Abstract
The management of human epidermal growth factor receptor (HER2)-positive breast cancer has improved over the past decade. However, despite improvements in systemic control, a substantial proportion of patients with advanced HER2-positive breast cancer suffer from central nervous system metastases and even intracranial progression after aggressive local treatment. There is paucity of data and no consensus on the systemic therapies for patients with intracranial progression. This review discusses both local and systemic treatments for HER2-positive breast cancer with brain metastases with a special focus on the response of central nervous system metastases. A recommended practical treatment algorithm to guide physicians in selecting the most appropriate anti-HER2 therapy for their patients is suggested.
Keywords: HER2+ breast cancer, HER2-targeted therapy, metastatic brain tumours, radiotherapy, tyrosine kinase inhibitor
Introduction
Metastatic brain tumours are the most common central nervous system (CNS) neoplasm in adults and metastatic breast cancer is the second most common cancer associated with brain involvement. The incidence of brain metastases ranges from 10% to 30% in patients with metastatic breast cancer.1–3 Major risk factors for brain metastases in patients with breast cancer include young age (<40 years old), active extra cranial disease, triple-negative and human epidermal growth factor receptor (HER2)-positive subtypes, presence of germline BRCA1/BRCA2 mutation, higher presenting stage, histological grade and Ki67 labelling index.4–7 Compared to other subtypes, HER2-positive breast cancer has a much higher incidence of brain metastases (up to 50% of autopsy cases) as around 10% of brain metastases are asymptomatic and are not diagnosed before death.8,9 Nearly half of patients with advanced HER2-positive breast cancer die from CNS progression.10 There are several factors attributed to the high risk of brain metastases in HER2-positive breast cancer, including the remarkable survival gain after patients receiving anti-HER2 therapies, allowing more time for brain relapse, the limited intracranial activity of the anti-HER2 therapies, and the propensity of HER2-positive breast cancer to spread to the brain.11,12
The current standard of care for brain metastases includes surgical resection, whole-brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS).13–15 For systemic treatment of brain metastases, it is recommended to follow the advanced breast cancer treatment algorithms in cases with extracranial progression. On the other hand, for patients developing brain metastases with stable extracranial disease, both European Society of Medical Oncology (ESMO) International Consensus Guidelines for Advanced Breast Cancer (ABC) and the National Comprehensive Cancer Network (NCCN) guidelines recommend no switching of systemic therapy after local treatment.16,17 Unlike other breast cancer subtypes, which often have brain metastases developed together with extracranial disease progression, patients with HER2-positive breast cancer can develop brain metastases with stable extracranial disease. A substantial proportion of patients also suffer from intracranial progression even after aggressive local therapies such as radiotherapy and surgical excision, while their extracranial diseases are stable. The reason for the intracranial progression is that brain metastases from HER2-positive breast cancers infiltrate brain tissue crossing the endothelial cells without disrupting the blood–brain barrier (BBB), subsequently leading to suboptimal doses of anti-HER2 agents reaching the CNS.18 Nevertheless, conflicting data also exists. For example, Dijkers et al. demonstrated uptake of 89Zr-trastuzumab [trastuzumab labelled with positron emission tomography (PET) isotope zirconium-89 (89Zr) for clinical HER2/neu immunoPET scintigraphy to determine HER2 expression and localization of HER2-overexpressing tumour lesions] in HER2-positive brain metastases.19
While there is an established standard treatment pathway for metastatic anti-HER2 breast cancer, consensus on the systemic therapies for patients with intracranial progression is lacking. Emerging evidence shows that some novel anti-HER2 agents can penetrate the BBB and effectively delay the development of brain metastases. Nevertheless, there are still unmet needs in systemic therapies for those with developed CNS metastases. In this review, we discuss both local and systemic treatments for HER2-positive breast cancer with brain metastases. The therapeutic benefits for CNS control of these anti-HER2 agents are focused on. We also provided a practical treatment algorithm to guide clinicians on the selection of therapeutic agents for each individual patient.
Local treatment for HER2-positive brain metastasis
Local treatment is usually given for HER2-positive brain metastases before systemic treatment. Local treatment includes steroids, WBRT, SRS and surgery.20–22 Optimal management depends on patient factors (e.g. age, performance status and comorbidities), tumour factors (e.g. number, size, location, extracranial control) and availability of treatment options (e.g. neurosurgical expertise, stereotactic radiotherapy service).
For patients with single brain metastasis measuring less than 3–4 cm and amenable to gross total resection, either surgery or radiosurgery can be considered. Surgery is preferred to radiosurgery in case biopsies are needed and the brain metastasis is causing a significant mass effect. WBRT/SRS is usually added after surgery to improve the intracranial control. Patchell et al.’s study demonstrated that postoperative WBRT lowered the brain recurrence at both the original site (10% versus 46%, p < 0.001) and distant sites in the brain (14% versus 37%, p < 0.01) compared with surgery alone.23 In the European Organization for Research and Treatment of Cancer 22952-26001 study, postoperative WBRT 30 Gy in 10 fractions given within 6 weeks of surgery reduced the 2-year brain relapse rate at both the initial site of surgery (27% versus 59%, p < 0.001) and new sites (23% versus 42%, p = 0.008).24 To limit the neurotoxicity associated with WBRT, postoperative SRS to the surgical bed is now replacing WBRT. Brown et al. performed a randomised controlled trial comparing SRS with WBRT for postoperative resected brain metastases.25 WBRT was associated with more frequent decline in cognitive function than SRS (85% versus 52%, p < 0.00031) but no significant difference in overall survival (OS). Similarly, in the Japan Clinical Oncology Group (JCOG0504) study, surgery followed by SRS alone had a significantly lower incidence of grade 2–4 neurocognitive function decline (7.7% versus 16.4%, p = 0.048) compared with surgery followed by WBRT but similar OS.26
For patients with multiple brain metastases (all <4 cm and up to four brain metastases) with good performance status, radiosurgery with or without WBRT is recommended. In the RTOG 9508 trial that examined WBRT + SRS compared with WBRT alone in patients with 1–3 newly diagnosed brain metastases, WBRT + SRS demonstrated better control of the treated lesions as compared with WBRT alone (82% versus 71%, p = 0.01).27 Aoyama’s study showed that WBRT + SRS had a lower 12-month brain tumour recurrence rate than the SRS alone arm (48.6% versus 76.4%, p < 0.001) but a higher risk of neurocognitive decline (39% versus 26%, p = 0.21).28 Cheng et al.’s study also showed that more patients had a decline in learning and memory after SRS + WBRT compared with SRS alone (52% versus 24%).29 The N0574 study re-examined this question and found consistent results of adding WBRT to SRS leading to a higher rate of deterioration of cognitive function (64% versus 92% at 3 months) as well as the quality of life.30 A meta-analysis evaluated SRS, WBRT or both for patients with a limited number of brain metastases and confirmed that WBRT improved local and distant brain control but not OS and it increased the rates of neurocognitive and functional deterioration.31 Therefore, SRS is now considered as the standard treatment for limited brain metastases, together with regular brain imaging for the early detection of any new brain relapse.
For patients with multiple brain metastases (more than 4), radiosurgery or WBRT are both reasonable options depending on the local institutional policy and resources availability. Radiosurgery applied to up to 5–10 brain metastases simultaneously has been proven safe if the total tumour volume is low.32 WBRT is usually given if there is leptomeningeal involvement.21 The common doses for WBRT include 30 Gy in 10 fractions or 20 Gy in 5 fractions. Steroids are usually given to reduce the cerebral oedema.
Options of systemic anti-HER2 agents for CNS metastases
Different types of anti-HER2 agents were developed in recent years, including monoclonal antibodies (trastuzumab, pertuzumab, margetuximab), antibody–drug conjugates [trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd)] and small-molecule tyrosine kinase inhibitors (lapatinib, neratinib, tucatinib and pyrotinib). These agents have different molecular sizes and ability in BBB penetration. Table 1 summarized the characteristics of different anti-HER2 agents and the level of evidence on the control of CNS metastases.
Table 1.
Summary on different anti-HER2 agents and their level of evidence on control of CNS metastases.
| Molecular weight | Level of evidence | CNS metastases control | Definition of CNS control | Remarks | |
|---|---|---|---|---|---|
| Monoclonal antibodies | |||||
| Trastuzumab | 148,531 g/mol | Retrospective study | CNS ORR: 74% when concurrent with WBRT33 | Not specific on CNS ORR definition | No report on trastuzumab alone |
| Pertuzumab | 148,556 g/mol | Retrospective study | Median PFS: 16 –20 months34,35
Median OS: Around 26 months |
No report on CNS ORR | |
| Margetuximab | 145,900 g/mol | Ongoing phase III RCT | Not yet reported36 | ||
| Antibody–drug conjugates | |||||
|
Trastuzumab emtansine
(T-DM1) |
T-DM1: 149,000 g/mol DM1 ×3.5: 737.5 g/mol ×3.5 |
Retrospective studies | CNS ORR: 20–40%37–40
CNS DCR: 50–60%37–40 |
RECIST v1.1 criteria as other non-CNS targets | No prospective studies to prove the efficacy Not mention the duration of disease control to define stable disease |
|
Trastuzumab deruxtecan
(T-DXd) |
T-DXd: 156,000 g/mol DXd ×8: 1000 g/mol ×8 |
phase II35 | No report on CNS ORR | ||
| Small-molecule tyrosine kinase inhibitors | |||||
| Lapatinib | 581 g/mol | Meta-analysis | CNS ORR: 21.4%41
CNS DCR: 65.1%41 |
RECIST criteria CNS ORR: rate of complete + partial response CNS DCR: rate of complete response, partial response or stable disease for at least 24 weeks |
Combined with capecitabine |
| phase II | CNS ORR: 65.9%42 | RANO criteria CNS ORR: either complete response or partial response [⩾50% reduction in the volumetric sum of all assessable (⩾10 mm diameter) lesions] without progression of non-measurable lesions, new lesions |
Combined with capecitabine | ||
| Neratinib | 557 g/mol | phase II | CNS ORR: 49%43 | RANO criteria | Combined with capecitabine |
| Tucatinib | 481 g/mol | phase III | CNS PFS: 9.9 months44
CNS ORR: 47.3% |
modified RECIST criteria (brain-specific criteria): - shrinkage target for partial response: ⩾30% |
Combined with trastuzumab and capecitabine |
| phase IB | CNS ORR: 36%45 | modified RECIST criteria (brain-specific criteria): - shrinkage target for partial response: ⩾30% |
Combined with T-DM1 | ||
| phase IB | CNS ORR: 42%46 | modified RECIST criteria (brain-specific criteria) | Combined with trastuzumab and capecitabine | ||
| Pyrotinib | 557 g/mol | Retrospective study | CNS ORR: 66.7%47 | RECIST v1.1 criteria | Combined with capecitabine or paclitaxel or vinorelbine or trastuzumab |
CNS, central nervous system; DCR, disease control rate; HER2, human epidermal growth factor receptor; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RCT, randomised controlled trial; RECIST, Response Evaluation Criteria in Solid Tumours; T-DXd, trastuzumab deruxtecan.
Monoclonal antibodies (trastuzumab, pertuzumab, margetuximab)
Trastuzumab is a recombinant monoclonal antibody that binds to the extracellular domain IV of HER2, which results in the downregulation of the PI3K/Akt pathway. It yields clinical benefits through different mechanisms of action, including antibody-dependent cell-mediated cytotoxicity, prevention of HER2 extracellular domain shedding and angiogenesis inhibition. Trastuzumab has a large molecular weight (148,531 g/mol), limiting its ability to cross the BBB. The cerebrospinal fluid (CSF)/serum drug concentration ratio was studied to predict the likelihood of drug penetration into the CNS and likelihood of efficacy. A previous study demonstrated that the trastuzumab level in CSF was 300-times lower than that in serum, implying poor penetration of trastuzumab through BBB, thereby limiting its CNS control.48 Nevertheless, animal and clinical studies provided evidences that brain radiotherapy increased the permeability of the BBB, increasing the CSF/serum drug concentration ratio. Stemmler et al. showed that the CSF/serum trastuzumab level in patients with brain metastases prior to any local therapy was 1:420, while the ratio increased to 1:79 after radiotherapy.49 Disruption of the BBB by radiotherapy might improve the penetration of trastuzumab in the CNS and enhance the intracranial control. In a retrospective study by Chargari et al., concurrent WBRT with trastuzumab could successfully control and treat the brain metastases with an objective response rate (ORR) of 74% and median OS of 18 months;33 however, there has been no prospective study to confirm the CNS control efficacy of trastuzumab.
In light of the poor BBB penetration, intrathecal administration of trastuzumab may be an effective treatment option for HER2-positive breast cancer with brain metastases. A systematic review by Zagouri et al. that involved 17 patients with HER2-positive breast cancer with brain metastases on intrathecal trastuzumab showed that the pooled CSF response rate (RR) was 66.7% and the median CNS progression-free survival (PFS) was 7.5 months.50 Intrathecal trastuzumab administration seems to be feasible and well tolerated but prospective controlled studies are highly desirable.
Pertuzumab is a humanised monoclonal antibody that binds to the extracellular domain II of HER2.51 Because pertuzumab and trastuzumab bind to different domains of HER2, they work synergistically, inhibiting ligand-dependent HER2–HER3 dimerization and reducing signalling via intracellular pathways. Its molecular weight is 148,000 g/mol.
In the phase III CLEOPATRA study which compared pertuzumab combined with trastuzumab and docetaxel (TTPH) with trastuzumab and docetaxel in treatment-naïve patients with advanced HER2-positive breast cancer, doublet anti-HER2 agents significantly improved both PFS (18.5 versus 12.4 months, p < 0.001) and OS (57.1 versus 40.8 months, p < 0.001).52 In the post hoc analysis, pertuzumab also delayed the time of onset of CNS metastasis from 11.9 months to 15.0 months.53 Since patients with baseline brain metastases were excluded in this study, the efficacy of intracranial control of pertuzumab cannot be evaluated.
Esin et al. performed a retrospective study on 317 patients with HER2-positive metastatic breast cancer using pertuzumab, trastuzumab and taxane-based treatment in the first-line setting. A total of 13 (4.1%) patients with brain metastases on presentation attained a median PFS of 16.7 months and median OS of 26.7 months.34 In another similar retrospective observational study on first-line TTPH in patients with advanced HER2-positive breast cancer, PFS was 20 months [95% confidence interval (CI) 13–27] in the group of patients with brain metastases at diagnosis (21of 264, 8%).35 Although both studies support the efficacy of TTPH in patients with brain involvement, they did not specifically report on the response of the CNS metastases.
Margetuximab is a novel anti-HER2 antibody that is directed against the same epitope as trastuzumab, with Fc engineering that is optimized for better antibody-dependent cellular cytotoxicity than trastuzumab. Its molecular weight is 1,459,000 g/mol. In the phase III randomised controlled trial SOPHIA, 536 patients who were previously treated with pertuzumab and trastuzumab were randomised to margetuximab or trastuzumab with chemotherapy (capecitabine, eribulin, navelbine or gemcitabine).36 The recently published second interim OS analysis demonstrated that margetuximab + chemotherapy significantly prolonged the median PFS [5.8 versus 4.9 months, hazard ratio (HR) = 0.76; 95% CI: 0.59 – 0.98, p = 0.033] but modestly in OS (median OS: 23.7 versus 19.4 months, HR 0.793; 95% CI 0.607–1.035; p = 0.087) compared with trastuzumab + chemotherapy. For those who carried the CD16A-158F allele (85% of the participants), the median OS was prolonged by 4.3 months (23.7 versus 19.4 months, p = 0.087). The efficacious data on patients with treated or stable brain metastases have not yet been presented. We hope the final report will provide more data on patients with existing brain metastases.
Antibody–drug conjugates: trastuzumab emtansine and trastuzumab deruxtecan
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of the cytotoxic agent emtansine (DM1) conjugated to trastuzumab via a stable linker, which facilitates intracellular delivery of DM1 to HER2-overexpressing tumour cells, resulting in inhibition of tubulin polymerization and cell death. The molecular weight of T-DM1 is 149,000 g/mol, while that of the drug linker (DM-1) is 737.5 g/mol. Evidences of T-DM1 in treating brain metastases are mainly from retrospective studies.
In the retrospective exploratory analysis of the phase III EMILIA study, 95 patients with baseline CNS metastases were randomised to either T-DM1 or lapatinib-capecitabine (XL).54 The percentages of patients with CNS progression were similar for the two regimens, regardless of any pre-existing baseline CNS metastases. Among the 95 patients with baseline CNS metastases, 22.2% (10 of 45) in T-DM1 and 16.0% (10 of 45) in the XL arm had CNS progression. The median OS in patients with CNS metastases at baseline was longer in the T-DM1 arm than in the XL arm (26.8 versus 12.9 months, HR 0.038, p = 0.008), while PFS was similar in both arms (5.9 versus 4.6 months, HR 0.78, 95% CI 0.44–1.39, p = 0.392). The safety profile was not significantly different in both arms.
In a multicentre retrospective study in Italy, which included 53 patients with HER2-positive brain metastases treated with T-DM1, 3.8% (2 of 53) had complete response, 20.7% (11 of 53) had partial response and 30.1% (16 of 53) had stable disease.37 In another multicentre study in France, which included 39 brain metastases patients on T-DM1 with a median follow up of 8.1 months, the CNS ORR was 59% (partial response: 44%, 17 of 39; stable disease: 15%, 6 of 39).38 Overall, 41.0% (16 of 39) had a first site of disease progression in the CNS and the CNS PFS was 8.6 months (95% CI 5.3–18.3 months). In Bartsch et al.’s study, which included 10 patients with HER2-positive brain metastases on T-DM1, the overall CNS clinical benefit rate (CBR) was 50% (3 patients with partial response, 2 patients with stable disease).39 Despite these encouraging results, data from the Royal Marsden Hospital were not so promising. Okines et al. reviewed all patients who received T-DM1 in the period of 2011–2016 in Royal Marsden Hospital.40 Overall, 16 out of 55 patients had baseline CNS involvement and all had received prior local therapy to the brain [whole brain (9), stereotactic radiotherapy (6) or both (1)]. None of these patients had radiological responses in the CNS after use of T-DM1. A total of 56.3% of the patients (9 of 16) had their first site of progression in the brain and the CNS PFS was 9.9 months (95% CI 3.9–12.2 months). With these conflicting results from the retrospective studies, prospective studies on T-DM1 in controlling brain metastases are warranted.
Trastuzumab deruxtecan (T-DXd, DS-8201) is a novel antibody–drug conjugate composed of a humanised monoclonal antibody specifically targeting HER2, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor (payload). Compared to T-DM1, T-DXd has a higher drug-to-antibody ratio (approximately 8 versus 3–4) making it more potent against cancer cells. It also has a released payload that can easily cross the cell membrane, which potentially allows for cytotoxic effect on neighbouring tumour cells regardless of target expression.55 The molecular weight of T-DXd is 156,000 g/mol, while that of drug linker DXd is 1000 g/mol.
In the phase II DESTINY-Breast01 study, 184 heavily pretreated patients with HER2-positive breast cancer (median previous treatments received: 6) received T-DXd. The efficacy of T-DXd was impressive with RR of 60.9% and median PFS of 16.4 months.56 Among 24 patients with baseline brain metastases, the ORR was 58% and the median PFS was 18.1 months (95% CI 6.4–18.1 months). The CNS ORR and CNS PFS were not presented in the final report. An important adverse event with T-DXd is interstitial lung disease, with an incidence rate of 13.6% and causing mortality in four patients. Close monitoring for signs and symptoms of interstitial lung disease (including fever, cough, or dyspnoea) is recommended. Other common grade 3 or higher adverse events include neutropenia (20.7%), anaemia (8.7%) and nausea (7.6%). Unlike other HER2-targeted therapies, such as trastuzumab and pertuzumab, which are associated with a risk of cardiomyopathy, particularly left ventricular dysfunction, clinically significant cardiotoxicity was not observed in T-DXd.
Small-molecule tyrosine kinase inhibitors: lapatinib, neratinib, tucatinib and pyrotinib
Lapatinib is a double-acting tyrosine kinase inhibitors (TKI) that inhibits phosphorylation and activation of the epthelial growth factor receptor (EGFR) and HER2 receptors. Lapatinib crosses the BBB because of its small molecular structure (molecular weight 581 g/mol). In the pivotal phase III randomised controlled trial on advanced HER2-positive breast cancer previously treated with anthracycline, taxane and trastuzumab, lapatinib-capecitabine combination improved the median PFS from 4.4 months to 8.4 months compared with capecitabine alone.57 Fewer patients in the lapatinib-capecitabine arm had CNS as the first site of disease progression than those in the capecitabine-alone arm (2.5% versus 6.8%, p = 0.10). The study did not report the CNS ORR or CNS PFS.
A meta-analysis that included 12 studies and 799 patients with HER2-positive breast cancer with brain metastases on lapatinib and capecitabine, provided good evidence of the two combinations on brain metastases control. In 9 out of 12 studies with data of brain metastases response, the pooled CNS ORR was 21.4% (95% CI 11.7–35.9%), while the intracranial disease control rate (DCR) was 65.1% (95% CI 47.1–79.7%). The pooled median PFS and OS were 4.1 (95% CI 3.1–6.7) and 11.2 (95% CI 8.9–14.1) months, respectively.41 In the single-arm, phase II, open-label LANDSCAPE study, which included 44 patients with HER-2 positive brain metastases without WBRT, a lapatinib-capecitabine combination achieved a CNS ORR of 65.9% (partial response: 29 of 44).42 Overall, 84% (35 of 44) had a reduction in tumour volume from baseline and 20% of the patients (9 of 44) even had >80% CNS volumetric reduction. The median time to documented response was 1.8 months (range 1.1–5.8 months). Overall, five patients (11.4%) had CNS progression and the median time of CNS progression was 5.5 months (range 4.5–6.1 months). Other smaller-scale phase I and II studies investigating adding lapatinib to WBRT also showed favourable results with CNS ORR at around 70–80%.
Neratinib, is a less-selective irreversible TKI, targeting EGFR, HER1, HER2 and HER4. Neratinib also has a lower molecular weight (557 g/mol). In the phase II Translational Breast Cancer Research Consortium Study 022, neratinib monotherapy for patients who had CNS progression after one or more CNS-directed local therapies including WBRT, SRS or surgical resection could attain only modest CNS ORR (n = 3 of 40, 8%, 95% CI: 2–22%).58 All the three responders had previously received lapatinib; however, in the same study of another cohort, neratinib-capecitabine combination improved the CNS ORR to 49% (RANO criteria, 95% CI 32–66%).43 The percentage with CNS response was higher in the lapatinib-naïve patients than those exposed to lapatinib before (49% and 33% respectively). The main concern with neratinib was grade 3 diarrhoea with an incidence rate around 30%.
Studies on neratinib in regard to reducing the incidence of brain relapse have exhibited contradictory results. In the phase III NEfERT-T study comparing neratinib-paclitaxel and trastuzumab-paclitaxel in patients with advanced HER-2 positive breast cancer, neratinib-paclitaxel was more effective than trastuzumab-paclitaxel in terms of CNS recurrence (8.3% versus 17.3%, HR 0.48, 95% CI 0.29–0.79, p = 0.002) and the time to brain metastases (not reached versus 18.3 months, HR 0.45, 95% CI 0.26–0.78, p = 0.004).59 The superiority of neratinib in controlling brain disease was seen in both patients with or without baseline CNS involvement. On the contrary, in the phase III ExteNet study, of which 12 months of adjuvant neratinib was given after chemotherapy and trastuzumab after operation, the 5-year cumulative incidence of CNS events was numerically less in the neratinib arm than the placebo arm but not statistically significant (1.3% versus 1.8%, p = 0.333).60 The total number of events was small, possibly accounting for the insignificant difference. Moreover, the benefit of 12 months’ extended adjuvant treatment with neratinib was seen largely in the subgroup of patients with hormone receptor-positive disease, the majority (93%) of whom were receiving concomitant endocrine therapy. The improved efficacy in the hormone receptor-positive disease may be due to the dual inhibition of estrogen receptor (ER)-HER2 crosstalk by neratinib and endocrine therapy and a longer duration of adjuvant treatment in hormone receptor-positive disease. In the ExteNet study, over 40% of the participants were hormone receptor-HER2-positive breast cancers. The effect of neratinib on CNS recurrence may be masked by the large proportion of hormone receptor-subgroup.
Tucatinib is a new oral TKI that is highly selective of the kinase domain of HER2 with minimal inhibition of EGFR, resulting in less diarrhoea and less skin toxicities. Its molecular weight is 480.5 g/mol. In the recently published HER2CLIMB study, adding tucatinib on top of trastuzumab and capecitabine improved both median PFS (7.8 versus 5.6 months, HR 0.54, p < 0.001) and OS (21.9 versus 17.4 months, HR 0.66, p = 0.005) compared with a trastuzumab-capecitabine combination in heavily pretreated patients with HER-2-positive metastatic breast cancer (who previously received trastuzumab, pertuzumab and T-DM1).61 Among 291 patients with brain metastases [tucatinib: 196 (48%); placebo: 93 (46%)], triplet therapy with tucatinib, trastuzumab and capecitabine demonstrated promising results both extracranially and intracranially. Adding tucatinib improved both PFS (7.6 versus 5.4 months, HR 0.48, p < 0.001) and OS (18.1 versus 12.0 months, HR 0.58, p = 0.005)), reduced risk of intracranial progression or death by 68% (HR 0.32, 95% CI 0.22–0.48, p < 0.0001), improved median CNS PFS (9.9 versus 4.2 months, p < 0.0001) and doubled intracranial ORR (47.3% versus 20.0%, p = 0.03).44
There were two other phase Ib studies providing evidence of the efficacy of tucatinib on brain metastases control. In the phase Ib study on tucatinib combined with T-DM1 on patients previously treated with trastuzumab and taxane, 14 patients with measurable brain metastases were included.45 The CNS ORR was 36% (complete response: two, partial response: three, stable disease: seven). In another phase Ib study on tucatinib, trastuzumab and capecitabine combination in patients who had progressed after trastuzumab, pertuzumab and T-DM1, 12 patients with measurable brain metastases were included.46 The CNS ORR was 42% (partial response: five, stable disease: five).
Pyrotinib is an oral, irreversible pan-ErbB receptor tyrosine kinase inhibitor that targets HER1, HER2 and HER4.62 Its molecular weight is 557 g/mol. In the pivotal randomised phase II study, 128 Chinese patients with HER2-positive relapsed or metastatic breast cancer previously treated with taxane, anthracyclines or trastuzumab were assigned to receive pyrotinib or lapatinib in combination with capecitabine.63 The median PFS was significantly prolonged with pyrotinib than with lapatinib (18.1 versus 7.0 months, HR 0.36, p < 0.001). The study did not include any patients with baseline brain metastases and did not report any data of brain relapses. Ying et al. performed a retrospective study on the real-world data on pyrotinib-based therapy in 113 patients with metastatic HER2-positive breast cancer.47 For patients with baseline brain metastases (n = 31), the median PFS was 6.7 months and intracranial ORR was 28%. When combined with radiotherapy/ brain surgery, the ORR was promising (66.7%) with three patients achieved complete response. However, without radiotherapy/surgery, the efficacy was limited with very low ORR (6.3%). More data with use of pyrotinib in non-Chinese patients are warranted.
Treatment algorithm with HER2-positive breast cancer with brain metastases
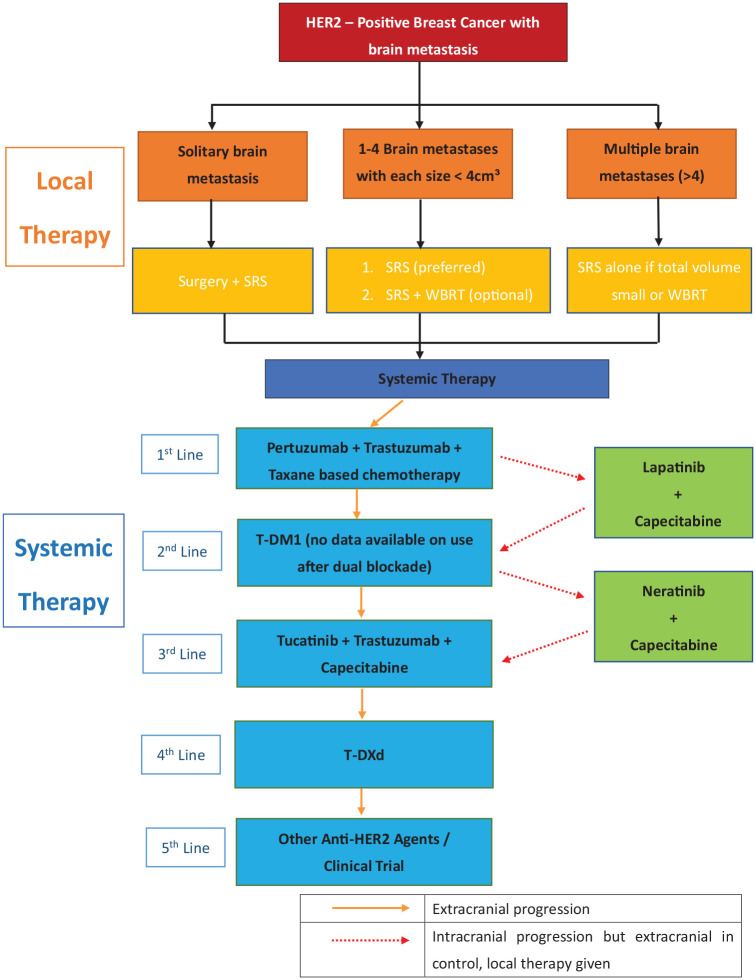
We have developed an algorithm for the treatment of HER2-positive breast cancer with brain metastases (Figure 1). Because of the increased permeability of the drugs through the BBB after local therapy and the uncertain CNS penetration of the anti-HER2 agents, local treatment with surgery, SRS or WBRT before systemic treatment is recommended. SRS is preferred over WBRT for limited brain metastases with consideration of the small reduction in the local and distant brain failure rate but higher risk of severe cognitive deterioration in WBRT. First-line treatment with doublet anti-HER2 agents with pertuzumab, trastuzumab and taxane chemotherapy should be used for systemic control. If there is isolated progression in the brain metastasis without extracranial progression, the systemic treatment can be continued after local CNS therapy; however, when there is CNS progression together with extracranial progression, the anti-HER2 agent has to be changed according to the routine HER2 treatment pathway. For cases with CNS progression only and already exhaustion of local treatment, a lapatinib-capecitabine combination, which provides the best evidence of CNS control, can be used. T-DM1 is suggested for the next line of treatment after a lapatinib-capecitabine combination. Otherwise, a neratinib-capecitabine combination can be considered. Further lines of treatment will follow the anti-HER2 pathway with newer agents such as tucatinib, T-DXd and margetuximab or enrolment in suitable clinical trials.
Figure 1.
Suggested treatment algorithm for patients with HER2-positive breast cancer with brain metastases.
HER2, human epidermal growth factor receptor.
Ongoing trials
The advances in anti-HER2 agents have brought new hope to patients with HER2-positive metastatic breast cancer with improvements in disease control and OS. Nevertheless, brain metastases in this group remain a therapeutic challenge. Despite the emerging evidence to support the efficacy of systemic anti-HER2 agents for intracranial control, there is lack of phase III completed trials dedicated to patients with HER2+ breast cancer with brain metastases. The good news is that a variety of clinical trials for the treatment of HER2-positive breast cancer with brain metastases have been planned, or are already in recruitment. Table 2 outlines in-progress and upcoming trials (phase II or III) targeting HER2-positive breast cancer with brain metastases.
Table 2.
Active in-progress or planned phase II or III trials targeting on HER2-positive breast cancer with brain metastases.
| Clinical trial | Phase | Intervention model | Treatment and comparator | End points |
|---|---|---|---|---|
| NCT04034823 | phase II, not yet recruiting | Single group | KN035 in Combination with Trastuzumab and Docetaxel in HER2-positive Breast Cancer | Objective response Adverse events |
| NCT03417544 | phase II, active not recruiting | Single group | Atezolizumab + Pertuzumab + Trastuzumab In CNS Mets in BC | CNS ORR; PFS; non-CNS ORR; OS; toxicity; patient-reported outcomes |
| NCT03765983 | phase II, recruiting | Single group | GDC-0084 in Combination with Trastuzumab for Patients With HER2-Positive Breast Cancer Brain Metastases | CNS ORR; adverse events; PFS; OS |
| NCT04334330 | phase II, not yet recruiting | Single group | Palbociclib, Trastuzumab, Lapatinib and Fulvestrant Treatment in Patients with Brain Metastasis from ER Positive, HER-2 Positive Breast Cancer: A Multicentre, Prospective Study in China | CNS ORR; OS; adverse events |
| NCT01494662 | phase II, recruiting | Four cohorts | HKI-272 for HER-2 Positive Breast Cancer and Brain Metastases | CNS ORR; PFS; OS; first site of disease progression; toxicity |
| NCT04420598 | phase II, active not recruiting | Four cohorts | DS-8201a for treatment of aBc, Brain Mets and Her2[+] Disease | CNS ORR; CBR; OS; adverse events |
| NCT04158947 | phase II, recruiting | Randomised two arms | A Study of HER-2 Positive Breast Cancer Patients with Active Brain Metastases Treated with Afatinib & T-DM1 versus T-DM1 Alone | Safety and tolerability of T-DM1 and afatinib; ORR; PFS |
| NCT04303988 | phase II, active not recruiting | Multi-cohort | A Multi-cohort phase II Study of HER2-positive and Triple-negative Breast Cancer Brain Metastases. Hormone Receptor-Positive/HER2+: pyrotinib + temozolomide |
CNS ORR; CNS-CBR; PFS; OS; safety |
| NCT02536339 | phase II, active not recruiting | Single group | A Study of Pertuzumab with High-Dose Trastuzumab for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer (MBC) With CNS Progression Post-Radiotherapy | CNS ORR; CNS-CBR; CNS PFS; PFS; OS |
| NCT01622868 | phase II, active not recruiting | Randomised | Whole-Brain Radiation Therapy or Stereotactic Radiosurgery with or Without Lapatinib Ditosylate in Treating Patients with Brain Metastasis From HER2-Positive Breast Cancer | CR of the brain metastases; CNS ORR; |
| NCT03933982 | phase II, recruiting | Single group | A Study of Pyrotinib Plus Vinorelbine in Patients with Brain Metastases from HER2-positive Metastatic Breast Cancer | CNS ORR; Time to progression; OS; time to radiotherapy |
| NCT03054363 | phase I/II, recruiting | Single group | Tucatinib, Palbociclib and Letrozole in Metastatic Hormone Receptor Positive and HER2-positive Breast Cancer | Adverse events; number of patients with PFS; pharmacokinetic properties of tucatinib and palbociclib |
| NCT03691051 | phase II, active not recruiting | Single group | A Study of Pyrotinib Plus Capecitabine in Patients with Brain Metastases from HER2-positive Metastatic Breast Cancer | CNS ORR; non-CNS ORR; CBR |
| NCT03501979 | phase II, recruiting | Single group | Tucatinib, Trastuzumab, and Capecitabine for the Treatment of HER2+ LMD | OS; PFS; adverse events; QoL assessment |
| NCT02260531 | phase II, active not recruiting | Single group | Cabozantinib ± trastuzumab In Breast Cancer Patients with Brain Metastases | CNS ORR; non-CNS ORR; PFS, CBR |
| NCT03190967 | phase I/II, recruiting | Randomised | T-DM1 and Tucatinib Compared With T-DM1 Alone in Preventing Relapses in People with High Risk HER2-Positive Breast Cancer, the Compass HER2 RD Trial | MTD of temozolomide when used with T-DM1 Median amount of time subject survives without disease progression after treatment adverse event frequency |
| NCT02947685 | phase III, recruiting | Randomised | Randomised, Open-Label, Clinical Study of the Targeted Therapy, Palbociclib, to Treat Metastatic Breast Cancer | PFS; OS; ORR; CBR; safety; patient-reported outcome |
| NCT02675231 | phase II, active not recruiting | Randomised | A Study of Abemaciclib (LY2835219) in Women with Hormone Receptor-Positive, HER2+ Locally Advanced or Metastatic Breast Cancer | PFS; OS; ORR; CBR; safety; QoL |
CNS, central nervous system; ER, estrogen receptor; HER2, human epidermal growth factor receptor; LMD, leptomeningeal metastases; MTD, maximum tolerated dose; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; QoL, quality of life; T-DM1, trastuzumab emtansine.
Conclusion
Brain metastasis in advanced HER2-positive breast cancer is a major cause of morbidity and mortality. Multimodality treatment with a combination of local therapy and systemic anti-HER2 agents should be given to attain the best control of the disease. Future studies on systemic therapies for HER2-positive breast cancer should no longer exclude patients with baseline brain metastases and should report on CNS metastases parameters, including CNS ORR and DCR, CNS PFS, median time to CNS progression, and time to CNS recurrence. These parameters will provide more guidance to support individualized treatment strategies for this particular type of patients. Furthermore, definition of CNS metastases response should be standardized to have a common language when comparing different agents across different trials.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Human rights statement and informed consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Wing-Lok Chan, Department of Clinical Oncology, Queen Mary Hospital, 1/F Professorial Block, 102 Pokfulam Road, Hong Kong.
Tai-Chung Lam, Department of Clinical Oncology, The University of Hong Kong, Hong Kong SAR.
Ka-On Lam, Department of Clinical Oncology, The University of Hong Kong, Hong Kong SAR.
Mai-Yee Luk, Department of Clinical Oncology, Queen Mary Hospital, Hong Kong SAR.
Roger Ngan Kai-Cheong, Department of Clinical Oncology, The University of Hong Kong, Hong Kong SAR.
Lai-Wan Dora Kwong, Department of Clinical Oncology, The University of Hong Kong, Hong Kong SAR.
References
- 1. Witzel I, Oliveira-Ferrer L, Pantel K, et al. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 2016; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol 2004; 22: 3608–3617. [DOI] [PubMed] [Google Scholar]
- 3. Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 2009; 27: 5278–5286. [DOI] [PubMed] [Google Scholar]
- 4. Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 1983; 52: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 5. Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 2006; 24: 5658–5663. [DOI] [PubMed] [Google Scholar]
- 6. Pestalozzi BC, Zahrieh D, Price KN, et al. ; International Breast Cancer Study Group (IBCSG). Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006; 17: 935–944. [DOI] [PubMed] [Google Scholar]
- 7. Song Y, Barry WT, Seah DS, et al. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer 2020; 126: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 2009; 45: 2792–2798. [DOI] [PubMed] [Google Scholar]
- 9. Lentzsch S, Reichardt P, Weber F, et al. Brain metastases in breast cancer: prognostic factors and management. Eur J Cancer 1999; 35: 580–585. [DOI] [PubMed] [Google Scholar]
- 10. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003; 97: 2972–2977. [DOI] [PubMed] [Google Scholar]
- 11. Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol 2013; 14: 244–248. [DOI] [PubMed] [Google Scholar]
- 12. Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol 2013; 24: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012; 2: 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014; 32: 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NCCN Clinical Practice Guidelines on Oncology: central nervous system cancers. Version 2.2020, https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (Accessed May 19, 2020)
- 16. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018; 29: 1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol 2018; 36: 2736–2740. [DOI] [PubMed] [Google Scholar]
- 18. Seoane J, De Mattos-Arruda L. Brain metastasis: new opportunities to tackle therapeutic resistance. Mol Oncol 2014; 8: 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010; 87: 586–592. [DOI] [PubMed] [Google Scholar]
- 20. Melisko ME, Moore DH, Sneed PK, et al. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol 2008; 88: 359–365. [DOI] [PubMed] [Google Scholar]
- 21. Mahmoud-Ahmed AS, Suh JH, Lee SY, et al. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002; 54: 810–817. [DOI] [PubMed] [Google Scholar]
- 22. Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 2008; 112: 2359–2367. [DOI] [PubMed] [Google Scholar]
- 23. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998; 280: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 24. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011; 29: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017; 18: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kayama T, Sato S, Sakurada K, et al. ; Japan Clinical Oncology Group. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol 2018: JCO2018786186. [DOI] [PubMed] [Google Scholar]
- 27. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with and without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 28. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006; 295: 2483–2491. [DOI] [PubMed] [Google Scholar]
- 29. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 30. Churilla TM, Ballman KV, Brown PD, et al. Stereotactic radiosurgery with or without whole-brain radiation therapy for limited brain metastases: a secondary analysis of the North Central Cancer Treatment Group N0574 (Alliance) randomized controlled trial. Int J Radiat Oncol Biol Phys 2017; 99: 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012; 118: 2486–2493. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014; 15: 387–395. [DOI] [PubMed] [Google Scholar]
- 33. Chargari C, Idrissi HR, Pierga JY, et al. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys 2011; 81: 631–636. [DOI] [PubMed] [Google Scholar]
- 34. Esin E, Oksuzoglu B, Bilici A, et al. ; Turkish Oncology Group. Pertuzumab, trastuzumab and taxane-based treatment for visceral organ metastatic, trastuzumab-naïve breast cancer: real-life practice outcomes. Cancer Chemother Pharmacol 2019; 83: 131–143. [DOI] [PubMed] [Google Scholar]
- 35. Gamucci T, Pizzuti L, Natoli C, et al. A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol Ther 2019; 20: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rugo HS, Im SA, Cardoso F, et al. Phase III SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: second interim overall survival analysis. 2019 San Antonio Breast Cancer Symposium Abstract GS1-02. Presented December 11, 2019. [Google Scholar]
- 37. Fabi A, Alesini D, Valle E, et al. T-DM1 and brain metastases: clinical outcome in HER2-positive metastatic breast cancer. Breast 2018; 41: 137–143. [DOI] [PubMed] [Google Scholar]
- 38. Jacot W, Pons E, Frenel JS, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat 2016; 157: 307–318. [DOI] [PubMed] [Google Scholar]
- 39. Bartsch R, Berghoff AS, Vogl U, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis 2015; 32: 729–737. [DOI] [PubMed] [Google Scholar]
- 40. Okines A, Irfan T, Khabra K, et al. Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: a single institution experience. Breast J 2018; 24: 253–259. [DOI] [PubMed] [Google Scholar]
- 41. Petrelli F, Ghidini M, Lonati V, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer 2017; 84: 141–148. [DOI] [PubMed] [Google Scholar]
- 42. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013; 14: 64–71. [DOI] [PubMed] [Google Scholar]
- 43. Freedman RA, Gelman RS, Anders CK, et al. ; Translational Breast Cancer Research Consortium. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 2019; 37: 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 2020: JCO2000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol 2018; 4: 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018; 19: 880–888. [DOI] [PubMed] [Google Scholar]
- 47. Lin Y, Lin M, Zhang J, et al. Real-world data of pyrotinib-based therapy in metastatic HER2-positive breast cancer: promising efficacy in lapatinib-treated patients and in brain metastasis. Cancer Res Treat. Epub ahead of print 24 April 2020. DOI: 10.4143/crt.2019.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol 2000; 18: 2349–2351. [DOI] [PubMed] [Google Scholar]
- 49. Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 2007; 18: 23–28. [DOI] [PubMed] [Google Scholar]
- 50. Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat 2013; 139: 13–22. [DOI] [PubMed] [Google Scholar]
- 51. Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68: 5878–5887. [DOI] [PubMed] [Google Scholar]
- 52. Swain SM, Miles D, Kim SB, et al. ; CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020; 21: 519–530. [DOI] [PubMed] [Google Scholar]
- 53. Swain SM, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol 2014; 25: 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 2015; 26: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016; 107: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Modi S, Saura C, Yamashita T, et al. ; DESTINY-Breast01 Investigators. trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2019;. [Google Scholar]
- 57. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733–2743. [DOI] [PubMed] [Google Scholar]
- 58. Freedman RA, Gelman RS, Wefel JS, et al. Translational breast cancer research consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 2016; 34: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol 2016; 2: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 60. Martin M, Holmes FA, Ejlertsen B, et al. ; ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1688–1700. [DOI] [PubMed] [Google Scholar]
- 61. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. Epub ahead of print 27 December 2019. . DOI: 10.1056/NEJMx190039. [DOI] [PubMed] [Google Scholar]
- 62. Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci 2017; 110: 51–61. [DOI] [PubMed] [Google Scholar]
- 63. Ma F, Ouyang Q, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol 2019; 37: 2610–2619. [DOI] [PubMed] [Google Scholar]