Abstract
Purpose and Methods:
To investigate the doses of total body (TBI) and whole abdominal irradiation (WAI) induced lethal intestinal injury, healthy C57BL/6 J mice were divided randomly into 7 groups: control group; 6, 7, and 8 Gy TBI groups; and 5, 10, and 15 Gy WAI groups. The survival length, general conditions, body weight, daily food and water intake of the mice and the histopathological changes of small intestine were observed.
Results:
Lethal injury among C57BL/6 J mice was caused by ≥6 Gy TBI and 15 Gy WAI. Their body weight and food intake decreased, the structure of their small intestinal villi was destroyed, and the number of surviving crypts per circumference of the jejunum decreased in ≥6 Gy TBI groups and 15 Gy WAI group. The mice in the 10 Gy WAI group significantly lost weight within 5 days but recovered slowly thereafter. They also had poor appetite and reversibly damaged intestinal mucosa.
Conclusions:
Nonlethal intestinal injury could be induced by 10 Gy WAI, whereas lethal intestinal injury could be triggered by ≥6 Gy TBI and >15 Gy WAI in mice. Our results provided a basis for establishing radiation-induced intestinal injury models with C57BL/6 J mice.
Keywords: irradiation dose, intestinal damage, lethal injury, C57BL/6 J mice
Introduction
With the rapid development and wide application of radiation, accidental acute radiation damages occur because of poor management or improper operation. Exposure to ionizing radiation (IR) from radioactive materials may cause total body irradiation (TBI), and radiotherapy as a treatment for abdominal cancer can increase the risk of whole abdominal irradiation (WAI) exposure. TBI and WAI can cause injury to the digestive system because the small intestine is highly sensitive to IR.1
Many studies have focused on the pathogenesis of radiation-induced intestinal injury, such as injury to intestinal epithelial2 and vascular endothelial cells3 and gut microbiota disorder.4 However, available information on TBI and WAI doses that cause lethal intestinal injury among mice is scarce and conflicting. For example, different doses of 7, 8 or 9 Gy TBI were used in previous studies for exploring radiation-induced intestinal injury on C57BL/6 mice.5-7 Moreover, doses between 12 and 20 Gy WAI were tested for mortality on C57BL/6 mice model, and the results show that animals started dying at doses of 12 Gy, 15 Gy or above 16 Gy.8 In all, the different irradiation techniques, dose rate, and the age of the mice could induce different biological outcomes among C57BL/6 mice.
In our study, the doses and effects of TBI and WAI modes on C57BL/6 J mice were explored and compared on the basis of the survival length, severity, and intestinal pathological characteristics of TBI- and WAI-induced injury. This study aimed to determine the optimal radiation method and dose for establishing intestinal injury models.
Materials and Methods
Animals
Forty-two 10-to-12-week-old male C57BL/6 J mice were obtained from Shanghai Laboratory Animal Center with average weight of (27±3)g (Chinese Academy of Science, Shanghai, China). All the mice were housed in a polycarbonate cage with a controlled environment (22°C±2°C, 60%±5% relative humidity, and 12 h light/dark cycle) and provided with standard mouse feed ad libitum throughout the experiment. The care and use of laboratory animals were in accordance with the guidelines for the Animal Experiments of Soochow University.
Groups
All the mice were subjected to acclimation for 1 week and assigned randomly to 7 groups with different treatments: (1) control group; (2) 6, 7, and 8 Gy TBI groups; and (3) 5, 10, 15 Gy WAI groups. For TBI and WAI groups, each mouse was intraperitoneally injected with 5% chloral hydrate before X-ray irradiation by using a linear accelerator (160 kV, 25 mA; RadSource, Suwanee, GA, USA) at a fixed dose rate of 1.103 Gy/min in the supine position. The focus skin distance was 40 cm. In WAI groups, the body of mice was shed by lead plates except the low xiphoid to the symphysis pubis, which were irradiated. All the mice were observed daily for any signs of radiation sickness, morbidity, and mortality. Their body weight and the number of deaths were recorded daily. The daily consumption of chow and water was measured by subtracting the amount consumed from the remaining food and water after 24 h.
Observed Indices
General status
Changes in mental state, survival, crissal condition, daily food intake, daily water intake, and body weight were observed.
Histopathological changes
The mice were sacrificed 14 days after IR, and the small intestine samples (1 cm segments at 5 cm proximal to the terminal ileum) were fixed in 10% buffered formaldehyde–saline solution. Then, 5 µm sections of paraffin-embedded samples were stained with hematoxylin and eosin (H and E, Sigma-Aldrich, USA) and examined under a microscope. The number of surviving crypts per circumference of the jejunum was assessed quantitatively by pathologists who were under blinded conditions. The nonirradiated jejunum sections were presented as controls. Ten circular transverse sections were blindly analyzed per mouse from photographs stained with H and E to measure the length of the villi and the infiltration of inflammatory situation.
Radiation injury score (RIS)
The overall severity of structural radiation injury was assessed using the RIS system.9 RIS is a composite histopathologic scoring system that provides a global measure of the severity of structural radiation injury. In brief, 7 histopathologic parameters of radiation injury (mucosal ulceration, epithelial atypia, subserosal thickening, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda, and lymph congestion) were assessed and graded from 0 to 3. The RIS is defined as the sum of the scores of the individual alterations.
Intestinal mucosal surface area
The mucosal surface area (MSA) was estimated using a projection and cycloid count method from vertical sections as described by Baddeley et al.10 The intestinal sections were prepared as described above with the cutting surface perpendicular to the mucosa and projected to a grid system containing cycloids (short curved lines in a systematic pattern). The number of intersections between the mucosal surface and cycloids was counted for 5 successive parts of the mucosal surface. The mean number from each specimen was used to calculate the surface area of a reference volume set to a 1 cm segment of the intestine. The intestinal surface area in all specimens was measured.
Intestinal wall and subserosal thickness
Intestinal wall thickening is a measure of reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia, while subserosal thickening mainly reflects reactive fibrosis. In this study, the intestinal wall thickness (encompassing the submucosa, muscularis externa, and subserosa) and subserosal thickness were measured via computerized image analysis (Image-Pro Plus, Media Cybernetics) in 10 fields per section (40× objective) with 3 measurements per field.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD) and analyzed using SPSS version 17.0 for Windows (SPSS Inc., USA). One-way ANOVA was used to determine differences among groups. The survival rate was analyzed with the log-rank test. P < 0.05 was considered statistically significant.
Results
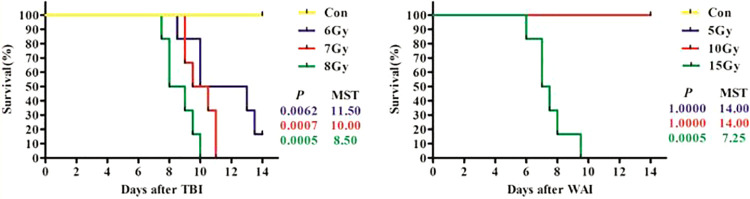
Radiation of TBI and WAI Reduced the Survival Length of Mice
All the mice in the control group and in the 5 and 10 Gy WAI groups remained alive 14 days after irradiation. The average survival periods of 6, 7, and 8 Gy TBI groups and 15 Gy WAI group were 11.5, 10, 8.7, and 7.5 days, respectively (Figure 1). The median survival time (MST) of 6, 7, and 8 Gy TBI groups and 15 Gy WAI group were 11.5, 10, 8.5, and 7.25 days. 6, 7, and 8 Gy TBI groups and 15 Gy WAI group showed increased mortality compare with the control group within 14 days (P < 0.01). The survival curve showed a dose-dependent decrease in the survival rates of mice in the TBI and WAI groups.
Figure 1.
The survival curve of the 7 groups during the experiment (n = 6).
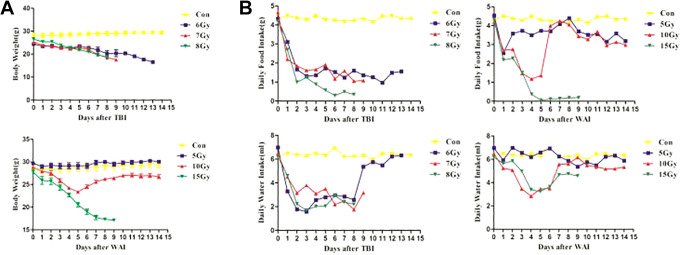
Radiation of TBI and WAI Worsened the General Status of Mice
Radiation reduced not only the food and water intake but also the body weight of the treated groups in a dose-dependent manner within 5 days compared with that in the untreated control (Figure 2). The food and water intake of the mice treated with 7 and 8 Gy TBI was low within 8–9 days; eventually, they died (Figure 2B). The water intake of the mice in the 6 Gy TBI group increased, but their food intake was low (Figure 2B). As a result, they continuously lost weight (Figure 2A). The mice had a short-term decrease in food and water intake 2 days after 5 Gy irradiation and 2–4 days after 10 Gy WAI. Then, the amount of water intake gradually returned to normal. However, the amount of food intake fluctuated between 3 and 15 days, indicating that 5 and 10 Gy WAI induced poor appetite. The body weight between the 5 Gy WAI group and the control group was similar, but it decreased obviously 2–4 days after 10 Gy WAI radiation and recovered afterward. However, the value was still lower than that of the control group. The mice in the 15 Gy WAI group had a sharp decrease in food intake and almost fasted 5 days after radiation (Figure 2B), which induced serious weight loss.
Figure 2.
General status of mice. (A)The body weight of mice during the experiment (n = 6). (B)Daily food intake and daily water intake of mice.
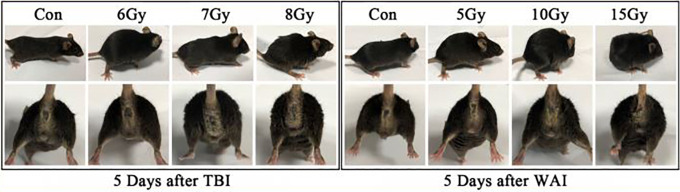
The mice in the TBI and WAI groups exhibited signs of radiation sickness 5 days after IR. The main symptoms included lack of movement, hump, hair ruffling, diarrhea, emaciation, and epilation. Diarrhea caused perianal swelling and rectal prolapse in 7 and 8 Gy TBI groups and in 10 and 15 Gy WAI groups. Gross blood stool was visible in the 15 Gy WAI group 5 days after irradiation, indicating severe intestinal injury (Figure 3).
Figure 3.
General status and crissal condition of mice.
Radiation of TBI and WAI Induced Damage to the Small Intestine
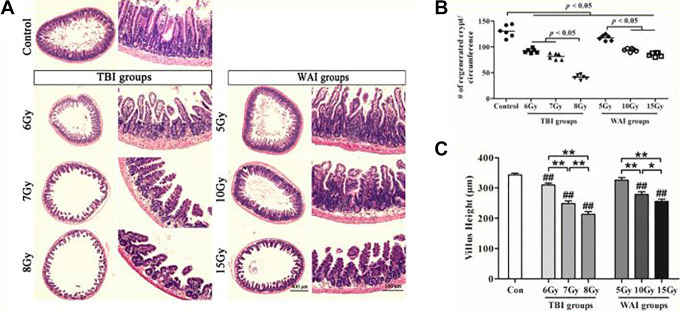
The intestinal villi of the treated groups were damaged by radiation compared with those of the untreated control (Figure 4A). The structures of the mucosa and submucosa were destroyed in the 6, 7, and 8 Gy TBI groups and in the 10 and 15 Gy WAI groups. The villus structure of the 10 Gy WAI group was wide and flat, and its epithelial layer was elevated. Moreover, hyperemia occurred in the intestinal submucosa, and this finding was accompanied with the loss of the small intestinal gland and the infiltration of inflammatory cells in the lamina propria in the 7 and 8 Gy TBI groups and the 15 Gy WAI group.
Figure 4.
Crypt and villus injury of the small intestine (n = 6). (A) The representative images of the H&E-stained sections of the jejunum are presented herein. Unirradiated jejunum sections were presented as a control. (B) A quantitation of the crypt numbers per circumference showed that a decrease in crypt numbers were observed dose-dependently in both TBI groups and WAI groups.(C) Villus height of the small intestine. The differences were evaluated by a one-way analysis of variance (ANOVA). # P < 0.05 as compared with control group; ## P < 0.01 as compared with control group; * P < 0.05; ** P < 0.01.
Quantitative analyses showed a dose-dependent decrease in the number of crypts in the TBI and WAI groups (Figure 4B). The degree of decrease in the number of crypts in the jejunum of the mice in the 8 Gy group was higher than that in the 6 and 7 Gy groups (P = 0.000). Similarly, the degrees of decrease in the number of crypts in the 10 and 15 Gy groups were higher than that in the 5 Gy group (P = 0.000).
The intestinal tissue was stained with HE to observe intestinal epithelial impairment 14 days after IR (Figure 4C). The villi of the IR groups were structurally disorganized and sloughed off. The heights of the villi of the mice in the different groups were as follows: 342.86 ± 18.90 µm in the control group; 309.64 ± 18.90, 248.57 ± 27.11, and 213.21 ± 28.77 µm in the 6, 7, and 8 Gy TBI groups, respectively; and 325.71 ± 27.76, 278.57 ± 27.20, and 255.00 ± 24.12 µm in the 5, 10, 15 Gy WAI groups, respectively. The heights of the villi between the 5 Gy group and the control group were not statistically different (P = 0.130). However, the intestinal villi of the 6, 7, and 8 Gy TBI groups (P < 0.01) and the 10 and 15 Gy WAI groups (P < 0.01) were shorter than those of the control group. This result revealed that the small intestine was damaged.
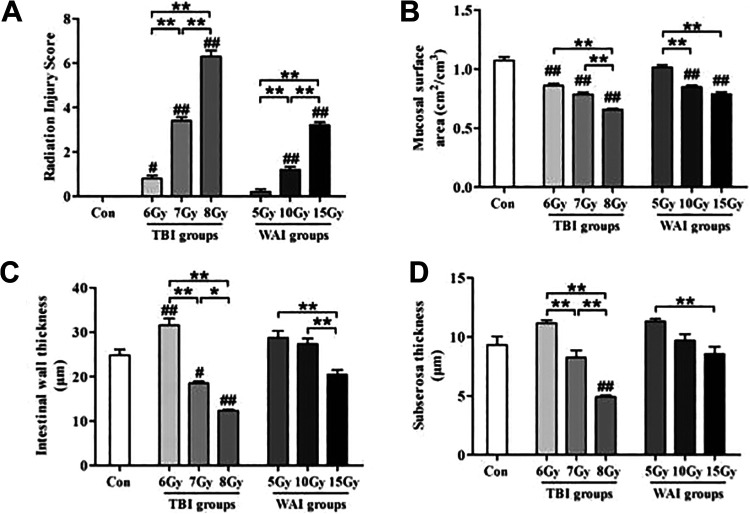
RIS system and MSA were used to evaluate the extent of intestinal injury in this study. Both TBI and WAI exposure can significantly increase RIS but reduce MSA at a dose-dependent manner (Figures 5A-B). The intestinal structure of the 5 Gy WAI group was not affected. The intestinal wall thickness in the 6 Gy TBI group increased significantly, whereas the intestinal wall thickness in the 7 and 8 Gy TBI groups decreased; furthermore, the intestinal wall thickness in the WAI groups did not clearly change compared with that of the control group (Figure 5C). This finding was similar to subserosal thickness (Figure 5D).
Figure 5.
Structural manifestations of radiation enteropathy (n = 6). The data were presented as the mean ± standard deviation of the 7 independent experiments. The differences were evaluated by a one-way analysis of variance (ANOVA). # P < 0.05 as compared with control group; ## P < 0.01 as compared with control group; * P < 0.05; ** P < 0.01.
Discussion
Approximately 60% of patients with gynecological and colorectal cancer receive abdominal radiation therapy (WAI) in clinical practice.11 Otherwise, the incidental leakage of radionuclides due to poor management or improper operation may make human body expose to the radiation environment and produce total-body irradiation (TBI). In both cases, gastrointestinal discomfort symptoms, including diarrhea, abdominal pain, rectal bleeding, and fecal incontinence, are observed.12 In this study, the biological effects of WAI and TBI were different and closely related to the irradiation dose. A low TBI dose (6, 7, and 8 Gy) and a high WAI dose (15 Gy) caused lethal injury among C57BL/6 J mice (Figure 1). Interestingly, the mice in the 6 Gy TBI and 15 Gy WAI groups recovered and drank water but did not eat food several days before they died, indicating the importance of nutrition for survival (Figure 2B). The WAI and TBI groups exhibited symptoms of acute radiation sickness (ARS), including lack of movement, hump, hair ruffling, diarrhea, emaciation, and epilation. Numerous studies have been conducted on ARS of human or other species in 1990s. According to authoritative reports (Hayes’ Principles and Methods of Toxicology Edt AW Hayes and CL Kruger, Chapter 18), whole body exposure of 4-6 Gy could induce severe ARS and that >8 Gy could induce lethal injury in human. Besides, the range of representative lethal dose 50% is remarkably narrow from 2.5 Gy to 7.5 Gy in species other than human, and the value is 6.38 Gy of 250KVP X-ray in mice model. We hypothesized that factors such as mice species and exposure parameters were the main reasons for the difference from our results.
Furthermore, diarrhea caused perianal swelling, rectal prolapse (10 and 5 Gy), and gross blood stool (15 Gy) in the high-dose WAI groups (Figure 3); thus, severe intestinal injury was proven by the pathological results (Figures 4 and 5), resulting in a significant increase in RIS and a significant reduction in MSA (Figures 5A and B). The intestinal wall thickness and the subserosal thickness in the 8 Gy TBI group significantly decreased probably because of the irreversible damage to the intestinal wall. In conclusion, our results revealed that death could be attributed to the loss of appetite and the damage to intestinal mucosa.
Previous studies have reported that irradiation induces significant structural alterations in the intestinal wall.13 The gut microbiota of mice are involved in regulating host immunity. The complex consortia of bacteria colonize the gut and constantly interact with the intestinal epithelium and the underlying immune cells. Intrinsic antimicrobials in the intestinal epithelium are upregulated in response to the presence of bacteria to protect host tissues from bacterial invasion.14 The small intestine is highly sensitive to IR.1 Injury to intestinal epithelial2 and vascular endothelial cells3 and gut microbiota disorder4 are the primary pathogenetic mechanisms of intestinal injury. IR gradually damages the normal barrier of the small intestine, and the lamina propria is exposed to the gut microbiota that induce acute inflammatory responses. This phenomenon causes symptoms, such as nausea, diarrhea, vomiting, bleeding, and intestinal perforation, leading to death caused by bacterial infection and septic shock.15 Thus, IR-induced injury to the gut and the immune system is essential for the lethality rate.
Radiation methods and doses reported in models of radiation intestinal injury vary. Lu et al.16 established a C57BL/6 J mouse model of total abdominal radiation intestinal injury with 15 Gy 137Cs γ-ray. Yang et al.17 studied the protective effect on Nrf2-deficient mice after receiving whole abdominal IR with 13 Gy γ-ray. Guo et al.5 used a model of 7 Gy whole body γ-ray to examine TLR4 agonist monophosphoryl lipid A-alleviated radiation-induced intestinal injury. In our study, the absence of crypts, a considerable loss of villi, and epithelial disruption were observed in the mouse models of radiation intestinal injury. Intestinal epithelial regeneration was more likely to happen in the WAI groups than in the TBI groups. Nevertheless, the lethal dose was lower and the damage was more serious in the TBI groups than in the WAI groups. Currently, with the deepening of the research on the damage effect of radiation, it was clear that the radiation could result in various tissues and organs faliure. For example, radiation induces lung toxicity which manifests acutely as radiation pneumonitis and chronically as radiation pulmonary fibrosis.18 Besides, clinical practice demonstrates the occurrence of radiation-induced peripheral neuropathies.19 Notably, it has been reported that radiation-associated pericardial disease can have devastating consequences.20 Since TBI exposure manner can have effect on organs or systems rather than intestine, we concluded that the serious response in the TBI group might be related to systemic injury and organ failure.21
Many clinical cases of radiation intestinal injury have been recorded, and their pathogenesis is complex and needs to be studied. The optimal method and radiation dose should be determined to establish intestinal injury models. The WAI of 10 Gy should be selected to create a C57BL/6 J mouse model of radiation intestinal injury and show nonlethal and similar clinical symptoms of radiation intestinal injury. Under abdominal radiation therapy or incidental leakage of radionuclides, the extent of intestinal injury and related symptomatic treatment methods should be explored. Adequate nutritional support can promote the fast recovery of patients. Given the lack of effective radiation protection agents,22 novel radioprotectors targeting intestinal damage with minimal toxicity and high efficacy should be developed in the future.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Natural Science Foundation of China (No. 81673101, 81703159, 81973024), China Postdoctoral Science Foundation (2015M571889), the Natural Science Foundation of the Jiangsu Higher Education institutions of China (18KJA310006), and the Postdoctoral research funding plan in Jiangsu province (1601121C), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
ORCID iD: Lin Zhao  https://orcid.org/0000-0002-5365-9779
https://orcid.org/0000-0002-5365-9779
References
- 1. Pierre JF, Busch RA, Kudsk KA. The gastrointestinal immune system: implications for the surgical patient. Curr Probl Surg. 2016;53(1):11–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirsch DG, Santiago PM, di Tomaso E, et al. P53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327(11):593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;316(5):293–297. [DOI] [PubMed] [Google Scholar]
- 4. Liu X, Zhou Y, Wang S, et al. Impact of low-dose ionizing radiation on the composition of the gut microbiota of mice. Toxicol Sci. 2019;171(1):258-268. [DOI] [PubMed] [Google Scholar]
- 5. Guo J, Liu Z, Zhang D, et al. TLR4 agonist monophosphoryl lipid A alleviated radiation-induced intestinal injury. J Immunol Res. 2019;(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li M, Lang Y, Gu MM, et al. Vanillin derivative VND3207 activates DNA-PKcs conferring protection against radiation-induced intestinal epithelial cells injury in vitro and in vivo. Toxicol Appl Pharmacol. 2020;387:114855. [DOI] [PubMed] [Google Scholar]
- 7. Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161(2):123–136. [DOI] [PubMed] [Google Scholar]
- 8. Brodin NP, Velcich A, Guha C, Tomé WA. A model for precise and uniform pelvic- and limb-sparing abdominal irradiation to study the radiation-induced gastrointestinal syndrome in mice using small animal irradiation systems. Dose-Response. 2017;15(1):155932581668579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Tolerance of rat small intestine to localized single dose and fractionated irradiation. Acta Oncol. 1992;31(7):781–787. [DOI] [PubMed] [Google Scholar]
- 10. Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142(Pt 3):259–276. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Z, Cheng W, Qu W, Shao G, Liu S. Antibiotic alleviates radiation-induced intestinal injury by remodeling microbiota, reducing inflammation, and inhibiting fibrosis. ACS Omega. 2020;5(6):2967–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19(2):185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67(19):9501–9506. [DOI] [PubMed] [Google Scholar]
- 14. Grizotte-Lake M, Guo Z, Duncan K, et al. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity. 2018;49(6):1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen W, Ju S, Lu T, et al. Directional delivery of RSPO1 by mesenchymal stem cells ameliorates radiation-induced intestinal injury. Cytokine. 2017;95:27–34. [DOI] [PubMed] [Google Scholar]
- 16. Lu L, Jiang M, Zhu C, He J, Fan S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3´-Diindolylmethane (DIM). Free Radic Biol Med. 2018;130:244–255. [DOI] [PubMed] [Google Scholar]
- 17. Yang W, Sun Z, Yang B, Wang Q. Nrf2-knockout protects from intestinal injuries in C57BL/6 J mice following abdominal irradiation with γ rays. Int J Mol Sci. 2017;18(8):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pradat PF, Delanian S. Late radiation injury to peripheral nerves. Handb Clin Neurol. 2013;115:743–758. [DOI] [PubMed] [Google Scholar]
- 20. Szpakowski N, Desai MY. Radiation-associated pericardial disease. Curr Cardiol Rep. 2019;21(9):97. [DOI] [PubMed] [Google Scholar]
- 21. Šinkorová Z, Filipová A, Vávrová J, et al. Investigation of the radioprotective effect of orthovanadate in mice after total body irradiation. Radiat Prot Dosimetry. 2019;186(2-3):149–154. [DOI] [PubMed] [Google Scholar]
- 22. Gosselin TK, Mautner B. Amifostine as a radioprotectant. Clin J Oncol Nurs. 2002;6(3):175–176. [DOI] [PubMed] [Google Scholar]