures of interval healing of split thickness skin graft beyond the 21-day mark.
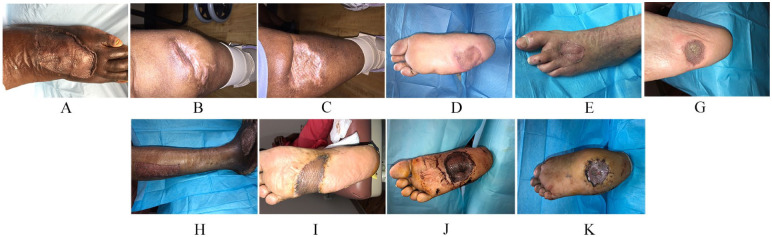
Figure 2.
(A-K) Clinical pictures of interval healing of split thickness skin graft 14 days postoperatively.
All wounds were the result of tissue loss secondary to SSTIs (Figures 1A-K). Diabetes mellitus type 2 was the major comorbidity for 11 wounds with average HbA1c of 8% (range 6.0% to 14%). The average albumin was 2.9 g/dL (range 2.4 to 3.7 g/dL). Five plantar midfoot wounds were secondary to Charcot neuroarthropathy–associated abscess (Figure 1D, F, G, J, and K). One forefoot plantar foot wound was the result of surgically treated diabetic foot abscess with concomitant osteomyelitis of part of the fourth and fifth metatarsals (Figure 1I). Two posterior leg wounds were secondary to infected hematoma formation following a motor vehicle accident (Figure 1B and C). One dorsal foot wound was secondary to necrotizing soft tissue infection (Figure 1A) and 2 dorsal foot wounds were secondary to surgically treated abscess associated with osteomyelitis of lesser metatarsals (Figure 1E and H).
The vascular status of all but 3 patients (27%) that required endovascular procedure was adequate with intact vasculotome to the area of interest. The other 3 required endovascular revascularization prior to reconstruction. This was confirmed with handheld Doppler ultrasound (Summit Doppler Bi-Directional Vascular Probe, VistaAVS/VistaABI 8 MHz) in all patients. Two (20%) of the patients were smokers. The wounds exposed to the level of subcutaneous tissue, fascia and muscle. No exposed periosteum or bone were noted during application of the STSG. The average follow-up was 13.3 months (range 1-33 months).
Statistical Analysis
Demographic and clinical characteristics in the study population are described using the mean for continuous variables; percentages were used for categorical variables.
Surgical Technique and Postoperative Protocol
Standard procedure was used with modification on the timing of the NPWT application and framing of the wound are noted as follows. The patient’s surgical wound is optimized for application of graft by ensuring systemic control of diabetes, vascular inflow is primed, appropriate granulation tissue with decrease in bacterial colonization, ensuring absence of infection, and no exposed osseous or non-viable structures. Debridement intraoperatively involves removing all devitalized tissue with sharp instrumentation, scalpel, and curettes. Additionally, irrigation of the wound with sterile saline using gravity irrigation while the wound is debrided meticulously with a curette to promote pin-point bleeding of all tissues. Standard donor site preparation and harvest techniques were employed for the STSG.
The graft is meshed in a 1:1.5 ratio using a commercially available mesher and then secured to the recipient site by skin staples ensuring good contact of the graft on the entire surface. The bolster dressing consists of a single layer of Adaptic nonadhering dressing placed directly over the graft (Figures 4-9), which is secured with staples in the periphery followed by negative pressure wound therapy using NPWT via Vacuum-Assisted Closure Device (VAC) Therapy System. The periphery of the wound is framed with duoDERM (Figure 4) prior to application of the sponge that allows the NPWT to stay uninterrupted for 7 days. Xeroform is sutured in place in the donor site followed by Adaptic nonadhering dressing and NPWT that is set at 75 mm Hg continuous mode high intensity. A well-padded posterior splint is placed on the operative lower extremity.
Figure 4.
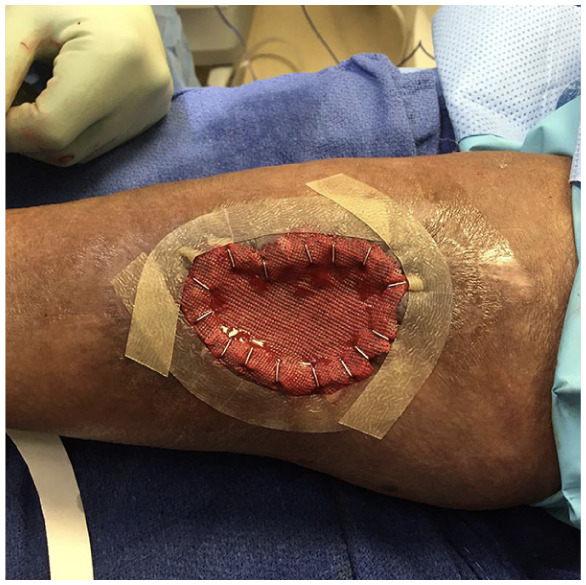
Intraoperative picture of a posterior leg wound with split thickness skin graft secured with staples, Adaptic nonadhering dressing secured with staples and DuoDERM framing the wound prior to application of negative pressure wound therapy.
Figure 5.
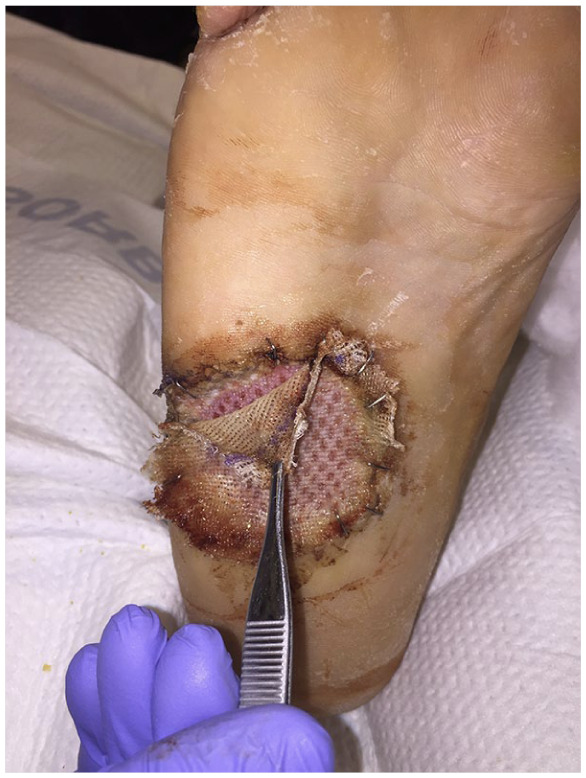
Clinical picture of interval healing of split thickness skin graft at 7 days postoperatively following removal of the negative pressure wound therapy bolster. Checking the edges for appropriate take.
Figure 6.
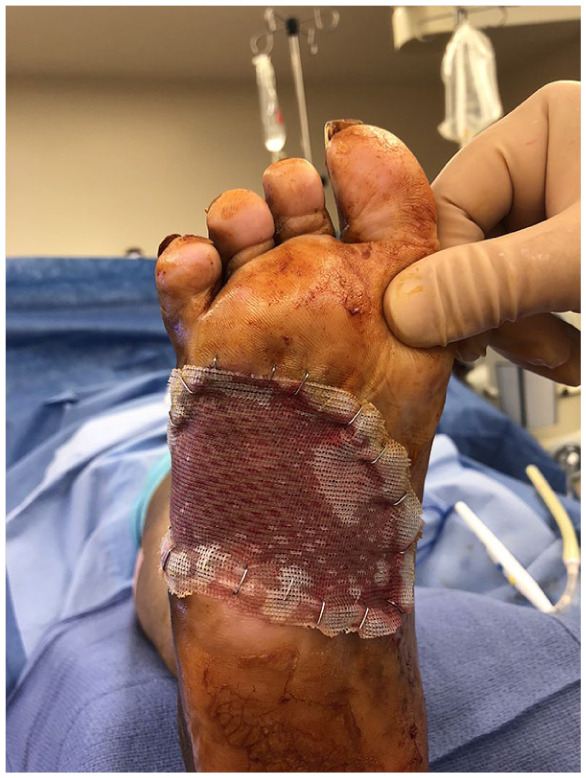
Intraoperative picture of a plantar foot wound with split thickness skin graft covered with Adaptic nonadhering dressing and secured with staples.
Figure 7.
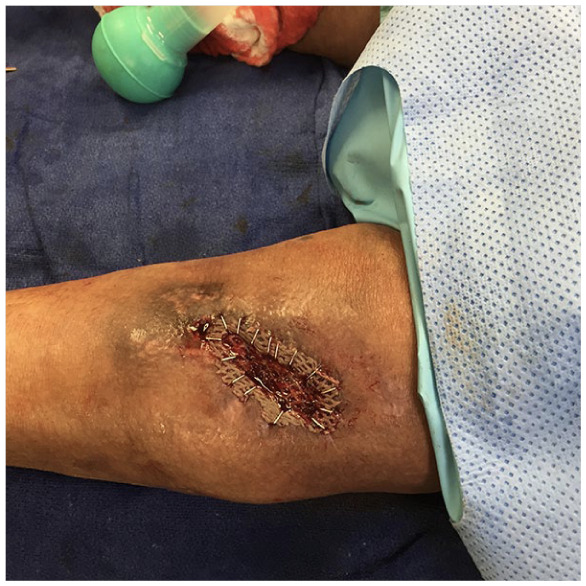
Intraoperative picture of a posterior leg wound with split thickness skin graft secured with staples.
Figure 8.
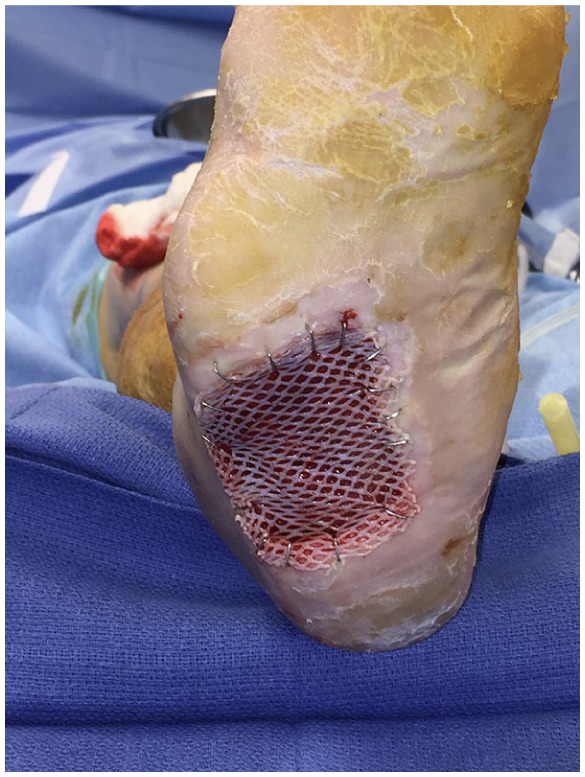
Intraoperative picture of a plantar foot wound with split thickness skin graft secured with staples.
Figure 9.
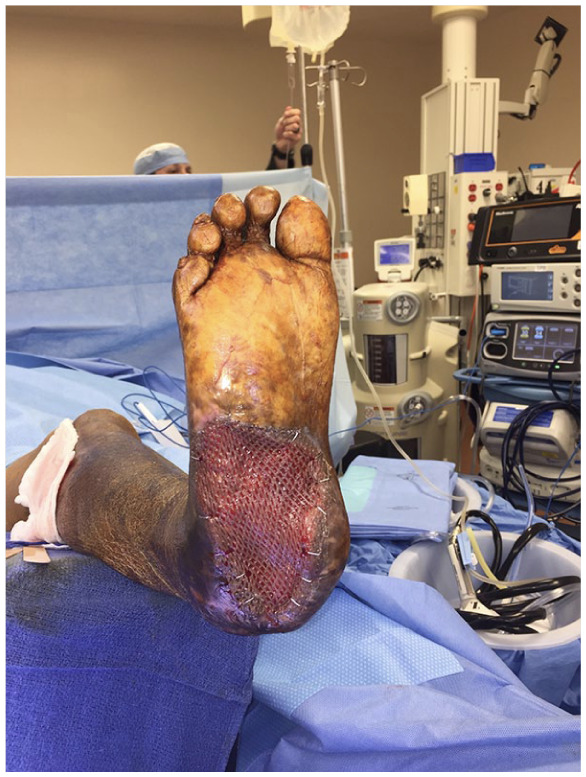
Intraoperative picture of a plantar wound with split thickness skin graft secured with staples.
The first postoperative dressing change is 7 days following initial graft application, which entails removal of the NPWT. At that point, the Adaptic on the recipient site is not removed. Two percent mupirocin ointment is applied on both donor and recipient sites followed by a well-padded posterior splint. At 14 days postoperatively, the Adaptic at the recipient site is removed with all staples (Figure 5). At the donor site, the Xeroform is removed. The donor is then allowed to heal by secondary intention by application of povidone/iodine 10% solution and Adaptic. The recipient site is dressed with Adaptic and Aquaphor ointment. For dorsal grafts, weightbearing begins the second week with a control ankle motion boot. For plantar grafts, weightbearing begins the third week postoperatively with a controlled ankle motion boot. Edema is controlled with Tubigrip. Patients are instructed to apply Aquaphor ointment on both sites every 2 to 3 days. For grafts on the dorsal aspect of the foot and ankle, patients begin weightbearing with supportive shoe gear once edema allows. For grafts on the plantar aspect of the foot, patients begin weightbearing with custom-made multidensity inserts and depth inlay commercially available shoes when their fabrication process is completed approximately 4 weeks following graft application.
Results
The average follow-up was 13 months. All surgical wounds demonstrated 100% take at 14 days and complete epithelialization by 21 days except 1 wound (9%) (Figure 2A-K). The specific wound healed at the fourth postoperative appointment in 30 days. The graft exhibited 100% take at 14 days (Figure 2A); however, a small area overlying the exposed inferior extensor retinaculum was slower to completely epithelialize at the recipient site. Epithelialization and healing were confirmed in the third and fourth week at 21 and 28 days, respectively (Figure 3A-K). Weightbearing was initiated in a control ankle motion boot after the second week for dorsal grafts and after the third week for plantar grafts due to graft immaturity.
The majority of the donor sites completely epithelialized at a later date when compared with the recipient sites. Time to ambulation out of the control ankle motion boot with supportive shoe gear was 3 weeks for STSG performed at the dorsal foot if edema of the foot allowed. Time to ambulation out of the control ankle motion boot with supportive shoe gear for STSG performed at the plantar foot was when fabrication of custom-made multidensity inserts was completed, which was approximately 4 to 5 weeks postoperatively. There was no difference in healing times between dorsal and plantar grafts (Table 2).
Complications
None of the surgically treated wounds became infected during the healing process. One wound (9%) located on the plantar surface of the foot STSG healing was complicated by recurring breakdown 3 weeks following complete healing. The patient was treated with total contact casting and healed without recurrence. One patient was lost to follow-up after complete healing. At an average follow-up of 13.3 months (range 1-33 months), the rest of the patients remained healed (Table 2).
Discussion
The diabetic population continues to increase and poses significant challenge to the foot and ankle surgeons given the presence of neuropathy, immunocompromised state, and compromised ability to heal.11-13 Failure to heal in a timely fashion increases the risk for infection, propagation of infection, further tissue damage, and ultimately higher level amputations. These are associated with increase in morbidity and overall mortality, emphasizing the importance of healing on timely fashion.15-19
STSG has been added on the armamentarium for reconstruction of diabetic foot and ankle wounds, as it can shorten the treatment duration and provide functional outcomes.5-10 A literature review (N = 229 patients) published in 2012, which included 4 studies, found that STSG are 78% successful at closing 90% of the wounds with 1 procedure by 8 weeks with no documented reulceration or infection to the recipient site at final follow-up.20
Anderson et al8 determined in their retrospective review of (N = 107) diabetic patients who received an STSG, that none of the comorbidities or risk factor variables had an independent effect on time to complete wound healing. Factors studied included smoking, peripheral arterial disease, end-stage renal disease, cardiac disease, and Charcot neuroarthropathy. Another important finding by Anderson et al8 was that mean healing time for patients with complications was 12 weeks while for those without complications was 4.9 weeks.8 The aforementioned study would have positively impacted the previous systematic review with mean healing times of 5.1 weeks and complication rate of 2.8%. Ramanujam et al9 discovered on their review of 83 diabetic patients that graft sizes are not associated with time to complete healing, postoperative complications significantly associated with current or previous smoking history (P = .016), but also validated significantly increased time to heal (χ2 = 6.79, P = .009) in patients with complications. The median time to complete healing for patients with complications 9.5 weeks versus 6.4 weeks for patients without complications.
Mahmoud et al10 prospectively studied patients with STSG versus conservative wound care for diabetic foot wounds and determined a statistically significant reduction (P < .001) in mean hospital stay and healing time for the patients belonging to the STSG group. Yet a recent retrospective review found no statistical difference in HbA1c between the group that healed the skin graft when compared with the group that the skin graft failed to adhere.21
In this study, the wounds were soft tissue defects resulting from the surgical treatment of SSTIs. NPWT was used as a bolster following STSG application for 7 days postoperatively in the hospital setting or at home setting when other patient-related factors permitted. Chiummariello et al22 calculated the economic impact when NPWT is used as a bolster for STSG in the hospital setting in Italy, but with different apparatus and dressing change protocol.
The greatest controversy in the literature seems to lie with the type of wound to treat; surgical versus conservative wound care. Lavery et al15 reported that a wound duration of >30 days is an independent risk factor for diabetic foot infection (odd ratio 4.7). The healing likelihood via secondary intention of the wounds above can be calculated based on the validated Stratification system wound healing index that can be applied in diabetic foot ulcers.23 The elaborate work performed by Fife et al24 based on 26 randomized controlled trials showed that 30.5% of diabetic foot wounds healed at 12 weeks, and 45.1% at indeterminate time, with mean follow-up of 19.7 weeks; data obtained from the US wound registry.
Healing in a timely manner is a crucial component for diabetic wounds independent of chronicity. This study focused on initial healing and immediate postoperative outcomes following STSG application with average time to heal of 17 days (range 14-30) days for all 11 surgical wounds averaging 57 cm2 (range 6.3-91 cm2) and demonstrated consistent results. These findings warrant further investigation given the difference in healing times and success rate when compared with alternative bolstering techniques for STSG in the diabetic population available in the literature.
The procedures performed by one foot and ankle surgeon with modification of the bolstering technique with standard operative technique and the postoperative course. Clinical evidence from a plethora of studies suggests NPWT to bolster STSG is superior to traditional bolstering techniques.22,25-35 At the physiological and molecular level notable studies included. Porcine experiment model with graft biopsies at days 2, 4, 6, 8, and 10 contradicts any difference in vascular ingrowth even though better subjective graft incorporation was noted in the NPWT samples and a nonsignificant trend toward improved graft survival in the NPWT group.36 On a different animal model, NPWT appears to improve angiogenesis and blood circulation occurred by increasing capillary caliber and blood volume, while also decrease in edema by narrowing endothelial spaces resulting in decrease permeability.37 Studies on human subjects demonstrated that NPWT applied to traumatic wounds increases levels of interleukin 8 (P < .001), and vascular endothelial growth factor (P < .05) measured with enzyme-linked immunosorbent assay suggestive of increase angiogenesis, but also histologic examination revealed increase neovascularization (P < .05) illustrated by CD31 and von Willenbrand factor.38 Other studies have demonstrated improved migration and proliferation of epithelial cells when NPWT is applied.39,40
The clinical effectiveness of NPWT in diabetic foot ulcers is undoubtedly based on a systematic review of 7 randomized controlled trials, in which STSG was not used as a bolster.41 Conflicting evidence from a different systematic review of randomized control trials found no clear indication that wounds heal any better or worse with NPWT than with conventional treatments.42 Worth mentioning is the latest systematic review that included only 1 study where NPWT was used as a bolster for STSG. That study included was focused on pretreatment.26,42 Randomized control and retrospective studies in mixed populations shows increase success in healing time greater than 95% when using NPWT as a bolster for STSG.22,25,26,31,32 An international panel has recommended the use for NPWT in traumatic wounds and reconstructive surgery with the highest evidence in fixation of STSG.43
Weaknesses of the study were that all cases were performed by single surgeon, relatively short follow-up period, the small number of patients, and retrospective design. Another shortcoming was that the patients were reviewed by a single reviewer, the author, and documented with clinical pictures with no other observer reviewing the cases.
This study suggests the use of NPWT used as a bolster in the diabetic population for quicker recovery and higher success at the graft site as measured by time to heal and STSG take. One graft (9%) exhibited a minor complication shortly after complete healing due to patient relates factors (inappropriate shoe gear early in the recovery), but eventually went to healing. At the time this study was concluded, 1 patient had been lost to follow-up. The remaining patients had no documented reulceration or breakdown at the recipient site. At present, there is no consensus as to the mechanism that NPWT improves outcomes currently exists, but some physiologic benefits exist and were mentioned above. The author advocates its use for surgical defects resulting from the treatment of diabetic infections on both the plantar and dorsal foot and attributes its success to factors that have already been identified, in addition to the reduction of shear forces between the graft and wound bed.32,44 There is mounting clinical evidence that outcomes are superior with its use. The results of this study are important in the diabetic population as they indicated quicker return to baseline activity. Subsequently, reduction in the number of visits to the physician for treatment of the wound, and ultimately reduction in the risk of amputation are potential outcomes with the technique.
Acknowledgments
The author would like to acknowledge Gundersen Health system in La Crosse, Wisconsin, Department of Orthopedics and Podiatry, Plastics and Reconstructive Surgery for their significant role in education. The author thanks Dr Joseph Benacci whom many parts of the technique used were adapted from. The author thanks Dr Michael Donnenwerth and Dr Christine Choi for proofreading our report.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Institutional review board approval was obtained from the Baylor College of Medicine (protocol H-44423).
Informed Consent: Not applicable.
Trial Registration: Not applicable.
ORCID iD: Efthymios Gkotsoulias  https://orcid.org/0000-0002-0506-9299
https://orcid.org/0000-0002-0506-9299
References
- 1. Davis JS. Address of the President: the story of plastic surgery. Ann Surg. 1941;113:641-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shapira J. Technique of split thickness skin grafts using Padgett-Hood dermatome. Med Rec Ann. 1947;41:377-379. [PubMed] [Google Scholar]
- 3. Tanner JC, Jr, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg. 1964;34:287-292. [PubMed] [Google Scholar]
- 4. Chick LR. Brief history and biology of skin grafting. Ann Plast Surg. 1988;21:358-365. [DOI] [PubMed] [Google Scholar]
- 5. Baumeister S, Dragu A, Jester A, Germann G, Menke H. The role of plastic and reconstructive surgery within an interdisciplinary treatment concept for diabetic ulcers of the foot [in German]. Dtsch Med Wochenschr. 2004;129:676-680. [DOI] [PubMed] [Google Scholar]
- 6. Roukis TS, Zgonis T. Skin grafting techniques for soft-tissue coverage of diabetic foot and ankle wounds. J Wound Care. 2005;14:173-176. [DOI] [PubMed] [Google Scholar]
- 7. Zgonis T, Stapleton JJ, Roukis TS. Advanced plastic surgery techniques for soft tissue coverage of the diabetic foot. Clin Podiatr Med Surg. 2007;24:547-568. [DOI] [PubMed] [Google Scholar]
- 8. Anderson JJ, Wallin KJ, Spencer L. Split thickness skin grafts for the treatment of non-healing foot and leg ulcers in patients with diabetes: a retrospective review. Diabet Foot Ankle. 2012;3. doi: 10.3402/dfa.v3i0.10204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramanujam CL, Stapleton JJ, Kilpadi KL, Rodriguez RH, Jeffries LC, Zgonis T. Split-thickness skin grafts for closure of diabetic foot and ankle wounds: a retrospective review of 83 patients. Foot Ankle Spec. 2010;3:231-240. [DOI] [PubMed] [Google Scholar]
- 10. Mahmoud SM, Mohamed AA, Mahdi SE, Ahmed ME. Split-skin graft in the management of diabetic foot ulcers. J Wound Care. 2008;17:303-306. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30:3058-3062. [DOI] [PubMed] [Google Scholar]
- 12. Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marston WA; Dermagraft Diabetic Foot Ulcer Study Group. Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia. Ostomy Wound Manage. 2006;52:26-28, 30, 32. [PubMed] [Google Scholar]
- 14. US Food and Drug Administration. Guidance for industry chronic cutaneous ulcer and burn wounds—developing products for treatment. https://www.fda.gov/media/71278/download. Published June 2006. Accessed June 27, 2019. [DOI] [PubMed]
- 15. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288-1293. [DOI] [PubMed] [Google Scholar]
- 16. Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013;46:124-131. [DOI] [PubMed] [Google Scholar]
- 17. Wukich DK, Ahn J, Raspovic KM, Gottschalk FA, La Fontaine J, Lavery LA. Comparison of transitibial amputations in diabetic patients with and without end-stage renal disease. Foot Ankle Int. 2017;38:388-396. [DOI] [PubMed] [Google Scholar]
- 18. Lavery LA, Hunt NA, Ndip A, Lavery DC, Van Houtum W, Boulton AJ. Impact of chronic Kidney disease on survival after amputation in individuals with diabetes. Diabetes Care. 2010;33:2365-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg. 2016;55:591-599. [DOI] [PubMed] [Google Scholar]
- 20. McCartan B, Dinh T. The use of split-thickness skin grafts on diabetic foot ulcerations: a literature review. Plast Surg Int. 2012;2012:715273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanniec K, Nguyen T, van Asten S, Fontaine J, Lavery LA. Split-thickness skin grafts to the foot ankle of diabetic patients. J Am Podiatr Med Assoc. 2017;107:365-368. [DOI] [PubMed] [Google Scholar]
- 22. Chiummariello S, Del Torto G, Lera M, Arleo S, Alfano C. Negative pressure dressing in split-thickness skin grafts: experience with an alternative method. Wounds. 2013;25:324-327. [PubMed] [Google Scholar]
- 23. Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson B. A predictive model for diabetic foot ulcer outcome: the Wound Healing Index. Adv Wound Care (New Rochelle). 2016;5:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fife CE, Eckert KA, Carter MJ. Publicly reported wound healing rates: the fantasy and the reality. Adv Wound Care (New Rochelle). 2018;7:77-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunn RM, Ignotz R, Mole T, Cockwill J, Smith JM. Assessment of gauze-based negative pressure wound therapy in the split-thickness skin graft clinical pathway—an observational study. Eplasty. 2011;11:e14. [PMC free article] [PubMed] [Google Scholar]
- 26. Saaiq M, Hameed-Ud-Din, Khan MI, Chaudhery SM. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak. 2010;20:675-679. [PubMed] [Google Scholar]
- 27. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917-922. [DOI] [PubMed] [Google Scholar]
- 28. Blume PA, Key JJ, Thakor P, Thakor S, Sumpio B. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C® therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7:480-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dissemond J, Körber A, Grabbe S. Take of mesh grafts in chronic leg ulcer patients improves by vacuum-assisted closure device [in German]. Zentralbl Chir. 2006;131(suppl 1):S165-S167. [DOI] [PubMed] [Google Scholar]
- 30. Egemen O, Ozkaya O, Ozturk MB, Aksan T, Orman Ç, Akan M. Effective use of negative pressure wound therapy provides quick wound bed preparation and complete graft take in the management of chronic venous ulcers. Int Wound J. 2012;9:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohsin M, Zargar HR, Wani AHet al. Role of customized negative-pressure wound therapy in integration of split-thickness skin grafts: a randomized control study. Indian J Plast Surg. 2017;50:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blackburn JH, 2nd, Boemi L, Hall WWet al. Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg. 1998;40:453-457. [DOI] [PubMed] [Google Scholar]
- 33. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137:930-934. [DOI] [PubMed] [Google Scholar]
- 34. Kim EK, Hong JP. Efficacy of negative pressure therapy to enhance take of 1-stage allodermis and a split-thickness graft. Ann Plast Surg. 2007;58:536-540. [DOI] [PubMed] [Google Scholar]
- 35. Schneider AM, Morykwas MJ, Argenta LC. A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg. 1998;102:1195-1198. [DOI] [PubMed] [Google Scholar]
- 36. Ward C, Ciraulo D, Coulter M, Desjardins S, Liaw L, Peterson S. Does treatment of split-thickness skin grafts with negative-pressure wound therapy improve tissue markers of wound healing in a porcine experimental model? J Trauma Acute Care Surg. 2012;73:447-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg. 2005;28:211-217. [DOI] [PubMed] [Google Scholar]
- 38. Labler L, Rancan M, Mica L, Härter L, Mihic-Probst D, Keel M. Vacuum-assisted closure increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma. 2009;66:749-757. [DOI] [PubMed] [Google Scholar]
- 39. Baldwin C, Potter M, Clayton E, Irvine L, Dye J. Topical pressure stimulates endothelial migration and proliferation: a suggested mechanism for improved integration of integra. Ann Plast Surg. 2009;62:92-96. [DOI] [PubMed] [Google Scholar]
- 40. Hsu CC, Tsai WC, Chen CP, Lu YM, Wang JS. Effects of negative pressure on epithelial tight junctions and migration in wound healing. Am J Physiol Cell Physiol. 2010;299:C528-C534. [DOI] [PubMed] [Google Scholar]
- 41. Xie X, McGregor M, Dendukuri N. The clinical effectiveness of negative pressure wound therapy: a systematic review. J Wound Care. 2010;19:490-495. [DOI] [PubMed] [Google Scholar]
- 42. Peinemann F, Sauerland S. Negative-pressure wound therapy-systematic review of randomized controlled trials. Dtsch Arztebl Int. 2011;108:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krug E, Berg L, Lee Cet al. ; International Expert Panel on Negative Pressure Wound Therapy [NPWT–EP]. Evidence-based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2011;42(suppl 1):S1-S12. [DOI] [PubMed] [Google Scholar]
- 44. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6:185-194. [DOI] [PubMed] [Google Scholar]