Abstract
Background
Liver disease is a major public health threat, particularly in developing countries. Several medicinal plants and formulations have been claimed to have liver protective activities. The present study aimed to evaluate in vitro antioxidant and in vivo hepatoprotective activities of root bark extracts of Croton macrostachyus (Euphorbiaceae).
Methods
Free radical scavenging activity of crude extract and solvent fractions of the plant was conducted using the DPPH assay method. Hepatoprotective activities of the crude extract and solvent fractions of the plant were carried out based on paracetamol-induced liver damage in mice. Serum biomarkers (AST, ALT, ALP, total bilirubin and total protein) were assessed to find out the effect. Histopathological examination was also carried out for all groups of mice to further confirm the findings.
Results
Antioxidant assay revealed that the crude extract, aqueous fraction and chloroform fraction of Croton macrostachyus exhibited free radical scavenging activity with IC50 values of 128.6, 168.9, and 406 µg/mL, respectively. Pretreatment of the mice with the crude extract and solvent fractions of Croton macrostachyus significantly reduced ALP (p<0.001), ALT (p<0.001), and AST (p<0.001) levels at all the administered doses compared to the toxic group. The crude extract and chloroform fraction decreased total bilirubin level at doses of 200 mg/kg (P<0.05) and 400 mg/kg (P<0.001). Pretreatment of the mice with 400 mg/kg of the crude extract and aqueous fraction elevated total protein value compared to the paracetamol treated group (P<0.05). The hepatoprotective activities of the plant extracts were confirmed by histopathological studies.
Conclusion
From this study, it can be concluded that the crude extract and solvent fractions of Croton macrostachyus demonstrated antioxidant and hepatoprotective activities.
Keywords: antioxidant, hepatoprotective, Croton macrostachyus, paracetamol, biochemical parameters, liver damage
Background
Liver diseases are among the global health problems; In which, liver cirrhosis is the ninth leading cause of death in western Nations.1 Toxic chemicals, xenobiotics, alcohol consumption, malnutrition, anemia, medications, autoimmune disorders, and viral infections2,3 are some major causes of liver disease; among which, medications are the most common contributing factors.4 Drug-induced liver damage accounts for more than 60% of all cases in the United States of America, and it is the leading cause of acute liver failure.5
Paracetamol is a commonly available over-the-counter analgesic and antipyretic drug which is associated with acute liver damage. The hepatotoxicity associated with paracetamol is mainly due to excessive accumulation of its toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which oxidizes liver tissue macromolecules such as lipid or -SH group of protein causing oxidative stress and hepatic necrosis.6–8
The modern drugs available for the treatment of liver ailments are less effective, less safe and, expensive.2,9 This indicates the need for new better drugs. Several medicinal plants and formulations have been claimed to have liver protective activities, about 160 phytoconstituents from 101 plants have been suggested to possess hepatoprotective activity.10 Among them, the methanol extract of Senna singueana demonstrated hepatoprotective activity against D-galactosamine-induced liver damage in rats by its antioxidant and anti-apoptotic effects.11 Additionally, tannin-containing extracts from Lannea stuhlmannii and Lannea humilis exhibit hepatoprotective activities against D-galactosamine-induced hepatic injury in rats. The hepatoprotective activity of the extracts was due to the antioxidant and anti-apoptotic effects of tannins and proanthocyanidins.12 As a result, highest priority has been given globally to investigate plant-based hepatoprotective drugs effective against different liver diseases.7
Croton macrostachyus Hochst. Ex Del. is one of the plants claimed traditionally for the treatment of liver ailments. It is a deciduous tree with a height of 3–25m which belongs to the family Euphorbiaceae. The plant is native to Ethiopia, Eritrea, Kenya, Nigeria, Tanzania, and Uganda. It is locally named as Bisana (Amharic) or Tambush, Tambuk (Tigrigna).13 Reviews of literature revealed that this plant has various traditional uses in different human diseases.14–21 The plant has been claimed traditionally to be used in the treatment of jaundice14,19 and hepatitis.16–18,21 Additionally, the plant has been investigated scientifically for various activities.13,22–25 But, its hepatoprotective activity has not been scientifically studied so far. Hence, this study was conducted to evaluate hepatoprotective activities of the crude extract and solvent fractions of root bark extracts of Croton macrostachyus on paracetamol-induced liver damage in mice.
Materials and Methods
Plant Material Collection
Fresh Croton macrostachyus root bark was collected from Ahsea, North Tigray region, Ethiopia. Identification and authentication of the plant were carried out at the Department of Biology, in Gondar University, and sample specimen was preserved with the Voucher number of GH0026/2010.
Experimental Animals
A total of 72 healthy Swiss albino mice of either sex weighing 25–35 g; aging 6–8 weeks were used for the study. All animals were housed under room temperature (at 22 ± 3°C), and 12 h light and dark cycles. Animals had free access to feed with a standard pellet diet and water ad libitum. Animals were acclimatized for one week prior to the experiment.
Plant Material Extraction
The root bark was washed with water to remove earthy materials, after air dried at room temperature under shade it was powdered using a mechanical grinder. The powdered material (1.46 kg) was then extracted by 70% ethanol using soxhlet apparatus by continuous hot extraction. Next, the extract was dried in an oven at 40 °C. The crude extract was packed in airtight amber colored glass and kept in a refrigerator at 4 °C for further experiments.
The crude extract was partitioned using different solvents (chloroform and distilled water) as described by Hossain et al, (2014).26 The two fractions (chloroform and aqueous fractions) were allowed to dry in drying oven at 40 °C.
Preliminary Phytochemical Screening
Preliminary phytochemical screening was carried out for the presence or absence of different phytoconstituents using standard procedures.27,28
Acute Oral Toxicity Study
Acute oral toxicity of the crude extract was carried out according to the organization of economic co-operation development (OECD)-425 guideline at a limit test dose of 2000 mg/kg.29 The test was conducted in adult nulliparous and non-pregnant female mice. The mice were randomly selected and acclimatized for 5 days prior to dose administration. The mice were fasted for food, but not water 3 hours prior to dosing and 1 hour after the administration of the extract. The dose was calculated using body weight of the mice just after fasting. A single dose of the extract was administered orally by oral gavage. Mice were observed individually for gross behavioral changes (locomotion, activity, hair texture, pupil size, and feeding) at least once during the first 30 minutes of dosing, periodically with special attention given during the first 4 hours of the 24 hours, and thereafter daily, for a total of 14 days.
Determination of Antioxidant Activity (DPPH) Assay
The free radical scavenging activity of the crude extract and solvent fractions of root bark extracts of Croton macrostachyus was determined in vitro by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay according to the standard method described by Braca et al, (2001).30 Initially, 3 mL of 0.004% DPPH in methanol was mixed with 1 mL of various concentrations (200, 100, 50, 25, 12.5 µg/mL) of the crude extract and solvent fractions of Croton macrostachyus separately. Mixtures were incubated for 30 minutes incubation at room temperature in a dark place. The absorbances of the mixtures in the samples were measured using a spectrophotometer at 517 nm against methanol as blank. The percentage of radical scavenging activities of the samples were evaluated compared with a control (3 mL DPPH solution and 1 mL methanol). Each sample was measured in triplicate and the average was calculated. The percentage of radical scavenging activity (RSA) was calculated using the following formula:
% RSA = [(A0 - A1)/A0] × 100
where A0 is the absorbance of the control, and A1 is the absorbance of samples after 30 minutes. The free radical scavenging activity of the plant extracts was expressed as IC50. The IC50 value is defined as the concentration (in µg/mL) of a sample that inhibits 50% of the DPPH radical.
Assessment of Hepatoprotective Activity
The in vivo hepatoprotective activity was evaluated on the basis of paracetamol-induced liver damage method as described in different studies with some modification.6,31,32
Grouping and Extract Administration
The animals were divided into six groups consisting of six animals in each. Animals were subjected to either one of the following treatments for 7 days. The Group I serve as a normal control group and received normal diet and 5% DMSO (10 mL/kg, p.o.) daily for 7 days. Group II was served as paracetamol control and treated with paracetamol dissolved in 5% DMSO (250 mg/kg, po) daily for 7 days, while group III, a standard group was treated with Silymarin (100 mg/kg/day, p.o.) for 7 days. Groups IV–VI were treated with the crude extract or solvent fractions at three different doses (100 mg/kg, 200 mg/kg and 400 mg/kg, p.o) for 7 days. Group III–VI animals were intoxicated with paracetamol (250 mg/kg, po) 3 hours after the administration of Silymarin or extracts daily for 7 days.
After 24 hours of the last treatment, all the mice were anesthetized with diethyl ether and blood was collected from each mouse through the cardiac puncture.
Serum Biochemical Analysis
Blood samples were taken and serum was separated by centrifugation at 3500 revolutions per minute (RPM) for 5 minutes. After that, it was used for the assay of marker enzymes such as AST, ALT, ALP, total protein (TP), and total bilirubin using blood chemistry analyzer.33
Histopathological Examination
After the collection of the blood, the mice were sacrificed and the liver was dissected out, and weighed. Then, it was preserved in a 10% formalin solution. A portion of liver tissue from the right lobe of the liver was taken and dehydrated in different grades of ethanol (40%, 70%, 80%, 95%, and 100%) and cleared with xylene. Xylene was also cleared using paraffin wax using an automatic tissue processing machine. The tissues were embedded with paraffin wax and blocked in the coronal plane. Sections of 4–5 micrometer thickness of the tissue was made using a microtome and stained with hematoxylin and eosin dye, and a histological observation was made under a light microscope.
Statistical Analysis
Results were expressed as means ± Standard Errors of Mean (SEM). The analysis was carried out using Graph pad instat 3 software. One-way analysis of variance (ANOVA) was applied to test for significance of biochemical data of the different groups. Significance was set at P ≤ 0.05.
Ethical Consideration
The animals were handled in accordance with guidelines for the care and use of animals for scientific purposes, which has been developed by the national advisory committee for laboratory animal research (NACLAR).34 Ethical approval for this study was obtained from the research ethics committee of college of health sciences, Mekelle University (1222/2018).
Results
Percentage Yield of Crude Extract and Solvent Fractions
The yields of crude extract, aqueous fraction, and chloroform fraction were found to be 11.6%, 61.67%, and 31.7%, respectively.
Preliminary Phytochemical Screening
The qualitative phytochemical screening reveals the presence of alkaloids, polyphenols, saponins, flavonoids, anthraquinones, and coumarins (Table 1).
Table 1.
Phytochemical Constituents of the Root Bark Extracts of Croton Macrostachyus
| Constituents | Test | Crude Extract | Aqueous Fraction | Chloroform Fraction | Indicator | |
|---|---|---|---|---|---|---|
| Alkaloids | Mayer’s test | + | + | + | Whitish opalescence | |
| Wagner’s test | + | + | + | Reddish brown | ||
| Anthraquinones | Borntrager’s Test | + | – | + | Pink to red | |
| Polyphenol | Ferric Chloride Test | + | + | + | Blue green | |
| Flavonoids | Shinoda reduction test | Magnesium | + | + | + | Orange |
| Zink | – | – | – | |||
| Saponins | Foam test | + | + | + | Foam persistence | |
| Tannins | Gelatin test | – | – | – | Precipitate formation | |
| Coumarins | Ammonia test | + | + | + | Intense fluorescence | |
Notes: +→ presence, – → absence.
Acute Toxicity Study
The 70% ethanol extract of Croton macrostachyus did not produce any behavioral changes (locomotion, activity, hair texture, pupil size, and feeding). Death was not observed at the dose of 2000 mg/kg. Hence, the LD50 of the extract is estimated to be greater than 2000mg/kg.
In vitro Antioxidant Activity Assays
The crude extract showed the highest free radical scavenging activity with 50% inhibitory concentration of 128.6 µg/mL compared to the aqueous fraction and chloroform fraction [IC50:168.9µg/mL and 406µg/mL], respectively. The maximum percentage of inhibition was observed with the crude extract (71%) at the highest concentration (200 µg/mL) (Table 2).
Table 2.
DPPH Scavenging Activities of Crude Extract and Solvent Fractions
| Concentration (µg/mL) | Percentage Radical Scavenging Activity | |||
|---|---|---|---|---|
| Ascorbic Acid | Crude Extract | Aqueous Fraction | Chloroform Fraction | |
| 12.5 | 40.9 | 17.7 | 6.6 | 5.8 |
| 25 | 47.5 | 21.8 | 8 | 6.4 |
| 50 | 58.7 | 26.5 | 17.5 | 9.7 |
| 100 | 70.7 | 40.6 | 34 | 17.1 |
| 200 | 86 | 71 | 57.2 | 26.2 |
| IC50 (µg/µL) | 30.8 | 128.6 | 168.9 | 406 |
Abbreviation: IC50, 50% inhibitory concentration.
Hepatoprotective Activity
Liver Weight
A significant increase in liver weight was observed in the mice administered with paracetamol compared to the normal control group (P<0.01). However, Pretreatment of the mice with 400 mg/kg of the crude extract and solvent fractions significantly (P<0.05) decreased liver weight compared to the toxic group as shown in Table 3.
Table 3.
Effects of Crude Extract and Solvent Fractions on Liver Weight Variation
| Treatment Group | Dose | Liver Weight (g) |
|---|---|---|
| Normal | 5%DMSO | 1.16±0.09 |
| Paracetamol | 250mg/kg | 1.47±0.07a |
| Silymarin | 100mg/kg | 1.17±0.06* |
| Crude | 100mg/kg | 1.30±0.03 |
| 200mg/kg | 1.23±0.04 | |
| 400mg/kg | 1.18±0.02* | |
| Aqueous | 100mg/kg | 1.33±0.06 |
| 200mg/kg | 1.28±0.05 | |
| 400mg/kg | 1.19±0.06* | |
| Chloroform | 100mg/kg | 1.33±0.10 |
| 200mg/kg | 1.26±0.08 | |
| 400mg/kg | 1.21±0.04* |
Notes: N= 6, Results were expressed as Mean ±SEM. aP<0.01Vs Normal; *P<0.05 Vs paracetamol. Data were analyzed by One-Way ANOVA followed by Dunnet multiple comparison test.
Abbreviation: DMSO, dimethyl sulfoxide.
Effects of the Crude Extract and Solvent Fractions on Liver Biomarkers
The analysis of biochemical enzymes AST and ALT levels were significantly increased with paracetamol administered group compared to the normal control group (P<0.001). But, pretreatment of the mice with the three different doses of the crude extract and solvent fractions of Croton macrostachyus decreased AST and ALT levels significantly (P<0.001).
The crude extract and solvent fractions reduced ALP level at all doses (P<0.001). Whereas, total bilirubin was reduced at 200 mg/kg (P<0.05) and 400 mg/kg (P<0.001) of the crude and chloroform fraction; and at doses of 200 mg/kg (P<0.01) and 400 mg/kg (P<0.001) with the aqueous fraction as compared to paracetamol only.
The crude extract and aqueous fraction showed an increased level of total protein (P<0.05) at 400 mg/kg as compared to the paracetamol treated group (Table 4).
Table 4.
Effects of Crude Extract and Fractions on Biochemical Parameters Against Paracetamol-Induced Hepatotoxicity in Mice
| Serum Enzyme Levels | |||||
|---|---|---|---|---|---|
| Treatment Group |
AST (U/L) | ALT(U/L) | ALP (U/L) | Total Bilirubin (mg/dl) | Total Protein (mg/dl) |
| Normal(5% DMSO) | 105.83±3.46 | 56.83±2.91 | 219.66±7.12 | 0.45±0.04 | 8.48±0.93 |
| Paracetamol (250mg/kg) | 313.50±13.75a | 164.06±6.95a | 506.72±16.38a | 1.27±0.07a | 4.68±0.48b |
| Silymarin (100mg/kg) | 161.50±5.60*** | 82.33±3.49*** | 268.83±6.26*** | 0.70±0.08*** | 8.00±0.57** |
| Crude(100mg/kg) | 250.55±9.49*** | 122.6±5.48*** | 381.50±10.86*** | 1.07±0.05 | 6.67±0.45 |
| Crude(200mg/kg) | 214.46±5.91*** | 103.43±6.87*** | 325.28±11.47*** | 0.99±0.06* | 6.91±0.37 |
| Crude (400mg/kg) | 183.42±5.86*** | 91.10±4.26*** | 303.98±8.47*** | 0.72±0.04*** | 7.90±0.55* |
| Aqueous(100mg/kg) | 242.03±7.35*** | 119.67±4.66*** | 402.04±9.28*** | 1.02±0.03 | 6.73±0.84 |
| Aqueous(200mg/kg) | 205.87±6.05*** | 98.72±5.08*** | 360.12±8.05*** | 0.96±0.0.07** | 7.16±0.45 |
| Aqueous (400mg/kg) | 197.65±5.68*** | 86.33±4.67*** | 317.21±10.72*** | 0.82±0.0.06*** | 7.86±0.37* |
| Chloroform(100mg/kg) | 256.47±8.14*** | 121.47±6.90*** | 416.32±12.72*** | 1.08±0.03** | 6.38±0.51 |
| Chloroform(200mg/kg) | 215.10±7.62*** | 104.57±5.48*** | 389.18±8.52*** | 0.98±0.05* | 6.93±0.49 |
| Chloroform(400mg/kg) | 196.55±4.84*** | 96.42±4.34*** | 321.50±9.35*** | 0.86±0.04*** | 7.29±0.68 |
Notes: N= 6; Results were expressed in mean ± SEM. aP<0.001;bP<0.01 Vs Normal group animals; ***P<0.001,**P<0.01, *P<0.05 vs paracetamol group animals. Data were analyzed by One-Way ANOVA followed by Tukey-Kramer multiple comparison test.
Abbreviation: DMSO, dimethyl sulfoxide.
Effects of the Crude Extract and Solvent Fractions on Liver Histology
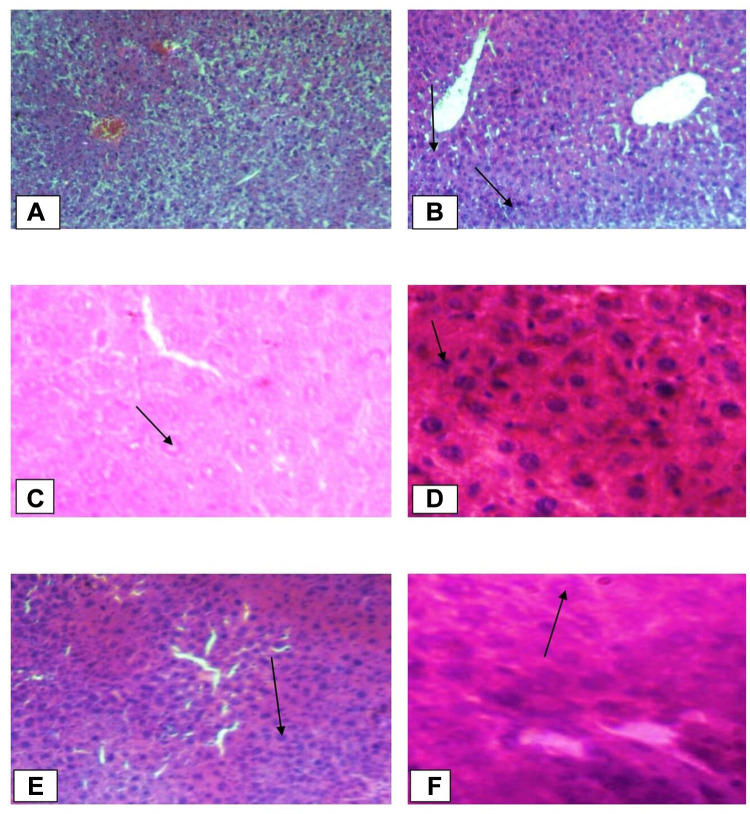
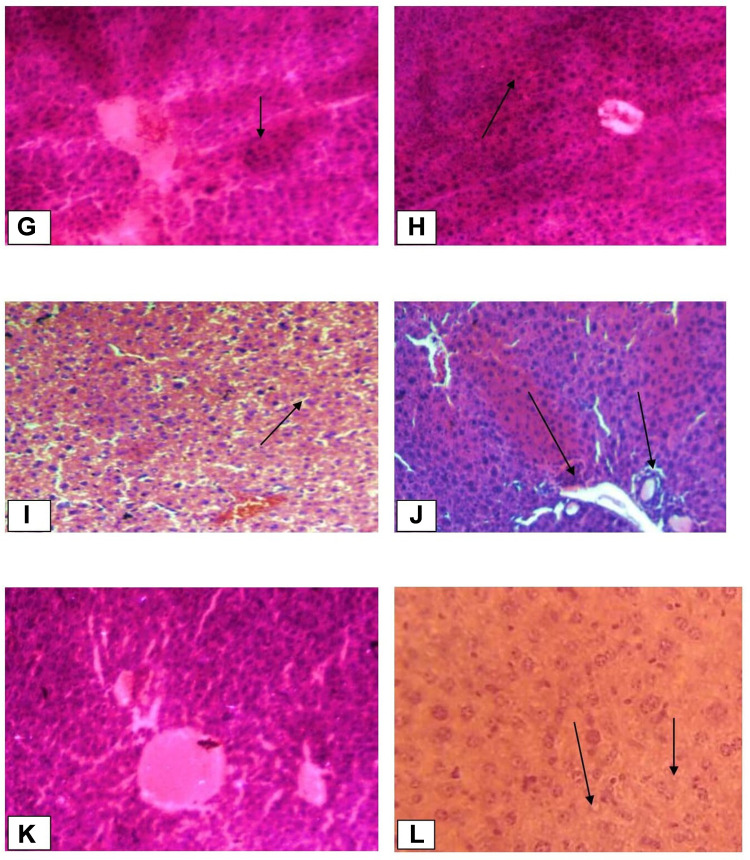
The liver tissues in the normal control group showed normal parenchymal architecture with no cellular necrosis (A), but the liver tissues of mice administered with paracetamol only, showed necrotic hepatocytes, hyperaemic/congested blood vessels and infiltration of inflammatory cells (B). Liver tissues of the mice treated with 400mg/kg showed normal hepatocytes and regeneration of liver cells were observed. Regeneration of the liver cells was evidenced by observing mitosis indicators in the liver tissues (Figure 1).
Figure 1.
Continued.
Figure 1.
Histopathological changes of liver tissues (10x and 40x). (A) Normal control group (Normal hepatic cells with normal liver architecture) (B) paracetamol group (Necrotic hepatocytes, severe degeneration, hyperaemic/condensed blood vessels and cells with inflammation); Black arrow shows damaged hepatocytes. (C) Standard group (Liver architecture normal, cells with mild inflammation); Black arrow shows normal hepatocytes (D) Crude extract 100 mg/kg (Aggravated necrotic cells); Black arrow shows necrotized cells. (E) Crude extract 200 mg/kg (More necrotic hepatocytes and regenerated cells); Black arrow shows regenerated cells. (F) Crude extract 400mg/kg (Hepatocytes are normal in appearance with some mitotic figures); Black arrow shows normal hepatocytes. (G) Aqueous fraction 100mg/kg (Necrotized cells are by far more than normal cells); Black arrow shows necrotized hepatocytes. (H) Aqueous fraction 200 mg/kg (Normal hepatocytes are fewer than in Aqueous fraction 400mg/kg and haemorrhage); Black arrow shows haemorrhages. (I) Aqueous fraction 400 mg/kg (Most cells are normal with some dead cells and regenerated cells); Black arrow shows regenerated cells. (J) Chloroform fraction 100mg/kg (Shows inflammation and degeneration of hepatocytes); Black arrow shows degenerated cells. (K) Chloroform fraction 200 mg/kg (Mild inflammation and degeneration; regeneration). (L) Chloroform fraction 400mg/kg (Almost normal hepatocytes with mitotic figures); Black arrow shows normal hepatocytes.
Discussion
As many of the chemicals pass through the liver to enter the general circulation; the liver is at a higher risk to be damaged than other organs.32 Hepatotoxicity is a significant problem in patients taking paracetamol intentionally or accidentally; causing acute liver failure.35
The evaluation of protective activity in liver damage induced by paracetamol has been widely used for hepatoprotective drug screening. Hepatotoxicity with paracetamol is due to its highly reactive metabolite, NAPQI.36 Increase in NAPQI quantity leads to glutathione depletion, which finally causes an alteration in homeostasis, an increase in the permeability of the cell membrane with a consequent cellular swelling, karyolysis, vacuolization of hepatocytes and an elevation of liver enzymes.2,37
The main enzyme levels elevated during liver injuries are ALT, AST, ALP, GGT and bilirubin. The reason is that these enzymes are mainly found in the liver and are released into the blood as a result of liver injury. There is also a reduction in the total protein and albumin levels due to disruption and dissociation of polyribosomes on endoplasmic reticulum resulting in decreasing the biosynthesis of protein.2
In the present study, the plant extracts showed a dose-dependent free radical scavenging activity; by which the crude extract showed antioxidant activity with IC50 of 128.6 µg/mL compared to the aqueous and chloroform fractions. Generally, the antioxidant activity of the plant extracts is in the order of crude extract, aqueous fraction, and chloroform fraction. The antioxidant activity may be attributed due to the presence of secondary metabolites including flavonoids, polyphenols, and coumarins. This is in agreement with the previous findings reported by Alghazeer et al (2018); Sharma et al (2016); Teshome et al (2015); Torres et al (2006).28,38–40 The variation in antioxidant activities of the plant extracts could be due to a difference in the amount and kind of phytochemicals present in the crude extract and solvent fractions of the plant. Thus, the highest free radical scavenging activity of the crude extract could be due to the ability of 70% ethanol to extract non-polar, medium polar and polar phytochemicals that can act synergistically.41
An increase in liver weight is an indication of liver injury.42 In the present study, liver weight of the mice administered with paracetamol alone significantly increased compared to the normal control group (P<0.01). The reason is that water is retained in the cytoplasm of hepatocytes leading to enlargement of liver cells, resulting in increased total liver mass.43 In our study, the crude extract and solvent fractions of Croton macrostachyus administered at a dose of 400 mg/kg reduced liver weight of the mice significantly (P<0.05).
Pretreatment of the mice with different doses of the crude extract and solvent fractions combined with paracetamol demonstrated hepatoprotective activities against paracetamol-induced liver injury; reducing the elevated levels of ALT, AST and ALP (P<0.001). Total bilirubin was reduced significantly in a dose dependent manner. The crude extract and the aqueous fraction showed a significant elevation in the total protein at a dose of 400 mg/kg compared to toxic group (P<0.05).
Suppression of the elevated serum levels of ALT and AST by the crude extract and solvent fractions of Croton macrostachyus is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damages caused by paracetamol. This is consistent with what has been found in previous studies done by Gutiérrez and Solís (2009).44 On the other hand, the suppression of increased ALP and the subsequent depletion of elevated bilirubin total level states that the crude extract and solvent fractions of the plant have the potential to stabilize biliary dysfunction. The negative effect of paracetamol on total protein was reversed with the administration of the crude extract and aqueous fraction which indicates an improvement of the functional status of the liver cells to synthesize proteins. These findings corroborate the ideas of Okokon et al (2017); Shrivastava et al (2017).45,46
As explained by Trifunschi et al (2015), the hepatoprotective activities of the plant extracts could be due to their free radical scavenging activity.47 Additionally, their anti-inflammatory activity could contribute to their hepatoprotective activity, as paracetamol toxicity produces inflammatory mediators such as monocytes, neutrophils and cytokines (interleukin-6 and tissue necrosis factor α).48
Other liver protective activities of the plant extracts could be; (1) inhibition of metabolism of paracetamol; because, metabolism of paracetamol with cytochrome P450 enzymes specifically, CYP2E1, is implicated in the hepatotoxicity of paracetamol by producing toxic metabolite, NAPQI. As a result, inhibition of this enzyme by the plant extracts could possibly reduce the toxic effects of paracetamol;49 and (2) an increase in glutathione level, which is reduced during paracetamol overdose due to production of excess NAPQI level. Excess NAPQI alkylates and oxidizes intracellular glutathione resulting in liver glutathione depletion subsequently leads to increased lipid peroxidation and liver damage.33,50 Thus, administration of the crude extract and solvent fractions might increase the glutathione level as a mechanism to protect paracetamol-induced liver damage in mice.
Hepatoprotective activities of the plant extracts could be due to the presence of phytochemicals such as polyphenols, alkaloids, flavonoids, saponins and coumarins which posses’ hepatoprotective activity either alone or in combination. These findings agree with other studies that have shown the hepatoprotective activity of these phytoconstituents.38,49,51–53
The mechanism of these phytochemicals for their hepatoprotective activity is mainly due to their free radical scavenging activity since the plant extracts contain different phytoconstituents which possess free radical scavenging activity. Additionally, the phytochemical flavonoid could maintain cell membrane stability or could protect cell membrane leakage up on damage by paracetamol as evidenced by a reduction in the liver biomarkers. This is in line with the findings of Tarahovsky et al (2014).54 Furthermore, the anti-inflammatory effect of the secondary metabolites such as alkaloids, saponins, coumarins and flavonoids could be the means for their hepatoprotective activity. This finding concurs with other studies that have shown the anti-inflammatory activity of these phytochemicals.52,55
On the other hand, various compounds have been isolated from the roots of Croton macrostachyus including, 3β-acetoxy taraxer-14-en-28-oic acid, trachyloban-19-oic acid, trachyloban-18-oic acid, neoclerodan-5, 10-en-19, 6β; 20, 12-diolide, 3α, 19-dihydroxytrachylobane, and 3α, 18, 19-trihydroxytrachylobane.56 Thus, the hepatoprotective activity of the extracts could also be due to the presence of these compounds.
Histopathologic findings revealed protective activities of the crude extract and solvent fractions against paracetamol-induced liver damage which is in good agreement with the results of the biochemical activities of the plant extracts and Silymarin. The section of the paracetamol intoxicated liver tissue demonstrates hepatic necrosis, condensed blood vessels and aggregation of inflammatory cells. This may be a result of the formation of free radicals and oxidative stress induced by paracetamol. These pathological changes were lesser in the mice administered with the different doses of the plant extracts and Silymarin followed by simultaneous administration of paracetamol. This indicates that treatment of the mice with the plant extracts and Silymarin may prevent paracetamol-induced liver damage.
In conclusion, the present results show that the crude extract and solvent fractions of Croton macrostachyus demonstrated antioxidant and hepatoprotective activities. The hepatoprotective activities of the plant extracts could be due to their free radical scavenging and antioxidant activities, resulting from the presence of some phytochemicals including polyphenols, flavonoids, saponins and alkaloids. Furthermore, the exact phytoconstituents and their mechanism of hepatoprotection should be studied. Additionally, these findings could justify the traditional use of the root bark of Croton macrostachyus in liver disorders; and suggest the possible utilization of the root bark of the plant as a source of new compounds for hepatoprotective activity.
Acknowledgments
Authors are thankful to school of graduate studies, Mekelle University, for sponsoring the work.
Funding Statement
This work was funded by Mekelle University.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; DPPH, 2, 2-diphenyl-1-picrylhydrazyl; NACLAR, National Advisory Committee for Laboratory Animal Research; NAPQI, N-acetyl-p-benzoquinone imine; OECD, Organization of Economic Co-operation Development; SEM, standard error of mean.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Park TY, Hong M, Sung H, Kim S, Suk KT. Effect of korean Red ginseng in chronic liver disease. J Ginseng Res. 2017;41(4):450–455. doi: 10.1016/j.jgr.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alqasoumi SI, Abdel-Kader MS. Screening of Some Traditionally Used Plants for Their Hepatoprotective Effect, Phytochemicals as Nutraceuticals - Global Approaches to Their Role in Nutrition and Health. InTech; 2012:256–278. [Google Scholar]
- 3.Kumar A. A review on hepatoprotective herbal drugs. Int J Res Pharm Chem. 2012;2(1):96–102. [Google Scholar]
- 4.Yin L, Wei L, Fu R, et al. Antioxidant and hepatoprotective activity of Veronica ciliata fisch. extracts against carbon tetrachloride-induced liver injury in mice. Molecules. 2014;19(6):7223–7236. doi: 10.3390/molecules19067223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H. Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis. 2015;33(4):464–471. doi: 10.1159/000374090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain L, Ikram J, Rehman K, Tariq M, Ibrahim M, Akash MSH. Hepatoprotective effects of Malva sylvestris l. against paracetamol-induced hepatotoxicity. Turk J Biol. 2014;38(3):396–402. doi: 10.3906/biy-1312-32 [DOI] [Google Scholar]
- 7.Jannu V, Baddam PG, Boorgula AK, Jambula SR. A review on hepatoprotective plants. Int J Drug Dev Res. 2012;4(3):1–8. [Google Scholar]
- 8.Sabiba E, Rasool M, Vedi M, et al. Hepatoprotective and antioxidant potential of Withania somnifera against paracetamol-induced liver damage in rats. Int J Pharm Pharm Sci. 2013;5(2):648–651. [Google Scholar]
- 9.Jain SK, Rajvaidy S, Desai P, Singh G, Nagori B. Herbal extract as hepatoprotective-a review. J Pharmacogn Phytochem. 2013;2(3):170–175. [Google Scholar]
- 10.Manjunath C, Balasubramannian T, Gnanasekaran D, Ashok Kumar U, Brahmaiah Y. Evaluation of hepatoprotective activity of Haldinia cordifolia against paracetamol induced liver damage in rats. Der Pharm Lett. 2012;4(3):768–774. [Google Scholar]
- 11.Sobeh M, Mahmoud MF, Hasan RA, Cheng H, El-Shazly AM, Wink M. Senna singueana: antioxidant, hepatoprotective, anti-apoptotic properties and phytochemical profiling of a methanol bark extract. Molecules. 2017;22(9):1502. doi: 10.3390/molecules22091502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobeh M, Mahmoud MF, Hasan RA, et al. Tannin-rich extracts from lannea stuhlmannii and lannea humilis (anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci Rep. 2018;8(1):1–6. doi: 10.1038/s41598-018-27452-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against plasmodium berghei in mice. BMC Complement Altern Med. 2014;14(1):1–10. doi: 10.1186/1472-6882-14-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araya S, Abera B, Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre district, Southern Tigray, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–25. doi: 10.1186/s13002-015-0015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekele G, Reddy PR. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo Tribes in Abaya district, Borana, Oromia, Ethiopia. J Plant Sci. 2015;3(1):1–8. [Google Scholar]
- 16.Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, Southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):1–10. doi: 10.1186/1746-4269-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: a comprehensive review. Evid Based Complement Alternat Med. 2017;2017:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekuanent T, Zebene A, Solomon Z. Ethnobotanical study of medicinal plants in Chilga District, Northwestern Ethiopia. J Nat Med. 2015;15(2):88–112. [Google Scholar]
- 19.Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):1–23. doi: 10.1186/1746-4269-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot District, Amhara, Northwestern Ethiopia. BMC Res Notes. 2017;10(1):1–13. doi: 10.1186/s13104-017-2482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yemane B, Berhane Y, Reddy KS. Ethnobotanical study of medicinal plants in Sub Region Logo Anseba, Region Gash Barka, Eritrea. J Pharm Biol Sci. 2016;11(4):63–73. [Google Scholar]
- 22.Aylate A, Agize M, Ekero D, Kiros A, Ayledo G, Gendiche K. In-vitro and in-vivo antibacterial activities of Croton macrostachyus methanol extract against E. Coli and S. Aureus. Adv Anim Vet Sci. 2017;5(3):107–114. [Google Scholar]
- 23.Degu A, Engidawork E, Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. Ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016;16(1):1–11. doi: 10.1186/s12906-016-1357-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teugwa M, Sonfack D, Fokom R, Penlap B, Amvam Z. Antifungal and antioxidant activity of crude extracts of three medicinal plants from cameroon pharmacopeia. J Med Plant Res. 2013;7(21):1537–1542. [Google Scholar]
- 25.Yassab M, Nedi T, Solomon D, Shibeshi W. Hepatoprotective effect of Croton macrostachyus Hochst. Ex Del leaves against CCl4-induced liver damage. Pharm J. 2016;32(1):23–36. doi: 10.4314/epj.v32i1.3 [DOI] [Google Scholar]
- 26.Hossain MA, Al-Hdhrami SS, Weli AM, Al-Riyami Q, Al-Sabahi JN. Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in Sultanate of Oman. Asian Pac J Trop Biomed. 2014;4(1):368–372. doi: 10.12980/APJTB.4.2014C1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harborne. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. United States of America: Chapman and Hall; 1973:1–276. [Google Scholar]
- 28.Teshome T, Sintayehu B, Yohannes H, et al. Radical scavenging activity and preliminary phytochemical screening on aerial part extracts of Cineraria abyssinica Sch. Bip. J Pharmacogn Phytochem. 2015;3(6):239–243. [Google Scholar]
- 29.OECD. Acute Oral Toxicity: Up and Down Procedure. France: Dixon and Mood; 2008:1–27. [Google Scholar]
- 30.Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–895. doi: 10.1021/np0100845 [DOI] [PubMed] [Google Scholar]
- 31.Qadir MI, Ali M, Saleem M, Hanif M. Hepatoprotective activity of aqueous methanolic extract of Viola odorata against paracetamol-induced liver injury in mice. Bangladesh J Pharmacol. 2014;9(2):198–202. [Google Scholar]
- 32.Saleem M, Ahmed B, Karim M, et al. Hepatoprotective effect of aqueous methanolic extract of Rumex dentatus in paracetamol-induced hepatotoxicity in mice. Bangladesh J Pharmacol. 2014;9(3):284–289. doi: 10.3329/bjp.v9i3.18874 [DOI] [Google Scholar]
- 33.Hanafy AA, Badr HM, Ibrahim JM, Amany K, El-Sayed S. Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid Based Complement Alternat Med. 2016;2016:1–7. doi: 10.1155/2016/4579149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard T Guidelines on the care and use of animals for scientific purposes. National advisory committee for laboratory animal research (NACLAR). Available from: www3. ntu. edu. sg/Research2/ … /NACLAR-guide% Z0Lines.pdf. 2004.
- 35.Uchida NS, Silva-Filho SE, Cardia GFE, et al. Hepatoprotective effect of Citral on acetaminophen-induced liver toxicity in mice. Evid Based Complement Alternat Med. 2017;2017:1–9. doi: 10.1155/2017/1796209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761–794. doi: 10.1016/j.mcna.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tittarelli R, Pellegrini M, Scarpellini M, et al. Hepatotoxicity of paracetamol and related fatalities. Eur Rev Med Pharmacol Sci. 2017;21(1):95–101. [PubMed] [Google Scholar]
- 38.Alghazeer R, Elgahmasi S, Elnfati AH, et al. Antioxidant activity and hepatoprotective potential of flavonoids from Arbutus pavarii against CCl4 induced hepatic damage. Biotechnol J Int. 2017;21(1):1–12. doi: 10.9734/BJI/2018/39528 [DOI] [Google Scholar]
- 39.Sharma S, Rana S, Patial V, Gupta M, Bhushan S, Padwad Y. Antioxidant and hepatoprotective effect of polyphenols from Apple pomace extract via apoptosis inhibition and Nrf2 activation in mice. Hum Exp Toxicol. 2016;35(12):1264–1275. doi: 10.1177/0960327115627689 [DOI] [PubMed] [Google Scholar]
- 40.Torres R, Faini F, Modak B, Urbina F, Labbé C, Guerrero J. Antioxidant activity of coumarins and flavonols from the resinous exudate of. Phytochem. 2006;67(10):984–987. doi: 10.1016/j.phytochem.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 41.Sintayehu B, Asres K, Raghavendra Y. Radical scavenging activities of the leaf extracts and a flavonoid glycoside isolated from Cineraria abyssinica Sch. Bip. Exa. rich. J Appl Pharm Sci. 2012;2(4):44–49. doi: 10.7324/JAPS.2012.2407 [DOI] [Google Scholar]
- 42.Mahmood N, Mamat S, Kamisan F, et al. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia Calabura L. leaves. Biomed Res Int. 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulla WA, Salunkhe VR, Bhise SB. Hepatoprotective activity of hydroalcoholic extract of leaves of Alocasia indica (Linn.). Indian J Exp Biol. 2009;47(10):816–821. [PubMed] [Google Scholar]
- 44.Gutiérrez RM, Solís RV. Hepatoprotective and inhibition of oxidative stress in liver of Prostechea michuacana. Rec Nat Prod. 2009;3(1):46. [Google Scholar]
- 45.Okokon JE, Simeon JO, Umoh EE. Hepatoprotective activity of the extract of Homalium letestui stem against paracetamol-induced liver injury. Avicenna J Phytomed. 2017;7(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 46.Shrivastava P. Hepatoprotective activity of Bridelia retusa leaves against paracetamol-induced liver damage in swiss albino mice. Drug Invent Today. 2017;9(1). [Google Scholar]
- 47.Trifunschi SI, Munteanu MFF, Ardelean DG, Orodan M, Osser GM, Gligor RI. Flavonoids and polyphenols content and antioxidant activity of Ficus carica L. extracts from romania. Matica Srpska J Nat Sci Novi Sad. 2015;128(128):57–65. doi: 10.2298/ZMSPN1528057T [DOI] [Google Scholar]
- 48.Yoshioka H, Usuda H, Fujii H, Nonogaki T. Sasa veitchii extracts suppress acetaminophen-induced hepatotoxicity in mice. Environ Health Prev Med. 2017;22(1):1–10. doi: 10.1186/s12199-017-0662-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma M, Sharma G, Vishal V, Ranjan B. Hepatotoxicity: a major complication with critical treatment. MOJ Toxicol. 2015;1(3):1–7. [Google Scholar]
- 50.Rao C, Singh A, Kumar G, Gupta S, Singh S, Rawat A. Hepatoprotective potential of Ziziphus oenoplia (l.) mill roots against paracetamol-induced hepatotoxicity in rats. Adv J Phytomed Clin Ther. 2015;3(1):064–078. [Google Scholar]
- 51.Abdel-Latif MS, K M E. Hepatoprotective effect of coumarin and chlorophyll against aflatoxicosis in rat. J Anim Poult Prod. 2016;7(12):483–490. doi: 10.21608/jappmu.2016.48813 [DOI] [Google Scholar]
- 52.Huang W, Wang Y, Jiang X, Sun Y, Zhao Z, Li S. Protective effect of flavonoids from Ziziphus jujuba cv. jinsixiaozao against acetaminophen-induced liver injury by inhibiting oxidative stress and inflammation in mice. Molecules. 2017;22(10):1–18. doi: 10.3390/molecules22101781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shanmugam B, Shanmugam KR, Doraswamy G, et al. Hepatoprotective effect of Phyllanthus niruri alkaloid fraction in D–galactosamine induced hepatitis in rats. Intl J Pharm Pharm Sci. 2016;8(5):158–161. [Google Scholar]
- 54.Tarahovsky YS, Kim YA, Yagolnik EA, Muzafarov EN. Flavonoid–membrane interactions: involvement of flavonoid–metal complexes in raft signaling. Biochim Biophys Acta. 2014;1838(5):1235–1246. doi: 10.1016/j.bbamem.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 55.Mohammed MS, Osman WJ, Garelnabi EA, et al. Secondary metabolites as anti-inflammatory agents. J Phytopharmacol. 2014;3(4):275–285. [Google Scholar]
- 56.Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: a comprehensive review. Evid Based Complement Alternat Med. 2017;1:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]