Abstract
Objective:
To provide real-world data and summarize current clinical evidence on the efficacy and safety of sirolimus in active systemic lupus erythematosus (SLE) patients.
Methods:
This was a prospective real-world clinical study. Included SLE patients should have Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) ⩾ 2. They were treated with sirolimus and followed up regularly. The SLEDAI-2K, Physician Global Assessment (PGA), serological activity indices, and remission of organ manifestations were evaluated. We also performed a meta-analysis to integrate current evidence of sirolimus in SLE.
Results:
A total of 49 patients were included in the final analysis. After treatment, the SLEDAI-2K (6.2 ± 3.1 versus 4.0 ± 3.4, p = 0.001) decreased significantly, and the prednisone dosage was tapered successfully (9.9 ± 8.8 mg/day versus 5.9 ± 4.0 mg/day, p = 0.002). Serological activity indices also improved [complement 3 (C3): 0.690 ± 0.209 g/l versus 0.884 ± 0.219 g/l, p < 0.001; complement 4: 0.105 ± 0.059 g/l versus 0.141 ± 0.069 g/l, p < 0.001; anti-dsDNA antibody, 200 ± 178 IU/ml versus 156 ± 163 IU/ml, p = 0.022]. The remission proportions of arthritis, skin rash, and thrombocytopenia were 100%, 88.8%, and 46.2%, respectively. A total of 41.2% of lupus nephritis (LN) patients achieved renal remission, but the average 24-h urine protein level was not significantly changed. Meta-analysis enrolled five studies with 149 patients included, and revealed similar results regarding the changes of SLEDAI-2K [−3.5 (−5.0, −2.1)], C3 [0.224 (0.136, 0.311) g/l] and daily dosage of prednisone [−12.7 (−19.9, −5.6) mg/day].
Conclusion:
Sirolimus might be effective and tolerated in SLE. The role of sirolimus in LN requires further study.
Keywords: lupus nephritis, mTOR inhibitor, sirolimus, systemic lupus erythematosus, thrombocytopenia, treatment
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that involves multiple organ systems and can cause organ damage. The mortality of SLE has declined greatly in recent years due to the application of glucocorticoids and antimalarial drugs and the development of immunosuppressants. However, considering its heterogeneity, relapsing-remitting course and great impact on quality of life, treatment options for SLE remain inadequate. For decades, only one new drug, belimumab, has been approved for the treatment of SLE.1,2
Mechanistic target of rapamycin (mTOR) is proved to be abnormally activated in both the immune system and non-traditional parenchymal organs in SLE patients.3,4 The mTOR inhibitor rapamycin (also called sirolimus) is reported to be effective in SLE, with limited adverse effects. Fernandez et al. were the first to successfully use sirolimus to treat nine SLE patients who failed with other immunosuppressive medications.5 Studies by Yap et al. focused on lupus nephritis (LN) and suggested that sirolimus was effective in decreasing 24-h urine protein as an induction therapy in active LN patients.6,7 In 2018, Lai et al. published a phase I/II, single-arm prospective clinical trial that included 29 SLE patients without renal involvement in the final analysis.8 They suggested that sirolimus could decrease SLE disease activity and improve arthritis and skin rash. Last year, Eriksson et al. retrospectively summarized their experience with long-term sirolimus as a treatment for non-renal manifestations of SLE, and indicated the acceptable tolerance and safety of sirolimus for a treatment period of nearly 4 years.9
To better clarify the efficacy and safety of sirolimus in SLE patients, we conducted a real-world study beginning January 2018. Furthermore, to integrate the current clinical evidence, we performed a meta-analysis of the literature.
Methods
Part I: real-world study
Patients
This was a prospective real-world study based on the Chinese SLE Treatment and Research group (CSTAR) registry conducted in a single centre, Peking Union Medical College Hospital (PUMCH), beginning January 2018. Included patients should be 18–65 years old, fulfill the Systemic Lupus International Collaborating Clinics (SLICC) 2012 classification criteria for SLE, and have at least mild disease activity [Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) ⩾ 2]. Patients were excluded for the following reasons: (1) being complicated with neuropsychiatric lupus, pregnancy, or concurrent infections; (2) incomplete baseline or follow-up data; (3) treatment time less than 1 month; or (4) receiving steroid pulse therapy, newly added immunosuppressants, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) within 2 months before the first dose of sirolimus. LN was defined as clinical and laboratory manifestations that meet American College of Rheumatology (ACR) criteria (persistent proteinuria >0.5 g per day or greater than 3+ by dipstick, and/or cellular casts including red cell, hemoglobin, granular, tubular, or mixed), or renal biopsy demonstrating immune complex-mediated glomerulonephritis compatible with LN.10 This study was approved by the Medical Ethics Committee of PUMCH (approval number: S-478), and written informed consent was obtained from all recruited patients.
Assessments and follow-up
Baseline information included demographics, family history, clinical manifestations, laboratory results, and disease activity. Laboratory tests were performed at each visit and included complete blood counts, urinalysis, 24-h urine protein (24 hUPro) levels, liver, and kidney function tests, and immunologic indices. Disease activity was assessed by Physician Global Assessment (PGA) and SLEDAI-2K scores.
Enrolled patients received sirolimus at a starting dose of 1 mg/day, and was adjusted according to tolerance. Patients were followed up at our clinic 1 month, 3 months, and 6 months after initial of sirolimus treatment. After 6 months, patients visited at a frequency suggested by their physicians. The visit during which new immunosuppressants, steroid pulse therapy, or ACEIs/ARBs were added to a patient’s therapy was considered the last follow up.
Remissions of arthritis and skin rash were defined as disappearance of the corresponding items from the SLEDAI-2K scoring system since the previous visit. According to the immune thrombocytopenia (ITP) International Working Group Criteria,11 the remission of thrombocytopenia was classified as complete remission (CR), which was defined as a platelet count ⩾100 × 109/l, and partial remission (PR), which was defined as a platelet count ⩾30 × 109/l and at least a 2-fold increase from the baseline count. According to criteria from the Aspreva Lupus Management Study,12 CR of LN was defined as 24 hUPro < 0.5 g/24 h, normal urinary sediment, and serum creatine within 15% of the baseline value. PR was defined as a 50% reduction in 24hUPro, urine protein <3.5 g/24 h, and serum creatine within 25% of the baseline value.
The primary endpoint was SLEDAI-2K. Secondary endpoints included PGA, serological activity indices (including serum complement and titre of anti-dsDNA antibody), glucocorticoids dose that was needed for controlling disease, and remissions of organ involvement.
Statistical analysis
Continuous variables were analysed by a repeated two-tailed paired t-test when comparing the data obtained at each visit with those obtained at baseline. Categorical variables were analysed by chi-square test or Fisher’s exact test, where appropriate. Two-tailed p < 0.05 was considered statistically significant. Analysis was performed using SPSS 21.0.0.0 (SPSS, Chicago, IL, USA) and pictured in Graph Pad Prism version 8.0.2.
Part II: meta-analysis
Literature search
This meta-analysis was completed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive literature search was conducted in MEDLINE (OVID from 1946 to December 2019), Cochrane (from 1997 to December 2019), and EMBASE (from 1980 to December 2019). We chose “sirolimus”, “rapamycin” and “systemic lupus erythematosus” as MeSH terms or keywords.
Study selection and data collection
Two researchers completed the study selection separately. The types of articles were restricted to randomized controlled trials (RCTs), retrospective or prospective clinical trials, and case series. Animal studies, cell studies, and reviews were excluded. Articles discussing the application of sirolimus in SLE patients in English underwent a further full-text evaluation. We also considered the references to avoid leaving out potential articles. Articles with data concerning the efficacy of sirolimus treatment in SLE (including the SLEDAI, level of complement, titer of antibodies, and 24-h urine protein) were ultimately included in the meta-analysis. Abstracts were also acceptable if they met the previously mentioned criteria. Data extraction was conducted by one researcher and checked by another researcher. Discrepancies were discussed and arbitrated with a third independent reviewer.
Statistical and bias analyses
To assess article quality, different methods, including the Cochrane risk of bias assessment tool for RCTs and the Newcastle-Ottawa quality assessment scale for nonrandomized clinical trials, were applied. Stata/SE 15.0 was used for the meta-analysis. Considering the existence of heterogeneity, we adopted the random pooling model to generate forest plots. Sensitivity analysis was performed by sequentially omitting included studies. Begg’s test and Egger’s test were used to detect possible publication bias.
Results
Part I: real-world study
Patient characteristics
A total of 49 patients were included in our study between January 2018 and December 2019. Baseline characteristics are shown in Table 1. The average follow-up time was 6.1 ± 3.6 months. Four SLE phenotypes were included in our study: mucocutaneous involvement, arthritis, hematologic disorder, and LN. Among 17 patients diagnosed with LN, 7 experienced renal biopsy. Three patients were LN Class V, one patient Class IV, two patients Class IV+V, and one patient Class III+V. As for disease activity, mildly and moderately active patients were included, and all had at least one point of clinical scores of SLEDAI-2K (i.e., excluding items for hypocomplementemia and positive anti-dsDNA antibody). The patients either had an intolerance or a poor response to the previous immunosuppressants. A total of 24 (49.0%) patients were switched from other immunosuppressants to sirolimus, and among other 25 (51.0%) patients, sirolimus was added on without suspending previous immunosuppressants (see “Concomitant medications” in Table 1).
Table 1.
Baseline characteristics of the included SLE patients receiving sirolimus treatment.
| Mean ± SD or n (%) n = 49 |
|
|---|---|
| Age (years) | 36.3 ± 9.9 |
| Female | 46 (93.9) |
| Duration of SLE (years) | 7.5 ± 5.1 |
| Clinical manifestations | |
| Mucocutaneous | 12 (24.5) |
| Arthritis | 3 (6.1) |
| Lupus nephritis | 24 (48.9) |
| Hematologic disorder | 23 (46.9) |
| Thrombocytopenia | 13 (26.5) |
| Immunologic indices | |
| ANA | 49 (100) |
| Anti-dsDNA | 40 (81.6) |
| Anti-Sm | 18 (36.7) |
| Anti-RNP | 24 (49.0) |
| Anti-SSA | 34 (69.4) |
| Anti-SSB | 38 (77.6) |
| Anti-rRNP | 16 (32.7) |
| Antiphospholipid antibody | 15 (30.6) |
| Hypocomplementemia | 33 (67.3) |
| SLEDAI-2K | 6.6 ± 3.1 |
| 2–4 | 14 (28.6) |
| 5–9 | 25 (51.0) |
| 10–13 | 10 (20.4) |
| PGA | 0.74 ± 0.39 |
| Concomitant medications | 25 (51.0) |
| Prednisone | 39 (79.6) |
| Daily dose (mg) | 12.6 ± 9.3 |
| Hydroxychloroquine | 44 (89.8) |
| ACEI/ARB | 13 (26.5) |
| Add-on therapy | 25 (51.0) |
| No previous IS agentsa | 9 (18.4) |
| Methotrexate | 2 (4.1) |
| Azathioprine | 5 (10.2) |
| Tacrolimus | 3 (6.1) |
| Mycophenolate mofetil | 3 (6.1) |
| Cyclophosphamide | 3 (6.1) |
| Switch therapy | 24 (49.0) |
| Tacrolimus | 9 (18.3) |
| Mycophenolate mofetil | 7 (14.2) |
| Cyclophosphamide | 4 (8.2) |
| Leflunomide | 3 (6.1) |
| Cyclosporin A | 1 (2.0) |
These patients were on prednisone and hydroxychloroquine before sirolimus was added on.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IS, immunosuppressive; PGA, physician global assessment; SD, standard deviation; SLE, systemic lupus erythematosus; SLEDAI-2K, systemic lupus erythematosus disease activity index 2000.
Immunosuppressants regime of all patients had been stable for at least 3 months before screening. After enrollment, the dose of concomitant immunosuppressants were not changed, except that two patients experienced withdrawal of cyclophosphamide because of disease improvement. The average dosage of sirolimus was 1.1 ± 0.3 mg/day, and the corresponding trough concentration was 4.5 (0.6–12.1) ng/ml.
Efficacy of sirolimus
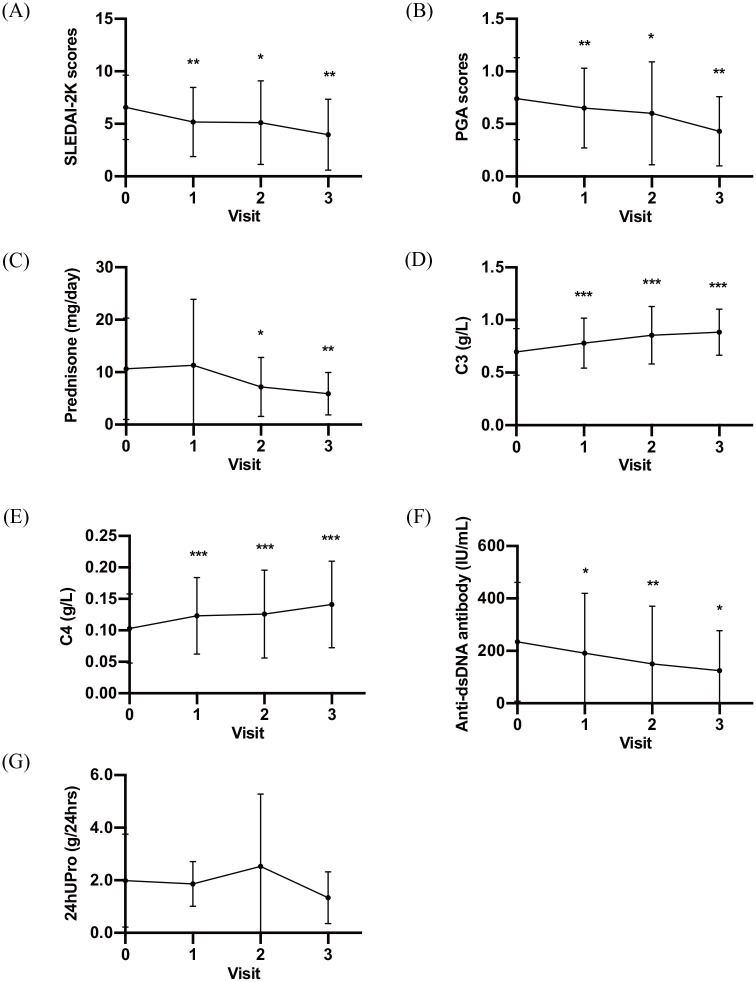
SLEDAI-2K scores decreased significantly over time (6.6 ± 3.1, 5.2 ± 3.3, 5.1 ± 4.0, and 4.0 ± 3.4 at baseline and at months 1, 3, and 6 after initial treatment, respectively; p = 0.001, 0.038, and 0.001 compared with baseline, respectively; Figure 1A), as did the PGA (0.74 ± 0.39, 0.65 ± 0.38, 0.60 ± 0.49, and 0.43 ± 0.33 at baseline and at months 1, 3, and 6 after initial treatment, respectively; p = 0.040, 0.041, and 0.002 compared with baseline, respectively; Figure 1B). Prednisone dosage, which was needed to control disease, tapered down smoothly (10.6 ± 9.7 mg/day, 11.3 ± 12.6 mg/day, 7.2 ± 5.6 mg/day, 5.9 ± 4.4 mg/day at baseline and at months 1, 3, and 6 after initial treatment, respectively; p = 0.929, 0.016, and 0.001 compared with baseline, respectively; Figure 1C).
Figure 1.
Efficacy of sirolimus in active SLE patients. Mean SLEDAI-2K score (A), PGA score (B), Daily dosage of prednisone that was required for controlling disease (C), Serum C3 (D), Serum C4 (E), Titer of anti-dsDNA antibody (F), and 24-hour urine protein (G), at baseline and at each visit (months 1, 3 and 6).
24hUPro, 24-h urine protein; C3, complement 3; C4, complement 4; PGA, physician global assessment; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
*p < 0.05; **p < 0.01; ***p < 0.001.
Sirolimus also showed an excellent effect in decreasing serological activity. Serum C3 (0.697 ± 0.222 g/l, 0.781 ± 0.238 g/l, 0.855 ± 0.274 g/l, and 0.884 ± 0.219 g/l at baseline and at months 1, 3, and 6 after initial treatment, respectively; p < 0.001, <0.001, and <0.001 compared with baseline, respectively; Figure 1D), as well as C4 (0.103 ± 0.055 g/l, 0.123 ± 0.061 g/l, 0.126 ± 0.070 g/l, and 0.141 ± 0.069 g/l at baseline and at months 1, 3, and 6 after initial treatment, respectively; p < 0.001, <0.001, and <0.001 compared with baseline, respectively; Figure 1E), increased significantly after treatment. Sirolimus returned low complement concentrations to normal in 60% of patients with low C3 levels (n = 25) and in 42.9% of patients with low C4 levels (n = 21) within 1 year. The anti-dsDNA antibody also decreased significantly over time (Figure 1F), and was converted to negative in 16.7% of patients with a positive anti-dsDNA antibody (n = 24).
In regard to organ remission, nine patients suffered from skin rash, and eight (88.8%) of them relieved rapidly within 4 months. All three patients who were complicated with arthritis relieved within 2 months; 13 patients had thrombocytopenia, 6 (46.2%) of whom achieved CR either by 2 months (4 patients) or by 12 months (2 patients).
Of 17 patients with urine protein ⩾0.5 g/24 h, 7 achieved renal remission (3 patients achieved CR, and 4 achieved PR) by 3 months (4 patients) or by 9 months (3 patients). However, there was no difference in 24-h urine protein before and after sirolimus treatment (Figure 1G).
Safety of sirolimus
Adverse events are summarized in Table 2. Drug discontinuation occurred in three patients due to allergic skin rash, ankle oedema and thrombocytopenia (n = 1 each). Dosage reduction was applied to two patients because of infection. Two patients developed mild infections (one Epstein-Barr virus infection and one varicella zoster virus infection), and one patient developed moderate infection (lung infection); all infections were relieved after treatment. Two patients developed leukopenia, one of whom was also prescribed mycophenolate mofetil and complicated with infection. Gastrointestinal symptoms, which occurred in one patient, were relieved very soon. One patient exhibited recurrent but mild mouth ulcers. Regarding renal function, five patients suffered from renal insufficiency [defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, which was calculated by CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation] at baseline, explaining why two patients switched to sirolimus from calcineurin inhibitors (CNIs). Among these individuals, renal function was recovered in four patients, and remained stable in the other one patient after treatment. The eGFR changed as follows: Patient_1, from 46 ml/min/1.73 m2 to 54 ml/min/1.73 m2; Patient_2, from 44 ml/min/1.73 m2 to 73 ml/min/1.73 m2; Patient_3, from 43 ml/min/1.73 m2 to 55 ml/min/1.73m2; Patient_4, from 43 ml/min/1.73m2 to 55 ml/min/1.73 m2; and Patient_5, from 54 ml/min/1.73 m2 to 52 ml/min/1.73 m2. Renal function in the remaining patients remained stable (62.1 ± 10.0 ml/min/1.73 m2 before sirolimus versus 60.1 ± 11.1 ml/min/1.73 m2 after sirolimus, p = 0.070).
Table 2.
Adverse events recorded in SLE patients who received sirolimus treatment.
| Adverse event | Incidence n (%)a |
|
|---|---|---|
| PUMCH n = 49 |
Literature review n = 127 |
|
| Infection | 3 (6.1) | 5 (3.9) |
| Leukopenia | 2 (4.1) | 4 (3.1) |
| Allergic symptoms | 2 (4.1) | 3 (2.4) |
| Thrombocytopenia | 1 (2.0) | 0 |
| Peripheral oedema | 1 (2.0) | 2 (1.6) |
| Gastrointestinal symptoms | 1 (2.0) | 9 (7.1) |
| Mucosal ulcer | 1 (2.0) | 1 (0.8) |
| Dyslipidaemia | NA | 10 (7.9) |
| Anaemia | 0 | 4 (3.1) |
| Elevated liver enzymes | 0 | 1 (0.8) |
| Malignant tumour | 0 | 3 (2.4) |
| Headache | 0 | 2 (1.6) |
Data from PUMCH and Literature review were compared by chi-square test or Fisher’s exact test, where appropriate, and the p values for each adverse event were all found to be >0.1.
NA, not applicable; PUMCH, Peking Union Medical College Hospital.
Part II: meta-analysis
Study characteristics
Five studies were included in the meta-analysis. A flowchart of article screening is illustrated in Figure 2. The quality assessment is shown in Supplemental Table S1. The characteristics of the five studies are summarized in Table 3. A total of 149 patients were involved in these studies, which were carried out in the United States and China. All of these studies had a follow-up time of at least 6 months. Yap et al. focused on LN,6 while the other four studies focused on SLE.
Figure 2.
Flowchart of studies included in the meta-analysis.
Table 3.
Characteristics of studies included in the meta-analysis.
| Study ID | Nation | Study type | Time span | No. of patients | Age (years) | Patients | Sirolimus dose (mg/day) | Follow-up time (months) |
|---|---|---|---|---|---|---|---|---|
| Fernandez et al. 5 | US | Prospective | NR | 9 | 37.4 ± 12.3 | SLE | 2 | 19.67 ± 15.08 |
| Yap et al. 6 | China | Retrospective | 2007–2016 | 16 | NA | LN | 2 or 1 | 36 |
| Lai et al. 8 | US | Prospective | 2009–2014 | 40 | 45.4 ± 14.3 | SLE | 2 | 12 |
| Chu et al. 13 | China | Prospective | 2016–2018 | 35 | 33.8 ± 10.1 | SLE | 0.25 | 6 |
| PUMCH | China | Prospective | 2018–2019 | 49 | 36.3 ± 9.9 | SLE | 1.1 ± 0.3 | 6.1 ± 3.6 |
Data are presented as mean ± SD, unless otherwise stated.
LN, lupus nephritis; NA, not applicable; NR, not reported; PUMCH, Peking Union Medical College Hospital; SD, standard deviation; SLE, systemic lupus erythematosus; US, United States.
Effectiveness of sirolimus
The pooled change in SLEDAI before and after sirolimus treatment was −3.5 (−5.0, −2.1) (p < 0.001); that in prednisone dose was −12.7 (−19.9, −5.6) mg/day (p < 0.001); and that in C3 was 0.224 (0.136, 0.311) g/l (p < 0.001) (Figure 3A–C).
Figure 3.
Forest diagrams of the meta-analysis assessing the efficacy of sirolimus on SLE patients. Pooled change in SLEDAI-2K scores (A), daily dose of prednisone required for controlling disease (B), and serum C3 (C).
C3, complement 3; PUMCH, Peking Union Medical College Hospital; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Sensitivity analysis suggested that the level of C3 was stable, and the deletion of a single study did not change the heterogeneity, while “Lai 2018”had an influence on heterogeneity for SLEDAI (I2 = 59% versus I2 = 43%), and the requirement of prednisone (I2 = 90% versus I2 = 82%).
Funnel plots did not reveal any obvious asymmetry when estimating the publication bias of the meta-analysis (Supplemental Figure S1). Furthermore, Begger’s and Egger’s tests confirmed that there was no publication bias (p > 0.05).
Safety of sirolimus
A literature review was also performed to thoroughly investigate the adverse events of sirolimus in SLE (Table 2). We included five studies by Lai et al.,8 Eriksson et al.,9 Fernandez et al.,5 Yap et al.,6 and Chu et al.13 with a total of 127 patients (Table 2). No severe adverse events were reported in these studies.
Discussion
This is the first meta-analysis exploring the efficacy and safety of sirolimus in SLE patients. Moreover, we added data from our prospective real-world study, which is the largest to date, to further support the results from previous studies. In this article, we proved that sirolimus was effective in decreasing the disease activity of SLE, especially in the recovery of hypocomplementemia. It was also helpful in the dosage tapering of prednisone and in treating to remission of arthritis, skin rash and thrombocytopenia. However, the role of sirolimus in LN requires further clinical evidence.
mTOR is a protein kinase that connects cellular metabolic processes with a wide range of environmental signals, such as proinflammatory cytokines, nutrients, and growth factors. The abnormal activation of mTOR drives the proinflammatory expansion of T helper (Th) type 1,14 Th17,15,16 and CD4–CD8– (double-negative, DN) T cells17 and promotes B cell proliferation/survival18 as well as the expansion of T-bet+CD11c+ atypical memory B cells,19 but inhibits the development of CD4+CD25+FoxP3+ T regulatory (Treg) cells,20 attributed to the pathogenesis of SLE and other autoimmune diseases.21–26 In fact, numerous studies indeed have shown that mTOR activation precedes the onset and flare of SLE.17,27–29 Rapamycin, the most commonly used inhibitor of mTOR, was first used to prevent organ transplantation rejection, but, in recent years, researchers have become aware of its great potency in blocking the development of SLE in lupus-like murine models.30–32 Clinical studies gave further credence to the effect and safety of rapamycin in SLE, as stated before. Confirmed by our meta-analysis, they all showed a significant reduction in disease activity, including serological activity, and the dosage tapering of glucocorticoids.
Our real-world study showed a significant decrease of SLEDAI-2K, further supporting the results of previous studies.8,9 Furthermore, improved serological activity, especially in C3 concentration, was prominent in our study after sirolimus treatment and even better than that reported in belimumab studies. In a belimumab trial, a 2.74–5.59% increase in the C3 concentration from baseline at week 52 was observed, and the low C3 level returned to normal in 23–34% of patients.1 As a comparison, our study illustrated an increase in C3 concentration by 28.1%, and the normalization of low C3 levels occurred in 60% of patients. The improvement in C4 and the anti-dsDNA antibody was similar between two studies (data not shown). Although it is difficult to compare a real-world study with a randomized clinical trial, these results are still encouraging and hold promise for sirolimus as an alternative treatment option for SLE.
Regarding the improvement in organ manifestations, the largest two studies by Lai et al.8 and Eriksson et al.9 focused on the mucocutaneous and musculoskeletal systems. Significant reductions in the PGA or BILAG scores in the mucocutaneous and musculoskeletal systems were observed in their studies. Similarly, the high remission proportions of skin rash (88.9%) and arthritis (100%) in our study further supports this conclusion.
Evidence for sirolimus in SLE-related thrombocytopenia in previous studies was limited. Only one clinical trial involved two SLE patients complicated by Evans syndrome, and both achieved complete response by 3 months and 12 months.33 However, many studies have demonstrated its efficacy and safety in ITP. In a randomized clinical trial, the response rate of ITP patients treated with sirolimus plus prednisone was similar to those treated with cyclosporine plus prednisone (58% versus 62%, p = 0.70).34 Even when used alone, sirolimus also achieved a high response rate in ITP patients.35,36 Similar to SLE, ITP is also characterized by an impairment in Treg cells, which can suppress self-reactive lymphocytes against platelets and the production of autoantibodies,37,38 and an increase in proinflammatory Th17 cells.38 Hence, we assumed that sirolimus could also aid in SLE-related thrombocytopenia. Our real-world study was the first to show a preliminary CR rate to sirolimus of 46.1% in SLE-related thrombocytopenia patients. Two-thirds of patients achieved quick remission within 2 months and maintained a stable platelet count during the subsequent visits.
Regarding LN, only one study by Yap et al. involved 16 patients with biopsy-proven LN, five of whom were active and 11 of whom were quiescent. Two active LN patients discontinued sirolimus because of adverse events, and the remaining three patients achieved CR. All 11 patients with quiescent disease remained stable without renal flare.6 Earlier evidence from lupus-prone mice also supported the role of sirolimus in LN.39 However, in our study, the role of sirolimus in proteinuria seemed illusive, and additional evidence is needed. Of the patients in our study, 7 achieved remission of proteinuria, whereas 10 patients experienced worsening of proteinuria. Since 1 mg/day sirolimus was used in our study, which was lower than 2 mg/day sirolimus used in the study by Yap et al., we believe that 1 mg/day sirolimus might be inadequate for the treatment of LN. On the other hand, mTOR inhibitors are indeed associated with a high incidence of new-onset proteinuria or worsening of pre-existing proteinuria in transplant recipients,40–42 but these effects might be dose-dependent. For example, everolimus, one of the most commonly used sirolimus analogues, showed a dose-dependent impact on proteinuria. Wiseman et al. found that 3.0 mg/day rather than 1.5 mg/day everolimus was correlated with proteinuria.42 Likewise, high sirolimus levels were found to induce de novo focal segmental glomerulosclerosis43 and were associated with reduced expression of key podocyte proteins.44 However, at a relatively lower dose (considerably lower than that required for the prevention of transplant rejection), sirolimus can markedly activate autophagy45 and protect proximal tubular cells against damage associated with proteinuria,46 thus exerting its beneficial effects in proteinuric nephropathy. In conclusion, the use of low doses (no more than 2 mg/day) of rapamycin might be related not to a high risk of proteinuria but to additional tubule-interstitial protection. However, based on our experience, a very low dose (e.g. 1 mg/day) may not be enough to control LN. Hence, more studies are required to prove our assumption and to determine a suitable dosage for the best application of sirolimus in LN. Regardless, to our delight, studies have also revealed that even if an mTOR inhibitor induces proteinuria, withdrawal of the inhibitor can reduce the urine protein level to near that before treatment.47 Thus, urine protein should be intensely monitored when sirolimus is administered. Moreover, considering the number of patients in different LN subgroups was limited in our study, it was hard for us to investigate the effect of sirolimus on different histopathological LN subtypes. Further studies are warranted in this respect.
All adverse events in our study and previous studies seemed tolerable and manageable. The most common adverse event was dyslipidaemia, which was reported to be easily controlled by statins. Infections, especially severe infections, were not common when using sirolimus. Our study indicated eGFR recovery after switching from CNIs to sirolimus, highlighting the advantage of sirolimus (i.e., lack of nephrotoxicity) from its long-term usage over CNIs. According to the literature review, one patient developed malignancy after being exposed to sirolimus for 31 months,9 which, however, might not be attributed to sirolimus. Sirolimus was actually reported to be associated with a lower incidence and lower recurrence of malignancy compared with other immunosuppressive regimens in kidney transplant recipients.48–50 This might give it an advantage over other immunosuppressive agents in SLE patients with previous cancer. Clinicians should also pay attention to possible drug allergies, although they rarely occur.
One of the limitations of this study was that we did not routinely monitor the lipid profiles of our patients. In addition, this was only a pilot study, with no comparison or control group. Further randomized clinical trials should be designed.
Conclusion
In summary, sirolimus was effective and tolerable in active SLE patients, either as a monotherapy or in combination with other immunosuppressive agents. It is promising in improving skin rash, arthritis, and thrombocytopenia, but its role in LN needs further investigation. The nonnephrotoxic property of sirolimus may make it a safe and unique option for immunosuppressive therapy in SLE. Further high-quality studies are required to further demonstrate its role in SLE and explore the optimal dose.
Supplemental Material
Supplemental material, Supplementary_Materials for Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: a real-world study and meta-analysis by Liying Peng, Chanyuan Wu, Ruping Hong, Yiduo Sun, Junyan Qian, Jiuliang Zhao, Qian Wang, Xinping Tian, Yanhong Wang, Mengtao Li and Xiaofeng Zeng in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to present our gratitude to the patients, nurses, and staff of the Rheumatology Department of Peking Union Medical College Hospital who participated in this study.
Footnotes
Author Contributions: Liying Peng, Chanyuan Wu, Mengtao Li and Xiaofeng Zeng contributed to the conception and design of the study; Liying Peng and Ruping Hong collected data from the CSTAR data collection system and patient medical charts; Mengtao Li, Chanyuan Wu, Xinping Tian, Jiuliang Zhao and Qian Wang contributed to patient recruitment; Liying Peng and Ruping Hong performed the literature review, literature screen, data collection, data analysis and the meta-analysis; Yanhong Wang helped maintain the CSTAR system and performed statistical analysis; Liying Peng, Ruping Hong, Chanyuan Wu and Mengtao Li were involved in the analysis and interpretation of the data; Liying Peng drafted the manuscript; Chanyuan Wu, Mengtao Li and Xiaofeng Zeng supervised the study and revised the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chinese National Key Research and Development Program [grant numbers 2017YFC0907600, 2008BAI59B02] and the Chinese National High Technology Research and Development Program, Ministry of Science and Technology [grant number 2012AA02A513].
ORCID iDs: Liying Peng  https://orcid.org/0000-0001-8447-8121
https://orcid.org/0000-0001-8447-8121
Chanyuan Wu  https://orcid.org/0000-0003-2710-9359
https://orcid.org/0000-0003-2710-9359
Mengtao Li  https://orcid.org/0000-0003-4252-2889
https://orcid.org/0000-0003-4252-2889
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Liying Peng, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Chanyuan Wu, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Ruping Hong, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Yiduo Sun, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Junyan Qian, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Jiuliang Zhao, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Qian Wang, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Xinping Tian, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Yanhong Wang, Department of Epidemiology and Bio-statistics (YW), Institute of Basic Medical Science, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China.
Mengtao Li, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, No.1 Shuai fu yuan, East City, Beijing 100730, China.
Xiaofeng Zeng, Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, No. 1 Shuaifuyuan, Beijing 100730, China.
References
- 1. Zhang F, Bae SC, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis 2018; 77: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 3. He J, Ma J, Ren B, et al. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin Arthritis Rheum. Epub ahead of print 11 November 2019. DOI: 10.1016/j.semarthrit.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 4. Oaks Z, Winans T, Huang N, et al. Activation of the mechanistic target of rapamycin in SLE: explosion of evidence in the last five years. Curr Rheumatol Rep 2016; 18: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez D, Bonilla E, Mirza N, et al. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum 2006; 54: 2983–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yap DYH, Tang C, Chan GCW, et al. Longterm data on sirolimus treatment in patients with lupus nephritis. J Rheumatol 2018; 45: 1663–1670. [DOI] [PubMed] [Google Scholar]
- 7. Yap DY, Ma MK, Tang CS, et al. Proliferation signal inhibitors in the treatment of lupus nephritis: preliminary experience. Nephrology (Carlton) 2012; 17: 676–680. [DOI] [PubMed] [Google Scholar]
- 8. Lai ZW, Kelly R, Winans T, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 2018; 391: 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eriksson P, Wallin P, Sjowall C. Clinical experience of sirolimus regarding efficacy and safety in systemic lupus erythematosus. Front Pharmacol 2019; 10: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hahn BH, McMahon MA, Wilkinson A, et al. American college of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009; 113: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 12. Sinclair A, Appel G, Dooley MA, et al. Mycophenolate mofetil as induction and maintenance therapy for lupus nephritis: rationale and protocol for the randomized, controlled aspreva lupus management study (ALMS). Lupus 2007; 16: 972–980. [DOI] [PubMed] [Google Scholar]
- 13. Chu Y, Zhao C, Zhang B, et al. Restoring T-helper 17 cell/regulatory T-cell balance and decreasing disease activity by rapamycin and all-trans retinoic acid in patients with systemic lupus erythematosus. Lupus 2019; 28: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 14. Ray JP, Staron MM, Shyer JA, et al. The Interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 2015; 43: 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4–CD8– double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol 2014; 192: 4134–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koga T, Hedrich CM, Mizui M, et al. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. J Clin Invest 2014; 124: 2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai ZW, Borsuk R, Shadakshari A, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol 2013; 191: 2236–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng Q, Qin S, Zhang H, et al. Rapamycin attenuates BAFF-extended proliferation and survival via disruption of mTORC1/2 signaling in normal and neoplastic B-lymphoid cells. J Cell Physiol 2018; 233: 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu C, Fu Q, Guo Q, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis 2019; 78: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kato H, Perl A. Blockade of treg cell differentiation and function by the interleukin-21-mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol 2018; 70: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crispin JC, Kyttaris VC, Terhorst C, et al. T cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010; 6: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Bavel CC, Dieker JW, Kroeze Y, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis 2011; 70: 201–207. [DOI] [PubMed] [Google Scholar]
- 23. Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 2005; 175: 8392–8400. [DOI] [PubMed] [Google Scholar]
- 24. Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2006; 27: 110–118. [DOI] [PubMed] [Google Scholar]
- 25. Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol 2020; 16: 100–112. [DOI] [PubMed] [Google Scholar]
- 26. Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol 2016; 12: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oaks Z, Winans T, Caza T, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol 2016; 68: 2728–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsokos GC. Systemic lupus erythematosus in 2015: cellular and metabolic requirements of effector T cells. Nat Rev Rheumatol 2016; 12: 74–76. [DOI] [PubMed] [Google Scholar]
- 29. Stylianou K, Petrakis I, Mavroeidi V, et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant 2011; 26: 498–508. [DOI] [PubMed] [Google Scholar]
- 30. Lui SL, Tsang R, Chan KW, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol Dial Transplant 2008; 23: 2768–2776. [DOI] [PubMed] [Google Scholar]
- 31. Ramos-Barron A, Pinera-Haces C, Gomez-Alamillo C, et al. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus 2007; 16: 775–781. [DOI] [PubMed] [Google Scholar]
- 32. Caza TN, Fernandez DR, Talaber G, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis 2014; 73: 1888–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood 2016; 127: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Wang Z, Dai L, et al. Effects of rapamycin combined with low dose prednisone in patients with chronic immune thrombocytopenia. Clin Dev Immunol 2013; 2013: 548085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jasinski S, Weinblatt ME, Glasser CL. Sirolimus as an effective agent in the treatment of immune thrombocytopenia (ITP) and evans syndrome (ES): a single institution’s experience. J Pediatr Hematol Oncol 2017; 39: 420–424. [DOI] [PubMed] [Google Scholar]
- 36. Miano M, Rotulo GA, Palmisani E, et al. Sirolimus as a rescue therapy in children with immune thrombocytopenia refractory to mycophenolate mofetil. Am J Hematol 2018; 93: E175–E177. [DOI] [PubMed] [Google Scholar]
- 37. Teke HU, Gunduz E, Akay OM, et al. Abnormality of regulatory T-cells in remission and non-remission idiopathic thrombocytopaenic purpura patients. Platelets 2013; 24: 625–631. [DOI] [PubMed] [Google Scholar]
- 38. Ji L, Zhan Y, Hua F, et al. The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS One 2012; 7: e50909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum 1994; 37: 289–297. [DOI] [PubMed] [Google Scholar]
- 40. Aliabadi AZ, Pohanka E, Seebacher G, et al. Development of proteinuria after switch to sirolimus-based immunosuppression in long-term cardiac transplant patients. Am J Transplant 2008; 8: 854–861. [DOI] [PubMed] [Google Scholar]
- 41. Asrani SK, Wiesner RH, Trotter JF, et al. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: the 2000-2003 phase II prospective randomized trial. Am J Transplant 2014; 14: 356–366. [DOI] [PubMed] [Google Scholar]
- 42. Wiseman AC, McCague K, Kim Y, et al. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. Am J Transplant 2013; 13: 442–449. [DOI] [PubMed] [Google Scholar]
- 43. Letavernier E, Bruneval P, Mandet C, et al. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2007; 2: 326–333. [DOI] [PubMed] [Google Scholar]
- 44. Stallone G, Infante B, Pontrelli P, et al. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation 2011; 91: 997–1004. [DOI] [PubMed] [Google Scholar]
- 45. Qi YY, Zhou XJ, Cheng FJ, et al. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann Rheum Dis 2018; 77: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vizza D, Perri A, Toteda G, et al. Rapamycin-induced autophagy protects proximal tubular renal cells against proteinuric damage through the transcriptional activation of the nerve growth factor receptor NGFR. Autophagy 2018; 14: 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wadei HM, Zaky ZS, Keaveny AP, et al. Proteinuria following sirolimus conversion is associated with deterioration of kidney function in liver transplant recipients. Transplantation 2012; 93: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 48. Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol 2006; 17: 581–589. [DOI] [PubMed] [Google Scholar]
- 49. Branco F, Cavadas V, Osorio L, et al. The incidence of cancer and potential role of sirolimus immunosuppression conversion on mortality among a single-center renal transplantation cohort of 1,816 patients. Transplant Proc 2011; 43: 137–141. [DOI] [PubMed] [Google Scholar]
- 50. Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014; 349: g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials for Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: a real-world study and meta-analysis by Liying Peng, Chanyuan Wu, Ruping Hong, Yiduo Sun, Junyan Qian, Jiuliang Zhao, Qian Wang, Xinping Tian, Yanhong Wang, Mengtao Li and Xiaofeng Zeng in Therapeutic Advances in Musculoskeletal Disease