Abstract
Up to 18000 ng of total chlorinated paraffins (CPs) was found in hand wipes of individual adult participants in a Norwegian cohort study (n = 60), with a geometric mean (SD) value of 870 (2700) ng. The CPs covered a wide range of alkane chain lengths from C7 to C48 with variable chlorine substitution. Complex mixtures of very-short-chain (vSCCPs, C<10), short-chain (SCCPs, C10–13), medium-chain (MCCPs, C14–17), and long-chain (LCCPs, C>17) CPs were found, contributing on average 0.3%, 20%, 58%, and 22%, respectively, of the total CPs. Significant positive correlations were found between CP levels and factors related to the indoor environment and product use, including living in a house/apartment built before the ban of SCCPs, having a sofa, the number of TVs in the home, and owning a car, which mirrors CP usage as flame retardants and/or plasticizers in consumer products. Compared to previous studies of other organic contaminants in hand wipe samples from the same cohort, CPs were the most abundant flame retardants. This is the first report of CPs in hand wipes, and dermal exposure based on these data suggested that hand contact could be an important human exposure pathway for LCCPs.
Introduction
Many additive, anthropogenic organic chemicals used as flame retardants, textile coatings, and plasticizers and in other applications are released from products found indoors, where they adhere to surfaces and dust. Humans come in contact with these chemicals via directly touching the products, the surfaces, and the dust gathered on surfaces.1−3 Inevitably, some of the chemicals adhere to the hands, leading to potential exposure through dermal absorption and/or hand-to-mouth contact.4,5 Particular concern has been raised about chemicals that are persistent and bioaccumulative and cause adverse health effects,6 necessitating relevant exposure assessment and biomonitoring. In the exposure scenario of hand contact, hand wipes have often been used as a non-invasive medium of sample collection.7 Good correlations have been found between hand wipe amounts and internal concentrations (i.e., human blood or urine) for several flame retardants (e.g., PentaBDEs,8 BDE153,9 TDCIPP,10 and TCPP11) and plasticizers (e.g., phthalates12), highlighting that hand contact may be an important source of human exposure.13
In 2013 and 2014, a sampling campaign including hand wipe collection was applied to a Norwegian cohort of 61 adults to study pathways of exposure to a large group of organic chemicals.14 These included perfluoroalkyl substances (PFASs),15 polybrominated diphenyl ethers (PBDEs), hexabromocyclododecanes (HBCDDs), emerging brominated flame retardants (EBFRs) such as tetrabromobisphenol A (TBBPA),16 organophosphate esters (OPEs),17 and phthalate diesters,18 with hand wipe mass levels ranging from picograms to micrograms per participant (Table S1). However, human exposure to the high-volume-use chemicals chlorinated paraffins (CPs) was not included, as the analysis of CPs was technically too challenging at the beginning of the project.19 Currently, there are still no reports about levels of CPs in hand wipes, and thus, no estimates of CP exposure from dermal contact exist.
CPs represent an extremely complex mixture of polychlorinated n-alkanes, which have a chemical formula of CnH2n+2–mClm (n ≥ 6; m ≥ 1).20 They are mostly applied in consumer products as flame retardants, fat liquors of leather, lubricants, and plasticizers. Current annual global production has exceeded 1 million tons.21 CP industrial products can be categorized as short-chain (SCCPs, C10–13), medium-chain (MCCPs, C14–17), or long-chain (LCCPs, C≥18) CPs on the basis of the chain length range of alkanes, and some CP products contain very-short-chain impurities (vSCCPs, C6–9).22 All CPs are persistent,23 and they are bioaccumulative in humans.24,25 Mammalian toxicity research has mostly focused on SCCPs26 and has identified SCCPs as possible carcinogens to humans.27 Although the toxicity of MCCPs and LCCPs has been studied less, MCCPs are considered to meet the REACH toxicity criteria,28 and MCCP exposure has been shown to cause hemorrhaging effects in rats.29 Liver is one of the target organs for CP toxicity,26 and increases in liver weight were observed for female rats exposed to LCCPs at a dose of 100 mg [kg of body weight (bw)]−1 day–1.30,31 However, only SCCPs have been under global regulation as Persistent Organic Pollutants (POPs) since 2017,32 while MCCPs and LCCPs, which contribute to >80% of the total CP production,21 are not regulated and still being used.
Recent developments in the atmospheric-pressure chemical ionization high-resolution mass spectrometry (APCI-HRMS) method make it feasible to measure CPs of all different categories.33 Using this method, CPs were found in the highest concentrations among the analyzed flame retardants in indoor dust from five countries on four continents,34 indicating significant near-field human exposure to CPs. We hypothesized that humans are in direct hand contact with high amounts of CPs. CPs were thus analyzed in hand wipes of the Norwegian cohort14 and were used to estimate human dermal exposure to CPs.
Materials and Methods
Study Cohort
Hand wipe samples were collected from 60 adults (45 women and 15 men, ages of 20–66) of the cohort from Oslo, Norway, between November 2013 and April 2014.14 The participants were advised to avoid hand washing for at least 60 min before the collection of the hand wipes. For each participant, two sterile gauze pads (3 in. × 3 in., Swift First Aid Inc., Valencia, CA) were collected, one for each hand, and stored together as one sample. Each pad was immersed in 3 mL of isopropanol and then used to wipe the palm and the back from wrist to fingertips.14,35 Detailed information about sample collection and storage is given in the Supporting Information. Each participant completed a questionnaire regarding age, gender, weight and height, type and number of home appliances, and other characteristics of the indoor home environment. This study was approved by the Regional Committees for Medical and Health Research Ethics in Norway (Case 2013/1269), and all participants completed a written informed consent form prior to participation. Approval for the chemical analyses carried out in Sweden was given by the Regional Ethics Committee in Stockholm, Sweden (Case 2014/624-31/1).
Analysis of CPs in Hand Wipes
The extraction and cleanup methods were introduced previously.16,36 In brief, hand wipe samples were extracted in a previous study for HFR analysis16 using ultrasonic extraction three times with 8 mL of a mixture of hexane and acetone (1:1, v:v). The extracts were reanalyzed for CPs. The extract was spiked with 10 ng of [13C10]-1,5,5,6,6,10-hexachlorodecane ([13C10]HCD, Cambridge Isotope Laboratories, Andover, MA) and then cleaned up on a multilayer SPE column. Twenty nanograms of Dechlorane 603 (Occidental Chemical Corp.) was added to the eluent as the volumetric standard before instrumental analysis.
CPs were measured on the basis of congener groups (denoted as CnClm, where n ≥ 6 and m ≥ 2) using direct injection dichloromethane (DCM)-enhanced APCI-Orbitrap-HRMS (Q Exactive, Thermo Fisher Scientific, San Jose, CA) in full-scan mode (m/z 250–2000) with a resolution of 120000 full width at half-maximum. For detailed instrumental settings, see the Supporting Information. A total of 444 congener groups (from C6Cl4 to C48Cl12) were considered to form a CnClm profile. SCCPs, MCCPs, and LCCPs in the samples were quantified using a CnClm-profile deconvolution method33 with five SCCP, seven MCCP, and five LCCP reference mixtures, respectively (Table S2), while vSCCPs were quantified on the basis of a Chinese CP mixture (CP-52).22
QA/QC
The mass resolution was sufficient to resolve the CnClm.37,38 The uncertainty test of CP quantification showed a mean deviation of 40% when R2 = 0.50 (Figure S1). The deviation was in a reasonable range,39 and thus, an R2 of 0.50 was used as a cutoff threshold. For detailed information about the uncertainty analysis and R2, see the Supporting Information. Quantification of SCCPs, MCCPs, and LCCPs of all samples fulfilled the R2 > 0.50 criterion (Table S2);40 for vSCCPs, the R2 ranged from 0.42 to 0.97 [median of 0.74 (Table S3)]. Method recovery was checked by the analysis of gauze pad blanks spiked with CP mixtures and [13C10]HCD, the recoveries of which were between 48% and 103%. If corrected with the recovery of [13C10]HCD (mean of 75%), measured levels of vSCCPs, SCCPs, MCCPs, and LCCPs were 100 ± 11%, 103 ± 12%, 96 ± 17%, and 96 ± 34% of the spiked levels, respectively (Table S4). Levels of CPs in the collected hand wipe samples were thus recovery-corrected. The mean recovery (±SD) of [13C10]HCD in the samples was 77 ± 24%. Thirteen field blanks were analyzed together with the hand wipe samples. Method detection limits (MDLs) and method quantification limits (MQLs) were defined as mean field blank values plus 3 and 5 times the SD, respectively. For vSCCPs, SCCPs, MCCPs, and LCCPs, the MDLs were 0.70, 27, 33, and 0.65 ng/participant, respectively, while the MQLs were 1.1, 42, 50, and 1.0 ng/participant, respectively. Levels of CPs were not blank corrected.
Statistical Analysis
Masses below the MDL were replaced with MDL/√2. Masses above the MDL but below MQL were replaced with MQL/√2. The distribution of CPs in the hand wipe samples was highly skewed. Therefore, the Mann–Whitney U and Kruskal–Wallis tests were used to explore differences between CP amounts in hand wipes (with a >80% detection frequency) and categorical indoor environment variables, while the Spearman’s rank correlation was used for continuous variables. The level of significance was set to p = 0.05.
Dermal Exposure Calculation
Hand skin surface area (SA, cm2) was first estimated using an equation adopted from the U.S. EPA Exposure Handbook41 based on the body weight (BW, kg) and height (cm) of the participants:
| 1 |
where a, b, and c are gender-specific constants (Table S5).41
Dermal exposure to CPs via hand contact [ng (kg of bw)−1 day–1] was then estimated for each participant:
| 2 |
where Chw is the surface area-normalized mass of CPs in hand wipes (ng/cm2), AF is the absorption fraction (unitless), ED is the exposure duration of 24 h (t/24, where t is assumed to be 24 h), and EF is the exposure frequency, which is assumed to be 1 event/day. Dermal AF was not available for any of the CP categories, so we thus referred to the values from ex vivo studies of the other halogenated flame retardants (HFRs)42 based on their correlations with log KOW(43) (Table S6).
Results and Discussion
Levels of CPs in Hand Wipes
The total individual masses of CPs measured in hand wipes ranged from 43 to 18000 ng/participant, with a median of 950 ng (Table 1). CPs were found to be the most abundant flame retardants analyzed in the same samples. As previously reported, the medians of PBDEs, HBCDDs, TBBPA,16 and OPEs17 were 2.9, 180, 570, and 192 ng/participant, respectively (Table S1). This is similar to results in indoor dusts from five countries where CPs were found in higher concentrations than several other flame retardants, including PBDEs, HBCDDs, EBFRs, and OPEs.34 On the basis of the reported positive association between hand wipe and indoor dust concentrations of other flame retardants,16 we assume that the CP hand wipe–indoor dust association would be positive, but this remains to be investigated.
Table 1. Descriptive Statistics for CPs Measured in Hand Wipe Samples (n = 60) and Estimated Daily Dermal Exposure to CPs for Adults Based on These Dataa.
| vSCCPs | SCCPs | MCCPs | LCCPs | sumCP | |
|---|---|---|---|---|---|
| DF | 40% | 97% | 100% | 100% | |
| Mass (ng/participant) | |||||
| geometric mean | <0.70 | 170 | 490 | 150 | 870 |
| median | <0.70 | 160 | 490 | 150 | 950 |
| range | <0.70–13 | 22–2400 | 33–7400 | 10–8500 | 43–18000 |
| Mass per Hand Surface Area (pg/cm2) | |||||
| geometric mean | <0.53 | 160 | 460 | 150 | 830 |
| median | <0.53 | 160 | 520 | 120 | 830 |
| range | 0.16–17 | 11–3400 | 16–11000 | 7.4–12000 | 35–26000 |
| Chlorine Content (w/w) | |||||
| geometric mean | 63% Cl | 58% Cl | 52% Cl | 46% Cl | 51% Cl |
| median | 63% Cl | 58% Cl | 52% Cl | 46% Cl | 51% Cl |
| range | 59–65% Cl | 56–60% Cl | 47–55% Cl | 40–52% Cl | 40–56% Cl |
| Estimated Daily Dermal Exposure [ng (kg of bw)−1 day–1] | |||||
| 5P | not available | 0.12 | 0.55 | 0.038 | 0.71 |
| median | not available | 0.62 | 2.4 | 0.3 | 3.8 |
| 95P | 0.045 | 3.6 | 11 | 5.5 | 17 |
| RfDb | not available | 2300 | 6000 | 71000 | not available |
The sums of CPs (sumCP) were calculated from individual results. The detection frequency (DF) is the percentage of samples with a mass above the MDLs.
Both MCCPs and LCCPs are current-use CPs and were detected in all of the hand wipes. MCCPs were the predominant CPs, measured in 50 of 60 samples, contributing on average 58% of the total CP amounts. In the other 10 samples, LCCPs were the most abundant and contributed up to 75% of the total CPs. There were no significant positive correlations between LCCP amounts and MCCP amounts (p > 0.05), which may indicate that MCCPs and LCCPs were not used simultaneously. Although the sale and use of SCCPs in consumer products has been banned in Norway since 2002,44 SCCPs were still above the MDL in 58 of 60 samples in the study presented here and contributed ≤40% of the total CPs (median of 20%). SCCP amounts were positively and significantly correlated to those of MCCPs (r2 = 0.75; p < 0.01), which may indicate SCCP impurities in MCCP products. Interestingly, vSCCPs were above the MDL in 24 samples, although they contributed a maximum of 2.1% of the total CPs. The amounts of vSCCPs significantly correlated with those of the C10 CPs (r2 = 0.83; p < 0.01), indicating that the vSCCPs were synthesized from C<10 alkane impurities in C10–13 alkanes used to synthesize SCCPs.
Associations between CPs in Hand Wipes and Personal Characteristics and Indoor Environment Information
No significant differences were observed between genders, between participant age groups, or between working conditions, yet positive and significant correlations [p < 0.05; Mann–Whitney test (Table S7)] were observed between CP ranked levels in hand wipes and owning a car (SCCPs, MCCPs, and LCCPs) or a sofa (SCCPs and MCCPs). Significantly smaller SCCP amounts in the hand wipes were observed for participants living in a house/apartment built after the 2002 ban (p < 0.05; Mann–Whitney test), which may suggest a successful enforcement of the regulation of SCCPs in Norway. LCCP amounts in hand wipes were correlated with the number of TVs (Spearman’s rank; r = 0.15; p < 0.05). Increasing concentrations of LCCPs in hand wipes were also observed with an increasing number of children in the household (p < 0.05; Kruskal–Wallis test). No significant differences were observed between home sizes or between how long the participant had lived in the home. No significant correlations were found between CP amounts in the hand wipes and home floor material (p > 0.05; Kruskal–Wallis test), although median amounts of MCCPs were relatively larger in the group having laminate flooring (Table S7). The correlations may reflect potential sources of CPs such as building materials, furnishings, and electronic home appliances, where CPs might be used as flame retardants and/or plasticizers.45
Dermal Exposure
The direct daily dermal exposure to CPs (Table 1) was estimated from hand wipe amounts. Arithmetic mean values were 0.013, 1.1, 4.3, and 1.1 ng (kg of bw)−1 day–1 for vSCCPs, SCCPs, MCCPs, and LCCPs, respectively. In a previous study, mean daily dietary intakes of CPs were estimated for adults in Sweden and were 18, 39, and 2.0 ng (kg of bw)−1 day–1 for SCCPs, MCCPs, and LCCPs, respectively.46 On the basis of the Swedish data, on average, dietary exposures to SCCPs and MCCPs were approximately 15 and 9 times higher than dermal exposure via hands, but for LCCPs, dermal exposure was up to half of dietary exposure (diet:dermal ratio of 1.9). Lower ratios between the two exposure pathways can be expected if bioaccessibility of CPs is considered in dietary intake estimation.47 The dermal exposure estimates for CPs were far smaller than the oral reference doses (RfDs) (Table 1), but the estimates consider only dermal exposure from the hands and may thus underestimate the total exposure from, e.g., air and clothing.16
Our study is subject to several limitations. Caution should be taken in interpreting the dermal exposure results, as the AFs of CPs were not available and were adapted from other halogenated flame retardants with similar log KOW values (Table S6). Levels of CPs and their dermal absorption efficiencies may be different between hand palm and back, which requires in-depth studies. Meanwhile, the total dermal exposure could be much more significant if the total body surface area is considered. Because levels of BDE-209 and phthalates in skin wipes from legs, forearms, and forehead were found to be similar to those of the hand wipes in other studies,50−52 it is likely that CPs are not only on the hands but also on other parts of the skin surface. Moreover, hand-to-mouth exposure to CPs was not assessed for the adults in this study, considering that mouthing hands might be more common for toddlers than adults. However, measurement of CPs on hands also suggests an ingestion pathway because CPs can be transferred from the hand surface to any finger foods consumed such as burgers and pizza or be ingested from activities like nail biting.4 In addition, we assumed that the levels of CPs measured at the time of sampling are representative of levels on skin surfaces over a 24 h period. Only one pair of hand wipes was collected per participant, which does not account for intraindividual variability over time and exposure in different microenvironments. However, statistically significant correlations were seen previously for some brominated flame retardants between the same handwipe samples used for CP analysis and dust samples from the home in this cohort.16 Other studies have indicated the reliability of using handwipes as significant correlations were found between concentrations of PBDEs in serum, handwipes, and dust in toddlers53 and in office workers.8 Significant correlations were also seen between handwipes and dust for PBDEs and organophosphate flame retardants in children, and little variability was seen in levels in handwipes between siblings in the same house.54 Finally, our participants were recruited from the Norwegian Institute of Public Health and were primarily women (45 of 60), which might limit our ability to generalize results to the broader Norwegian population.
Complex Compositions of CPs
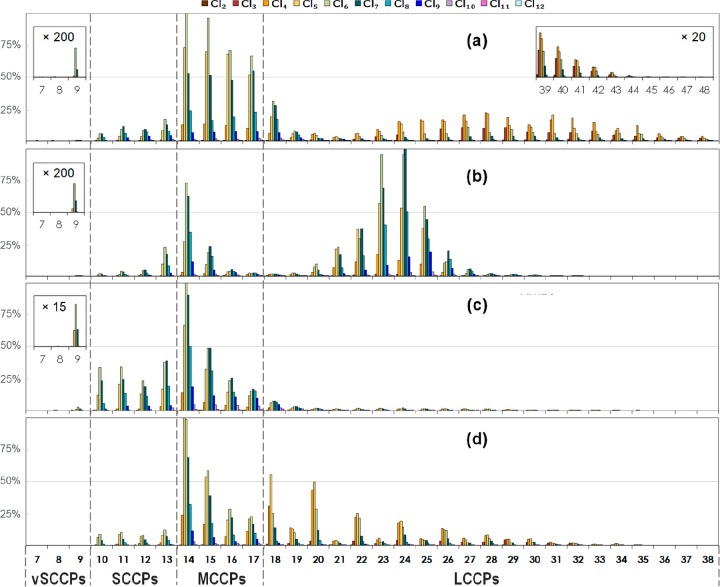
Some examples of CP compositions in the hand wipes of this study are shown in Figure 1 and Figure S2 based on relative abundances of CP congener groups. The CPs in the samples were complex mixtures with variable chain lengths from C7 to C48 with chlorine contents ranging from 40% to 56% Cl (Table 1). This is the first observation of CPs with chain lengths of longer than C40.
Figure 1.
Selected congener group profiles of chlorinated paraffins in hand wipes from four different individuals in the Norwegian cohort to show the variability of CP patterns found. The vertical axis represents the percent relative abundance (semiquantified on the basis of the instrumental response); all of the horizontal axes represent carbon chain length.
In indoor environments, CPs have been found in electronics46,55 and building materials.56 The chain length distributions can be used as fingerprints of CPs for tracking possible uses of CP commercial products.57 C14 CPs were the predominant chain length in 57 of 60 samples, with a mean contribution of 33% to the total instrumental responses of CPs. C14 CPs stand out in four samples (Figure S2a), which contributed more than half of the total CP abundance. This may indicate direct contact with chlorinated tetradecane products.58 Similar profiles were found in two sediment core sections from the Swedish coast representing the years 1976 and 2015 in which C14 CPs contributed up to 89% to the total instrumental responses of CPs.23 Meanwhile, even-numbered chain length CPs such as C18 and C20 stand out in five of 60 samples (Figure 1d). A similar profile was recently reported in an indoor dust sample from South Africa.59 The profiles suggest the use of CP commercial products in indoor environments that have been synthesized by ethylene oligomerization.60
C13 CPs were the predominant SCCPs in 51 of 58 samples, followed by C12 or C11 CPs. This may indicate that some of the SCCPs were impurities in MCCP products. C11 CPs were the most or the second most abundant SCCPs in 34 of 58 samples, indicating potential sources of SCCP commercial products. These SCCPs could be legacy chemicals present in items produced before SCCPs were banned in 2002 in Norway. As shown in a previous study, SCCPs were approximately 50 mg/g in the sealing material between concrete piers and walls as well as the material between the floor plate and walls in an old office building in Germany while no MCCPs were detected.56 SCCPs in building materials may lead to long-term emissions.61 Another possible source of SCCP commercial products could be items with CPs of unspecified compositions. For example, SCCPs predominated in the components of several kitchen hand blenders sold on the Swedish market between 2014 and 2016.46 SCCP commercial products may also be a source of vSCCPs detected in the study presented here (Figure 1c), as small amounts of C9 CPs were previously found in a legacy SCCP commercial product from the EU market.62
For SCCPs, Cl6 homologues predominated, while for MCCPs, Cl6 and then Cl5 and Cl7 were predominant. LCCPs showed a wide range of chlorine contents from 40% Cl to 52% Cl (Table 1), predominantly Cl4 and Cl5 (Figure 1a,d) or Cl6 and Cl7 (Figure 1b,c). For each chain length, CPs consisted of a large number of positional isomers, in particular for longer-chain CPs. For example, there are 327 theoretical isomers for C10 CPs and more than half a billion for C30 CPs, by assuming no more than one bound chlorine atom on any carbon atom.63 As such, CP congener group profiles in the hand wipes (Figure 1 and Figure S1) indicate human exposure to extremely complex mixtures of CPs, but identification of the individual CP isomers from the mixtures cannot be achieved at the moment,64−66 leaving a question mark over the possibility of mixture effects67 from the exposures.
This first report on CPs in hand wipes, where dermal exposure to CPs was identified as an important human exposure pathway for LCCPs, demonstrates the need for additional studies exploring the relative contribution of various exposure pathways to these ubiquitous environmental contaminants.
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007–2013 for research, technological development and demonstration under Grant Agreement 316665 (A-TEAM project). The CP analyses were funded by the Nordic Council of Ministers (Project 2019-008). However, the content of this publication does not necessarily reflect the Nordic Council of Ministers’ views, opinions, attitudes, or recommendations. All participants are acknowledged for their contribution. The authors also acknowledge A-TEAM’s Ph.D. fellows for their contributions during the A-TEAM sampling campaign. Merle Plassmann (ACES) is acknowledged for her support with the Orbitrap measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.0c00090.
Sample collection and storage, instrumental analysis, uncertainty analysis, previous hand wipe results (Table S1), profile deconvolution results (Tables S2 and S3), method recoveries (Table S4), gender-specific constants for SA (Table S5), AF estimations (Table S6), statistical analysis (Tables S7), uncertainty analysis (Figure S1), and CP congener group profiles (Figure S2) (PDF)
Author Present Address
§ J.H.T.: Faculty of Industrial Sciences & Technology (FIST), Universiti Malaysia Pahang (UMP), Lebuhraya Tun Razak, 26300 Gambang, Kuantan, Pahang, Malaysia.
The authors declare no competing financial interest.
Supplementary Material
References
- Hoffman K.; Garantziotis S.; Birnbaum L. S.; Stapleton H. M. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ. Health Perspect. 2015, 123 (2), 160–165. 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. L.; Hammel S. C.; Hoffman K.; Lorenzo A. M.; Chen A.; Webster T. F.; Stapleton H. M. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int. 2018, 116, 176–185. 10.1016/j.envint.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S.; de Wit C. A.; Abdallah M. A.-E.; Bergh C.; Björklund J. A.; Covaci A.; Darnerud P. O.; de Boer J.; Diamond M.; Huber S.; Leonards P.; Mandalakis M.; Östman C.; Haug L. S.; Thomsen C.; Webster T. F. Indoor Contamination with Hexabromocyclododecanes, Polybrominated Diphenyl Ethers, and Perfluoroalkyl Compounds: An Important Exposure Pathway for People?. Environ. Sci. Technol. 2010, 44 (9), 3221–3231. 10.1021/es903476t. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Kelly S. M.; Allen J. G.; McClean M. D.; Webster T. F. Measurement of Polybrominated Diphenyl Ethers on Hand Wipes: Estimating Exposure from Hand-to-Mouth Contact. Environ. Sci. Technol. 2008, 42 (9), 3329–3334. 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- Zartarian V. G.; Ozkaynak H.; Burke J. M.; Zufall M. J.; Rigas M. L.; Furtaw Jr E. J. A modeling framework for estimating children’s residential exposure and dose to chlorpyrifos via dermal residue contact and nondietary ingestion. Environ. Health Perspect. 2000, 108 (6), 505–514. 10.1289/ehp.00108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S.; Staskal D. F. Brominated flame retardants: cause for concern?. Environ. Health Perspect. 2004, 112 (1), 9–17. 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Cao Z.; Yu G.; Wu M.; Li X.; Zhang Y.; Wang B.; Huang J. Estimation of Exposure to Organic Flame Retardants via Hand Wipe, Surface Wipe, and Dust: Comparability of Different Assessment Strategies. Environ. Sci. Technol. 2018, 52 (17), 9946–9953. 10.1021/acs.est.8b02723. [DOI] [PubMed] [Google Scholar]
- Watkins D. J.; McClean M. D.; Fraser A. J.; Weinberg J.; Stapleton H. M.; Sjödin A.; Webster T. F. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect. 2011, 119 (9), 1247–1252. 10.1289/ehp.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J. H.; Sellström U.; Papadopoulou E.; Padilla-Sánchez J. A.; Haug L. S.; de Wit C. A. Serum concentrations of legacy and emerging halogenated flame retardants in a Norwegian cohort: Relationship to external exposure. Environ. Res. 2019, 178, 108731. 10.1016/j.envres.2019.108731. [DOI] [PubMed] [Google Scholar]
- Hammel S. C.; Hoffman K.; Webster T. F.; Anderson K. A.; Stapleton H. M. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 2016, 50 (8), 4483–4491. 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Eulaers I.; Alves A.; Papadopoulou E.; Padilla-Sanchez J. A.; Lai F. Y.; Haug L. S.; Voorspoels S.; Neels H.; Covaci A. Human exposure pathways to organophosphate flame retardants: Associations between human biomonitoring and external exposure. Environ. Int. 2019, 127, 462–472. 10.1016/j.envint.2019.03.053. [DOI] [PubMed] [Google Scholar]
- Hammel S. C.; Levasseur J. L.; Hoffman K.; Phillips A. L.; Lorenzo A. M.; Calafat A. M.; Webster T. F.; Stapleton H. M. Children’s exposure to phthalates and non-phthalate plasticizers in the home: The TESIE study. Environ. Int. 2019, 132, 105061. 10.1016/j.envint.2019.105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Arnot J. A.; Wania F. Model for Screening-Level Assessment of Near-Field Human Exposure to Neutral Organic Chemicals Released Indoors. Environ. Sci. Technol. 2014, 48 (20), 12312–12319. 10.1021/es502718k. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E.; Padilla-Sanchez J. A.; Collins C. D.; Cousins I. T.; Covaci A.; de Wit C. A.; Leonards P. E. G.; Voorspoels S.; Thomsen C.; Harrad S.; Haug L. S. Sampling strategy for estimating human exposure pathways to consumer chemicals. Emerging Contaminants 2016, 2 (1), 26–36. 10.1016/j.emcon.2015.12.002. [DOI] [Google Scholar]
- Poothong S.; Padilla-Sánchez J. A.; Papadopoulou E.; Giovanoulis G.; Thomsen C.; Haug L. S. Hand Wipes: A Useful Tool for Assessing Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) through Hand-to-Mouth and Dermal Contacts. Environ. Sci. Technol. 2019, 53 (4), 1985–1993. 10.1021/acs.est.8b05303. [DOI] [PubMed] [Google Scholar]
- Tay J. H.; Sellström U.; Papadopoulou E.; Padilla-Sánchez J. A.; Haug L. S.; de Wit C. A. Assessment of dermal exposure to halogenated flame retardants: Comparison using direct measurements from hand wipes with an indirect estimation from settled dust concentrations. Environ. Int. 2018, 115, 285–294. 10.1016/j.envint.2018.03.038. [DOI] [PubMed] [Google Scholar]
- Xu F.; Giovanoulis G.; van Waes S.; Padilla-Sanchez J. A.; Papadopoulou E.; Magnér J.; Haug L. S.; Neels H.; Covaci A. Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy. Environ. Sci. Technol. 2016, 50 (14), 7752–7760. 10.1021/acs.est.6b00246. [DOI] [PubMed] [Google Scholar]
- Giovanoulis G.; Bui T.; Xu F.; Papadopoulou E.; Padilla-Sanchez J. A.; Covaci A.; Haug L. S.; Cousins A. P.; Magnér J.; Cousins I. T.; de Wit C. A. Multi-pathway human exposure assessment of phthalate esters and DINCH. Environ. Int. 2018, 112, 115–126. 10.1016/j.envint.2017.12.016. [DOI] [PubMed] [Google Scholar]
- van Mourik L. M.; Leonards P. E. G.; Gaus C.; de Boer J. Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere 2015, 136, 259–272. 10.1016/j.chemosphere.2015.05.045. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Short-Chain Chlorinated Paraffins (SCCPs) and Other Chlorinated Paraffins Action Plan. 2009.https://www.epa.gov/sites/production/files/2015-09/documents/sccps_ap_2009_1230_final.pdf (accessed 2019-11-09).
- Glüge J.; Wang Z.; Bogdal C.; Scheringer M.; Hungerbühler K. Global production, use, and emission volumes of short-chain chlorinated paraffins–A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. 10.1016/j.scitotenv.2016.08.105. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; de Wit C. A.; Yin G.; Du X.; Yuan B. Shorter than short-chain: Very short-chain chlorinated paraffins (vSCCPs) found in wildlife from the Yangtze River Delta. Environ. Int. 2019, 130, 104955. 10.1016/j.envint.2019.104955. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Brüchert V.; Sobek A.; de Wit C. A. Temporal Trends of C8–C36 Chlorinated Paraffins in Swedish Coastal Sediment Cores over the Past 80 Years. Environ. Sci. Technol. 2017, 51 (24), 14199–14208. 10.1021/acs.est.7b04523. [DOI] [PubMed] [Google Scholar]
- Li T.; Wan Y.; Gao S.; Wang B.; Hu J. High-Throughput Determination and Characterization of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Human Blood. Environ. Sci. Technol. 2017, 51 (6), 3346–3354. 10.1021/acs.est.6b05149. [DOI] [PubMed] [Google Scholar]
- Qiao L.; Gao L.; Zheng M.; Xia D.; Li J.; Zhang L.; Wu Y.; Wang R.; Cui L.; Xu C. Mass Fractions, Congener Group Patterns, and Placental Transfer of Short-and Medium-Chain Chlorinated Paraffins in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2018, 52 (17), 10097–10103. 10.1021/acs.est.8b02839. [DOI] [PubMed] [Google Scholar]
- Ei-Sayed Ali Y.; Legler J.. Overview of the Mammalian and Environmental Toxicity of Chlorinated Paraffins. In Handbook of Environmental Chemistry; Boer J., Ed.; Springer-Verlag: Berlin, 2010; Vol. 10, pp 135–154. [Google Scholar]
- IARC . IARC Monographs Volume 48: Chlorinated paraffins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; 1990; Vol. 48, p 55. [PMC free article] [PubMed] [Google Scholar]
- Gluge J.; Schinkel L.; Hungerbuhler K.; Cariou R.; Bogdal C. Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol. 2018, 52 (12), 6743–6760. 10.1021/acs.est.7b06459. [DOI] [PubMed] [Google Scholar]
- DanishEPA . Survey of short-chain and medium-chain chlorinated paraffins. 2014. http://www2.mst.dk/Udgiv/publications/2014/11/978-87-93283-19-0.pdf (accessed 2019-11-09).
- Bucher J. R.; Alison R. H.; Montgomery C. A.; Huff J.; Haseman J. K.; Farnell D.; Thompson R.; Prejean J. D. Comparative toxicity and carcinogenicity of two chlorinated paraffins in F344N rats and B6C3F1 mice. Toxicol. Sci. 1987, 9 (3), 454–468. 10.1093/toxsci/9.3.454. [DOI] [PubMed] [Google Scholar]
- Nilsen O.; Toftgård R.; Glaumann H. Effects of chlorinated paraffins on rat liver microsomal activities and morphology. Importance of the length and the degree of chlorination of the carbon chain. Arch. Toxicol. 1981, 49 (1), 1–13. 10.1007/BF00352066. [DOI] [PubMed] [Google Scholar]
- UNEP . UNEP/POPS/COP.8/SC-8/11. Listing of short-chain chlorinated paraffins. 2017. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf (accessed 2019-02-18).
- Bogdal C.; Alsberg T.; Diefenbacher P. S.; MacLeod M.; Berger U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87 (5), 2852–60. 10.1021/ac504444d. [DOI] [PubMed] [Google Scholar]
- Wong F.; Suzuki G.; Michinaka C.; Yuan B.; Takigami H.; de Wit C. A. Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere 2017, 168, 1248–1256. 10.1016/j.chemosphere.2016.10.074. [DOI] [PubMed] [Google Scholar]
- A-TEAM. https://www.facebook.com/ateamresearch/videos/678237242256284/ (accessed 2020-02-07).
- Yuan B.; Benskin J. P.; Chen C.-E. L.; Bergman Å. Determination of Chlorinated Paraffins by Bromide-Anion Attachment Atmospheric-Pressure Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett. 2018, 5 (6), 348–353. 10.1021/acs.estlett.8b00216. [DOI] [Google Scholar]
- Schinkel L.; Lehner S.; Heeb N. V.; Lienemann P.; McNeill K.; Bogdal C. Deconvolution of Mass Spectral Interferences of Chlorinated Alkanes and Their Thermal Degradation Products: Chlorinated Alkenes. Anal. Chem. 2017, 89 (11), 5923–5931. 10.1021/acs.analchem.7b00331. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Lehner S.; Heeb N. V.; Marchand P.; Cariou R.; McNeill K.; Bogdal C. Dealing with strong mass interferences of chlorinated paraffins and their transformation products: An analytical guide. TrAC, Trends Anal. Chem. 2018, 106, 116–124. 10.1016/j.trac.2018.07.002. [DOI] [Google Scholar]
- Krätschmer K.; Schächtele A. Interlaboratory studies on chlorinated paraffins: Evaluation of different methods for food matrices. Chemosphere 2019, 234, 252–259. 10.1016/j.chemosphere.2019.06.022. [DOI] [PubMed] [Google Scholar]
- Brandsma S. H.; van Mourik L.; O’Brien J. W.; Eaglesham G.; Leonards P. E.; de Boer J.; Gallen C.; Mueller J.; Gaus C.; Bogdal C. Medium-Chain Chlorinated Paraffins (CPs) Dominate in Australian Sewage Sludge. Environ. Sci. Technol. 2017, 51 (6), 3364–3372. 10.1021/acs.est.6b05318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Exposure Factors Handbook; EPA/600/R-09/052F; 2011.
- Abdallah M. A.-E.; Pawar G.; Harrad S. Evaluation of 3D-human skin equivalents for assessment of human dermal absorption of some brominated flame retardants. Environ. Int. 2015, 84, 64–70. 10.1016/j.envint.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Frederiksen M.; Vorkamp K.; Jensen N. M.; Sørensen J. A.; Knudsen L. E.; Sørensen L. S.; Webster T. F.; Nielsen J. B. Dermal uptake and percutaneous penetration of ten flame retardants in a human skin ex vivo model. Chemosphere 2016, 162, 308–314. 10.1016/j.chemosphere.2016.07.100. [DOI] [PubMed] [Google Scholar]
- UNEP . Draft technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with short-chain chlorinated paraffins. 2018. http://www.basel.int/Portals/4/download.aspx?d=UNEP-CHW-SUBM-POPs-TGs-Comment-SCCP-Canada-201801.English.docx (accessed 2019-09-19).
- De Boer J.; El-Sayed Ali T.; Fiedler H.; Legler J.; Muir D. C.; Nikiforov V. A.; Tomy G. T.; Tsunemi K.. The handbook of environmental chemistry 10: Chlorinated paraffins; Springer-Verlag: Berlin, 2010. [Google Scholar]
- Yuan B.; Strid A.; Darnerud P. O.; de Wit C. A.; Nyström J.; Bergman Å. Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ. Int. 2017, 109, 73–80. 10.1016/j.envint.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Cui L.; Gao L.; Zheng M.; Li J.; Zhang L.; Wu Y.; Qiao L.; Xu C.; Wang K.; Huang D. Bioaccessibility of short chain chlorinated paraffins in meat and seafood. Sci. Total Environ. 2019, 668, 996–1003. 10.1016/j.scitotenv.2019.03.043. [DOI] [PubMed] [Google Scholar]
- EFSA . Scientific opinion on the risk for animal and human health related to the presence of chlorinated paraffins in feed and food. 2019. https://www.efsa.europa.eu/sites/default/files/consultation/consultation/EFSA_CONTAM_Chlorinated_paraffins.pdf (accessed 2020-02-05).
- EnvironmentCanada . Canadian Environmental Protection Act: priority substances list assessment report chlorinated paraffins. 1993. http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/psl1-lsp1/paraffins-paraffines/paraffins-paraffines-eng.pdf (accessed 2019-02-27).
- Gong M.; Weschler C. J.; Zhang Y. Impact of Clothing on Dermal Exposure to Phthalates: Observations and Insights from Sampling Both Skin and Clothing. Environ. Sci. Technol. 2016, 50 (8), 4350–4357. 10.1021/acs.est.6b00113. [DOI] [PubMed] [Google Scholar]
- Gong M.; Zhang Y.; Weschler C. J. Measurement of Phthalates in Skin Wipes: Estimating Exposure from Dermal Absorption. Environ. Sci. Technol. 2014, 48 (13), 7428–7435. 10.1021/es501700u. [DOI] [PubMed] [Google Scholar]
- Cao Z.; Chen Q.; Zhu C.; Chen X.; Wang N.; Zou W.; Zhang X.; Zhu G.; Li J.; Mai B.; Luo X. Halogenated Organic Pollutant Residuals in Human Bared and Clothing-Covered Skin Areas: Source Differentiation and Comprehensive Health Risk Assessment. Environ. Sci. Technol. 2019, 53 (24), 14700–14708. 10.1021/acs.est.9b04757. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Eagle S.; Sjödin A.; Webster T. F. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 120 (7), 1049–1054. 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Misenheimer J.; Hoffman K.; Webster T. F. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, 116, 54–60. 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistl C.; Sprengel J.; Vetter W. High levels of medium-chain chlorinated paraffins and polybrominated diphenyl ethers on the inside of several household baking oven doors. Sci. Total Environ. 2018, 615, 1019–1027. 10.1016/j.scitotenv.2017.09.112. [DOI] [PubMed] [Google Scholar]
- Hilger B.; Fromme H.; Volkel W.; Coelhan M. Occurrence of chlorinated paraffins in house dust samples from Bavaria, Germany. Environ. Pollut. 2013, 175, 16–21. 10.1016/j.envpol.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Du X.; Yuan B.; Zhou Y.; Benskin J. P.; Qiu Y.; Yin G.; Zhao J. Short-, Medium-, and Long-Chain Chlorinated Paraffins in Wildlife from Paddy Fields in the Yangtze River Delta. Environ. Sci. Technol. 2018, 52 (3), 1072–1080. 10.1021/acs.est.7b05595. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . TSCA new chemicals review program standard review risk assessment on medium-chain chlorinated paraffins (PMN P-14-0683, P-14-0684). 2015. https://www.epa.gov/sites/production/files/2015-12/documents/standard_review_risk_assessment_p-14-683-684_qualice_docket.pdf (accessed 2019-09-19).
- Brits M.; de Boer J.; Rohwer E. R.; De Vos J.; Weiss J. M.; Brandsma S. H. Short-, medium-, and long-chain chlorinated paraffins in South African indoor dust and cat hair. Chemosphere 2020, 238, 124643. 10.1016/j.chemosphere.2019.124643. [DOI] [PubMed] [Google Scholar]
- Chibwe L.; Myers A. L.; De Silva A. O.; Reiner E. J.; Jobst K.; Muir D.; Yuan B. C12–30 α-Bromo-Chloro “Alkenes”: Characterization of a Poorly Identified Flame Retardant and Potential Environmental Implications. Environ. Sci. Technol. 2019, 53 (18), 10835–10844. 10.1021/acs.est.9b03760. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Ye W.; Little J. C. Predicting emissions of volatile and semivolatile organic compounds from building materials: A review. Building and Environment 2013, 64, 7–25. 10.1016/j.buildenv.2013.02.012. [DOI] [Google Scholar]
- Castro M.; Breitholtz M.; Yuan B.; Athanassiadis I.; Asplund L.; Sobek A. Partitioning of Chlorinated Paraffins (CPs) to Daphnia magna Overlaps between Restricted and in-Use Categories. Environ. Sci. Technol. 2018, 52 (17), 9713–9721. 10.1021/acs.est.8b00865. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.; Stern G. A.; Muir D. C. G.; Fisk A. T.; Cymbalisty C. D.; Westmore J. B. Quantifying C-10-C-13 polychloroalkanes in environmental samples by high-resolution gas chromatography electron capture negative ion high resolution mass spectrometry. Anal. Chem. 1997, 69 (14), 2762–2771. 10.1021/ac961244y. [DOI] [Google Scholar]
- van Mourik L. M.; Lava R.; O’Brien J.; Leonards P. E. G.; de Boer J.; Ricci M. The underlying challenges that arise when analysing short-chain chlorinated paraffins in environmental matrices. Journal of Chromatography A 2020, 1610, 460550. 10.1016/j.chroma.2019.460550. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Bogdal C.; Canonica E.; Cariou R.; Bleiner D.; McNeill K.; Heeb N. V. Analysis of Medium-Chain and Long-Chain Chlorinated Paraffins: The Urgent Need for More Specific Analytical Standards. Environ. Sci. Technol. Lett. 2018, 5 (12), 708–717. 10.1021/acs.estlett.8b00537. [DOI] [Google Scholar]
- Yuan B.; Muir D.; MacLeod M. Methods for trace analysis of short-, medium-, and long-chain chlorinated paraffins: Critical review and recommendations. Anal. Chim. Acta 2019, 1074, 16–32. 10.1016/j.aca.2019.02.051. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A.; Faust M.; Scholze M.; Backhaus T. Low-Level Exposure to Multiple Chemicals: Reason for Human Health Concerns?. Environ. Health Perspect. 2007, 115 (Suppl. 1), 106–114. 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.