Abstract

Halides of ns2 metal ions have recently regained broad research interest as bright narrowband and broadband emitters. Sb(III) is particularly appealing for its oxidative stability (compared to Ge2+ and Sn2+) and low toxicity (compared to Pb2+). Square pyramidal SbX5 anion had thus far been the most common structural motif for realizing high luminescence efficiency, typically when cocrystallized with an organic cation. Luminescent hybrid organic–inorganic halides with octahedral coordination of Sb(III) remain understudied, whereas fully inorganic compounds show very limited structural engineerability. We show that the host–guest complexation of alkali metal cations with crown ethers fosters the formation of zero-dimensional Sb(III) halides and allows for adjusting the coordination number (5 or 6). The obtained compounds exhibit bright photoluminescence with quantum yields of up to 89% originating from self-trapped excitons, with emission energies, Stokes shifts, and luminescence lifetimes finely-adjustable by structural engineering. A combination of environmental stability and strong, intrinsic temperature-dependence of the luminescence lifetimes in the nanosecond-to-microsecond range nominate these compounds as highly potent luminophores for remote thermometry and thermography owing to their sensitivity range of 200–450 K and high specific sensitivities of 0.04 °C–1.
Organic–inorganic hybrid compounds with structurally and electronically isolated (0D) metal halide anions and organic cations are gaining increasing attention since the past few years.1−5 The interest stems from their color-tunable emission from self-trapped excitons (STE), often with high photoluminescence quantum yield (PL QY) already at room temperature (RT). Bright STE emission is commonly reported for metal ions with ns2 electron configuration, for example, Ge2+, Sn2+, Pb2+, Sb3+, Bi3+, Te4+, and Cu+ with halide ligands.6−23 Since STE is the result of exciton interaction with lattice and large structural reorganization in the excited state, emission in prevailing majority of such materials is spectrally broad and exhibits large Stokes shift. Depending upon the depth of trapping, PL QY of up to 100% at RT is not uncommon and, unlike, for example, semiconductor quantum dots, PL QY is rather insensitive to the surface states and density of carriers and traps. At present, STE emissive halides are researched for applications in solid-state lighting,24,25 remote thermography,26 and X-ray scintillation.8,27
Generally, the coordination of ns2 ions by halides adopts diverse geometries, such as pyramidal, tetrahedral, disphenoidal, square pyramidal, and octahedral, which, in turn, determine the luminescence characteristics. The same metal ion may assume several of these coordinations and rational control of the preferred structure remains a formidable challenge. While adjusting the choice of bulky organic cations, typically quaternary ammonium or phosphonium salts, seems to offer a vast space for structure engineering, the outcome of such trials remains rather unpredictable with regard to the resulting coordination geometry. In this study, we sought of devising an alternative structure engineering strategy and showcase it for one specific ns2-ion case—Sb(III) halides. The latter are appealing for their environmental stability and low toxicity. We also note that hybrid Sb(III) halides, all comprising SbX52– anion, are known to exhibit bright PL at RT.16,28−32 On the other hand, fully inorganic Sb halides, such as A3Sb2X9(where M = Rb, Cs, methylammonium, X = Cl, Br, I), do not yield discrete 0D anions, but rather condensed, non- or poorly luminescent structures (dimers, 1D-chains, 2D-layers).33−36 By adopting a well-known supramolecular concept of host-guest coordination of alkali and alkaline earth metal cations (Cs+, Rb+, Ba2+, etc.) with crown ethers, we could deliberately form either SbX52– or SbX63– ions. We have then crystallized a library of Sb-halides, with bright PL spanning the visible spectral range, and rationalized their PL properties considering the STE geometries in relation to their crystal structures. We also show that some of these halides, such as [C@Ba]4[SbCl6]2[Sb2Cl8], [C@Ba]4[SbBr6]2[Sb2Br8], [C@Cs]2CsSbBr6, [C@Rb]2SbBr5 (with C being 18-crown-6), are promising thermoluminophores for applications in remote thermometry and thermography.26
Typical 0D hybrids feature SbX52– square pyramidal anions (Figure 1A).16,28−31,37,38 Discrete SbX63– octahedra (Figure 1B) are very seldom.38−41 Indeed, common organic cations are single charged (Ph4P+, Et4N+, etc.) and, hence, favor the lower charge on the halometalate anion. Our supramolecular approach readily serves both needs—controlled bulkiness and adjustable charge of the cation—as 18-crown-6 ether readily coordinates alkali metal (K+, Rb+, Cs+) or alkaline earth metal (Ba2+, Figure 1C). Consequently, the coordination number around Sb(III) could be adjusted in the obtained compounds (Figure 2), concomitantly with the tailoring of the emission color. The approach also allows for combining differently charged inorganic cations in one structure, such as in [C@Rb][C@Ba][SbBr6] and [C@Cs][C@Ba][SbBr6] (Figure 2). In the following, we correlate the structure of the obtained compounds with the PL characteristics. In this context, we would like to emphasize that such host-guest chemistry engineering is interesting not only for controlling formability of specific halometalate ions, as in this study, but also because it can alter the optical and other properties in non-obvious ways. An example of the latter is [K(dibenzo-18-crown-6)]2MnX4 (X = Cl, Br), which exhibits circularly polarized emission owing to the difference in the symmetry of the crystal structure.42 We would also note that the supramolecular approach, despite integration of crown molecules, still leads to a rather compact packing of Sb-halide anions. For instance, the molar volume of [C@Rb]2SbCl5 is just 70% of that of (Ph4P)2SbCl5 (457 vs 655 cm3 mol–1, respectively), while both compounds exhibit high PL QY.
Figure 1.
(A) Square pyramidal coordination of Sb3+ ion with C4v point symmetry. (B) Octahedral coordination of Sb3+ ion with Oh point symmetry. (C) General chemical formula of the 18-crown-6 complex with alkali or alkaline-earth metal ions. (D) Photo of several obtained 0D Sb(III) halide compounds with a complex crown-ether/metal cation, namely, [C@Rb]2SbBr5, [C@Cs]2CsSbBr6, [C@Ba]4[SbBr6]2[Sb2Br8], and [C@Ba]4[SbCl6]2[Sb2Cl8] under UV (365 nm) excitation.
Figure 2.
(A) Synthetic approach towards obtaining compounds with SbX52– anionic species that results in various crystal structures motives (half-filled atoms represent 50% static disorder). (B) Several synthetic approaches that result in SbX63– anionic species and local geometry of the obtained compounds.
Two equivalents of single-charged complex cations result solely in square-pyramidal SbX52– (Figure 2A). The SbBr52– anion is “sandwiched” between two crown-ether metal complexes, yielding short distances between halide ions and inorganic cations. The crystal structure usually contains also solvent molecules, which is typical also for many 0D organic–inorganic hybrids. There are also several modes for solvent molecule coordination, of which one could highlight the appearance of an oxygen atom from DMF molecule in the Sb coordination sphere ([C@Cs]2SbBr5·DMF).
Discrete SbX63– octahedra were successfully obtained in several ways, namely by having (i) three equivalents of A+ cation, (ii) one equivalent of A2+, and (iii) one equivalent of each A2+ and A+ (see Supporting Information, SI, for details). The first scenario results in a compound [C@Cs]2CsSbBr6, where only two Cs atoms form complex with the crown ether. This also leads to the packing of [C@Cs]2CsSbBr6 units in 1D fashion along the b direction (Figure S1). This compound can be viewed as the first antimony-based hybrid in between fully inorganic (e.g., Cs3Sb2Br9) and compounds with organic cations. Scenario 2 yields compounds [C@Ba]4[SbX6]2[Sb2X8] (X = Cl or Br, isostructural compounds, Figure S2D) that features two 0D Sb(III) halide species (Figure S3, partially shown in Figure 2). Apart from octahedral species (Figure S3B), the overall charge neutrality is maintained by the dimer [Sb2Br8]2– species, which are built from two edge-sharing square pyramids (Figure S3C). Such edge-sharing SbX5 pyramids are known from the literature; however, they display no emission at RT.43 The scenario (iii) yields compounds [C@Rb][C@Ba][SbBr6] and [C@Cs][C@Ba][SbBr6], crystallizing in a high symmetry space group, R3a. This is due to the fact that the cumulative positive charge of 3+ from two cations is matching the negative charge of the anionic species. Crystal data and structure refinement details for all crystallized compounds as well as photos of crystalline samples can be found in Tables S1–9 and Figure S4.
Having a library of novel 0D Sb(III)-halides allows for correlating the anion geometry and other structural details with the PL characteristics (see optical properties on Figures 3 and S5–S7 and in Table S10). All obtained compounds are luminescent at RT, exhibiting broad emission bands (full width at half maxima, fwhm, of 350-510 meV) and large Stokes shifts for both square pyramidal (Figure 3A) or octahedral (Figure 3B) anions. While PL bands are located at 550–750 nm, PL excitation (PLE) spectra appears below 450 nm and contains several bands. The optical properties of isolated Sb3+ ions, as well as any ns2 ion, can be described in terms of transitions between the states derived from Sb 5s and 5p states hybridized with ligand p states. Despite significant contributions from the ligand atomic orbitals, some features of the PLE and PL can still be simply interpreted using Seitz model.44,45 In this model, the ground state (5s2) is a singlet denoted with atomic term 1S0 and the excited states (5s15p1) consist of triplets and a singlet with corresponding atomic terms 3P0, 3P1, 3P2 and 1P1, respectively.
Figure 3.
(A) Normalized PLE and PL spectra of the compounds with square pyramidal coordination, [C@A]2SbX5, where A = Rb, Cs and X = Cl, Br. (B) Normalized PLE and PL spectra of the compounds with octahedral coordination, [C@Ba]4[SbBr6]2[Sb2Br8], [C@Rb][C@Ba][SbBr6], [C@Cs][C@Ba][SbBr6], and [C@Cs]2CsSbBr6. (C) Single-coordinate diagram depicting the excitation and emission processes from of the localized states. (D) PL peak, (E) Stokes shift, and (F) fwhm trends across different compounds at RT.
For 0D ns2 group 4 metal halides, it has been recently demonstrated that various RT emission bands and their components can be ascribed to transitions between these states.8 A similar study has also been conducted on (Et4N)2SbCl5 proving that the model is valid in case of Sb.29 Seitz model can also be extended with molecular orbital theory to account for the ligand field influence.46 Upon hybridization with molecular orbitals of the ligands, resulting molecular states are derived from respective atomic states (3P0, 3P1, 3P2, and 1P1) and preserve multiplicity (singlet or triplet). Increasingly common in the recent literature is to outline the optical properties of the STE in ns2 metal halides using a simple configurational diagram (Figure 3C). In this diagram, the ground and excited states are represented by potential energy curves. The abscissa axis in this diagram represents the change in geometry: zero point is taken as the ground state equilibrium geometry. The further on this coordinate is the excited state minimum, the bigger is the difference between the ground and excited-state geometries. This depiction readily visualizes the large PL Stokes shift. SbX52– ions display emission shifted to the red region of the visible spectrum, unlike to emission from SbX63– occurring at higher energies. Likewise, also the PLE onset for SbX52– is found at lower energies. This suggests that higher coordination number leads to larger HOMO-LUMO gaps. For SbX52–, the Stokes shift is much larger for chlorides than bromides (Figure 3E), which results from greater structural rearrangement in the excited state for chlorides. Interestingly, RT broadening has two contributors: intrinsic, related to the variation in the local environment of different emitting centers and extrinsic, caused by the energy states broadening at higher temperatures due to activation of vibrational states. The trends obtained from experimental data evidence that square pyramidal coordination results in narrower emission as compared to octahedral. The fwhm in SbX52– compounds scale with the average Sb–X bond length for the same halide (Figure S8), which could be explained by stronger hybridization between the central ion and ligands causing larger thermal broadening. At the same time, no such correlation with the average bond length could be seen for SbX63–, suggesting a dynamic effect discussed below.
Further insight into the structure-property relation was obtained with the aid of Density Functional Theory (DFT) calculations as implemented in the cp2k code (see SI for details). We have performed both ground state and excited state geometry optimization. Generally, the electronic structure of all ns2 metal halides is similar in terms of atomic contributions. Overall, the electronic structure is highly localized, and the typical band structure diagram features flat non-dispersed bands (Figure S9). Because of this, we only concentrate on the analysis of the density of states (DOS). The highest occupied molecular orbital (HOMO) has an antibonding character and is derived from Sb-p and X-p states, while the lowest unoccupied molecular orbital (LUMO) also has an antibonding character and is derived from Sb-p and X-p states. A significant difference between square pyramidal and octahedral coordination is the stereoactivity of the lone pair (Figure 4A). In the case of SbX5, the lone pair occupies a vacant axial position, stabilizing the square pyramidal geometry. There is no apparent evidence of the lone pair stereoactivity in SbX63– from the structure determination (systematic axial bond elongation or Sb atom off-centering for stabilizing HOMO, e.g., first and second-order Janh-Teller distortions).46 A less obvious player could be a dynamic distortion,47,48 which may be a hidden cause for a broader emission from SbX6 compounds. Figures 4B and S10 displays typical element- and orbital-decomposed DOS plots for SbX5 and SbX6.
Figure 4.
(A) Atomically projected molecular orbitals for SbX5 (top) and SbBr6 (bottom) molecular geometries. The HOMO in both cases has antibonding σ* nature, whereas LUMO has antibonding π* nature. (B) Cumulative DOS of [C@Rb]2SbBr5(top) and [C@Cs][C@Ba][SbBr6] (bottom); other atomic contributions (O, C, H, etc.) are omitted for clarity. (C) The distortion of the ground state molecular geometry in the excited state for SbX5 (top) and SbX6 (bottom) geometries showcasing an axial metal halide bond extension.
In accordance with experimental PLE data, calculated HOMO–LUMO gap is smaller for square pyramidal geometry. SbX5 LUMO has two nondegenerate energy states as expected for C4v symmetry, whereas three excited states in SbX6 LUMO are all degenerate due to Oh symmetry. In agreement, SbCl5 compounds typically exhibit one additional emission band in the blue region, unlike SbCl63–.29,39 Typically for an STE, a large structural change occurs once electron is promoted to LUMO. This structural change has been previously accessed with DFT for various 0D Sn and Sb emitters.8,10−12,16,49 We have simulated the structural change by relaxing the triplet geometry in unrestricted Kohn-Sham method as implemented in the cp2k code (details can be found in SI). Figure 4C depicts the geometry change in the excited state. In both SbX5 and SbX6, the axial Sb–X bonds are elongating by the significant value of about 0.5 Å, while the equatorial Sb–X bonds are shortened by about 0.1 Å. In addition, in SbX5, there is a unique mode of distortion: out-of-plane movement of Sb atom above the axial halides plane (Figure S11). While the relative change in bond length is comparable for Cl and Br ligands, the Sb displacement is stronger for Cl, explaining a larger difference in Stokes shift for SbBr5 and SbCl5(from 1.4 to 1.8 eV) in comparison to SbBr6 and SbCl6(1.45 to 1.4–1.3 eV). The energy states of both isolated octahedral and dimer units in [C@Ba]4[SbBr6]2[Sb2Br8] lie close together in DOS (Figure S12). The HOMO states that originate from SbBr63– are at 0.59 eV in the DOS, while LUMO is at 3.95 eV. The HOMO–LUMO difference equates to 3.36 eV (370 nm). For the Sb2Br8 dimeric unit, HOMO–LUMO gap is 2.95 eV (420 nm).
The onset of the experimental PLE spectrum is at 3.22 eV, rather close to the HOMO–LUMO gap of SbBr6. In the literature, Sb2Br82– units were found to luminesce at cryogenic temperatures.43 Taken together, these considerations suggest that the RT emission in [C@Ba]4[SbBr6]2[Sb2Br8] can be assigned to SbBr63– centers. DFT analysis also points to insignificant electronic hybridization with the DMF solvent molecule, commonly found to coordinate SbBr5 anion in one of the obtained compounds (Figure S13).
We have recently demonstrated that STE-emissive
ns2 metal
halides are highly promising luminophores for remote thermometry and
thermography, as demonstrated with Cs4SnX6 and
Sn(II) hybrids.26 Luminescent Sb(III) halides
make for a highly appealing alternative due to their much higher environmental
stability (Sn2+ readily oxidizes). A candidate STE emitter
must exhibit the following PL characteristics: strong temperature-dependence
of the PL lifetime in the desired (for sensing) temperature range
and variation of this lifetime in the nanosecond-to-microsecond range.
The measured PL decays (Figure 5A and B; see also Table S11) can
be fitted with either a monoexponential function or a biexponential
with a shorter component. The corresponding radiative and nonradiative
processes can be outlined with the configuration coordinate diagram
(Figure 5C). The slower,
radiative decay in this model (kr) occurs
from the center of the excited state parabola and the ground state.
A competing, faster nonradiative decay path (knr) is a phonon-assisted de-trapping of the STE. This process
has an activation barrier ΔE and, hence, accelerates
at higher temperatures. Furthermore, such an increase in knr starts earlier (i.e., at lower temperatures) for compounds
with larger Stokes shift (see a general correlation trend between
the Stokes shifts in Table S10 and PL lifetimes
at RT in Table S11). Consequently, at sufficiently
low temperatures, STE-emitters shall ideally exhibit PL QY of 100%
(in the absence of additional absorptive processes like the presence
of impurities, etc., at the excitation wavelengths). At higher temperatures,
there is a temperature range in which the PL QY and measured PL lifetime 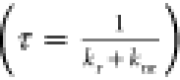 must rapidly drop. Remote
measurement/mapping
of the PL lifetime by, for instance, TCSPC or time-of-flight techniques,
is the physical basis for remote thermometry/thermography. Structure-engineering
of STE-emitters allows tuning of ΔE and hence
the temperature range of PL lifetime sensitivity. Already RT values
of PL QY and PL lifetime provide initial guidance as to the PL lifetime
sensitivity range. For instance, moderate PL QY (20-60%), along with
a PL lifetime of 0.1–1 μs, may indicate a partially quenched
state at RT, and hence, such a compound may be suited as a thermoluminophore
in a temperature range of approximately RT ± 50 °C. A material
with a near-unity RT PL QY and a long PL lifetime (1–10 μs)
is likely sensitive to the elevated temperatures (within its thermal
durability window). Conversely, a compound with RT PL QY of just a
few% or less and PL lifetime of few tens of ns is expected to exhibit
a sensitivity range upon cooling or even at cryogenic temperatures.
For Sb–X bond series, the iodides have the lowest vibrational
frequency and are activated already at very low temperatures, thereby
explaining why SbI52– or SbI63– compounds usually not emissive at RT.41,50 On the contrary, chlorides have the highest vibrational frequency
(Figure S14), which means that RT modes,
responsible for emission quenching, are not sufficiently populated
and this results in almost unity PL QY at RT.
must rapidly drop. Remote
measurement/mapping
of the PL lifetime by, for instance, TCSPC or time-of-flight techniques,
is the physical basis for remote thermometry/thermography. Structure-engineering
of STE-emitters allows tuning of ΔE and hence
the temperature range of PL lifetime sensitivity. Already RT values
of PL QY and PL lifetime provide initial guidance as to the PL lifetime
sensitivity range. For instance, moderate PL QY (20-60%), along with
a PL lifetime of 0.1–1 μs, may indicate a partially quenched
state at RT, and hence, such a compound may be suited as a thermoluminophore
in a temperature range of approximately RT ± 50 °C. A material
with a near-unity RT PL QY and a long PL lifetime (1–10 μs)
is likely sensitive to the elevated temperatures (within its thermal
durability window). Conversely, a compound with RT PL QY of just a
few% or less and PL lifetime of few tens of ns is expected to exhibit
a sensitivity range upon cooling or even at cryogenic temperatures.
For Sb–X bond series, the iodides have the lowest vibrational
frequency and are activated already at very low temperatures, thereby
explaining why SbI52– or SbI63– compounds usually not emissive at RT.41,50 On the contrary, chlorides have the highest vibrational frequency
(Figure S14), which means that RT modes,
responsible for emission quenching, are not sufficiently populated
and this results in almost unity PL QY at RT.
Figure 5.

RT emission decay curves (355 nm laser excitation) for the selected compounds with (A) square pyramidal and (B) octahedral coordinations. (C) Single coordinate diagram model to account for the non-radiative (knr) exciton recombination pathway, showing excited states with different Stokes shift, corresponding to different temperatures of PL quenching. (D) Temperature-dependent average radiative decay time τ dependencies for the selected compounds: [C@Ba]4[SbCl6]2[Sb2Cl8] (green), [C@Ba]4[SbBr6]2[Sb2Br8] (yellow), [C@Cs]2CsSbBr6 (orange), and [C@Rb]2SbBr5 (red).
Figure 5D exemplifies PL lifetime vs T dependencies, showing, for instance, that [C@Cs]2CsSbBr6 can measure temperature upon cooling, whereas [C@Ba]4[SbCl6]2[Sb2Cl8] is operational at higher temperatures. On the basis of these PL lifetime vs temperature curves, specific sensitivity α can be calculated (Figure S15), which is, for instance, up to 0.04 °C–1 for [C@Cs]2CsSbBr6. This value is comparable with 0.05 °C–1 for (C4N2H14I)4SnI6 and Cs4SnBr6 compounds in our earlier study26 and higher than that of commonly reported thermoluminophores (0.005–0.030).51
In conclusion, we have employed supramolecular chemistry strategy to selectively access a specific molecular geometry of antimony halide 0D centers, SbX5 or SbX6. This has enabled us to reveal the trends in compounds with a broad, largely Stokes shifted and highly efficient emission. The PL of synthesized compounds covers a major part of the visible spectrum (550–750 nm), with large Stokes shifts from 1 to 2 eV, while RT PL QY varies from 11 to 89%. Understanding the optical properties trends allows for the choice of the emitter, aiming for certain PL QY, Stokes shift, or spectral color of the emission. Furthermore, we have assessed the potential of such stable Sb-based emitters for a thermography application, revealing that by tuning the coordination and composition, thermoluminophores covering a broad temperature range (from 200 K to above 450 K) with good specific sensitivity (up to 0.04 °C–1) were designed and synthesized.
Acknowledgments
The authors are indebted to Dr. Yevhen Shynkarenko for assistance with PL QY measurements, Dr. Michael Woerle for assistance with crystal structure solution, and Dr. Kyle Mccall for performing Raman experiments. The authors acknowledge the support of the Scientific Center for Optical and Electron Microscopy (ScopeM) of the Swiss Federal Institute of Technology (ETHZ) for the Raman spectroscopy measurements. This work was financially supported by the European Union through Horizon 2020 research and innovation programme (Grant Agreement 819740, Project SCALE-HALO).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmaterialslett.0c00174.
Materials, synthesis, characterization, computational details, packing in [C@Cs]2CsSbBr6, experimental and simulated PXRD patterns, packing of the [C@Ba]4[SbBr6]2[Sb2Br8], optical microscope images of crystalline samples, PL and PLE of the [C@Ba]4[SbCl6]2[Sb2Cl8], comparison of PLE and Kubelka–Munk spectra, 2D PL/PLE maps, optical properties (PL peak position, fwhm, PLE peak, Stokes shift, PLQY) for the obtained antimony(III) halide hybrids, PL fwhm dependence on the average Sb–X bond, orbital-projected band structure plots, orbital-decomposed cumulative DOS, structural change from ground to the excited state, DOS of [C@Ba]4[SbBr6]2[Sb2Br8] and [C@Cs]2SbBr5, results of mono- or biexponential fitting of the RT decay times, Raman spectra of [C@Ba]4[SbCl6]2[Sb2Cl8] and [C@Ba]4[SbBr6]2[Sb2Br8], specific sensitivity, and photostability test (PDF)
Crystallographic data for [C@K]2SbBr5, [C@Ba]4[SbBr6]2[Sb2Br8], [C@Cs][C@Ba][SbBr6], [C@Rb]2SbCl5, [C@Cs]2SbCl5, [C@Rb]2SbBr5, [C@Cs]2SbBr5, [C@Cs]2CsSbBr6, and [C@Rb][C@Ba][SbBr6] (CCDC 1997955) (ZIP)
The authors declare no competing financial interest.
Notes
Experimental and computational methods and crystallographic data (CIF files) for [C@Cs]2SbBr5, [C@Cs]2SbCl5, [C@Rb]2SbBr5, [C@Rb]2SbCl5, [C@Ba]4[SbBr6]2[Sb2Br8], [C@Rb][C@Ba][SbBr6], [C@Cs][C@Ba][SbBr6], [C@Cs]2CsSbBr6 and [C@K]2SbBr5 are deposited in the Cambridge Crystallographic Data Centre database (CCDC) with the following deposition numbers: 1997940, 1998078, 1997957, 1998034, 1998064, 1997955, 1998033, 1997953, 1998071.
Supplementary Material
References
- Lin H. R.; Zhou C. K.; Tian Y.; Siegrist T.; Ma B. W. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018, 3, 54–62. 10.1021/acsenergylett.7b00926. [DOI] [Google Scholar]
- Zhou G.; Su B.; Huang J.; Zhang Q.; Xia Z. Broad-Band Emission in Metal Halide Perovskites: Mechanism, Materials, and Applications. Mater. Sci. Eng., R 2020, 141, 100548. 10.1016/j.mser.2020.100548. [DOI] [Google Scholar]
- Smith M. D.; Connor B. A.; Karunadasa H. I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. 10.1021/acs.chemrev.8b00477. [DOI] [PubMed] [Google Scholar]
- Li S. R.; Luo J. J.; Liu J.; Tang J. Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. 10.1021/acs.jpclett.8b03604. [DOI] [PubMed] [Google Scholar]
- Zhou C. K.; Lin H. R.; He Q. Q.; Xu L. J.; Worku M.; Chaaban M.; Lee S.; Shi X. Q.; Du M. H.; Ma B. W. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng., R 2019, 137, 38–65. 10.1016/j.mser.2018.12.001. [DOI] [Google Scholar]
- Dohner E. R.; Jaffe A.; Bradshaw L. R.; Karunadasa H. I. Intrinsic White-Light Emission from Layered Hybrid Perovskites. J. Am. Chem. Soc. 2014, 136, 13154–13157. 10.1021/ja507086b. [DOI] [PubMed] [Google Scholar]
- Yangui A.; Roccanova R.; Wu Y. T.; Du M. H.; Saparov B. Highly Efficient Broad-Band Luminescence Involving Organic and Inorganic Molecules in a Zero-Dimensional Hybrid Lead Chloride. J. Phys. Chem. C 2019, 123, 22470–22477. 10.1021/acs.jpcc.9b05509. [DOI] [Google Scholar]
- Morad V.; Shynkarenko Y.; Yakunin S.; Brumberg A.; Schaller R. D.; Kovalenko M. V. Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-ray Scintillation. J. Am. Chem. Soc. 2019, 141, 9764–9768. 10.1021/jacs.9b02365. [DOI] [PubMed] [Google Scholar]
- Zhou C. K.; Tian Y.; Wang M. C.; Rose A.; Besara T.; Doyle N. K.; Yuan Z.; Wang J. C.; Clark R.; Hu Y. Y.; Siegrist T.; Lin S. C.; Ma B. W. Low-Dimensional Organic Tin Bromide Perovskites and Their Photoinduced Structural Transformation. Angew. Chem., Int. Ed. 2017, 56, 9018–9022. 10.1002/anie.201702825. [DOI] [PubMed] [Google Scholar]
- Benin B. M.; Dirin D. N.; Morad V.; Worle M.; Yakunin S.; Raino G.; Nazarenko O.; Fischer M.; Infante I.; Kovalenko M. V. Highly Emissive Self-Trapped Excitons in Fully Inorganic Zero-Dimensional Tin Halides. Angew. Chem., Int. Ed. 2018, 57, 11329–11333. 10.1002/anie.201806452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. K.; Lin H. R.; Shi H. L.; Tian Y.; Pak C.; Shatruk M.; Zhou Y.; Djurovich P.; Du M. H.; Ma B. W. A Zero-Dimensional Organic Seesaw-Shaped Tin Bromide with Highly Efficient Strongly Stokes-Shifted Deep-Red Emission. Angew. Chem., Int. Ed. 2018, 57, 1021–1024. 10.1002/anie.201710383. [DOI] [PubMed] [Google Scholar]
- Fu P. F.; Huang M. L.; Shang Y. Q.; Yu N.; Zhou H. L.; Zhang Y. B.; Chen S. Y.; Gong J. K.; Ning Z. J. Organic-Inorganic Layered and Hollow Tin Bromide Perovskite with Tunable Broadband Emission. ACS Appl. Mater. Interfaces 2018, 10, 34363–34369. 10.1021/acsami.8b07673. [DOI] [PubMed] [Google Scholar]
- Mao L. L.; Guo P. J.; Kepenekian M.; Hadar I.; Katan C.; Even J.; Schaller R. D.; Stoumpos C. C.; Kanatzidis M. G. Structural Diversity in White-Light-Emitting Hybrid Lead Bromide Perovskites. J. Am. Chem. Soc. 2018, 140, 13078–13088. 10.1021/jacs.8b08691. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Zhou C. K.; Tian Y.; Shu Y.; Messier J.; Wang J. C.; van de Burgt L. J.; Kountouriotis K.; Xin Y.; Holt E.; Schanze K.; Clark R.; Siegrist T.; Ma B. W. One-Dimensional Organic Lead Halide Perovskites with Efficient Bluish White-Light Emission. Nat. Commun. 2017, 8, 14051. 10.1038/ncomms14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. K.; Lin H. R.; Neu J.; Zhou Y.; Chaaban M.; Lee S.; Worku M.; Chen B. H.; Clark R.; Cheng W. H.; Guan J. J.; Djurovich P.; Zhang D. Z.; Lu X. J.; Bullock J.; Pak C.; Shatruk M.; Du M. H.; Siegrist T.; Ma B. W. Green Emitting Single-Crystalline Bulk Assembly of Metal Halide Clusters with Near-Unity Photoluminescence Quantum Efficiency. ACS Energy Lett. 2019, 4, 1579–1583. 10.1021/acsenergylett.9b00991. [DOI] [Google Scholar]
- Zhou C. K.; Worku M.; Neu J.; Lin H. R.; Tian Y.; Lee S. J.; Zhou Y.; Han D.; Chen S. Y.; Hao A.; Djurovich P. I.; Siegrist T.; Du M. H.; Ma B. W. Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency. Chem. Mater. 2018, 30, 2374–2378. 10.1021/acs.chemmater.8b00129. [DOI] [Google Scholar]
- Jing Y. Y.; Liu Y.; Zhao J.; Xia Z. G. Sb3+ Doping-Induced Triplet Self-Trapped Excitons Emission in Lead-Free Cs2SnCl6 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7439–7444. 10.1021/acs.jpclett.9b03035. [DOI] [PubMed] [Google Scholar]
- Shen N. N.; Cai M. L.; Song Y.; Wang Z. P.; Huang F. Q.; Li J. R.; Huang X. Y. Supramolecular Organization of [TeCl6]2- with Ionic Liquid Cations: Studies on the Electrical Conductivity and Luminescent Properties. Inorg. Chem. 2018, 57, 5282–5291. 10.1021/acs.inorgchem.8b00297. [DOI] [PubMed] [Google Scholar]
- Creason T. D.; Yangui A.; Roccanova R.; Strom A.; Du M. H.; Saparov B. Rb2CuX3 (X = Cl, Br): 1D All-Inorganic Copper Halides with Ultrabright Blue Emission and Up-Conversion Photoluminescence. Adv. Opt. Mater. 2020, 8, 1901338. 10.1002/adom.201901338. [DOI] [Google Scholar]
- Roccanova R.; Yangui A.; Nhalil H.; Shi H.; Du M.-H.; Saparov B. Near-Unity Photoluminescence Quantum Yield in Blue-Emitting Cs3Cu2Br5–xIx (0 ≤ x ≤ 5). ACS Appl. Electron. Mater. 2019, 1, 269–274. 10.1021/acsaelm.9b00015. [DOI] [Google Scholar]
- Roccanova R.; Yangui A.; Seo G.; Creason T. D.; Wu Y. T.; Kim D. Y.; Du M. H.; Saparov B. Bright Luminescence from Nontoxic CsCu2X3 (X = Cl, Br, I). Acs Mater. Lett. 2019, 1, 459–465. 10.1021/acsmaterialslett.9b00274. [DOI] [Google Scholar]
- Tan Z. F.; Li J. H.; Zhang C.; Li Z.; Hu Q. S.; Xiao Z. W.; Kamiya T.; Hosono H.; Niu G. D.; Lifshitz E.; Cheng Y. B.; Tang J. Highly Efficient Blue-Emitting Bi-Doped Cs2SnCl6 Perovskite Variant: Photoluminescence Induced by Impurity Doping. Adv. Funct. Mater. 2018, 28 (29), 1801131. 10.1002/adfm.201801131. [DOI] [Google Scholar]
- Kshirsagar A. S.; Nag A. Synthesis and Optical Properties of Colloidal Cs2AgSb1-xBixCl6 Double Perovskite Nanocrystals. J. Chem. Phys. 2019, 151 (16), 161101. 10.1063/1.5127971. [DOI] [PubMed] [Google Scholar]
- Worku M.; Tian Y.; Zhou C. K.; Lee S.; Meisner Q.; Zhou Y.; Ma B. W. Sunlike White-Light-Emitting Diodes Based on Zero-Dimensional Organic Metal Halide Hybrids. ACS Appl. Mater. Interfaces 2018, 10, 30051–30057. 10.1021/acsami.8b12474. [DOI] [PubMed] [Google Scholar]
- Jun T.; Sim K.; Iimura S.; Sasase M.; Kamioka H.; Kim J.; Hosono H. Lead-Free Highly Efficient Blue-Emitting Cs3Cu2I5 with 0D Electronic Structure. Adv. Mater. 2018, 30, 1804547. 10.1002/adma.201804547. [DOI] [PubMed] [Google Scholar]
- Yakunin S.; Benin B. M.; Shynkarenko Y.; Nazarenko O.; Bodnarchuk M. I.; Dirin D. N.; Hofer C.; Cattaneo S.; Kovalenko M. V. High-Resolution Remote Thermometry and Thermography Using Luminescent Low-Dimensional Tin-Halide Perovskites. Nat. Mater. 2019, 18, 846–852. 10.1038/s41563-019-0416-2. [DOI] [PubMed] [Google Scholar]
- Cao J.; Guo Z.; Zhu S.; Fu Y.; Zhang H.; Wang Q.; Gu Z. Preparation of Lead-free Two-Dimensional-Layered (C8H17NH3)2SnBr4 Perovskite Scintillators and Their Application in X-ray Imaging. ACS Appl. Mater. Interfaces 2020, 12, 19797–19804. 10.1021/acsami.0c02116. [DOI] [PubMed] [Google Scholar]
- Zhou C. K.; Lin H. R.; Tian Y.; Yuan Z.; Clark R.; Chen B. H.; van de Burgt L. J.; Wang J. C.; Zhou Y.; Hanson K.; Meisner Q. J.; Neu J.; Besara T.; Siegrist T.; Lambers E.; Djurovich P.; Ma B. W. Luminescent Zero-Dimensional Organic Metal Halide Hybrids with Near-Unity Quantum Efficiency. Chem. Sci. 2018, 9, 586–593. 10.1039/C7SC04539E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Y.; Li Y.; Liang P.; Zhou T. L.; Wang L.; Xie R. J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. 10.1021/acs.chemmater.9b02935. [DOI] [Google Scholar]
- Zhou C.; Lee S.; Lin H.; Neu J.; Chaaban M.; Xu L.-J.; Arcidiacono A.; He Q.; Worku M.; Ledbetter L.; Lin X.; Schlueter J. A.; Siegrist T.; Ma B. Bulk Assembly of Multicomponent Zero-Dimensional Metal Halides with Dual Emission. ACS Mater. Lett. 2020, 2, 376–380. 10.1021/acsmaterialslett.0c00011. [DOI] [Google Scholar]
- Sedakova T. V.; Mirochnik A. G. Luminescence of Antimony(III) Halogenides Complexes with 2-and 4-Benzylpyridine. Russ. J. Phys. Chem. A 2017, 91, 791–795. 10.1134/S0036024417030256. [DOI] [Google Scholar]
- Zhou J.; Li M.; Molokeev M. S.; Sun J.; Xu D.; Xia Z. Tunable Photoluminescence in Sb3+-Doped Zero-Dimensional Hybrid Metal Halides with Intrinsic and Extrinsic Self-Trapped Excitons. J. Mater. Chem. C 2020, 8, 5058. 10.1039/D0TC00391C. [DOI] [Google Scholar]
- Saparov B.; Hong F.; Sun J. P.; Duan H. S.; Meng W. W.; Cameron S.; Hill I. G.; Yan Y. F.; Mitzi D. B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632. 10.1021/acs.chemmater.5b01989. [DOI] [Google Scholar]
- Hebig J. C.; Kuhn I.; Flohre J.; Kirchartz T. Optoelectronic Properties of (CH3NH3)2Sb2I9 Thin Films for Photovoltaic Applications. ACS Energy Lett. 2016, 1, 309–314. 10.1021/acsenergylett.6b00170. [DOI] [Google Scholar]
- Stercho I. P.; Barchii I. E.; Malakhovskaya T. A.; Pogodin A. I.; Sidei V. I.; Solomon A. M.; Peresh E. Y. Physicochemical Interaction in the Cs3Sb2Br9-Cs2TeBr6 system: The Phase Diagram and the Nature of the Interaction of Components. Russ. J. Inorg. Chem. 2015, 60, 225–229. 10.1134/S0036023615020163. [DOI] [Google Scholar]
- Timmermans C. W. M.; Cholakh S. O.; Blasse G. The Luminescence of Cs3Bi2Cl9 and Cs3Sb2Cl9. J. Solid State Chem. 1983, 46, 222–233. 10.1016/0022-4596(83)90146-9. [DOI] [Google Scholar]
- Wang Z. P.; Wang J. Y.; Li J. R.; Feng M. L.; Zou G. D.; Huang X. Y. [Bmim]2SbCl5: a Main Group Metal-Containing Ionic Liquid Exhibiting Tunable Photoluminescence and White-Light Emission. Chem. Commun. 2015, 51, 3094–3097. 10.1039/C4CC08825E. [DOI] [PubMed] [Google Scholar]
- Petrochenkova N. V.; Storozhuk T. V.; Mirochnik A. G.; Karasev V. E. Antimony(III) Complexes with Quaternary Ammonium Bases: Synthesis, Spectral, and Luminescent properties. Russ. J. Coord. Chem. 2002, 28, 468–472. 10.1023/A:1016245126807. [DOI] [Google Scholar]
- Wang Z. P.; Zhang Z. Z.; Tao L. Q.; Shen N. N.; Hu B.; Gong L. K.; Li J. R.; Chen X. P.; Huang X. Y. Hybrid Chloroantimonates(III): Thermally Induced Triple-Mode Reversible Luminescent Switching and Laser- Printable Rewritable Luminescent Paper. Angew. Chem., Int. Ed. 2019, 58, 9974–9978. 10.1002/anie.201903945. [DOI] [PubMed] [Google Scholar]
- Chen D.; Dai F.; Hao S.; Zhou G.; Liu Q.; Wolverton C. M.; Zhao J.; Xia Z. Crystal Structure and Luminescence Properties of Lead-Free Metal Halides: (C6H5CH2NH3)3MBr6 (M = Bi and Sb). J. Mater. Chem. C 2020, 8, 7322–7329. 10.1039/D0TC00562B. [DOI] [Google Scholar]
- Sedakova T. V.; Mirochnik A. G.; Karasev V. E. Structure and Luminescence Properties of Antimony(III) Complex Compounds. Opt. Spectrosc. 2008, 105, 517–523. 10.1134/S0030400X08100056. [DOI] [Google Scholar]
- Zhao J.; Zhang T. J.; Dong X. Y.; Sun M. E.; Zhang C.; Li X. L.; Zhao Y. S.; Zang S. Q. Circularly Polarized Luminescence from Achiral Single Crystals of Hybrid Manganese Halides. J. Am. Chem. Soc. 2019, 141, 15755–15760. 10.1021/jacs.9b08780. [DOI] [PubMed] [Google Scholar]
- Wojciechowska M.; Szklarz P.; Bialonska A.; Baran J.; Janicki R.; Medycki W.; Durlak P.; Piecha-Bisiorek A.; Jakubas R. Enormous Lattice Distortion Through an Isomorphous Phase Transition in an Organic-Inorganic Hybrid Based on Haloantimonate(III). CrystEngComm 2016, 18, 6184–6194. 10.1039/C6CE01008C. [DOI] [Google Scholar]
- Seitz F. Interpretation of the Properties of Zinc Sulphide Phosphors. J. Chem. Phys. 1938, 6, 454–461. 10.1063/1.1750291. [DOI] [Google Scholar]
- Jacobs P. W. M. Alkali-Halide Crystals Containing Impurity Ions with the ns2 Ground-State Electronic Configuration. J. Phys. Chem. Solids 1991, 52, 35–67. 10.1016/0022-3697(91)90059-9. [DOI] [Google Scholar]
- Vogler A.; Nikol H. The Structures of s2 Metal Complexes in the Ground and sp Excited States. Comments Inorg. Chem. 1993, 14, 245–261. 10.1080/02603599308048663. [DOI] [Google Scholar]
- Oomen E. W. J. L.; Smit W. M. A.; Blasse G. Jahn-Teller Effect in the Sb3+ Emission in Zircon-Structured Phosphates. Chem. Phys. Lett. 1984, 112, 547–550. 10.1016/0009-2614(84)85775-9. [DOI] [Google Scholar]
- Oomen E. W. J. L.; Smit W. M. A.; Blasse G. The Luminescence of Cs2NaSbCl6 and Cs2NaSbBr6: a Transition from a Localized to a Delocalized Excited State. Chem. Phys. Lett. 1987, 138, 23–28. 10.1016/0009-2614(87)80336-6. [DOI] [Google Scholar]
- Han D.; Shi H. L.; Ming W. M.; Zhou C. K.; Ma B. W.; Saparov B.; Ma Y. Z.; Chen S. Y.; Du M. H. Unraveling Luminescence Mechanisms in Zero-Dimensional Halide Perovskites. J. Mater. Chem. C 2018, 6, 6398–6405. 10.1039/C8TC01291A. [DOI] [Google Scholar]
- McCall K. M.; Stoumpos C. C.; Kostina S. S.; Kanatzidis M. G.; Wessels B. W. Strong Electron–Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. 10.1021/acs.chemmater.7b01184. [DOI] [Google Scholar]
- Savchuk O. A.; Haro-Gonzalez P.; Carvajal J. J.; Jaque D.; Massons J.; Aguilo M.; Diaz F. Er:Yb:NaY2F5O Up-Converting Nanoparticles for Sub-Tissue Fluorescence Lifetime Thermal Sensing. Nanoscale 2014, 6, 9727–9733. 10.1039/C4NR02305F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






