Abstract
Background:
Teriflunomide 14 mg significantly reduced brain volume loss (BVL) and confirmed disability worsening (CDW) compared with placebo in the TEMSO core study.
Objective:
To investigate the relationship between BVL from Baseline to Year 2 in the TEMSO core study and long-term CDW (Year 7) in the TEMSO long-term extension (NCT00803049).
Methods:
Structural Image Evaluation using Normalization of Atrophy determined BVL. Long-term CDW was assessed by Expanded Disability Status Scale confirmed for 12 and 24 weeks. An additional analysis evaluated the relative contribution of BVL (Year 2) and other outcomes as potential mediators of the effect of teriflunomide 14 mg on 12-week CDW.
Results:
Patients with the least BVL were significantly less likely to have 12- and 24-week CDW at Year 7 compared with patients with the most BVL. A mediation analysis revealed that BVL (Year 2) explained 51.3% of the treatment effect on CDW; new or enlarging T2w lesions over 2 years explained 30.8%, and relapses in the first 2 years explained 38.5%.
Conclusions:
These results highlight the potential predictive value of BVL earlier in the disease course on long-term disability outcomes. The mediation analysis suggests that teriflunomide may prevent disability worsening largely through its effects on BVL.
Keywords: Teriflunomide, multiple sclerosis, brain volume loss, disability worsening, TEMSO, mediation analysis
Introduction
Brain volume loss (BVL) is accelerated in patients with MS and occurs early in the disease course. It continues throughout the disease course1–3 and is associated with disability worsening.1,4–7 A meta-analysis of 13 clinical trials of several disease-modifying therapies (DMTs) carried out in 2014 showed a relationship between treatment effect on BVL and disability worsening, at a trial level, over 2 years.5
Teriflunomide is a once-daily oral immunomodulator approved for the treatment of relapsing forms of MS. In two large, placebo-controlled phase 3 studies, TEMSO (NCT00134563) and TOWER (NCT00751881), teriflunomide demonstrated efficacy on clinical endpoints, and magnetic resonance imaging (MRI) outcomes (TEMSO only). In both studies, teriflunomide 14 mg significantly reduced the risk of disability worsening in patients with relapsing forms of MS compared with placebo.8,9 When TEMSO MRI data were evaluated post hoc in a blinded evaluation performing Structural Image Evaluation using Normalization of Atrophy (SIENA),10 teriflunomide 14 mg was shown to significantly slow the rate of BVL over 2 years compared with placebo.11,12
The present post hoc analysis investigates the association of BVL (quantified using annualized percent brain volume change (PBVC) from Baseline to Year 2) with confirmed disability worsening (CDW) at Year 7 in patients with MS treated with teriflunomide 14 mg in the TEMSO long-term extension (NCT00803049).13 Using mediation analysis,14,15 we aimed to determine the proportion of teriflunomide’s effect on disability worsening that is mediated by its effects on BVL, as well as on other outcomes.
Materials and methods
Study design and patients
TEMSO was a multinational, multicenter, randomized, placebo-controlled, double-blind, parallel-group phase 3 study in patients with relapsing MS. Details of the patient population and study design have been published previously.8 Eligible patients were aged 18–55 years, had an Expanded Disability Status Scale (EDSS) score of 0–5.5, and had at least two clinical relapses in the previous 2 years or one relapse during the previous year. A total of 1088 patients were randomized 1:1:1 to receive once-daily oral teriflunomide 7 mg (n = 365) or 14 mg (n = 358), or placebo (n = 363), for 108 weeks (two patients were excluded from treatment due to protocol violations).
Upon core study completion, patients were eligible to enter the long-term, double-blind extension. Patients already receiving teriflunomide 7 or 14 mg remained on their original dose, while those previously receiving placebo were re-randomized 1:1 to teriflunomide 7 or 14 mg.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by central and local ethics committees and each site’s institutional review board. Patients gave written informed consent before entering the study.
MRI analysis
In the TEMSO study, naïve and contrast-enhanced brain MRI was acquired using a standardized sequence protocol. Semi-automated processing was used to extract the various tissue compartment volumes. Serial registered sets of fully processed proton-density/T2-weighted (T2w) dual fast spin echo, FLAIR, as well as naïve and contrast-enhanced T1-weigthed (T1w) datasets with segmentations underwent expert review to enumerate new or substantially enlarged T2w-hyperintense lesions that lacked contrast enhancement.16
Blinded post hoc SIENA analysis was performed using MRI data collected at baseline, Week 48 (Year 1), and Week 108 (Year 2) during the core study, as published before.11 SIENA was applied to naïve T1w images of 3 mm slice thickness without gap in a 70 mm central brain area section (Montreal Neurological Institute Z coordinates −10 to +60 mm), an area selected for optimal reproducibility and comparability to previous trials of other oral DMTs.17–22 MRI of two time points was co-registered, and surface changes were determined using SIENA to estimate BVL (quantified using annualized PBVC).10 SIENA was used without lesion filling because it has been shown that it is relatively insensitive to the presence of white matter lesions, with the exception of cases in which the lesion load exceeds 60 mL and lesions have CSF-like intensity.23
At all stages, rigorous quality control of the SIENA analysis was done. MRI scans were excluded where atrophy calculation was not possible, for example, due to lack of complete coverage, quality of MRI sequence(s) was insufficient for the evaluation, or a reference scan required for evaluation was not available. Baseline demographics and clinical characteristics of the cohort included in the SIENA analysis were similar to those previously published for the entire TEMSO MRI cohort.8
Statistical analysis
Association between BVL and disability worsening
The present analysis is limited to patients with valid SIENA data who were treated with teriflunomide 7 or 14 mg, or placebo, during the core study period. To evaluate the association of BVL with CDW, the SIENA population (teriflunomide 7 or 14 mg, or placebo) was categorized into three groups using BVL thresholds generated from the interquartile range of change from Baseline to Year 2 in the placebo group. Group 1 included patients in the quartile with least BVL (⩽0.52% reduction), Group 2 included patients in the two intermediate BVL quartiles (i.e. the interquartile range; >0.52% to 2.18% reduction), and Group 3 included patients in the quartile with most BVL (>2.18% reduction). The middle quartiles were combined to focus on the two extreme quartiles (Groups 1 and 3), as this contrast is likely to be the most informative.
CDW was defined as ⩾1-point increase for patients with a baseline EDSS of ⩽5.5, or ⩾0.5-point increase for patients with a baseline EDSS of >5.5, that persisted for 12 and 24 weeks. Risk of 12-week CDW was the primary outcome measure, as in the original TEMSO core study.8 Risk of 12- and 24-week CDW and reaching EDSS milestones of ⩾4 or ⩾6 confirmed for 12 weeks was derived from Kaplan–Meier estimates until the end of Year 7, stratified by baseline EDSS strata and region. Comparisons between BVL groups were made using a Cox proportional hazards model with EDSS strata at baseline and region as adjustment variables. These analyses were irrespective of treatment.
Mediation analysis
Mediation analysis was conducted comparing 14 mg teriflunomide to placebo during the core study period (108 weeks). Teriflunomide 7 mg was excluded as this failed to reach significance in the TEMSO study.8 The four Prentice criteria14,15 were used to identify the proportion of treatment effect (PTE) on CDW mediated through three potential surrogates: BVL at Year 2; new or enlarging T2w lesions over 2 years; and relapses in the first 2 years. The four Prentice criteria are as follows:
Criterion 1 requires the potential surrogate to be significantly associated with treatment. The effect of teriflunomide on BVL was assessed using an analysis of variance (ANOVA) model and expressed as a mean difference in BVL between the teriflunomide and placebo groups. The effect of teriflunomide on new or enlarging T2w lesions was evaluated using negative binomial (NB) regression and was expressed as a relative risk (RR) comparing the number of new or enlarging T2w lesions in the teriflunomide arm relative to the placebo arm. The effect of teriflunomide on annual relapse rate (ARR) was similarly analyzed using NB regression, with the association between relapses in the teriflunomide arm relative to the placebo arm expressed as a RR.
Criterion 2 requires the clinical outcome to be associated with treatment. The effect of teriflunomide on the risk of 12-week CDW was assessed using logistic regression and expressed as an odds ratio (OR) comparing the odds of 12-week CDW in the teriflunomide arm to the odds of 12-week CDW in the placebo arm.
Criterion 3 requires the clinical outcome to be associated with the potential surrogate. The association between BVL, new or enlarging T2w lesions, and relapses with EDSS worsening was assessed using logistic regression, with probability of EDSS worsening as the dependent variable, and with BVL, new or enlarging T2w lesions, and relapses as the covariates.
Criterion 4 requires, for an ideal surrogate, that the association of treatment with the clinical outcome (criterion 2) disappears when adjusting for the potential surrogate. The association of teriflunomide with probability of EDSS worsening, adjusted for BVL, new or enlarging T2w lesions, and relapses, was assessed by evaluating whether the treatment effect is attenuated when adding these variables to the logistic regression model described for criterion 2.
The PTE on 12-week CDW that could be explained by treatment effect on surrogates was estimated as the percent attenuation in the adjusted (criterion 4) versus the unadjusted (criterion 2) association between treatment and 12-week CDW. Each potential surrogate was evaluated separately and in combination.
Results
Baseline characteristics
Patient demographics and baseline clinical characteristics according to BVL groups and treatment groups are shown in Tables 1 and 2, respectively. Across BVL groups, patient demographics were similar, while some clinical and MRI outcomes were better in the groups with less BVL. The group with least (Group 1) and intermediate (Group 2) BVL had a lower EDSS score compared with the group with most BVL (Group 3) (mean (standard deviation (SD)): Group 1: 2.4 (1.2), Group 2: 2.5 (1.3), Group 3: 2.8 (1.3). Number of contrast-enhancing lesions and baseline T2 lesion volume (mL) was lower in the group with least (Group 1) and intermediate (Group 2) BVL compared with the group with most BVL (Group 3) (mean (SD)): Group 1: 0.5 (1.2), Group 2: 1.2 (2.8), Group 3: 4.0 (7.9); mean (SD): Group 1: 10.1 (11.1), Group 2: 14.8 (14.8), Group 3: 25.5 (17.7), respectively. Baseline normalized brain volume (cm3) was higher in the group with least (Group 1) and intermediate (Group 2) BVL compared with the group with most BVL (Group 3) (mean (SD)): Group 1: 1514.6 (74.8), Group 2: 1509.5 (77.7), Group 3: 1476.3 (85.1). Placebo patients were 54% less likely to be in the group with the least BVL, and 39% less likely to be in the group with intermediate BVL compared to teriflunomide-treated patients (OR = 0.46, p = 0.0007; OR = 0.61, p = 0.0189, respectively).
Table 1.
Patient demographics and baseline clinical characteristics according to BVL group for the ITT population with valid scan at Baseline and Year 2a.
| Group 1 Least BVL (⩽0.52% reduction) n = 221 |
Group 2 Intermediate BVL (>0.52%–2.18% reduction) n = 357 |
Group 3 Most BVL (>2.18% reduction) n = 131 |
|
|---|---|---|---|
| Age, mean (SD), years | 38.4 (8.0) | 37.8 (8.8) | 36.1 (9.3) |
| Female sex, no. (%) | 152 (68.8) | 272 (76.2) | 99 (75.6) |
| Time since diagnosis of MS, mean (SD), years | 5.2 (5.8) | 5.3 (5.5)b | 4.3 (4.8) |
| Time since first symptoms of MS, mean (SD), years | 8.6 (7.2) | 8.9 (7.0) | 7.7 (6.5) |
| Time since most recent relapse onset, mean (SD), months | 6.6 (3.4) | 6.8 (3.7) | 6.8 (4.0) |
| Relapses, mean (SD), no. | |||
| In previous year | 1.3 (0.7)c | 1.3 (0.7)d | 1.4 (0.7)e |
| In previous 2 years | 2.1 (0.9) | 2.2 (0.9) | 2.3 (1.0) |
| MS subtype, n (%) | |||
| Relapsing remitting | 204 (92.3) | 336 (94.1) | 122 (93.1) |
| Use of previous MS treatment in the last 2 years, n (%) | 47 (21.3) | 83 (23.2) | 35 (26.7) |
| Baseline EDSS score, mean (SD) | 2.4 (1.2) | 2.5 (1.3) | 2.8 (1.3) |
| Number of contrast-enhancing lesions, mean (SD) | 0.5 (1.2)f | 1.2 (2.8) | 4.0 (7.9) |
| Baseline normalized brain volume, cm3, mean (SD) | 1514.6 (74.8) | 1509.5 (77.7) | 1476.3 (85.1) |
| Baseline T2-weighted lesion volume, mL, mean (SD) | 10.1 (11.1) | 14.8 (14.8) | 25.5 (17.7) |
BVL: brain volume loss; EDSS: Expanded Disability Status Scale; ITT: intent-to-treat; SD: standard deviation.
The ITT population represents pooled data from placebo and active treatment groups.
n = 356.
n = 172.
n = 277.
n = 103.
n = 220.
Table 2.
Patient demographics and baseline clinical characteristics according to treatment group for the ITT population with valid scan at Baseline and Year 2.
| Placebo, n = 234 | 7 mg, n = 240 | 14 mg, n = 235 | |
|---|---|---|---|
| Age, mean (SD), years | 37.6 (8.7) | 37.5 (8.9) | 37.9 (8.5) |
| Female sex, no. (%) | 184 (78.6) | 171 (71.3) | 168 (71.5) |
| Time since diagnosis of MS, mean (SD), years | 4.6 (5.1) | 5.1 (5.4)a | 5.7 (5.9) |
| Time since first symptoms of MS, mean (SD), years | 8.3 (6.8) | 8.5 (7.0) | 9.0 (7.1) |
| Time since most recent relapse onset, mean (SD), months | 6.7 (3.8) | 6.5 (3.3) | 6.9 (3.8) |
| Relapses, mean (SD), no. | |||
| In previous year | 1.4 (0.7)b | 1.4 (0.6)c | 1.3 (0.7)c |
| In previous 2 years | 2.1 (0.8) | 2.2 (1.0) | 2.2 (0.9) |
| MS subtype, n (%) | |||
| Relapsing remitting | 216 (92.3) | 220 (91.7) | 226 (96.2) |
| Use of previous MS treatment in the last 2 years, n (%) | 50 (21.4) | 57 (23.8) | 58 (24.7) |
| Baseline EDSS score, mean (SD) | 2.5 (1.3) | 2.5 (1.3) | 2.5 (1.2) |
| Number of contrast-enhancing lesions, mean (SD) | 1.3 (3.1) | 1.5 (3.7) | 1.7 (5.4)d |
| Baseline normalized brain volume, cm3, mean (SD) | 1506.3 (80.3) | 1508.9 (82.6) | 1499.6 (74.8) |
| Baseline T2-weighted lesion volume, mL, mean (SD) | 15.2 (15.7) | 16.6 (16.4) | 14.2 (13.5) |
EDSS: Expanded Disability Status Scale; ITT: intent-to-treat; SD: standard deviation.
n =239.
n = 180.
n = 186.
n = 234.
Patient demographics and baseline clinical/MRI characteristics were similar across treatment groups.
Association between BVL and disability worsening
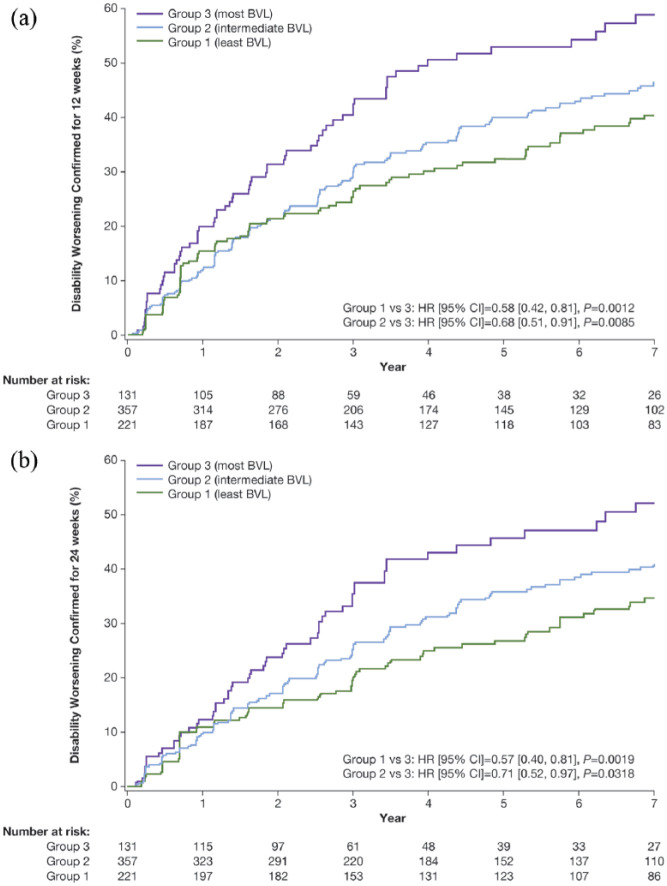
Irrespective of treatment, risk of 12-week CDW at Year 7 was lower in patients with the least and intermediate BVL between Baseline and Year 2 compared with patients with the most BVL (Group 1 vs Group 3, hazard ratio (HR) (95% confidence interval (CI)) =0.58 (0.42, 0.81), p = 0.0012; Group 2 vs Group 3, HR (95% CI) = 0.68 (0.51, 0.91), p = 0.0085; Figure 1(a)). This effect was evident at all time points between Years 3 and 7 (Group 1 vs Group 3, all p ⩽ 0.0033; Group 2 vs Group 3, all p ⩽ 0.0117; Supplementary Table S1). No differences were revealed between the least and intermediate BVL groups. Similar results were observed for risk of 24-week CDW (Figure 1(b) and Supplementary Table S2).
Figure 1.
Time to disability worsening up to Year 7 confirmed for 12 (a) and 24 (b) weeks in BVL groupsa (Baseline to Year 2).
aAnalyses based on pooled data from placebo and active treatment groups (total population); Group 1 (least BVL): ⩽0.52% reduction; Group 2 (intermediate BVL): >0.52% to 2.18% reduction; Group 3 (most BVL): >2.18% reduction.
BVL: brain volume loss.
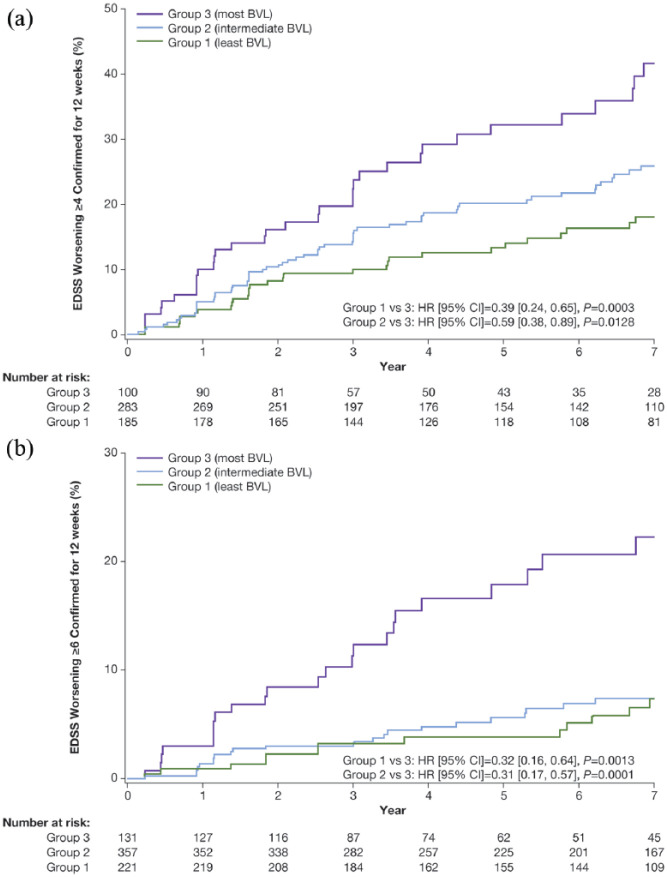
Irrespective of treatment, the risk of reaching EDSS milestones of ⩾4 and ⩾6 confirmed for 12 weeks at Year 7 was lower in patients with the least and intermediate BVL during Years 1–2 compared with patients with the most BVL; EDSS milestone of ⩾4: Group 1 versus Group 3, HR (95% CI)= 0.39 (0.24, 0.65), p = 0.0003; Group 2 versus Group 3, HR (95% CI)= 0.59 (0.38, 0.89), p = 0.0128; Figure 2(a); EDSS milestone of ⩾6: Group 1 versus Group 3, HR (95% CI)= 0.32 (0.16, 0.64), p = 0.0013; Group 2 versus Group 3, HR (95% CI)= 0.31 (0.17, 0.57), p = 0.0001; Figure 2(b). No differences were revealed between the least and intermediate BVL groups.
Figure 2.
Time to post-baseline EDSS milestones of ⩾4 (a) and ⩾6 (b) confirmed for 12 weeks up to Year 7 categorized by BVL groupa (Baseline to Year 2).
aAnalyses based on pooled data from placebo and active treatment groups (total population); Group 1 (least BVL): ⩽0.52% reduction; Group 2 (intermediate BVL): >0.52% to 2.18% reduction; Group 3 (most BVL): >2.18% reduction.
BVL: brain volume loss; EDSS: Expanded Disability Status Scale.
BVL as a mediator of teriflunomide effect on disability worsening
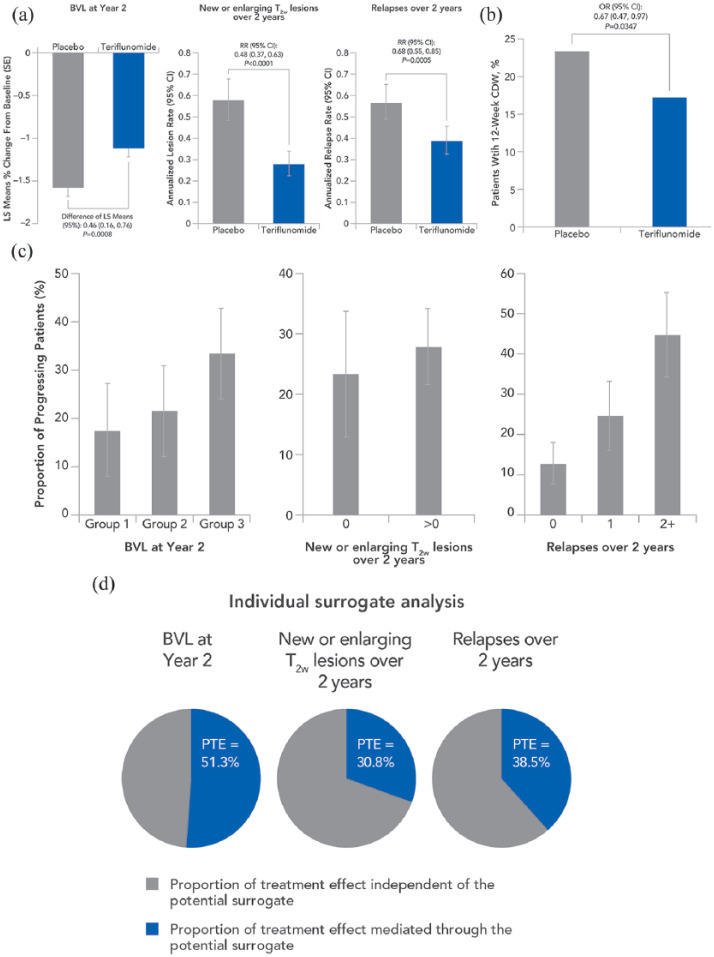
Teriflunomide was associated with significant reductions in all surrogate markers of disability worsening (Prentice criterion 1; Figure 3(a)). The least squares (LS) mean difference in BVL from Baseline to Year 2 was 0.46% lower in the teriflunomide group compared with placebo (95% CI = 0.16, 0.76, p = 0.0008). The annualized lesion rate over 2 years of treatment was reduced in the teriflunomide group by 52% compared with placebo (RR (95% CI) = 0.48 (0.37, 0.63), p < 0.0001). Teriflunomide also reduced the ARR over 2 years by 32% compared with placebo (RR (95% CI) =0.68 (0.55, 0.85), p = 0.0005).
Figure 3.
Satisfaction of Prentice criteria: (a) Criterion 1 requires the potential surrogate to be significantly associated with treatment. (b) Criterion 2 requires the clinical outcome to be associated with treatment. (c) Criterion 3 requires the clinical outcome to be associated with the potential surrogate. (d) Criterion 4 requires the association of treatment with the clinical outcome (criterion 2) to be no longer statistically significant when adjusting for the potential surrogate (p-values for the treatment effect, adjusted for the surrogate: BVL at Year 2 p=0.3911; New or enlarging T2w lesions over 2 years p=0.2033; Relapses over 2 years p=0.2064).
Group 1 (least BVL): ⩽0.52% reduction; Group 2 (intermediate BVL): >0.52% to 2.18% reduction; Group 3 (most BVL): >2.18% reduction. BVL: brain volume loss; CDW: confirmed disability worsening; CI: confidence interval; LS: least squares; OR: odds ratio; RR: relative risk; T2w: T2-weighted.
Teriflunomide significantly reduced the risk of 12-week CDW by 33% compared with placebo (OR (95% CI) =0.67 (0.47, 0.97), p = 0.0347; Figure 3(b); Prentice criterion 2). The surrogate markers were significantly associated with the risk of disability worsening (Prentice criterion 3; Figure 3(c)). Patients were 19% more likely to experience 12-week CDW for each additional percentage of brain volume decrease (OR (95% CI) =1.19 (1.04–1.35), p = 0.0095). For every additional T2w lesion, patients were 20% more likely to experience 12-week CDW (OR (95% CI) =1.20 (1.05–1.38), p = 0.0086). For each additional relapse, patients were 68% more likely to experience 12-week CDW (OR (95% CI) =1.65 (1.44–1.95), p < 0.0001).
The reduction in risk of disability worsening with teriflunomide was no longer statistically significant when adjusting for the surrogate markers (Prentice criterion 4; Figure 3(d) and Supplementary Table S3). The unadjusted association of teriflunomide on 12-week CDW was attenuated from OR = 0.67 (95% CI: 0.47–0.97) to OR = 0.92 (95% CI: 0.58–1.45) in the model adjusting for BVL, T2w lesions, and relapses together. This demonstrates that the combination of the three potential surrogate markers explained 76.9% of the treatment effect on 12-week CDW. When surrogate markers were analyzed separately in the model, BVL explained the greatest PTE on 12-week CDW (51.3%), followed by relapses (38.5%) and new or enlarging T2w lesions (30.8%). Pairwise combination of the surrogate markers indicated that the highest proportion of the treatment effect was explained by the combination of BVL and relapses (82.1%).
Discussion
BVL in patients with MS has been consistently associated with disability worsening and cognitive impairment.1,4–7 Teriflunomide 14 mg has been shown to have positive effects on BVL11,24,25 and disability worsening independently of each other.8,9,12,26
In the present analysis, we showed that the extent of BVL impacts the risk of long-term disability worsening. A lower amount of BVL (quantified using annualized PBVC from Baseline to Year 2) was associated with a reduced risk of 12-week CDW at both Years 2 and 7. Less BVL was also associated with a reduced risk of 24-week CDW, and reaching EDSS milestones of ⩾4 or ⩾6 (confirmed for 12 weeks) at Year 7. Thus, our analysis supports the accumulating evidence for a correlation between BVL and CDW in patients with MS4–7,12,24,26 and highlights the potential predictive value of BVL early in the disease course for longer-term disability worsening.
These findings highlight the importance of early effective treatment to minimize disability worsening. Accumulating evidence has shown that intervention during an early period in the MS disease course is crucial to optimize outcomes, including prevention of disability worsening and loss of neurological function.27,28 The benefits of early intervention with teriflunomide were demonstrated in post hoc analysis from the TOWER core and extension study up to 5.5 years. Earlier (on entering the core study) versus delayed (following receipt of placebo for ⩾48 weeks during the core study) teriflunomide 14 mg treatment significantly reduced risk of ⩾12-week CDW.29
Treatment effect on BVL in the teriflunomide group in TEMSO using the initially applied magnetic resonance imaging analysis package (MRIAP) did not reach statistical significance.16 However, due to the strong disability benefit seen with teriflunomide, the decision was made to further study potential effects of teriflunomide on BVL with SIENA, as a longitudinal and thus potentially more sensitive analysis. Although no systematic comparison between MRIAP and SIENA is available, generally lower brain volume change error is observed with dedicated longitudinal measurement techniques such as SIENA, compared with cross-sectional measurement techniques.10
The mediation analysis revealed that the teriflunomide treatment effect on BVL at Year 2 may explain the greatest proportion of its effect on 12-week CDW (51.3%), greater than that explained by its effect on relapses in the first 2 years (38.5%), and on new or newly enlarging T2w lesions over 2 years (30.8%). As BVL likely represents total, cumulative injury, derived from both focal and non-focal (diffuse) disease mechanisms in patients with MS,5 it is expected to be associated more closely with CDW than relapses or focal brain parenchymal pathology such as MRI lesions.
A previously published study of fingolimod on disability progression that used similar methods as in the current mediation analysis found relapses at 1 year to explain the largest proportion of fingolimod’s effect on disability (60%), when surrogates were analyzed separately; combining relapses and BVL better explained treatment effect on disability (73%).14
In the present analysis, the effect of teriflunomide on BVL/relapses combined explained the greatest PTE on 12-week CDW (82.1%), compared with all three variables combined (BVL/new or enlarging T2w lesions/relapses; 76.9%). This could be due to the loss of precision when adding multiple correlated variables into the model, or because lesions were only weakly associated with CDW. The residual treatment effect when adjusting for BVL/relapses combined, and BVL/new or enlarging T2w lesions/relapses combined, is reduced compared with the residual treatment effect when each surrogate is adjusted for individually. This finding is in line with the mediation analysis done for fingolimod.14 The fact that, in comparison to fingolimod, more of the teriflunomide effect on CDW is explained by its effect on BVL, when surrogates were analyzed individually, may indicate that―relative to its overall effect―teriflunomide targets diffuse disease processes in the central nervous system (CNS) more strongly. However, exact biological mechanisms of such putative diffuse CNS effects remain unclear.
A limitation of this analysis was that both those patients who received teriflunomide or placebo during the TEMSO core study were included in the long-term extension analysis. The study did not include adjustment for lesions in the spinal cord or cortex, which may cause BVL.
In conclusion, lower BVL from Baseline to Year 2 (analyzed using blinded post hoc SIENA analysis and quantified using annualized PBVC) was associated with a reduced risk of long-term (Year 7) CDW and reaching EDSS milestones of ⩾4 and ⩾6. These findings highlight the fact that patients with more BVL earlier in the MS disease course may have higher subsequent long-term disability worsening. The mediation analysis suggests that teriflunomide may prevent disability worsening largely through its effects on BVL.
Supplemental Material
Supplemental material, MSJ855722_supplementary_materials for Association of brain volume loss and long-term disability outcomes in patients with multiple sclerosis treated with teriflunomide by Till Sprenger, Ludwig Kappos, Ernst-Wilhelm Radue, Laura Gaetano, Nicole Mueller-Lenke, Jens Wuerfel, Elizabeth M Poole and Steven Cavalier in Multiple Sclerosis Journal
Acknowledgments
The authors would like to thank the patients, their families, and all investigators who provided data for this report. Additional statistical support was provided by Karthinathan Thangavelu, PhD, of Sanofi. Medical writing support under the direction of the authors was provided by Beth Fisher, PhD (Onyx, Knutsford, UK), funded by Sanofi, according to Good Publication Practice guidelines (https://annals.org/aim/fullarticle/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3). The manuscript was reviewed for scientific accuracy by Darren P Baker, PhD, Jonathan Valenzano, PharmD, and Karyn Liu, PhD, of Sanofi. The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as verification of the data presented in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.S.: Till Sprenger’s previous and/or current institution received payments for steering committee/consultation and speaking activities from Mitsubishi Pharma, Desitin, Eli Lilly, Sanofi Genzyme, Novartis, ATI, Actelion, Merck Serono, Electrocore, Biogen Idec, Roche, and TEVA. T.S. has received grants from the Swiss MS Society, Swiss National Research Foundation, EFIC-Grünenthal, and Novartis Pharmaceuticals Switzerland. L.K.: Ludwig Kappos’ Institution (University Hospital Basel) received in the last 3 years and used exclusively for research support at the Department: steering committee, advisory board and consultancy fees from Actelion, Alkermes, Almirall, Bayer, Biogen, Celgene/Receptos, df-mp, Excemed, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi Pharma, Novartis, Roche, Sanofi-Aventis, Santhera, Teva, Vianex, and license fees for Neurostatus-UHB products. The Research of the MS Center in Basel has been supported by grants from Bayer, Biogen, Novartis, the Swiss MS Society, the Swiss National Research Foundation, the European Union, and Roche Research Foundations. E.-W.R.: Received speaker honoraria and travel compensation from Bayer, Biogen, Fondazione Italiana Sclerosi Multipla, Genzyme, Novartis, Merck Serono, MorphoSys, and Synthon; consultancy fees from Bayer, Biogen, Fondazione Italiana Sclerosi Multipla, Genzyme, Novartis, Merck Serono, Synthon, MorphoSys; institutional research support from Novartis, Biogen, Actelion, Basilea, SAKK, and Synarc. L.G.: Received temporary salary from Novartis AG, currently employed by F. Hoffman-La Roche (her current institution was not involved at any time with the preparation of this manuscript). N.M.-L.: Nothing to disclose. J.W.: CEO of the MIAC AG in Basel, Switzerland and has served on scientific advisory boards of Actelion, Biogen, Sanofi Genzyme, Novartis, and Roche. E.M.P.: Employee of Sanofi, with ownership interest. S.C.: Employee of Sanofi, with ownership interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Development of this manuscript was supported by Sanofi.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Contributor Information
Till Sprenger, University Hospital Basel, Basel, Switzerland/ Department of Neurology, DKD Helios Klinik Wiesbaden, Wiesbaden, Germany.
Ludwig Kappos, Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland.
Ernst-Wilhelm Radue, Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland.
Laura Gaetano, Medical Image Analysis Center, Basel, Switzerland/ Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland.
Nicole Mueller-Lenke, Medical Image Analysis Center, Basel, Switzerland.
Jens Wuerfel, Medical Image Analysis Center, Basel, Switzerland/ Department of Biomedical Engineering, University of Basel, Basel, Switzerland.
Elizabeth M Poole, Global Scientific Communications, Sanofi, Cambridge, MA, USA.
Steven Cavalier, Global Scientific Communications, Sanofi, Cambridge, MA, USA.
References
- 1. De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010; 74(23): 1868–1876. [DOI] [PubMed] [Google Scholar]
- 2. Fisher E, Lee JC, Nakamura K, et al. Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann Neurol 2008; 64: 255–265. [DOI] [PubMed] [Google Scholar]
- 3. Giorgio A, Battaglini M, Smith SM, et al. Brain atrophy assessment in multiple sclerosis: Importance and limitations. Neuroimaging Clin N Am 2008; 18(4): 675–686, xi. [DOI] [PubMed] [Google Scholar]
- 4. Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 2009; 66(9): 1144–1150. [DOI] [PubMed] [Google Scholar]
- 5. Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014; 75: 43–49. [DOI] [PubMed] [Google Scholar]
- 6. Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84(10): 1082–1091. [DOI] [PubMed] [Google Scholar]
- 7. Kappos L, Sprenger T, Radue EW, et al. Brain volume loss correlates with long-term disability worsening in patients with MS: SIENA analysis of TEMSO MRI data (P733). Mult Scler J 2016; 22: 88–399. [Google Scholar]
- 8. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365(14): 1293–1303. [DOI] [PubMed] [Google Scholar]
- 9. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(3): 247–256. [DOI] [PubMed] [Google Scholar]
- 10. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002; 17(1): 479–489. [DOI] [PubMed] [Google Scholar]
- 11. Radue EW, Sprenger T, Gaetano L, et al. Teriflunomide slows BVL in relapsing MS: A reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm 2017; 4(5): e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sprenger T, Gaetano L, Mueller-Lenke N, et al. Correlation between brain volume loss and long-term disability worsening in patients with MS: SIENA analysis of TEMSO MRI data (P-22). Mult Scler J 2018; 24: 368–423. [Google Scholar]
- 13. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: Nine-year follow-up of the randomized TEMSO study. Neurology 2016; 86: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sormani MP, De Stefano N, Francis G, et al. Fingolimod effect on brain volume loss independently contributes to its effect on disability. Mult Scler 2015; 21(7): 916–924. [DOI] [PubMed] [Google Scholar]
- 15. Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med 1989; 8(4): 431–440. [DOI] [PubMed] [Google Scholar]
- 16. Wolinsky JS, Narayana PA, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler 2013; 19(10): 1310–1319. [DOI] [PubMed] [Google Scholar]
- 17. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 18. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(6): 545–556. [DOI] [PubMed] [Google Scholar]
- 19. Arnold DL, Gold R, Kappos L, et al. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol 2014; 261(9): 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 21. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 22. Miller DH, Fox RJ, Phillips JT, et al. Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology 2015; 84(11): 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 2012; 33(9): 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sprenger T, Kappos L, Radue EW, et al. Teriflunomide significantly slows brain volume loss in MS patients irrespective of disability progression (P3.047). Neurology 2016; 86(Suppl. 16). [Google Scholar]
- 25. Zivadinov R, Dwyer MG, Carl E, et al. Evaluating the effect of teriflunomide on whole brain atrophy in the phase 3 TOPIC study (P870). Mult Scler 2018; 24(Suppl. 2): 328–529. [Google Scholar]
- 26. Sormani MP, Radue EW, Sprenger T, et al. Incorporating the TEMSO SIENA analysis improves correlation of brain atrophy and disability progression. Eur J Neurol 2016; 23: 546. [Google Scholar]
- 27. Tintore M. Early MS treatment. Int MS J 2007; 14: 5–10. [PubMed] [Google Scholar]
- 28. Ziemssen T, Derfuss T, deStefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol 2016; 263(6): 1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Connor P, Thangavelu K, Rufi P, et al. Early vs delayed treatment with teriflunomide 14 mg results in reduced risk of disability progression in patients with MS (P3.021). Neurology 2016; 86(Suppl. 86). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ855722_supplementary_materials for Association of brain volume loss and long-term disability outcomes in patients with multiple sclerosis treated with teriflunomide by Till Sprenger, Ludwig Kappos, Ernst-Wilhelm Radue, Laura Gaetano, Nicole Mueller-Lenke, Jens Wuerfel, Elizabeth M Poole and Steven Cavalier in Multiple Sclerosis Journal