Abstract
Background and Aims
Oral systemic pan-Janus kinase [JAK] inhibition is effective for ulcerative colitis [UC] but is limited by toxicities. We describe preclinical to clinical translation of TD-1473—an oral gut-selective pan-JAK inhibitor—from in vitro characterization through a Phase 1b study in patients with UC.
Methods
TD-1473 JAK inhibition potency was evaluated in vitro; plasma pharmacokinetics, safety and efficacy were assessed in mice. In a first-time-in-human study, plasma pharmacokinetics and safety were assessed after single and multiple [14 days] ascending doses administered orally to healthy subjects. The Phase 1b study randomized patients with moderately to severely active UC to receive once-daily oral TD-1473 20, 80 or 270 mg, or placebo for 28 days. Plasma and colonic tissue concentrations were measured; safety was assessed; and efficacy was evaluated by UC clinical parameters, disease-surrogate biomarkers, endoscopy, histology and colonic tissue JAK signalling.
Results
TD-1473 exhibited potent pan-JAK inhibitory activity in vitro. Oral TD-1473 administration to mice achieved high, biologically active colonic tissue concentrations with low plasma exposure and decreased oxazolone-induced colitis activity without reducing blood cell counts vs placebo. TD-1473 administration in healthy human subjects and patients with UC yielded low plasma exposure and was generally well tolerated; treatment in patients with UC resulted in biologically active colonic tissue concentrations and descriptive trends toward reduced clinical, endoscopic and histological disease activity vs placebo.
Conclusion
Gut-selective pan-JAK inhibition with TD-1473 administration resulted in high intestinal vs plasma drug exposure, local target engagement, and trends toward reduced UC disease activity. [Clinicaltrials.gov NCT02657122, NCT02818686]
Keywords: Ulcerative colitis, gut-selective, JAK inhibitor
1. Introduction
Oral Janus kinase [JAK] inhibitors offer a promising treatment option for patients with moderate to severe ulcerative colitis [UC]. The JAK family comprises four tyrosine kinases, JAK 1–3 and tyrosine kinase 2 [TYK2], which associate with intracellular domains of the class I and II cytokine receptor superfamily.1 Upon binding their cytokine ligands, these receptors activate specific JAK pairings, resulting in phosphorylation-mediated activation of signal transducer and activator of transcription [STAT] proteins, which regulate expression of genes that drive immune cell activation.1 The JAK proteins thereby mediate proinflammatory responses to cytokines implicated in UC, including interleukin [IL]-6, IL-23, IL-13, IL-15 and interferon [IFN]γ.2
Tofacitinib, a systemically active pan-JAK inhibitor, is approved for induction and maintenance therapy for patients with moderate to severe UC.3 However, tofacitinib use is associated with systemic adverse events [AEs]. Reductions in leukocyte subset numbers, serious and/or opportunistic infections, elevations in low-density lipoprotein [LDL] cholesterol, non-melanoma skin cancer and other neoplasms have been observed in patients receiving chronic tofacitinib treatment.4–6 In an ongoing safety trial, pulmonary embolism and death were reported in patients with rheumatoid arthritis taking tofacitinib 10 mg twice daily.7 Based on these data, a recent black box warning was issued, and tofacitinib use for UC in the USA is now restricted to patients who have failed tumour necrosis factor [TNF] antagonists.3 Despite early results suggesting enhanced efficacy of tofacitinib 15 mg dosed twice daily in Phase 2 and 3 induction clinical trials for UC, tofacitinib 15 mg was not pursued further due to safety signals.8,9 Thus, the currently approved induction [10 mg twice daily] and maintenance [5 or 10 mg twice daily] doses of tofacitinib3 may not achieve maximal efficacy.
We previously demonstrated that tofacitinib dosed directly to the large intestine in mice limited colitis with minimal systemic exposure.10 These data provided proof-of-concept for the potential therapeutic value of a gut-selective JAK inhibitor in patients with UC. To capture the efficacy of JAK inhibition in UC treatment while minimizing systemic toxicity, we designed TD-1473 as a novel, orally administered, gut-selective pan-JAK inhibitor. The goal of TD-1473 treatment is to inhibit inflammatory bowel disease [IBD]-related proinflammatory cytokine signalling locally in the gastrointestinal tract with minimal systemic exposure. Here we report the results of a preclinical and translational medicine programme describing the preclinical pharmacology, pharmacokinetics [PK], efficacy results and safety profile for TD-1473 from preclinical studies, a first-time-in-human [FTIH] Phase 1 study in healthy subjects, and a first-in-patient Phase 1b study in patients with moderately to severely active UC.
2. Materials and Methods
2.1. Study materials
TD-1473 and tofacitinib sources and formulation are detailed in the Supplementary methods.
2.2. In vitro and human tissue ex vivo studies
Human and mouse recombinant JAK biochemical kinase assays used LanthaScreen assays with recombinant human JAK1, JAK2, JAK3 and TYK2, and mouse JAK1 proteins [Life Technologies; details in Supplementary methods]. Measurement of JAK inhibitory potency in cell-based assays is described in the Supplementary methods. Briefly, test compound potencies were assessed using a variety of cytokine/phosphorylated STAT [pSTAT] pairings to provide comprehensive characterization of JAK inhibitory activity. Inhibition of JAK-mediated cytokine signalling was tested in human and mouse colonic epithelial cells (human colorectal adenocarcinoma-derived HT-29 or Balb/C mouse primary colonic epithelial cells [Cell Biologics]), human monocytic THP-1 cells [ATCC], human peripheral blood mononuclear cells [PBMCs; Stanford Blood Center] and human whole blood [Stanford Blood Center]. Cells were preincubated with test compounds for 1 h, then stimulated with cytokines at 10× the predetermined concentration producing 50% maximal activity [EC50]. Target levels were quantified after an assay-specific incubation period. Compound potencies for all in vitro functional assays were expressed as −log10 of the concentration required for half-maximal inhibition [pIC50] via iterative curve fitting [GraphPad Prism, GraphPad Software]. In vitro measurement of TD-1473 off-target activity, activity in colonic tissue from patients with IBD, and bidirectional transport of TD-1473 and tofacitinib in Caco-2 cell monolayers are described in the Supplementary methods.
2.3. Mouse studies
All ex vivo and in vivo mouse studies were conducted in 15–27 g Balb/C males [aged approximately 6–8 weeks] [Envigo] at Theravance Biopharma US, Inc., using protocols approved by its Institutional Animal Care and Use Committee. Mice were group housed in individually ventilated cages with pellet paper bedding and mouse igloos for enrichment; Envigo Teklad Global 18% protein rodent diet and water were provided ad libitum. All interventions were performed during the light cycle, and mice were not fasted for this study. Measurement of inhibition of JAK-mediated signalling in mouse colon is described in the Supplementary methods.
2.3.1. Oral pharmacokinetics in mice
These procedures are described in the Supplementary methods. Briefly, mice were orally dosed with TD-1473 or tofacitinib [10 mg/kg]. At 0.25, 1, 2, 4, 6 and 24 h after dosing, animals were anaesthetized, and terminal blood samples and colon tissue samples were collected and processed to plasma and colon homogenate, respectively. Plasma and colon concentrations of TD-1473 and tofacitinib were determined by liquid chromatography-tandem mass spectrometry [LC-MS/MS] analysis [details in the Supplementary methods].
2.3.2. Oxazolone-induced colitis model
Mice were anaesthetized with isoflurane [2%], and oxazolone [4%, 150 µL] or vehicle [4:1 acetone/olive oil] was applied to interscapular skin. Seven days later, mice were anaesthetized with isoflurane [2–4%], and oxazolone [1%, 50 µL] or vehicle [1:1 ethanol/water] was injected intrarectally. Oral TD-1473 (0.1–30 mg/kg twice daily [BID]) or tofacitinib (5–30 mg/kg three times daily [TID]) or vehicle (0.5% carboxymethylcellulose [CMC]) was initiated 1 day before intrarectal oxazolone challenge. Two days after intrarectal oxazolone, colitis disease activity index [DAI] and its subscores [stool consistency, gross bleeding, weight loss] were measured as previously described.10 Assessment of target engagement in mouse colon is described in the Supplementary methods.
2.3.3. Splenocyte studies
Mice were dosed orally with TD-1473 [1, 10 and 100 mg/kg, BID], tofacitinib [10, 15 and 30 mg/kg, TID] or vehicle [0.5% CMC] BID or TID for 3 days. In an effort to demonstrate the gastrointestinal selectivity of TD-1473 following oral administration, higher doses than those used in the oxazolone model were chosen for the splenocyte studies. The mice were killed via CO2 inhalation, and then spleens were removed and processed for counting of natural killer [NK] and T and B cells by flow cytometry [BD LSR II, FCS Express 4 Flow Research program, Becton Dickinson], as previously described.10
2.4. Clinical studies
2.4.1. Study design and treatment
The FTIH trial [clinicaltrials.gov NCT02657122] was a double-blind, placebo-controlled, randomized, single-ascending dose [SAD] and multiple-ascending dose [MAD] study in healthy subjects conducted at the Phase 1 unit (Celerion). Participants were sequentially randomized to five single-dose cohorts receiving TD-1473 10, 30, 100, 300 or 1000 mg, or placebo once, and four multiple-dose cohorts receiving TD-1473 10, 30, 100 or 300 mg, or placebo once daily for 14 consecutive days. The study drug was administered orally under fasting conditions. Participants remained in the Phase 1 unit throughout study drug administration. Each cohort included eight participants randomized to active drug or placebo [3:1], for a total of 40 SAD and 32 MAD subjects.
The Phase 1b study [clinicaltrials.gov NCT02818686] was a multicentre, randomized, double-blind, placebo-controlled study evaluating three doses of TD-1473 [20, 80 and 270 mg] administered orally once daily after an overnight fast for 28 days in patients with moderately to severely active UC [Supplementary Figure 1]. The selection of these dose levels was based on [1] pre-clinical models, which provided an estimated human efficacious dose range of 30–60 mg once daily and [2] the safety and PK data from the Phase 1 study in healthy subjects, with TD-1473 administered at doses up to 300 mg once daily. Three doses were selected in order to establish a dose–response relationship to inform the next stage of clinical development.
The study was conducted at six study centres, three in the US and three in Eastern Europe [Georgia, Moldova and Romania; one each]. Patients were enrolled sequentially into three cohorts randomized to receive TD-1473 20 mg vs placebo [10:3], TD-1473 80 mg vs placebo [10:4], or TD-1473 270 mg vs placebo [10:3]. Endoscopy was performed at screening [colonoscopy or sigmoidoscopy] and day 28 [sigmoidoscopy only], following two enemas without oral bowel preparation to minimize drug washout. There was an end-of-study safety and biomarker assessment 14 days after the last dose of TD-1473 or placebo [day 42].
Both studies were approved by the Institutional Review Board and were conducted in agreement with the principles of the Declaration of Helsinki. Participants gave written consent. All authors had access to the study data and reviewed and approved the final manuscript.
2.4.2. Participants
In the FTIH study, healthy male and female adults [19–55 years old] were eligible to enroll subject to standard inclusion and exclusion criteria [Supplementary methods]. Eligible patients for the Phase 1b study were male and female adults [18–75 years old] with UC diagnosed by endoscopy ≥3 months before screening and moderately to severely active disease (Mayo rectal bleeding subscore ≥1, Mayo stool frequency subscore ≥1, and modified Mayo endoscopic subscore ≥2 [presence of any degree of friability was scored as 2 points]) [Supplementary Table 1] with ≥10 cm of disease extent. Patients who received immunomodulators ≤28 days, tofacitinib or biologics ≤60 days [120 days for vedolizumab], or intravenous or rectal corticosteroids or rectal mesalamines ≤14 days before screening were ineligible.
2.4.3. Samples collected
In the FTIH study, blood samples were collected from predose to 72 h postdose for the SAD portion; in the MAD portion, blood was collected predose to 24 h after the day 1 dose; predose on days 1, 2, 3, 5, 7, 9 and 11; and predose to 72 h after the day 14 dose. In the Phase 1b study, blood samples were collected predose and 0.5, 1, 2 and 4 h postdose on days 1 and 14; and once on day 28 after the patient took TD-1473 at home, without regard to time of day. Sigmoid colon and rectal tissues were obtained at screening and from day 28 endoscopies. Stool samples were collected on days 1 and 28.
2.4.4. Pharmacokinetic analysis
Plasma and colonic tissue concentrations of TD-1473 were determined using high-performance LC-MS/MS methods [details in Supplementary methods].
2.4.5. Pharmacodynamic analysis
Frozen biopsy tissues were lysed and levels of pSTAT1 and pSTAT3 were measured by enzyme-linked immunosorbent assay as previously described.11
2.4.6. Efficacy assessments
Phase 1b study clinical efficacy assessments included changes in total and partial Mayo scores and rates of clinical response, clinical remission, improvement in Mayo subscores, and endoscopic improvement assessed on days 1, 14 and 28 [endoscopy on day 1 and 28 only] [Supplementary Table 1]. Stool frequency and rectal bleeding subscores on days 1, 14 and 28 were determined from patient daily diaries. Luminal and systemic signs of inflammation were assessed via faecal calprotectin [predose and day 28] and serum C-reactive protein [CRP; screening part 2 and days 1, 14, 28 and 42] levels, respectively. Histological disease activity, measured by the Robarts Histopathology Index [RHI] and Geboes score,12 was assessed in colonic biopsies at screening and day 28. Endoscopic subscore and histological indices were evaluated by central readers [Robarts Clinical Trials]. Summaries of clinical response, endoscopic response (terminology updated from “mucosal healing” in study protocol), improvement in rectal bleeding subscore, and improvement in endoscopic subscore (terminology updated from “endoscopic response” in study protocol) imputed missing data as treatment failure. Otherwise, summaries (including stool frequency and Physician Global Assessment [PGA] subscores, partial Mayo Score, and total Mayo score) were based on collected data.
2.4.7. Safety and tolerability
In both studies, safety and tolerability were monitored at screening, during study drug administration and at follow up 2 weeks after the last dose of the study drug. Safety assessments in both human studies included: serum electrolytes, blood urea nitrogen, creatinine, hepatic panel and creatinine phosphokinase; complete blood count with differential; fasting lipid panel; vital signs; and 12-lead electrocardiograms [ECGs]. In the Phase 1b study, peripheral blood immunophenotyping was performed to assess absolute numbers of NK cells and T and B lymphocytes.
2.5. Statistical analysis
In vitro and ex vivo assay results and clinical PK parameters were summarized using descriptive statistics; no formal hypothesis testing was performed. Composite PK parameters for TD-1473 and tofacitinib in mice and PK parameters for TD-1473 in human samples were determined by non-compartmental analysis using Phoenix WinNonlin Version 6 [Certara USA, Inc.].
In mouse oxazolone-induced colitis studies, DAI and subscores for each animal were normalized to the control mean [i.e. vehicle/vehicle and vehicle/oxazolone] in each study to facilitate combining data and comparing activities of TD-1473 and tofacitinib. The DAI and subscores of the vehicle/vehicle and vehicle/oxazolone groups were compared using Student’s unpaired t-test. Scores were compared between the vehicle/oxazolone and TD-1473 or tofacitinib/oxazolone groups using one-way analysis of variance [ANOVA] with a Fisher’s Least Significant Difference [LSD] post hoc test. Splenic cell numbers were compared using one-way ANOVA with Fisher’s LSD post hoc test. For all mouse studies, statistical significance was defined as p ≤ 0.05.
The Phase 1b clinical study efficacy and safety results in patients with UC were summarized using descriptive statistics. No formal hypothesis testing was performed.
3. Results
3.1. Preclinical studies
3.1.1. Potency at human recombinant Janus kinases
The mean ± standard deviation [SD] TD-1473 inhibitory potency (−log10 dissociation constant [pKi] values) for human recombinant JAK1, JAK2, JAK3 and TYK2, respectively, were 10.0 ± 0.1, 10.0 ± 0.1, 8.8 ± 0.2 and 9.5 ± 0.1 [Supplementary Table 2]. The corresponding potencies for tofacitnib for human recombinant JAK1, JAK2, JAK3 and TYK2, respectively, were 9.1 ± 0.1, 9.1 ± 0.1, 9.5 ± 0.2 and 7.9 ± 0.2 [all n = 10] [Supplementary Table 2]. The mean ± SD pKi values for mouse recombinant JAK1 for TD-1473 [10.2 ± 0.1, n = 2] and tofacitinib [8.9 ± 0.1, n = 3] were similar to those for human JAK1.
3.1.2. Human and mouse cellular Janus kinase inhibitory potency
TD-1473 produced potent JAK inhibition in a variety of functional assays comprising different cytokine/pSTAT combinations in human THP-1 monocytes, PBMCs or whole blood [Table 1]. TD-1473 and tofacitinib potencies were generally similar except for 10-fold higher potency for TD-1473 vs tofacitinib for inhibition of IL-12-induced phosphorylation of STAT4 in human CD3+ T cells [pIC50 6.9 and 5.9, respectively] [Table 1]. TD-1473 and tofacitinib inhibited cytokine-induced phosphorylation of STAT1 in mouse primary colonic epithelial cells; pIC50 values for inhibition against IL-6, IFNα and IFNγ were 7.3, 6.8 and 7.0 for TD-1473, and 7.9, 6.7 and 6.9 for tofacitinib, respectively [Table 1]. Off-target activity of TD-1473 in vitro is described in the Supplementary results and Supplementary Table 3. Results of screening for the potential of limited gastrointestinal absorption in polarized Caco-2 cell monolayer assays are described in the Supplementary results and Supplementary Table 4. TD-1473 demonstrated JAK inhibitory activity in ex vivo mouse colon and human IBD patient colonic tissue [Supplementary results].
Table 1.
Inhibitory potency for TD-1473 and tofacitinib with respect to cytokine-induced pSTAT, IL-8 or IFNγ elevation in human epithelial cells, monocytes, PBMCs or whole blood
| Cell type | Cytokine stimulus/end point | JAK pairing | Mean ± SD inhibitory potency [pIC50] | |
|---|---|---|---|---|
| TD-1473 | Tofacitinib | |||
| Human epithelial cells [HT-29] | IL-13/pSTAT6 | JAK1/JAK2 | 7.1 ± 0.2 [n = 9] | 7.3 ± 0.1 [n = 14] |
| IL-13/IL-8 | JAK1/JAK2 | 7.1 ± 0.3 [n = 4] | 7.6 ± 0.2 [n = 12] | |
| Human monocytes [THP1] | IL-4/pSTAT6 | JAK1/JAK3 | 7.5 ± 0.1 [n = 2] | 7.4 ± 0.1 [n = 12] |
| IL-6/pSTAT3 | JAK1/JAK2 | 7.3 ± 0.0 [n = 2] | 7.5 ± 0.1 [n = 10] | |
| GM-CSF/pSTAT5 | JAK2/JAK2 | 7.0 ± 0.1 [n = 2] | 6.8 ± 0.1 [n = 4] | |
| Human PBMCs [T cells] | IL-2 [+ anti-CD3]/pSTAT5 | JAK1/JAK3 | 7.5 ± 0.4 [n = 5] | 7.7 ± 0.3 [n = 5] |
| IL-4/pSTAT6 | JAK1/JAK3 | 7.5 ± 0.3 [n = 8] | 7.8 ± 0.3 [n = 8] | |
| IL-6/pSTAT3 | JAK1/JAK2 | 7.1 ± 0.3 [n = 4] | 7.2 ± 0.3 [n = 4] | |
| IFNα/pSTAT1 | JAK1/TYK2 | 7.5 ± 0.1 [n = 6] | 7.5 ± 0.2 [n = 6] | |
| GM-CSF/pSTAT5 | JAK2/JAK2 | 7.2 ± 0.3 [n = 2] | 6.6 ± 0.2 [n = 3] | |
| IL-12/pSTAT4 | JAK2/TYK2 | 6.9 ± 0.1 [n = 4] | 5.9 ± 0.2 [n = 4] | |
| IL-2 [+ anti-CD3]/IFNγ | JAK1/JAK3 | 7.2 ± 0.3 [n = 8] | 7.5 ± 0.3 [n = 8] | |
| Human whole blood | GM-CSF/pSTAT5 | JAK2/JAK2 | 6.7 ± 0.4 [n = 4] | 6.9 ± 0.4 [n = 4] |
| Mouse colonic epithelial cells | IL-6/pSTAT1 | JAK1/JAK2 | 7.3 ± 0.0 [n = 2] | 7.9 ± 0.3 [n = 3] |
| IFNα/pSTAT1 | JAK1/TYK2 | 6.8 ± 0.1 [n = 2] | 6.7 ± 0.1 [n = 3] | |
| IFNγ/pSTAT1 | JAK1/JAK2 | 7.0 ± 0.1 [n = 2] | 6.9 ± 0.0 [n = 3] | |
CD, cluster of differentiation; GM-CSF, granulocyte-monocyte colony stimulating factor; JAK, Janus kinase; IFN, interferon; IL, interleukin; PBMCs, peripheral blood mononuclear cells; pIC50, −log10 concentration producing 50% maximal inhibition; pSTAT, phosphorylated signal transducer and activator of transcription; SD, standard deviation; TYK, tyrosine kinase 2.
3.1.3. Absorption of TD-1473 and tofacitinib: Evidence of gut selectivity of TD-1473
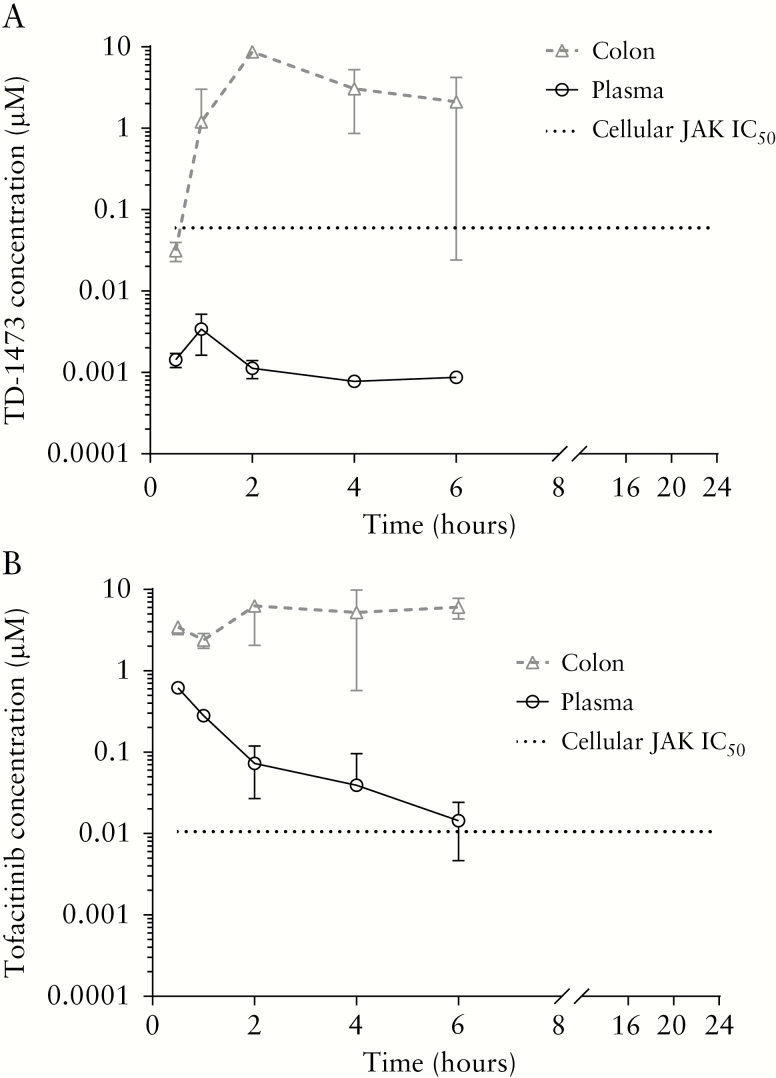
Following oral dosing of TD-1473 [10 mg/kg] in mice, plasma exposure was limited at 0.5–6 h and below the limit of quantification [BLQ; <0.00025 µM] by 24 h postdose. The maximal plasma concentration [Cmax] of 0.003 µM [0.00140 µg/mL] was observed 1 h postdose [Figure 1A]. Colonic exposure was comparatively high, with Cmax of 9.168 µM [3.69 µg/g] at 3 h postdose [Table 2]; the colonic concentration was above the mouse colonic epithelial IL-6/pSTAT1 IC50 [0.05 µM, 0.020 µg/g] [Table 1], whereas plasma levels were approximately 100-fold below the IC50, from 1 to 6 h postdose. In contrast, tofacitinib [10 mg/kg] was rapidly absorbed following oral dosing, with plasma Cmax of 0.62 µM [0.193 µg/mL] at 30 min and colonic Cmax of 8.25 µM [2.57 µg/mL] at 2 h postdose [Figure 1B, Table 2]. Plasma and colonic tofacitinib levels were detectable from 0.5 to 6 h but BLQ [0.00025 µM, 0.000078 µg/mL] by 24 h postdose. Tofacitinib colonic and plasma exposure were above the mouse colonic epithelial IL-6/pSTAT1 IC50 [0.013 µM, 0.00406 µg/g] [Table 1] from 1 to 6 h postdose. The Cmax and area under the concentration–time curve [AUC] from 0 to 24 h colon/plasma exposure ratios for TD-1473 [2636 and 3883, respectively] were markedly higher compared with corresponding values for tofacitinib [13 and 41, respectively].
Figure 1.
Plasma and colon tissue homogenate levels of [A] TD-1473 and [B] tofacitinib following oral administration of TD-1473 [10 mg/kg] or tofacitinib [10 mg/kg] to mice. Data are expressed as mean ± SD [n = 4]. Samples collected from 0 to 24 h; lower limit of quantification = 0.00025 µM plasma or colon. IC50, concentration producing 50% maximal inhibition of mouse colonic epithelium interleukin-6/phosphorylated signal transducer and activator of transcription [STAT]-1 signalling; JAK, Janus kinase; SD, standard deviation.
Table 2.
Composite TD-1473 and tofacitinib plasma and colonic tissue pharmacokinetic parameters [n = 4 per time point] following oral administration of TD-1473 [10 mg/kg] or tofacitinib [10 mg/kg] in male Balb/c mice.
| Analyte | TD-1473 | Tofacitinib | ||
|---|---|---|---|---|
| Dose | 10 mg/kg | 10 mg/kg | ||
| Matrix | Plasma | Colon | Plasma | Colon |
| C max [µM] | 0.003 ± 0.002 | 9.168 ± 10.293 | 0.62 ± 0.075 | 8.252 ± 2.740 |
| AUC [µM·h] | 0.006 ± 0.002 | 22.186 ± 21.888 | 0.720 ± 0.117 | 29.385 ± 14.597 |
| Tissue/plasma ratio [Cmax] | 2636 | 13 | ||
| Tissue/plasma ratio [AUC] | 3883 | 41 |
Cmax and AUC values are expressed as the mean ± SD.
AUC, area under the concentration–time curve; Cmax, maximal plasma concentration; SD, standard deviation.
3.1.4. Efficacy in the oxazolone-induced colitis model
Oral dosing of TD-1473 [0.3–10 mg/kg, BID] and tofacitinib [10 and 15 mg/kg, TID] attenuated the oxazolone-induced elevated DAI, reduction in body weight and stool consistency, and increase in stool blood content [Figure 2 and Supplementary Figure 2A–C]. TD-1473 [1 mg/kg BID] and tofacitinib [10 and 15 mg/kg TID] achieved similar maximum inhibition, with U-shaped dose–response curves; the highest doses of tofacitinib and TD-1473 tested [i.e. 30 mg/kg] did not significantly attenuate the effects of oxazolone [Figure 2 and Supplementary Figure 2A–C]. Orally administered TD-1473 and tofacitinib [both 30 mg/kg BID] vs vehicle engaged JAK targets and significantly inhibited pSTAT3 levels in colonic mucosa of oxazolone-treated mice while preventing T cell infiltration into the mucosa [Supplementary results and Supplementary Figures 3 and 4].
Figure 2.
Effects of orally dosed [A] TD-1473 [0.1–30 mg/kg BID] and [B] tofacitinib [5–30 mg/kg TID] on the disease activity index following Oxa challenge to sensitized mice. Data are expressed as mean ± SD [n = 5–34]. ****p < 0.0001 vs Veh/Veh using Student’s unpaired t-test; #p < 0.05 vs Veh/Oxa using one-way ANOVA with Fisher’s LSD; ##p < 0.01 vs Veh/Oxa using one-way ANOVA with Fisher’s LSD; ###p < 0.001 vs Veh/Oxa using one-way ANOVA with Fisher’s LSD; ####p < 0.0001 vs Veh/Oxa using one-way ANOVA with Fisher’s LSD. ANOVA, analysis of variance; BID, twice daily; LSD, least significant difference; Oxa, oxazolone; SD, standard deviation; TID, three times daily; Veh, vehicle.
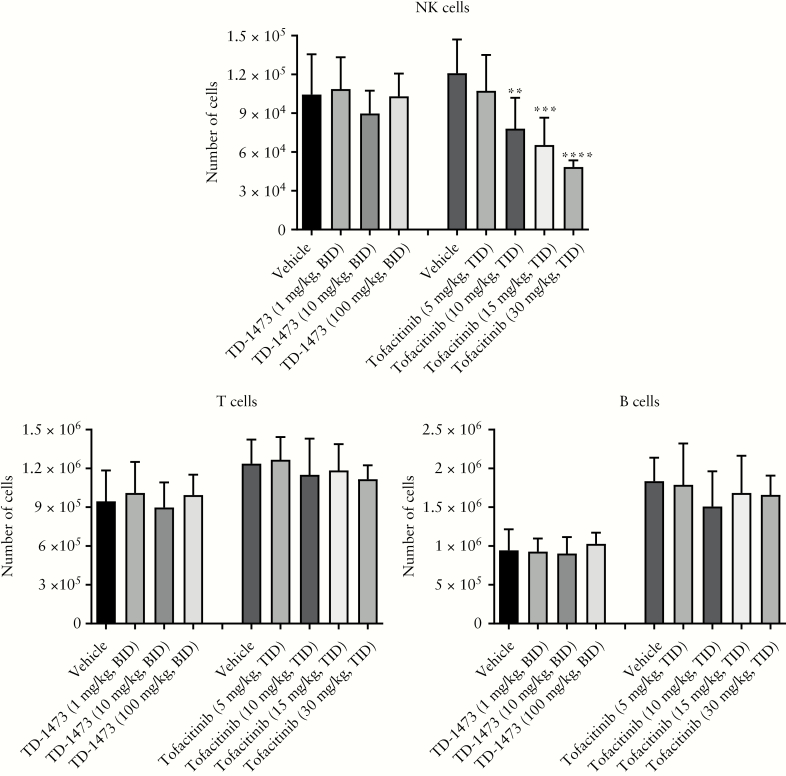
3.1.5. Effect of TD-1473 on splenic NK, B and T cell populations
Oral dosing of TD-1473 [1, 10 and 100 mg/kg BID; i.e. up to 100-fold higher relative to the maximally effective dose in the oxazolone model] vs vehicle did not affect numbers of splenic NK, B or T cells, with statistically non-significant variations ranging from −4% to 14% [Figure 3]. Tofacitinib treatment [10, 15 and 30 mg/kg TID] did not affect splenic T and B cell numbers but resulted in a dose-dependent reduction in NK cell counts relative to vehicle by up to 60% [Figure 3].
Figure 3.
Effects of orally dosed TD-1473 [1, 10 and 100 mg/kg BID] and tofacitinib [5, 10, 15 and 30 mg/kg TID] on splenic NK, T and B cells following 3 days of dosing to mice. All data are expressed as mean ± SD [n = 5–8]. All statistical analyses used one-way ANOVA with Fisher’s LSD. **p = 0.003 vs vehicle; ***p = 0.0004 vs vehicle; ****p < 0.0001 vs vehicle. ANOVA, analysis of variance; BID, twice daily; LSD, least significant difference; NK, natural killer; SD, standard deviation; TID, three times daily.
3.2. Clinical studies
3.2.1. Disposition and baseline demographics
In the FTIH study in healthy subjects, 39/40 enrolled SAD participants and 31/32 enrolled MAD participants completed the study; two participants assigned to placebo withdrew from the study for reasons unrelated to treatment. In the SAD portion, 55% of participants were male with a mean ± SD age of 32 ± 10.25 years and mean body mass index [BMI] of 25.9 kg/m2. In the MAD portion, 78% of participants were male with mean ± SD age of 31.7 ± 8.66 years and mean BMI of 26.3 kg/m2. Baseline demographics are shown in Supplementary Table 5.
In the Phase 1b study in patients with moderately to severely active UC, 39/40 enrolled patients completed the study; one patient discontinued TD-1473 20 mg on day 5 due to lack of efficacy and was hospitalized for UC exacerbation on day 7. The majority were male [65%] and White [95%] [Supplementary Table 6]. Mean ± SD age was 43.8 ± 12.28 years and mean BMI was 27.1 kg/m2 across treatment arms. Median time since UC diagnosis ranged from 2.9 to 5.0 years across treatment arms; individual patient range was 0.3–21.4 years [Supplementary Table 6]. None of the recruited patients received immunomodulators or anti-TNF therapies ≤60 days before screening. However, 5/40 patients previously received immunomodulators and 2/40 underwent anti-TNF antibody therapy; all were randomized to TD-1473. Concomitant steroid treatment was used in 60% of patients receiving TD-1473 80 mg and 22%–30% of patients across the remaining treatment arms. Baseline disease extent and biomarker levels varied, with a tendency toward longer extent of disease in those receiving TD-1473 270 mg vs other treatment arms.
3.2.2. Pharmacokinetics of TD-1473
In the FTIH study, plasma TD-1473 after single dosing was low but detectable at 12 h postdose in all but one participant receiving TD-1473 10 mg, and detectable at 72 h postdose in one participant receiving TD-1473 30 mg and all participants receiving TD-1473 100–1000 mg. Plasma PK parameters are shown in Supplementary Table 7. After multiple dosing, plasma TD-1473 concentrations were low but detectable at 12 h postdose on days 1 and 14 [Figure 4A] except in two participants receiving TD-1473 10 mg on day 1. All available plasma samples from participants receiving TD-1473 30–300 mg had detectable TD-1473 concentrations at 72 h postdose on days 1 and 14. PK parameters after the initial MAD dose were comparable to SAD results; TD-1473 Cmax accumulation ratio [day 14/day 1] ranged from 0.516 to 2.27, and TD-1473 AUC accumulation ratio ranged from 1.34 to 1.69 [Supplementary Table 7].
Figure 4.
Concentrations of TD-1473 following oral administration. [A] Steady-state plasma concentrations on day 14 in first-time-in-human study; [B] steady-state plasma concentrations on day 14 in Phase 1b UC study; [C] colonic tissue concentrations on day 28 in Phase 1b study. Geometric mean [95% confidence interval] TD-1473 colonic tissue concentration was 4090 [2270–7380] pg/g, 65 300 [28 200–151 000] pg/g, and 43 500 [15 200–124 000] pg/g for TD-1473 20, 80 and 270 mg, respectively. Grey shading represents IC50 values [nM] of cellular cytokine/pSTAT assays in human epithelial and immune cells representing all JAK pairings [compare to Table 1]. IC50, 50% of the concentration producing half-maximal inhibition; JAK, Janus kinase; QD, once daily; SD, standard deviation; UC, ulcerative colitis.
In the Phase 1b study in UC patients, plasma TD-1473 levels were also low following oral administration at all dose levels, with a median time to maximal plasma concentration of approximately 1 h [Figure 4B]. Plasma concentrations of TD-1473 following once-daily oral TD-1473 20, 80 or 270 mg for 28 days were similar to FTIH study results and low relative to JAK IC50 values [Figure 4B]. Individual plasma trough concentrations on day 14 were BLQ in 4/9 patients receiving TD-1473 20 mg, and geometric mean trough concentrations on day 14 in subjects receiving TD-1473 80 and 270 mg were 3.57% and 5.79%, respectively, of the corresponding Cmax values [Supplementary Table 8]. Little to no plasma accumulation of TD-1473 was observed after once-daily multiple dosing; geometric mean Cmax and AUC from 0 to 4 h [AUC0-4] values in subjects receiving TD-1473 20 and 270 mg were similar or slightly lower on day 14 relative to day 1 [Supplementary Table 8]. In subjects receiving TD-1473 80 mg, geometric mean AUC0-4 was similar between days 1 and 14, but geometric mean Cmax was approximately 40% higher on day 14 relative to day 1 [Supplementary Table 8].
Geometric mean colonic tissue concentrations [average of sigmoid and rectum samples] on day 28 were 4090, 65 300 and 43 500 pg/mL in patients receiving TD-1473 20, 80 and 270 mg, respectively [Figure 4C]. Subjects receiving TD-1473 80 and 270 mg had high tissue/plasma concentration ratios [day 28 tissue relative to day 14 plasma trough concentration] of approximately 802 and 62. The tissue/plasma ratio for 20 mg TD-1473 was not estimable due to BLQ plasma trough concentrations.
3.3. Pharmacodynamic effects of TD-1473
Levels of pSTAT1 and pSTAT3 in lysate prepared from tissue biopsy at baseline and week 4 were used as pharmacodynamic markers for JAK inhibition. Because pSTAT expression levels generally vary among individuals and over time, the treatment effect for each cohort was assessed using the geometric mean ± 95% confidence interval. In TD-1473-treated patients, pSTAT levels at week 4 were either stable [following TD-1473 80 mg] or reduced [following TD-1473 20 and 270 mg] relative to baseline; in contrast, pSTAT levels increased from baseline to week 4 in placebo-treated patients [Supplementary Figure 5].
3.3.1. Descriptive analyses of the efficacy of TD-1473
As the Phase 1b study was not powered for efficacy analyses, all comparisons are descriptive, and no formal statistical analyses were performed. For this reason, all efficacy data are exploratory. With this caveat, the descriptive analyses demonstrated numerically higher rates of clinical response by total Mayo score, endoscopic response and endoscopic improvement in patients receiving TD-1473 [all doses] vs placebo at day 28 [Figure 5A]; numerically more frequent improvement in rectal bleeding following TD-1473 80 and 270 mg at day 28 vs placebo [Figure 5B]; numerically larger reductions from baseline in rectal bleeding in patients receiving TD-1473 [all doses] at day 14 and TD-1473 80 and 270 mg at day 28 vs placebo [Supplementary Table 9]; and numerically greater decreases in the modified Mayo endoscopic subscores from baseline to day 28 in patients receiving TD-1473 20, 80 and 270 mg vs placebo [Supplementary Table 9]. Reduction in stool frequency from baseline was similar or smaller in patients receiving all doses of TD-1473 compared with placebo-treated patients [Supplementary Table 9]. Only patients receiving TD-1473 270 mg had numerically larger reduction in PGA subscore compared with patients receiving placebo [Supplementary Table 9]. Similarly, numerically greater mean improvement in composite clinical scores [including total and partial Mayo scores] over time occurred following TD-1473 270 mg vs placebo [Supplementary Table 10]. No patient achieved clinical remission after the 28-day treatment. Both Geboes score and RHI showed trends for larger reductions from baseline following treatment with TD-1473 20 and 270 mg, but not TD-1473 80 mg, vs placebo [Figure 5C and Supplementary Table 11]; notably, 3/10 patients receiving TD-1473 80 mg had RHI scores of 0 and Geboes’ major grade of 2A or less at screening, inconsistent with their respective screening endoscopic subscores of 2.
Figure 5.
Efficacy of TD-1473 in subjects with moderately to severely active ulcerative colitis. [A] Rates of clinical response and endoscopic response on day 28; [B] rates of modified Mayo endoscopic and Mayo rectal bleeding subscore improvement from baseline at day 28; [C] change in Robarts Histopathology Index from baseline to day 28. Clinical response, decrease in total Mayo score of ≥3 points and ≥30% with decrease in rectal bleeding subscore by ≥1 or an absolute rectal bleeding subscore of ≤1. Endoscopic response, endoscopic subscore of ≤1. Endoscopic and rectal bleeding subscore improvement, reduction by ≥1 point. SD, standard deviation.
The proportion of patients demonstrating a reduction in faecal calprotectin level—either ≥75% reduction or faecal calprotectin level reduced from >150 mg/L on day 1 to ≤150 mg/L on day 28—was numerically higher in patients receiving TD-1473 80 or 270 mg [38% and 45%, respectively] vs placebo or TD-1473 20 mg [25% and 22%, respectively] [Figure 6A]. Serum CRP levels numerically decreased from baseline during treatment with TD-1473 vs placebo; a treatment effect was evident by day 14 and increased further by day 28, followed by a rebound in serum CRP levels 14 days after the last dose [day 42] [Figure 6B]. Reductions in serum CRP were largest in patients receiving TD-1473 270 mg relative to other treatments.
Figure 6.
Placebo-adjusted changes in disease surrogate biomarkers of ulcerative colitis. [A] Change in faecal calprotectin level at day 1 and day 14; [B] change in serum C-reactive protein by visit and dose. Gray dots and dashed line, subject[s] with faecal calprotectin level ≤150 at day 28; open squares and gray line, subject[s] with faecal calprotectin level reduced by ≥75% from baseline to day 28. CI, confidence interval.
3.3.2. Safety of TD-1473
In the FTIH study, TD-1473 was generally well tolerated as a single dose up to 1000 mg and continuous daily doses up to 300 mg for 14 days. There were no clinically meaningful treatment-emergent AEs [TEAEs] or changes in vital signs, clinical laboratory or ECG parameters in participants receiving TD-1473 vs placebo; details are presented in the Supplementary results and Supplementary Table 12.
In the Phase 1b study, TD-1473 was generally well tolerated. TEAEs were reported in <50% of patients, with similar incidence between TD-1473 treatment arms [38.7% overall] and placebo [44.4%] [Supplementary results and Supplementary Table 13].
There were no vital sign or ECG changes related to study treatment. TD-1473 treatment did not adversely affect laboratory parameters, including haematological [haemoglobin, reticulocytes, leukocytes and leukocyte subsets], renal and hepatic parameters; creatinine phosphokinase; and lipid parameters including total cholesterol and triglyceride levels. High variation in high-density lipoprotein [HDL] and LDL cholesterol levels was observed between biweekly measurements in patients receiving placebo [Supplementary Figure 6A, E]. Per-patient changes in HDL cholesterol levels following treatment with TD-1473 were within the biological variation for HDL cholesterol informed by placebo-treated patients, except in one patient treated with TD-1473 270 mg who demonstrated an increase in HDL cholesterol beyond normal variation on day 14 but not day 28 or 42 [Supplementary Figure 6B–D]. One patient each receiving placebo and TD-1473 80 mg had an increase in LDL cholesterol beyond the upper limit of biological variation [Supplementary Figure 6F, G]. No patient receiving TD-1473 270 mg had post-treatment change in LDL cholesterol greater than biological variation in placebo-treated patients [Supplementary Figure 6H].
4. Discussion
TD-1473 was designed as an orally administered, gut-selective, potent, pan-JAK inhibitor to treat IBD locally while minimizing potential for AEs associated with systemic exposure. This was achieved through application of structure-based drug design, in conjunction with a physicochemical property-focused strategy to optimally balance cellular penetration and inhibition of the JAK targets in gastrointestinal tissue, while simultaneously minimizing the potential for systemic absorption [Supplementary results and Supplementary Table 4].
Like tofacitinib, TD-1473 inhibited JAK1, JAK2, JAK3 and TYK2 in vitro, decreased JAK-dependent responses in mouse and human cells, and, consistent with its PK profile, reduced JAK-mediated signalling in inflamed colonic tissue from mice and from human patients with IBD.
Compared with tofacitinib, TD-1473 demonstrated approximately 40-fold stronger potency for TYK2. Because IL-23 and IL-12 signal via JAK2 and TYK2 but not JAK1 or JAK3,2 a pan-JAK inhibitor with higher potency for TYK2 might provide additional clinical benefits. These include potential for modulation of IL-12/IL-23-mediated inflammation through TYK2, which raises the possibility for TD-1473 to be effective in treating Crohn’s disease as seen for ustekinumab, an anti-IL-12/IL-23 monoclonal antibody.13 As expected, TD-1473 reduced JAK-dependent responses in mouse and human cells, with approximately 10-fold higher potency over tofacitinib for inhibiting IL-12-induced pSTAT4 via JAK2/TYK2 signalling in human whole blood.
Oral administration to mice yielded markedly higher colon/plasma exposure ratios for TD-1473 vs tofacitinib; TD-1473 concentrations were well above the ex vivo IC50 values in colon but 100-fold below plasma levels. Consistent with low systemic exposure, treatment with TD-1473, unlike tofacitinib, did not reduce NK cell numbers in mice compared with vehicle. The highest dose of TD-1473 evaluated in the splenocyte model [i.e. 100 mg/kg] was 100-fold higher than the maximally efficacious dose in the mouse oxazolone colitis model.
As these preclinical results supported the anticipated gut selectivity, potency and anti-inflammatory activity of TD-1473, the development programme progressed to an FTIH SAD–MAD study to assess PK and safety in healthy human subjects. Plasma concentrations of TD-1473 were low and a favourable safety profile was observed following individual doses up to 1000 mg and multiple doses up to 300 mg.
Proceeding with the planned translational progression, the Phase 1b study examined TD-1473 activity in patients with moderately to severely active UC. Plasma PK in these patients was consistent with results from healthy subjects, and colonic tissue exposure was within the range of cellular JAK IC50 values. Despite the 28-day study period, which may be too short to allow clinical remission, TD-1473 demonstrated descriptive evidence of numerical trends toward reduced colonic and systemic inflammation vs placebo. The safety profile remained favourable, with no change from baseline in leukocyte or reticulocyte counts and a reassuring lipid profile. Collectively, these results validate TD-1473 as a gut-selective pan-JAK inhibitor that can accumulate in the colon of patients with UC at concentrations sufficient to inhibit pSTAT signalling. These preclinical results and Phase 1 clinical data in healthy subjects and patients with UC provide a rationale for further evaluation of TD-1473 in patients with IBD. Phase 2/3 clinical trials of TD-1473 in patients with UC and a Phase 2 study in Crohn’s disease are now underway to better characterize the clinical efficacy of this novel therapeutic approach.
Successful approaches to gut-selective drug action in IBD treatment include delayed release or rectal administration of 5-aminosalicylates14 or steroids such as budesonide,15 and biochemical targeting of vedolizumab to a gut-restricted integrin.16 Limiting bioavailability by decreasing gastrointestinal absorption and/or increasing susceptibility to gastrointestinal or hepatic metabolism is also feasible.17 TD-1473 was discovered through application of structure-based drug design, in conjunction with a physicochemical property-focused strategy to afford a gut-selective profile. In both mice and humans, oral administration of TD-1473 achieved high colon tissue exposures with low systemic concentrations, even in the setting of colonic inflammation.
Consistent with preclinical results, descriptive efficacy results of the Phase 1b study showed numerical trends toward higher rates of clinical response, endoscopic response and endoscopic improvement, and decreased serum CRP levels, following all doses of TD-1473 vs placebo at day 28. Rectal bleeding subscores also decreased to a numerically greater extent from baseline to day 14 following all doses of TD-1473 vs placebo, suggesting potential onset of TD-1473 action within 2 weeks. Mean changes in clinical scores from baseline numerically favoured TD-1473 270 mg vs placebo. In patients receiving lower doses of TD-1473 vs placebo, changes from baseline were generally favourable for rectal bleeding and endoscopy subscores but mixed for stool frequency and PGA subscores. Of note, rectal bleeding has been shown to correlate better than stool frequency with endoscopic response and remission, presumably due to multiple potential factors beyond UC activity that could affect stool frequency.18 Mixed results for stool frequency and PGA in the current study are likely to be due to high rates of placebo response and small sample size. These results demonstrate that these subscores may not be useful end points in early-phase clinical trials with small populations. An inconsistent dose effect was observed in histological end points, which trended toward greater improvement with TD-1473 20 and 270 mg but not 80 mg vs placebo. Histological results for patients receiving TD-1473 80 mg were potentially confounded by low pretreatment histological scores inconsistent with pretreatment endoscopic scores in 3/10 patients, which suggested their screening endoscopy biopsies were possibly not taken from the area of worst inflammation. Overall, despite the limitations of the Phase 1b UC study—including small sample size, which precluded formal statistical analysis, and short treatment duration—the data generally showed numerical trends toward TD-1473 clinical activity after only 4 weeks of treatment.
Chronic tofacitinib use is associated with reduced haemoglobin level and immune cell counts, and increased infection risk.5 TD-1473 treatment did not decrease immune cell counts in mice or humans. There were no reductions in leukocytes or leukocyte subsets or haemoglobin level in patients treated with TD-1473 compared with placebo. Systemically available JAK inhibitors, including tofacitinib, baricitinib, upadacitinib and filgotinib, have been associated with increases in total, LDL and/or HDL cholesterol levels within 2–4 weeks of treatment in patients with rheumatoid arthritis.4,5,19–21 The present study showed no dose-related adverse changes in total cholesterol, HDL cholesterol, LDL cholesterol or triglyceride levels after treatment with TD-1473 for up to 4 weeks. In the Phase 1b study, mean HDL cholesterol seemed to increase from baseline to day 28 with increasing TD-1473 dose. However, changes in most individuals were within expected biological variation in HDL cholesterol based on placebo-treated patients, and changes in LDL cholesterol levels were also within the range of intrastudy variability. Taken together, clinical study data suggest the low plasma exposure of TD-1473 limits changes in lipid parameters observed with systemically bioavailable JAK inhibitors.
In conclusion, TD-1473 exhibited gut-selective pharmacology with low systemic oral bioavailability, and high colon tissue exposures within the range of cellular JAK IC50 values. Oral administration of TD-1473 decreased oxazolone-induced colitis disease activity in mice, and descriptive analyses suggested numerical trends toward clinical efficacy in patients with UC. TD-1473 treatment did not reduce leukocyte subset counts in mice and was generally well tolerated in healthy human participants and patients with UC for up to 4 weeks, with no clinically significant changes in haematological or lipid parameters. These results demonstrate the potential of gut-selective pan-JAK inhibition for treatment of IBD. Clinical studies are underway to evaluate the clinical efficacy and safety of TD-1473 in UC and Crohn’s disease.
Supplementary Material
Acknowledgments
The authors would like to acknowledge contributions from Bruce Sands and Brian Feagan in the study design; Arensia Exploratory Medicines and Celerion for subject/patient recruitment; Anshin BioSolutions for initial preparation of the manuscript; and AlphaBioCom, LLC, for writing and editorial support.
Funding
This work was supported by Theravance Biopharma R&D, Inc. The sponsor was involved in collection, analysis and interpretation of the data; authors had full access to the data and approved the final manuscript for submission. Theravance Biopharma Ireland Limited and Janssen Biotech, Inc., have entered into a global co-development and commercialization agreement for TD-1473 and related back-up compounds for inflammatory intestinal diseases, including UC and Crohn’s disease. Writing and editorial support was provided by Judy Phillips, DVM, PhD, of AlphaBioCom, LLC, and by Anshin BioSolutions, and was funded by Theravance Biopharma R&D, Inc.
Data Sharing
Theravance Biopharma [and its affiliates] will not be sharing individual de-identified participant data or other relevant study documents.
Conflict of Interest
W.J.S.: research grants from AbbVie, Amgen, Atlantic Healthcare Limited, Celgene/Receptos, Eli Lilly, Genentech, Gilead Sciences, Janssen, Pfizer, Prometheus Laboratories [owned by Precision IBD] and Takeda; consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Eli Lilly, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Oppilan Pharma, Otsuka, Pfizer, Precision IBD [owns Prometheus Laboratories], Progenity, Prometheus Laboratories [owned by Precision IBD], Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials [owned by Health Academic Research Trust, HART], Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD [owns Prometheus Laboratories], Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech – consultant, stock options; Progenity – consultant, stock; Oppilan Pharma – employee, stock options; Escalier Biosciences – employee, stock options; Precision IBD [also owns Prometheus Laboratories] – employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options. J.P.: Consulting fees from AbbVie, Arena, Boehringer Ingelheim, Celgene, GoodGut, GSK, Janssen, MSD, Nestlé, Oppilan, Pfizer, Takeda, Theravance Biopharma and TiGenix. Unrestricted research grants from AbbVie, MSD and Pfizer. R.B., R.G.: Nothing to disclose. J.A.L.: Consulting fees from Medtronic and research funds from Medtronic, Pfizer and Shire. D.L.B.: Research funding from Theravance Biopharma US, Inc. D.D.N., R.A.G., P.B., J.W., R.S., E. Sandvik, M.T.P.R., M.A.K.: Employees of Theravance Biopharma US, Inc., and shareholders of Theravance Biopharma, Inc. D.T.B., W.K., E. Situ: Former employees of Theravance Biopharma US, Inc., and shareholders of Theravance Biopharma, Inc. B.A.: Employee of Theravance Biopharma Ireland Limited and shareholder of Theravance Biopharma, Inc.
Author Contributions
W.J.S., J.P., D.D.N., B.A., D.T.B., P.B., W.K., J.W., J.A.L. and R.A.G. wrote the article; D.T.B., M.T.P.R., W.K., M.A.K., P.B., W.J.S., R.B., D.D.N., R.A.G., D.L.B. and J.P. designed the research; R.B., J.A.L., M.T.P.R., W.K., R.S., E. Sandvik, E. Situ, D.L.B. and R.A.G. performed the research; and W.J.S., D.D.N., B.A., R.A.G., J.W., D.T.B., P.B., M.A.K., M.T.P.R., W.K., E. Sandvik, E. Situ, D.L.B. and J.P. analysed the data. All authors had full access to all of the data in the study, reviewed and edited the manuscript for intellectual content, and had final responsibility for the decision to submit for publication.
References
- 1. Boland BS, Sandborn WJ, Chang JT. Update on Janus kinase antagonists in inflammatory bowel disease. Gastroenterol Clin North Am 2014;43:603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Vries LCS, Wildenberg ME, De Jonge WJ, D’Haens GR. The future of Janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis 2017;11:885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xeljanz® (tofacitinib) Prescribing Information. New York: Pfizer Labs; 2019. [Google Scholar]
- 4. Fleischmann R, Kremer J, Cush J, et al.; ORAL Solo Investigators Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 5. Kivitz AJ, Cohen S, Keystone E, et al.. A pooled analysis of the safety of tofacitinib as monotherapy or in combination with background conventional synthetic disease-modifying antirheumatic drugs in a Phase 3 rheumatoid arthritis population. Semin Arthritis Rheum 2018;48:406–15. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Panés J, D’Haens GR, et al.. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. https://www.fda.gov/drugs/drug-safety-and-availability/safety-trial-finds-risk-blood-clots-lungs-and-death-higher-dose-tofacitinib-xeljanz-xeljanz-xr. Accessed June 10, 2019.
- 8. Sandborn WJ, Su C, Sands BE, et al.; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Ghosh S, Panes J, et al.; Study A3921063 Investigators Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 10. Beattie DT, Pulido-Rios MT, Shen F, et al.. Intestinally-restricted Janus Kinase inhibition: a potential approach to maximize the therapeutic index in inflammatory bowel disease therapy. J Inflamm [Lond] 2017;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyle DL, Soma K, Hodge J, et al.. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis 2015;74:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosli MH, Feagan BG, Zou G, et al.. Development and validation of a histological index for UC. Gut 2017;66:50–8. [DOI] [PubMed] [Google Scholar]
- 13. Feagan BG, Sandborn WJ, Gasink C, et al.; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 14. Kam L, Cohen H, Dooley C, Rubin P, Orchard J. A comparison of mesalamine suspension enema and oral sulfasalazine for treatment of active distal ulcerative colitis in adults. Am J Gastroenterol 1996;91:1338–42. [PubMed] [Google Scholar]
- 15. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 16. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009;330:864–75. [DOI] [PubMed] [Google Scholar]
- 17. Filipski KJ, Varma MV, El-Kattan AF, et al.. Intestinal targeting of drugs: rational design approaches and challenges. Curr Top Med Chem 2013;13:776–802. [DOI] [PubMed] [Google Scholar]
- 18. Colombel JF, Keir ME, Scherl A, et al.. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017;66:2063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremer JM, Emery P, Camp HS, et al.. A Phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol 2016;68:2867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kremer JM, Genovese MC, Keystone E, et al.. Effects of baricitinib on lipid, apolipoprotein, and lipoprotein particle profiles in a phase IIb study of patients with active rheumatoid arthritis. Arthritis Rheumatol 2017;69:943–52. [DOI] [PubMed] [Google Scholar]
- 21. Vanhoutte F, Mazur M, Voloshyn O, et al.. Efficacy, safety, pharmacokinetics, and pharmacodynamics of filgotinib, a selective JAK-1 inhibitor, after short-term treatment of rheumatoid arthritis: results of two randomized phase IIa trials. Arthritis Rheumatol 2017;69:1949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.