Abstract
Aims:
This study aimed to assess the associations between clinical parameters, long-term outcomes, and expression of chemokine receptor CXCR2 in patients with acute myeloid leukemia (AML).
Methods:
From May 2013 to May 2017, 83 adult patients newly diagnosed with AML in the Affiliated Hospital of BeiHua University and Jilin Chemical Hospital, were enrolled in this study. The expression of CXCR2 in bone marrow mononuclear cells was determined by quantitative real-time polymerase chain reaction (qRT-PCR). Clinical information and RNA-sequencing datasets of The Cancer Genome Atlas (TCGA) (n = 136) were obtained. The associations between clinical parameters, prognosis, and CXCR2 expression were analyzed.
Results:
From both cohorts, patients with AML with M4 and M5 subtypes showed higher CXCR2 expression levels than those with other French-American-British (FAB) subtypes. Patients with extramedullary leukemia infiltration had higher CXCR2 levels than those without. In our cohort, patients with high CXCR2 levels (⩾2.099) had lower relapse-free survival (RFS) (p < 0.000001) and overall survival (OS) (p = 0.000107) than those with low levels (<2.099). High CXCR2 levels (⩾2.082) also indicated a poor OS in the TCGA cohort but only in patients younger than 65 years (5-year OS: 7.7% versus 29.9% in those with CXCR2 levels < 2.082). High CXCR2 levels independently predicted poor prognosis in AML patients, as determined by Cox proportional hazards models.
Conclusion:
Our results suggest that high CXCR2 expression associates with the monocytic lineage of AML and is an independent risk factor for poor patient prognosis.
Keywords: acute myeloid leukemia, clinical features, correlations, CXCR2 expression, prognosis
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disorder characterized by the accumulation of poorly differentiated leukemic cells in the bone marrow (BM) and extramedullary organs. The French-American-British (FAB) classification recognizes eight AML subtypes (M0–M7), based on morphological features. Patients with M3, or acute promyelocytic leukemia (APL), have a very favorable prognosis due to the discovery of arsenic trioxide and all-trans retinoic acid.1,2 However, clinical presentation, treatment response, and prognosis of patients with non-APL AML are highly heterogeneous. Thus, the National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) have established guidelines to classify newly diagnosed AML patients.3,4 The presence of specific chromosomal abnormalities and gene mutations is used to stratify the patients into different risk groups (low, intermediate, or high), which help assess prognosis and post-remission treatment-chemotherapy, or allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although the NCCN and ELN guidelines are used widely in clinical practice for managing AML patients, heterogeneity still exists in certain risk groups. For example, in AML with CEBPA double mutations, a low-risk group, some patients experience continuous remission, while others relapse after cycles of consolidation chemotherapy or stem cell transplantation.5,6 Studies from different medical centers have attempted to explore new markers for re-stratifying these patients.7,8 Therefore, there is an urgent need to uncover potential markers for AML stratification. Many studies have attempted to dissect the AML heterogeneity with different molecular markers,9–11 but it is more convenient to regulate gene expression than to correct gene mutations.
CXCR2 is a seven-transmembrane-domain G-protein-coupled receptor (GPCR) expressed in multiple cell types such as neutrophils, monocytes, eosinophils, and tumor cells.12 It plays an important role in immunocyte migration and angiogenesis.12 Furthermore, its upregulation in tumor cells is a poor prognostic factor in various cancers such as prostate, lung, and breast cancer.13–15 Schinke et al. reported that CXCR2 expression is high in different leukemia cell lines (e.g., KG-1, MOLM-13, HL-60, U937, and THP-1) and primary AML samples. Moreover, using the Cancer Genome Atlas (TCGA) dataset, the authors found that higher levels of CXCR2 expression led to worse clinical outcomes.16 Inhibiting or downregulating CXCR2, conversely, leads to decreased viability and clonogenic capability of AML cells.16 Another group also used TCGA database but failed to establish an association between high CXCR2 expression and poor patient prognosis.17 Since the correlation between CXCR2 expression levels and clinical parameters of AML patients is also unclear, further studies are needed to associate CXCR2 expression with AML clinical phenotypes. Here, two independent patient cohorts were analyzed, one from our hospitals and the other from the TCGA database, to study the relationships between patient clinical parameters, long-term outcomes, and CXCR2 expression.
Materials and methods
Patients and controls
From May 2013 to May 2017, 83 patients newly diagnosed with AML in the Affiliated Hospital of BeiHua University and Jilin Chemical Hospital were enrolled in this study. Patients were diagnosed based on the FAB classification (M0–M7), immunological phenotypes, cytogenetics, and gene mutation analyses. All patients received the standard 3+7 regimen for induction therapy (daunorubicin or idarubicin with cytarabine). Some elderly patients in our patient cohort were also treated with the CAG regimen (aclarubicin, cytarabine, and granulocyte colony-stimulating factor). The first consolidation regimen was always similar to that achieved in remission, and the patients were administered a scheduled 3–4 courses of intermediate- or high-dose cytarabine (1.5–2.0 g/m2) for subsequent therapies. High-risk or relapsed patients underwent allo-HSCT if an appropriate donor was available. Peripheral blood samples from healthy volunteers were collected as controls. Before enrollment, the volunteers, patients, and their relatives gave written informed consent. The ethics committee of the Affiliated Hospital of BeiHua University (No. 2013-003) and Jilin Chemical Hospital (No. 2014-028) approved the study, and it followed the principles outlined in the Declaration of Helsinki.
Cytogenetic analysis and screening of somatic gene mutations
Standard leukemia cell culturing and chromosome-banding techniques determined the karyotypes of AML patients. The results were defined and described according to the International System for Human Cytogenetic Nomenclature 2013.18 NPM1, FLT3-ITD, c-kit, and CEBPA mutations were detected by polymerase chain reaction (PCR).
Quantitative real-time PCR (qRT-PCR)
Bone marrow mononuclear cells (BMMCs) and peripheral blood mononuclear cells (PBMCs) were isolated from the patients and volunteers by density gradient centrifugation. Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To synthesize cDNA, the RNA was reverse-transcribed with the PrimeScript RT kit (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. The CXCR2 expression was determined by qRT-PCR using the SYBR Premix Ex Taq II kit (TaKaRa). GAPDH transcript levels were an endogenous control that normalized the variance between the samples. The comparative ΔΔCt method allowed the calculation of relative gene expression values. The CXCR2 and GAPDH primer sequences (Sangon Biotech, Shanghai, China) are listed in Table 1.
Table 1.
Primer sequences for CXCR2 and GAPDH..
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| CXCR2 | CCTGTCTTACTTTTCCGAAGGAC | TTGCTGTATTGTTGCCCATGT |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
Data mining of public and well-documented datasets
Normalized CXCR2 and CXCL1/2/3/5/6/7/8 expression data and clinical information of 136 AML patients were retrieved from the Genomic Data Commons (GDC) TCGA database and downloaded from the website of the University of California, Santa Cruz (UCSC) at http://xena.ucsc.edu/welcome-to-ucsc-xena/. The expression data and clinical information were combined according to patient sample numbers.
Statistical analysis
Statistical Package for Social Sciences (SPSS) software (Version 20, SPSS Inc., Chicago, IL, USA) and Prism 8.0 (GraphPad Software, San Diego, CA, USA) were used to process the data. For continuous variables with normal distribution and homogeneity of variance, independent sample t-test (for two groups) or one-way analysis of variance (ANOVA) (for three or more groups) were used to compare differences. Alternatively, the Mann–Whitney U-test compared the differences between two groups; the Kruskal–Wallis H test, three or more groups. For further two-group comparisons, the least significant difference or Tamhane test was used. Correlation analysis was performed using Pearson’s or Spearman correlation. When CXCR2 and its ligand expression levels were AML prognosticators, a receiver operating characteristic (ROC) curve evaluated the distribution of specificity and sensitivity. The Kaplan–Meier method was employed for survival analysis, and the log-rank test was used to compare differences between groups. The Cox proportional hazard model was used for multivariate analysis, including the low p-value variables (p < 0.1). A p value < 0.05 was considered significant for all tests.
Results
Characteristics of AML patients and controls
A total of 219 adult AML patients participated in this study, 83 from our hospital and 136 from the TCGA dataset; 16 healthy volunteers were included as controls, consisting of 10 males and 6 females, with a median age of 42 years (range, 32–58 years). While patients from the TCGA cohort were of advanced age, the median age was 57 years, patients from our hospital were younger with a median age of 46 years (t = 5.002; p = 0.000001). Concerning FAB classifications, acute myeloblastic leukemia (M2) was the most common subtype in both patient cohorts. Our patients also carried significantly more CEBPA mutations (χ2 = 5.349; p = 0.021) and had lower hemoglobin levels (u = 5.900; p < 0.000001) than those in the TCGA cohort. Detailed information is summarized in Table 2.
Table 2.
Basic characteristics of patients with AML.
| Our cohort (n = 83) | TCGA cohort (n = 136) | |
|---|---|---|
| Age, median (range) | 46.0 (18.0–65.0) | 57.0 (21.0–88.0) |
| Gender | ||
| Male | 40 (48.19%) | 76 (55.88%) |
| Female | 43 (51.81%) | 60 (44.12%) |
| FAB classification | ||
| M0 | 0 (0.00%) | 15 (11.03%) |
| M1 | 5 (6.02%) | 35 (25.74%) |
| M2 | 33 (39.76%) | 38 (27.94%) |
| M4 | 27 (32.53%) | 29 (21.32%) |
| M5 | 16 (19.28%) | 15 (11.03%) |
| M6 | 2 (2.41%) | 2 (1.47%) |
| M7 | 0 (0.00%) | 1 (0.70%) |
| Others | 0 (0.00%) | 1 (0.70%) |
| Cytogenetics | ||
| Low-risk | 11 (16.92%) | 17 (12.69%) |
| Intermediate-risk | 49 (75.38%) | 81 (60.45%) |
| High-risk | 5 (7.69%) | 36 (26.87%) |
| NPM1 mutation | ||
| Yes | 20 (24.10%) | 38 (28.57%) |
| No | 63 (75.90%) | 95 (71.43%) |
| FLT3 mutation* | ||
| Yes | 24 (28.92%) | 37 (27.82%) |
| No | 59 (71.08%) | 96 (72.18%) |
| c-kit mutation | ||
| Yes | 6 (7.23%) | 7 (5.26%) |
| No | 77 (92.77%) | 126 (94.74%) |
| CEBPA mutations # | ||
| Yes | 18 (21.69%) | 13 (9.77%) |
| No | 65 (78.31%) | 120 (90.23%) |
| White blood cells (×109/l) | 21.26 (4.33, 61.15) | 24.5 (6.00, 56.00) |
| Hemoglobin (g/l) | 75.80 ± 28.02 | 90.00 (90.00, 100.00) |
| Platelets(×109/l) | 43.00 (19.00, 89.00) | 50.00 (30.00, 87.75) |
| BM blasts (%) | 67.5 (43.00, 82.50) | 65.74 ± 21.77 |
Information for FLT3-ITD or FLT3-TKD mutations is lack in TCGA dataset.
Information for CEBPA single or double mutations is lacking in the TCGA dataset. Successful cytogenetic analysis was completed in 78.31% (65/83) of our patients and 98.53% (134/136) of TCGA cohort. There is a lack of information for molecular mutations in some patients from TCGA dataset, so the number of patients with NPM1, FLT3, c-kit, and CEBPA mutations is less than the total patient number.
AML, acute myeloid leukemia; BM, bone marrow; FAB, French-American-British; TGCA, the Cancer Genome Atlas.
CXCR2 expression in AML patients and its association with clinical factors
Compared with healthy controls, AML patients showed higher levels of CXCR2 expression (Figure 1). There was no significant association between age, sex, white blood cell (WBC) counts, BM blast percentages, and CXCR2 levels (Table 3 and supplemental Table S1) in both patient cohorts. In both cohorts, patients with acute myelomonocytic leukemia (M4) and acute monocytic leukemia (M5) showed higher CXCR2 levels than those with other FAB subtypes (Table 3 and supplemental Table S2). Patients with extramedullary leukemia infiltration (EMLI) (lymph nodes, liver, spleen, skin, testicles, Waldeyer ring, or central nervous system) had higher CXCR2 levels (1.906 ± 0.795, n = 32) than those without (1.567 ± 0.660, n = 51) (t = 2.106, p = 0.038). There was no significant difference among patients in different cytogenetic risk groups in our and TCGA cohorts. The number of patients in the low-risk, intermediate-risk, and high-risk groups were 34, 22, and 23, respectively, in our patient cohort according to the NCCN guidelines for AML (version 2.0 2020). Patients in the low-risk group (1.450 ± 0.652) had lower CXCR2 levels compared with those in the intermediate-risk (1.848 ± 0.689) (p = 0.048) and high-risk (1.931 ± 0.843) (p = 0.016) groups. We also found no significant association between NPM1, c-kit mutations, and CXCR2 levels in either patient cohort. In the TCGA cohort, nonetheless, patients carrying the FLT3 mutation had higher CXCR2 levels than those with wild-type FLT3, and a tendency of high CXCR2 expression also could be observed in FLT3 mutated patients in our cohort. Patients with CEBPA mutations (single plus double) showed lower CXCR2 levels than those without (p = 0.000394) in our cohort. Although we observed no significant difference in CXCR2 expression between patients with and without CEBPA mutations in the TCGA cohort, we noticed a tendency of lower CXCR2 expression in patients with CEBPA mutations than in those without (Table 3). Furthermore, patients with CEBPA double mutations also showed lower CXCR2 expression (0.861 ± 0.488) than those without (1.825 ± 0.676) in our cohort (t = 4.545; p = 0.000019).
Figure 1.
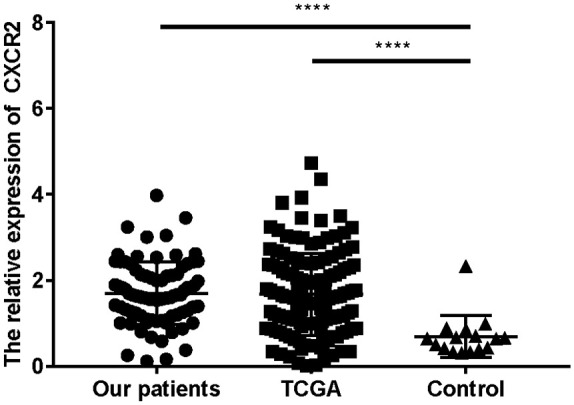
CXCR2 expression is high in AML patients compared with healthy controls.
****p < 0.0001.
AML, acute myeloid leukemia; TCGA, the Cancer Genome Atlas.
Table 3.
The relationships between clinical factors and CXCR2 expression.
| Our cohort |
TCGA |
|||
|---|---|---|---|---|
| CXCR2 levels | p | CXCR2 levels | p | |
| Gender | 0.318 | 0.927 | ||
| Male | 1.614 ± 0.769 | 1.682 ± 0.968 | ||
| Female | 1.775 ± 0.691 | 1.663 ± 0.961 | ||
| FAB classification* | 0.007 | <0.001 | ||
| M0 | – | 1.129 ± 0.640 | ||
| M1 | 1.093 ± 0.427 | 1.295 ± 0783 | ||
| M2 | 1.474 ± 0.713 | 1.478 ± 1.002 | ||
| M4 | 1.872 ± 0.663 | 2.257 ± 0.871 | ||
| M5 | 2.042 ± 0.752 | 2.528 ± 0.630 | ||
| Cytogenetic risk groups | 0.841 | 0.259 | ||
| Low-risk | 1.696 ± 0.488 | 1.408 ± 0.636 | ||
| Intermediate-risk | 1.665 ± 0.809 | 1.786 ± 1.038 | ||
| High-risk | 1.467 ± 0.661 | 1.582 ± 0.905 | ||
| NPM1 mutation | 0.390 | 0.163 | ||
| Yes | 1.821 ± 0.627 | 1.858 ± 1.069 | ||
| No | 1.658 ± 0.760 | 1.600 ± 0.913 | ||
| FLT3 mutations | 0.299 | 0.009 | ||
| Yes | 1.829 ± 0.638 | 2.022 ± 1.048 | ||
| No | 1.644 ± 0.763 | 1.539 ± 0.899 | ||
| c-kit mutations | 0.884 | 0.508 | ||
| Yes | 1.740 ± 0.569 | 1.687 ± 0.970 | ||
| No | 1.694 ± 0.744 | 1.38 ± 0.861 | ||
| CEBPA mutations | <0.001 | 0.494 | ||
| Yes | 1.174 ± 0.609 | 1.499 ± 0.928 | ||
| No | 1.842 ± 0.697 | 1.692 ± 0.969 | ||
Due to limited patient number, the CXCR2 expression level was not calculated in patients with M6, M7 and others.
AML, acute myeloid leukemia; FAB, French-American-British; TGCA, the Cancer Genome Atlas.
The relationship between complete remission and CXCR2 expression
Achieving complete remission (CR) after one course of chemotherapy is an important indicator of chemosensitivity. The CXCR2 expression levels in our patients who achieved CR after one course of chemotherapy were 1.731 ± 0.719, which was similar to those who did not (1.704 ± 0.835) (t = 1.123; p = 0.902).
High CXCR2 expression associates with poor prognosis
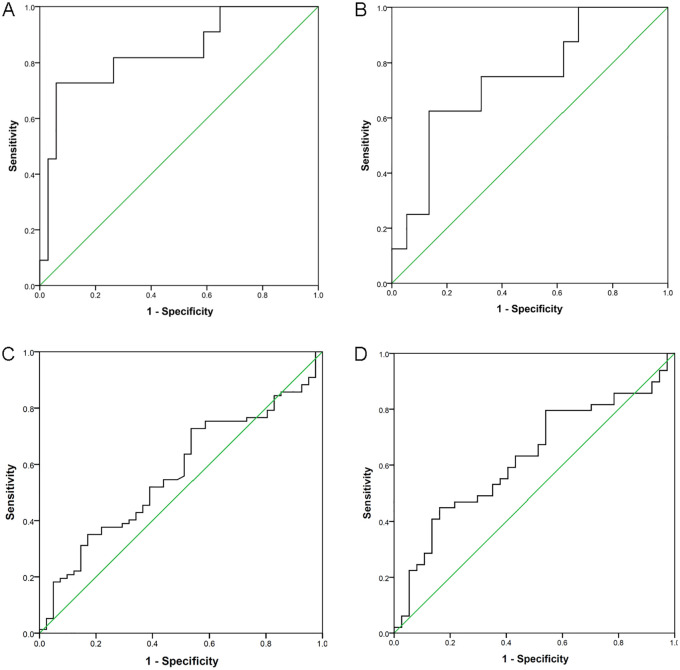
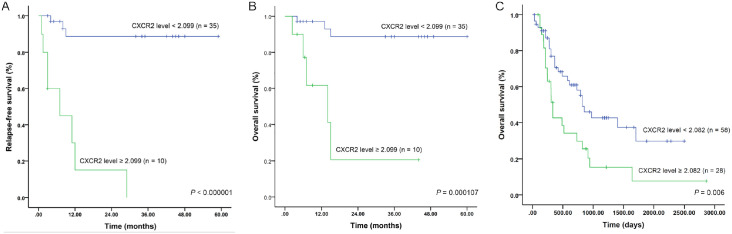
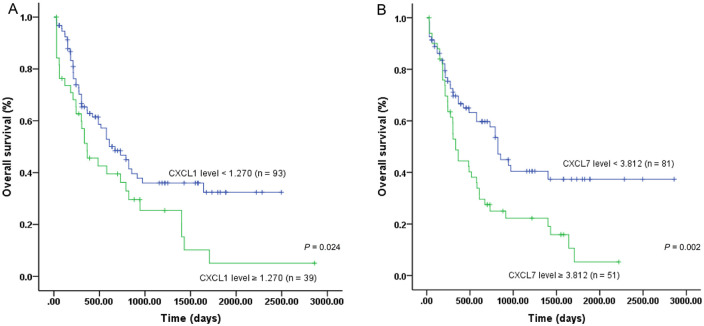
We used ROC curves to predict relapse-free survival (RFS) and overall survival (OS) of patients with CXCR2 expression levels. In our patient cohort, we selected an optimal cutoff value of 2.099 for CXCR2 expression to predict both RFS and OS. The area under the curve (AUC) was 0.837 (p = 0.001), and the sensitivity (Se) and specificity (Sp) were 72.7% and 94.1%, respectively, (Figure 2A). For OS, the AUC was 0.740 (p = 0.035), and Se and Sp were 62.5% and 86.5%, respectively (Figure 2B). Patients with high CXCR2 expression levels (⩾2.099) showed significantly inferior RFS (p < 0.000001) and OS (p = 0.000107) than those with low levels (<2.099) (Figure 3A,B). In the TCGA cohort, however, we could not determine the optimal cutoff value in the whole patient cohort (Figure 2C). Nonetheless, in those younger than 65 years old, a level of 2.082 was found to be an optimal cutoff value (Figure 2D) (AUC = 0.625; Se = 44.9%; Sp = 83.8%; p = 0.048). The 5-year OS was 7.7% in patients with high CXCR2 expression levels (⩾2.082), which was significantly lower than that in patients with low levels (<2.082) (29.9%) (p = 0.006) (Figure 3C). The influence of expression levels of CXCR2 ligands on prognosis of patients was also analyzed in this study with TCGA dataset. Among them, both CXCL1 and CXCL7 expression levels were associated with outcomes of AML patients (supplemental Figure S1). The 5-year OS in patients with low (<1.270) and high (⩾1.270) CXCL1 expression levels were 32.4% and 5.1%, respectively (p = 0.024) (Figure 4A). Patients with high CXCL7 expression levels (⩾3.812) had an inferior 5-year OS than those with low expression levels (<3.812) (5.3% versus 37.4%) (p = 0.003) (Figure 4B).
Figure 2.
ROC curve was used to predict survival of AML patients with CXCR2 expression levels. (A) CXCR2 expression levels predicted RFS in our patient cohort; (B) CXCR2 expression levels predicted OS in our patient cohort; (C) CXCR2 expression levels predicted OS in the whole cohort of TCGA patients; (D) CXCR2 expression levels predicted OS in patients ⩽65 years old in TCGA cohort.
AML, acute myeloid leukemia; OS, overall survival; RFS, relapse-free survival; ROC, receiver operating characteristic; TCGA, the Cancer Genome Atlas.
Figure 3.
Survival of AML patients based on CXCR2 expression levels. (A) RFS of our patient cohort; (B) OS of our patient cohort; (C) OS of TCGA patient cohort (⩽65 years old).
AML, acute myeloid leukemia; OS, overall survival; RFS, relapse-free survival; TCGA, the Cancer Genome Atlas.
Figure 4.
Survival of AML patients based on CXCL1 and CXCL7 expression levels. (A) OS of patient with low and high CXCL1 levels; (B) OS of patient with low and high CXCL7 levels.
AML, acute myeloid leukemia; OS, overall survival.
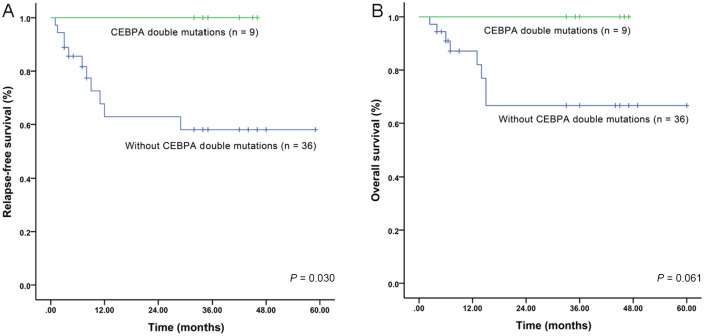
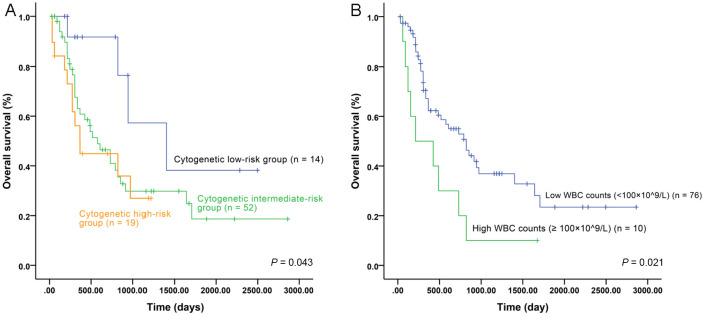
Next, using univariate analysis, we estimated the prognostic significance of cytogenetic risk groups, molecular markers, and high WBC counts (⩾100 × 109/l) (Figures 5, 6 and supplemental Figures S2, S3), and then used these results for multivariate analysis. In our patient cohort, both CEBPA double mutations (Figure 5) and CXCR2 expression associated with RFS and OS. High CXCR2 expression, furthermore, was the only independent factor for RFS and OS in Cox proportional hazard models (Table 4). In the TCGA cohort, cytogenetic risk groups (Figure 6A), high WBC counts (Figure 6B), and CXCR2 expression all associated with OS and high CXCR2 expression was an independent risk factor for unfavorable prognosis (Table 5). Due to the relatively small number of patients receiving allo-HSCT (eight in our cohort) or lack of information for allo-HSCT in the TCGA dataset, we could not analyze the influence of CXCR2 expression on the survival in the allo-HSCT setting. Taken together, these results suggest that high CXCR2 expression is an independent unfavorable risk factor for AML patients.
Figure 5.
Influence of CEBPA double mutations on survival of AML patients from our cohort. (A) RFS of patients with and without CEBPA double mutations; (B) OS of patients with and without CEBPA double mutations.
AML, acute myeloid leukemia; OS, overall survival; RFS, relapse-free survival.
Figure 6.
Influence of cytogenetic risk groups and WBC counts on survival of AML patients from TCGA cohort. (A) OS of patients with different cytogenetic risk groups; (B) OS of patients with high (⩾100 × 109/L) and low (<100 × 109/l) WBC counts.
AML, acute myeloid leukemia; OS, overall survival; TCGA, the Cancer Genome Atlas; WBC, white blood cell.
Table 4.
Cox proportional hazard models for RFS and OS in our cohort of patients.
| RFS |
OS |
|||
|---|---|---|---|---|
| CEBPA dm | CXCR2 levels ⩾ 2.099 | CEBPA dm | CXCR2 levels ⩾ 2.099 | |
| B | 11.902 | −2.509 | 12.389 | −1.918 |
| SE | 296.253 | 0.690 | 374.877 | 0.740 |
| Wald | 0.002 | 13.231 | 0.001 | 6.720 |
| Exp (B) | 147513.441 | 0.081 | 240051.89 | 0.147 |
| CI | 0.000–UD | 0.021–0.314 | 0.000–UD | 0.034–0.626 |
| p | 0.968 | 0.000275 | 0.974 | 0.010 |
B, regression coefficient; CEBPAdm, CEBPA double mutations; CI, confidence interval; Exp (B), odds ratio; OS, overall survival; RFS, relapse-free survival; SE, standard error; UD, undetermined; Wald, statistical value.
Table 5.
Cox proportional hazard model for OS in TCGA dataset.
| B | SE | Wald | Exp (B) | CI | p | |
|---|---|---|---|---|---|---|
| CXCR2 levels ⩾ 2.082 | –0.864 | 0.296 | 8.494 | 0.422 | 0.236–0.754 | 0.004 |
| Low-risk cytogenetics | 4.754 | 0.093 | ||||
| Intermediate-risk cytogenetics | –1.234 | 0.581 | 4.512 | 0.291 | 0.093–0.909 | 0.034 |
| High-risk cytogenetics | –0.482 | 0.362 | 1.771 | 0.618 | 0.304–1.133 | 1.256 |
| High WBC counts (⩾100 × 109/l) | –0.800 | 0.413 | 3.753 | 0.449 | 0.200–1.009 | 0.053 |
B, regression coefficient; CI, confidence interval; Exp (B), odds ratio; OS, overall survival; SE, standard error; TGCA, the Cancer Genome Atlas; Wald, statistical value; WBC, white blood cell.
Discussion
Chemokine receptor CXCR2 is expressed in a variety of tumor cells, and plays an important role in their proliferation, metastasis, and chemoresistance.19–21 The CXCR2 pathway is also involved in angiogenesis and the activation of cancer stem cells (CSCs).16,22,23 Although high CXCR2 expression is an indicator of poor prognosis in many solid tumors,13–15 its role in AML still needs to be elucidated as limited studies are available and controversial results exist. Therefore, in this study, we assessed the associations between clinical parameters, prognosis, and CXCR2 expression in a Chinese patient cohort with AML. To overcome problems related to the small sample size, we verified our findings with a well-documented public database (TCGA).
Patients with AML showed higher CXCR2 expression levels compared with healthy controls, which was consistent with the results of a previous study.16 We also found a significant association between CXCR2 levels and the FAB subtypes. In patients with M4 and M5 subtypes, the CXCR2 levels were similar and higher than those with other FAB subtypes in both cohorts. Previous study reported that CXCR2 played a critical role in the development of monocytes/macrophages, and CXCR2 deficiency reduces the ability of granulocyte and macrophage progenitor cells to give rise to macrophage and dendritic cell progenitor cells.24 Therefore, high CXCR2 expression in patients with monocytic lineage of AML indicate that CXCR2 may contribute to leukemogenesis. Moreover, high CXCR2 levels in patients with the monocytic lineage of AML may partly contribute to the high incidence of leukemic cell infiltration to extramedullary sites. The CXCR2 receptor is expressed in monocytes and it may account for their enhanced migration to the lungs in chronic obstructive pulmonary disease.25 Similarly, during the early phases of nephrotoxic nephritis, CXCR2 promotes glomerular monocyte recruitment.26 In our patient cohort, we found that high CXCR2 levels were related with EMLI. Furthermore, the frequency of EMLI in patients with monocytic lineage of AML was higher (44.19%, 19/43) than in those with other FAB subtypes (32.5%, 13/40), although no statistical difference was reached (χ2 = 1.195, p = 0.274). Correlation analysis showed that CXCR2 levels associated with EMLI (r = 0.217, p = 0.048). However, when FAB subtype was set as a control variable, no correlation between EMLI and CXCR2 levels could be observed (r = 0.200, p = 0.071). Collectively, these results indicated that CXCR2 may not only participate in leukemogenesis, but also may be associated with extramedullary infiltration in patients with monocytic lineage of AML.
Interestingly, we found that patients with CEBPA mutations had lower CXCR2 levels than those without in our cohort. A previous study reported that the majority of patients with CEBPA double mutations had the M1 and M2 subtypes,6 which also could be observed in our patients (7/10). This may account for the low CXCR2 levels observed in patients with CEBPA mutations. In the TCGA cohort, patients with FLT3 mutations had higher CXCR2 levels than those without. Tendencies of high CXCR2 expression also could be found in patients with FLT3 mutations and without CEBPA mutation in our and TCGA cohorts, respectively. Thus, further studies are needed to confirm the correlation between CXCR2 levels and the above AML marker mutations, and to elucidate its biological significance.
The prognostic significance of high CXCR2 expression was controversial in two previous studies.16,17 We also could not find an optimal cutoff value to predict survival with the CXCR2 levels in the whole cohort of TCGA dataset. Nonetheless, in patients younger than 65 years, high CXCR2 levels were associated with an unfavorable outcome. In our patient cohort, high CXCR2 levels were an indicator of poor prognosis. Moreover, multivariate analyses demonstrated that high CXCR2 levels were an independent risk factor for poor prognosis. CXCR2 ligands were reported to be related with proliferation of leukemia cells.27 Hence, the prognostic significance of these ligands was analyzed as well. High expression levels of CXCL1 and CXCL7 associated with poor prognosis could be observed with TCGA cohort. Accordingly, CXCR2 expression is a potential marker for risk stratification of AML patients.
We could not observe significant associations between NPM1 mutation, FLT3-ITD mutation, cytogenetic risk groups, and survival in our patient cohort. Furthermore, the TCGA dataset lacks the information for FLT3-ITD or FLT3-TKD mutations, and CEBPA single or double mutations. Therefore, further research on the AML marker mutations and a larger sample are needed to verify our findings.
CSCs are rare immortal cells within a tumor that can both self-renew and give rise to many cell types that constitute the tumor, and can therefore form tumors. The regulatory effect of CXCR2 on CSCs activity have been reported in previous studies.16,23 With in vitro and in vivo models, inhibition of CXCR2 by genetic and pharmacologic means leads to decreased viability in AML/MDS stem cells.16 Reparixin is a small molecular weight antagonist of CXCR1/2. In a phase Ib study [ClinicalTrials.gov identifier: NCT02001974], 33 patients with metastatic breast cancer were treated with paclitaxel plus escalating doses of reparixin.28 Finally, 27 patients were evaluated for antitumor activity, and 8 patients had a confirm RECIST response. The median time to progression in low (400 mg), intermediate (800 mg), and high dose (1200 mg) of reparixin groups were 58 days, 67 days, and 162 days, respectively. Three patients achieved long-term remission.28 In another clinical trial [ClinicalTrials.gov identifier: NCT02001974], patients with untreated operable breast cancer not eligible for neoadjuvant treatment were treated with reparixin for 21 consecutive days. Signal of activity was defined as a ⩾20% reduction of CSCs in tumor tissue from baseline values determined by flow cytometry. A total of 20 patients were enrolled, and signal of activity was detected in the majority of patients.29 In patients with metastatic castration-resistant prostate cancer, the safety and toxicity of CXCR2 antagonist AZD5069 in combination with enzalutamide are evaluated in a multi-center Phase I/II trial [ClinicalTrials.gov identifier: NCT03177187]. Therefore, clinical study is needed to explore the efficacy of CXCR2 blockade in AML.
In summary, using two independent AML patient cohorts, we found that CXCR2 expression is associated with FAB subtypes. Patients with the monocytic lineage of AML showed higher CXCR2 levels than those with other FAB subtypes. High CXCR2 expression was associated with extramedullary leukemia infiltration and an independent risk factor for poor prognosis in AML patients. Accordingly, CXCR2 may be used as a prognostic indicator or therapeutic target for AML.
Supplemental Material
Supplemental material, Supplementary_materials_2 for High CXCR2 expression predicts poor prognosis in adult patients with acute myeloid leukemia by Wei Tang, Zunyan Li, Xian Li and Zhonghua Huo in Therapeutic Advances in Hematology
Acknowledgments
We thank Department of Blood Transfusion, the affiliated Hospital of BeiHua University, and Department of Hematology, Jilin Chemical Hospital, for their assistance in this work.
Footnotes
Author’s note: The results of this study have been submitted to the 25th European Hematology Association Annual Congress, Frankfurt, Germany.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Jilin Medical and Health Guiding Plan Project (grant numbers 201900977, 201900979, 201900987).
ORCID iD: Xian Li  https://orcid.org/0000-0002-6884-3843
https://orcid.org/0000-0002-6884-3843
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wei Tang, Department of Blood Transfusion, the Affiliated Hospital of BeiHua University, Jilin, Jilin Province, China.
Zunyan Li, Department of Blood Transfusion, the Affiliated Hospital of BeiHua University, Jilin 132011, Jilin Province, China.
Xian Li, Department of Hematology, Jilin Chemical Hospital, Jilin 132021, Jilin Province, China.
Zhonghua Huo, Department of Blood Transfusion, the Affiliated Hospital of BeiHua University, Jilin, Jilin Province, China.
References
- 1. Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 1996; 88: 1052–1061. [PubMed] [Google Scholar]
- 2. Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988; 72: 567–572. [DOI] [PubMed] [Google Scholar]
- 3. Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol 2018; 93: 1267–1291. [DOI] [PubMed] [Google Scholar]
- 4. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlenk RF, Taskesen E, van Norden Y, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood 2013; 122: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 6. Su L, Tan YH, Lin H, et al. Mutational spectrum of acute myeloid leukemia patients with double CEBPA mutations based on next-generation sequencing and its prognostic significance. Oncotarget 2018; 9: 24970–24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theis F, Corbacioglu A, Gaidzik VI, et al. Clinical impact of GATA2 mutations in acute myeloid leukemia patients harboring CEBPA mutations: a study of the AML study group. Leukemia 2016; 30: 2248–2250. [DOI] [PubMed] [Google Scholar]
- 8. Su L, Gao SJ, Tan YH, et al. CSF3R mutations were associated with an unfavorable prognosis in patients with acute myeloid leukemia with CEBPA double mutations. Ann Hematol 2019; 98: 1641–1646. [DOI] [PubMed] [Google Scholar]
- 9. Buccisano F, Maurillo L, Del Principe MI, et al. Minimal residual disease as a biomarker for outcome prediction and therapy optimization in acute myeloid leukemia. Expert Rev Hematol 2018; 11: 307–313. [DOI] [PubMed] [Google Scholar]
- 10. Patel SS, Kuo FC, Gibson CJ, et al. High NPM1-mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML. Blood 2018; 131: 2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dillon R, Hills R, Freeman S, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 2020; 135: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng Y, Ma XL, Wei YQ, et al. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer 2019; 1871: 289–312. [DOI] [PubMed] [Google Scholar]
- 13. Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res 2013; 73: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maxwell PJ, Gallagher R, Seaton A, et al. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene 2007; 26: 7333–7345. [DOI] [PubMed] [Google Scholar]
- 15. Nannuru KC, Sharma B, Varney ML, et al. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog 2011; 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schinke C, Giricz O, Li W, et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood 2015; 125: 3144–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen XM, Zhang HM, Yang B, et al. [Analysis of unfavorable prognosis gene markers in patients with acute myeloid leukemia by multiomics]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019; 27: 331–338. [DOI] [PubMed] [Google Scholar]
- 18. Shaffer LG, McGowan-Jordan J, Schmid M. (eds). ISCN 2013. An international system for human cytogenetic nomenclature (2013). Basel: Karger, 2013. [Google Scholar]
- 19. Chen H, Sun Y, Wu C, et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocr Relat Cancer 2012; 19: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shao N, Chen LH, Ye RY, et al. The depletion of interleukin-8 causes cell cycle arrest and increases the efficacy of docetaxel in breast cancer cells. Biochem Biophys Res Commun 2013; 431: 535–541. [DOI] [PubMed] [Google Scholar]
- 21. Itoh Y, Joh T, Tanida S, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine 2005; 29: 275–282. [DOI] [PubMed] [Google Scholar]
- 22. Põld M, Zhu LX, Sharma S, et al. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res 2004; 64: 1853–1860. [DOI] [PubMed] [Google Scholar]
- 23. Singh JK, Simões BM, Clarke RB, et al. Targeting IL-8 signalling to inhibit breast cancer stem cell activity. Expert Opin Ther Targets 2013; 17: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 24. Han X, Shi H, Sun Y, et al. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis 2019; 10: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Traves SL, Smith SJ, Barnes PJ, et al. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J Leukoc Biol 2004; 76: 441–450. [DOI] [PubMed] [Google Scholar]
- 26. Zernecke A, Weber KS, Erwig LP, et al. Combinatorial model of chemokine involvement in glomerular monocyte recruitment: role of CXC chemokine receptor 2 in infiltration during nephrotoxic nephritis. J Immunol 2001; 166: 5755–5762. [DOI] [PubMed] [Google Scholar]
- 27. Katsumura KR, Ong IM, DeVilbiss AW, et al. GATA Factor-dependent positive-feedback circuit in acute myeloid leukemia cells. Cell Rep 2016; 16: 2428–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schott AF, Goldstein LJ, Cristofanilli M, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res 2017; 23: 5358–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldstein LJ, Perez RP, Yardley DA, et al. A single-arm, preoperative, pilot study to evaluate the safety and biological effects of orally administered reparixin in early breast cancer patients who are candidates for surgery. Cancer Res 2016; 76(Suppl. 14): Abstract CT057. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_materials_2 for High CXCR2 expression predicts poor prognosis in adult patients with acute myeloid leukemia by Wei Tang, Zunyan Li, Xian Li and Zhonghua Huo in Therapeutic Advances in Hematology