Abstract
Background
Treatment of MS often begins with low-efficacy injectable disease-modifying therapy (iDMT).
Objectives
To compare the effect of fingolimod 0.5 mg/day on clinical, MRI, patient-reported, and safety outcomes, in treatment-naïve and previously treated (≥1 iDMT) patients with early MS.
Methods
EARLIMS was a multicentre, open-label, non-randomized, parallel-group phase 3 b/4 study in Australia and Spain. Patients with relapsing–remitting MS, Expanded Disability Status Scale (EDSS) score <4.0, and ≥1–5 years since diagnosis, received daily fingolimod for 48 weeks. The primary endpoint was annualized relapse rate (ARR).
Results
Of 347 patients enrolled at 51 sites (treatment-naïve, 200 [57.6%]; previously treated, 147 [42.4%]), 320 completed the study (treatment-naïve, 184 [92.0%]; previously treated, 136 [92.5%]), but the study remained underpowered (planned enrolment, n = 432). Fingolimod reduced ARR to similar levels in both treatment-naïve (mean ARR [95% confidence interval], 0.21 [0.14, 0.29]) and previously treated groups (0.30 [0.20, 0.41]; p = 0.1668). There were no new safety signals.
Conclusions
Fingolimod appeared equally effective as first- or second-line therapy in relapsing MS. There was a trend for better outcomes with fingolimod in treatment-naïve patients than in those previously treated with >1 iDMT.
Keywords: Beta-interferon, clinical trial, disease-modifying therapies, glatiramer acetate, outcome measurement, relapsing/remitting
Introduction
Early treatment in relapsing multiple sclerosis (MS) is associated with improved long-term outcomes.1,2 Typically, patients receive injectable disease-modifying therapy (iDMT; glatiramer acetate; interferon beta-1a or -1b) or oral agents first line, then switch to another first-line therapy or escalate to high-efficacy therapy if disease breakthrough occurs.3 However, there is an argument that treatment should be initiated with high-efficacy therapy, if justified based on assessment of benefit and risk.4,5 A consideration is that acceleration of brain tissue loss early in MS6 correlates with long-term accrual of disability.7High-efficacy immunomodulatory therapy slows accumulation of disability in moderately advanced and advanced relapsing MS,8 therefore, early treatment with high-efficacy therapies could be strategically preferable.
Fingolimod is a high-efficacy oral sphingosine 1-phosphate receptor modulator,9 indicated second line in the EU in adults with relapsing forms of MS or first line in patients with rapidly evolving severe relapsing MS.10 In several countries, including the USA and Australia, fingolimod is approved for first-line use.11 Fingolimod demonstrated better outcomes than placebo or intramuscular interferon beta-1a in the pivotal FREEDOMS12 and TRANSFORMS13 trials, respectively. Post hoc analyses of these trials showed that treatment-naïve patients receiving fingolimod derived greater benefit than those previously treated with an iDMT,14 including patients with highly active disease.15,16 An observational study also showed benefits among those receiving first-line fingolimod compared with those previously on an iDMT.17 Here, we report findings from EARLIMS, a 48-week phase 3 b/4 study of patients with early-stage relapsing MS in Australia and Spain that examined whether outcomes with first-line fingolimod were superior to those with fingolimod administered second line after iDMT treatment.
Methods
Study oversight
EARLIMS (ClinicalTrials.gov: NCT01498887; European Union Clinical Trials Register: 2011-003484-30) complied with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practices guidelines.18,19 The study protocol and all amendments were reviewed by an Independent Ethics Committee or Institutional Review Board representing each study site. Written informed consent was obtained from each patient before any evaluations were undertaken. Amendments to the study protocol are listed in the Appendix.
Study design
EARLIMS was a multicentre, open-label, non-randomized, parallel-group study in Australia and Spain in patients diagnosed with relapsing–remitting MS.20,21 Following a 1-month screening period, all eligible patients were assigned to receive fingolimod 0.5 mg/day for 48 weeks (336 days). Within this group, outcomes were compared between patients who were treatment naïve and those previously treated with a first-line iDMT (glatiramer acetate, interferon beta-1a or -1b). Scheduled visits were at screening (day −30 to −1), day 0, then (±15 days) at weeks 12, 24 and 48. On day 0, patients initiated fingolimod treatment and were observed for at least 6 hours to ensure management of any cardiac side effects. As an open-label study, randomization and blinding were not applicable. In Australia, study medication was dispensed according to standard clinical practice. In Spain, study drug was dispensed at the day-0, week-12 and week-24 visits in packs bearing the study code, the investigator’s name, and the centre, patient and visit numbers. Patients were asked to return all unused study drug at the week-48 visit for assessment of adherence. Dose adjustment was not permitted. Treatment interruption was only permitted for safety reasons. Patients who withdrew from the study were treated according to local best clinical practice.
Participants
At screening, eligible patients were 18–50 years of age, had been diagnosed with relapsing–remitting MS for at least 1 year and for no more than 5 years, were treatment naïve or had been treated continuously with a first-line iDMT for at least 1 year, had at least nine brain lesions on T2-weighted magnetic resonance imaging (MRI), and an Expanded Disability Status Scale (EDSS)22 score less than 4.0 (see Appendix for all eligibility criteria). An additional requirement in Spain was at least two relapses in the preceding 2 years and, in Australia, patients were required to be eligible for the Pharmaceutical Benefits Scheme, under which the Australian Government subsidizes the cost of certain drugs. Key exclusion criteria were a history of chronic immune disease, malignant disease (except localized basal-cell carcinoma of the skin), diabetes mellitus, severe hepatic impairment, and prior treatment with fingolimod. Patients with cardiac risk factors, suspected macular oedema, active infection, or who tested seronegative for varicella-zoster, rubella and measles at screening, and women of child-bearing age who did not wish to use effective contraception, were also excluded.
Outcome measures and assessments
The primary endpoint was annualized relapse rate (ARR; the number of relapses in 12 months), comparing patients who were treatment-naïve with patients previously treated with first-line iDMTs. Relapse information was recorded at each visit, and confirmed by a neurologist if accompanied by an increase in EDSS score of at least 0.5 points, or by a 1.0-point increase in two functional systems, or a 2.0-point increase in one functional system, unless sphincter- or cognition-related.
Secondary endpoints were: time to first relapse from day 0; severity of relapse (‘mild’ if neither steroid treatment nor hospitalization were needed, otherwise ‘moderate–severe’); percentage of relapse-free patients at week 48; percentage brain volume change (PBVC) at week 48; number of active (new or enlarged) T2 lesions on MRI at week 48; disability worsening at each visit by change in EDSS score (range, 0 to 10; higher scores indicate greater disability); treatment satisfaction at week 48, using the patient-reported outcome indices for multiple sclerosis (PRIMUS) scale;23 and safety and tolerability by assessment of adverse events (AEs) and serious AEs (SAEs), vital signs, and laboratory parameters at each visit, by ophthalmological examination (screening and week 12) and electrocardiogram (screening and day 0).
Total PBVC on study was measured using T1-weighted MRI and Structural Image Evaluation using Normalization of Atrophy.24 All MRI analyses were undertaken at the Sydney Neuroimaging Analysis Centre. Patients rated satisfaction on the three PRIMUS subscales: symptoms (22 items; score 0–22); activity limitation (15 items; score, 0–30); and quality-of-life (QoL; 22 items; score 0–22). An increase in score indicates worsening in that domain; PRIMUS has been validated in Spanish.25 Clinical disease activity (relapses and disability progression) and overall disease activity (clinical disease activity, active T2 lesions and PBVC ≥0%) were also assessed.
Statistical analyses
The safety population consisted of all patients who received at least one dose of fingolimod. The intention-to-treat (ITT) population consisted of all patients in the safety population with information relating to the primary endpoint from at least one study visit. The per protocol (PP) population consisted of all patients in the ITT population with no major protocol deviations. Study power was calculated based on ARR outcomes in the TRANSFORMS13 and FREEDOMS trials of fingolimod.12 Assuming an ARR of 0.11 in the treatment-naïve group and of 0.25 in the previously treated group, 216 patients in each group would yield 90% statistical power to demonstrate the superiority of fingolimod at a significance level of 5%, with an estimated loss to follow-up of 5%.
The primary endpoint was analysed in the ITT population and a sensitivity analysis was conducted in the PP population to examine the effect of protocol violations and early withdrawal from the study; all secondary endpoints were analysed in the ITT population. The primary endpoint was the difference in ARR between the treatment-naïve and previously treated groups.
Analyses were also conducted in the subgroup of patients previously treated with one iDMT (single-iDMT), in the subgroup previously treated with more than one iDMT (multi-iDMT), and in the total population. The Mann–Whitney–Wilcoxon test was used for comparisons between two groups and the Kruskal–Wallis test for comparisons among more than two groups. All statistical tests were two-sided at a significance level of 0.05. Time to first relapse was calculated using the Kaplan–Meier method, between-group comparisons of the proportions of relapse-free patients used the χ2 or Fisher exact test as appropriate. Between-group comparisons of PBVC used analysis of covariance adjusted for total cerebral volume at screening, and comparisons of change in EDSS score used analysis of covariance adjusted for EDSS score on day 0.
For analyses of active T2 lesions, PBVC, EDSS and PRIMUS, patients were only included if they had non-missing values both at baseline (screening or day 0) and at week 48; no missing values were imputed. Patient disposition, demographics, baseline characteristics and safety assessments are reported descriptively; AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 19.0) and were summarized for the first 24 hours, and after the first 24 hours.
Results
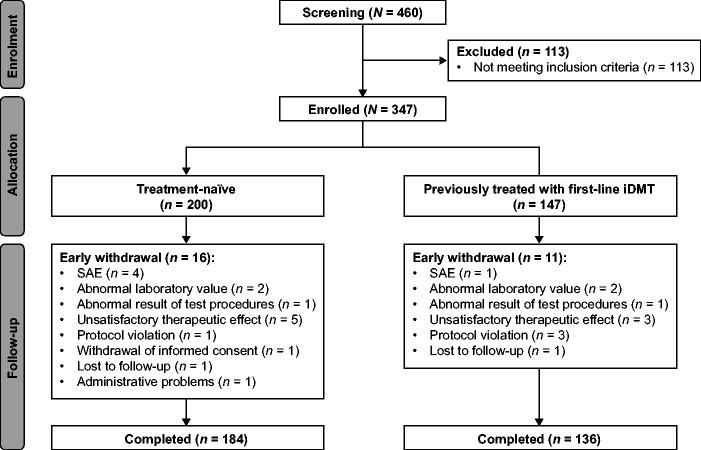
EARLIMS was conducted between 21 December 2011 and 23 December 2015 at 34 hospitals in Spain and 17 in Australia. In total, 460 patients were screened and 347 were enrolled in the safety population (treatment naïve, 200 [57.6%]; previously treated, 147 [42.4%]); 320 (92.2%) completed the study (treatment naïve, 184 [92.0%]; previously treated, 136 [92.5%]). Fewer patients than planned participated in the study owing to difficulties in recruitment driven primarily by the timing of fingolimod’s second-line approval in Europe (see Appendix for further information).
Patient disposition is summarized in Figure 1. The most frequent reasons for withdrawing early from the study were: unsatisfactory therapeutic effect (8 [2.3%]), SAE (5 [1.4%]), abnormal laboratory values (4 [1.2%]) and protocol violations (4 [1.2%]). Among 320 patients in the ITT population, 70 (21.9%) were excluded, mostly for non-compliance with treatment (39 [12.2%]), low lymphocyte count (15 [4.7%]) or protocol violations (6 [1.9%]), resulting in a PP population of 250 patients. In the two patient groups, mean treatment compliance on study was approximately 98% and mean exposure to study drug was similar (treatment-naïve, 335 days; previously treated, 332 days).
Figure 1.
Patient disposition in EARLIMS.
iDMT: injectable disease-modifying therapy; SAE: serious adverse event.
Patient characteristics at baseline were generally similar in the treatment-naïve and previously treated groups (Table 1). There were differences in disease history: the average times since onset of MS symptoms, since diagnosis of MS, and since first relapse were shorter among treatment-naïve patients than among those previously treated (p < 0.0001, all). On average, the number of relapses in the 2 years before enrolment was slightly lower (p = 0.0749), but ARR was higher in the same period (p < 0.0001) and in the year before enrolment (p = 0.0074) in the treatment-naïve than in the previously treated group. Proportionally more treatment-naïve than previously treated patients had experienced severe relapses (p = 0.0318) or a relapse requiring hospitalization (p = 0.0183). Comparison of 95% confidence intervals (CI) suggests that treatment-naïve patients had a lower mean EDSS score at baseline than those previously treated (Table 1).
Table 1.
Baseline demographic and disease characteristics (ITT population).
| Characteristic | Treatment-naïve patients (n = 185) | Previously treated patients (n = 135) | Total (n = 320) | p-valuea |
|---|---|---|---|---|
| Age, years | 33.1 (32.0, 34.3) | 34.0 (32.7, 35.3) | 33.5 (32.6, 34.4) | 0.3023 |
| Women, n (%) | 137 (74.1) | 88 (65.2) | 225 (70.3) | 0.0864b |
| Education, n (%) | 0.0323b | |||
| Primary | 21 (11.4) | 19 (14.1) | 40 (12.5) | |
| Secondary | 46 (24.9) | 53 (39.3) | 99 (30.9) | |
| Higher | 91 (49.2) | 53 (39.3) | 144 (45.0) | |
| Time since first symptoms of MS, years | 2.4 (2.2, 2.5) (n = 178) | 3.2 (3.0, 3.4) (n = 128) | 2.7 (2.6, 2.9) (n = 306) | <0.0001 |
| Time since diagnosis of MS, years | 0.6 (0.5, 0.7) (n = 182) | 2.5 (2.3, 2.7) (n = 135) | 1.4 (1.3, 1.6) (n = 317) | <0.0001 |
| Time since first relapse, years | 2.0 (1.8, 2.2) (n = 184) | 2.9 (2.7, 3.2) (n = 135) | 2.4 (2.3, 2.6) (n = 319) | <0.0001 |
| Relapses in the 2 years before screening, n | 2.6 (2.5, 2.7) (n = 183) | 3.0 (2.7, 3.2) (n = 133) | 2.8 (2.6, 2.9) (n = 316) | 0.0749 |
| ARR in the 2 years before screening | 2.3 (1.9, 2.8) (n = 184) | 1.4 (1.3, 1.5) (n = 134) | 1.9 (1.6, 2.2) (n = 318) | <0.0001 |
| ARR in the year before screening | 2.1 (1.8, 2.4) (n = 184) | 1.6 (1.4, 1.7) (n = 134) | 1.9 (1.7, 2.1) (n = 318) | 0.0074 |
| Patients experiencing relapses requiring hospitalization, n (%) | 0.0183c | |||
| 0 relapses | 123 (66.5) | 108 (80.0) | 231 (72.2) | |
| 1 relapse | 56 (30.3) | 23 (17.0) | 79 (24.7) | |
| 2 relapses | 4 (2.2) | 2 (1.5) | 6 (1.9) | |
| Maximum severity of relapses before enrolment, n (%) | 0.0318c | |||
| Mild | 67 (36.2) | 34 (25.2) | 101 (31.6) | |
| Moderate | 102 (55.1) | 93 (68.9) | 195 (60.9) | |
| Severe | 13 (7.0) | 5 (3.7) | 18 (5.6) | |
| EDSS score | 1.6 (1.4, 1.7) (n = 179) | 2.0 (1.9, 2.2) (n = 130) | 1.8 (1.7, 1.9) (n = 309) | NR |
| Normalized brain volume, mL | 1548 (1532, 1565) (n = 92) | 1532 (1508, 1557) (n = 43) | 1543 (1530, 1557) (n = 135) | 0.1998 |
| T2 lesion volume, mL | 7.0 (5.6, 8.4) (n = 163) | 6.9 (5.1, 8.6) (n = 106) | 6.9 (5.9, 8.0) (n = 269) | 0.3764 |
| T2 lesions at screening, n | 41.6 (36.1, 47.2) (n = 163) | 40.2 (33.9, 46.6) (n = 106) | 41.1 (36.9, 45.2) (n = 269) | 0.6872 |
Note: Data are mean (95% confidence interval) unless specified otherwise.
ARR: annualized relapse rate; EDSS: Expanded Disability Status Scale; ITT: intention-to-treat; NR: not reported.
aTreatment-naïve patients vs previously treated patients determined using the Mann–Whitney–Wilcoxon test unless noted otherwise.
bχ2test.
cFisher’s exact test.
Longitudinal comparison of on-study ARR with ARR in the year before enrolment demonstrated substantial, significant reductions in ARR after patients initiated fingolimod (p < 0.0001, both groups). The mean (95% CI) reduction was greater in treatment-naïve (n = 162) patients (−88.7% [−93.1%, −84.2%]) than in previously treated (n = 114) patients (−76.8% [−85.7%, −67.8%]; p = 0.0408). The ARR reduction was smallest in the multi-iDMT subgroup (treatment naïve, −88.7% [−93.1%, −84.2%]; single-iDMT [n = 87], −82.4% [−91.1%, −73.8%]; multi-iDMT [n = 24], −53.3% [−81.7%, −24.9%]; p = 0.0037), even when compared only with the single-iDMT subgroup (p = 0.0183; Figure 2(a)).
Figure 2.
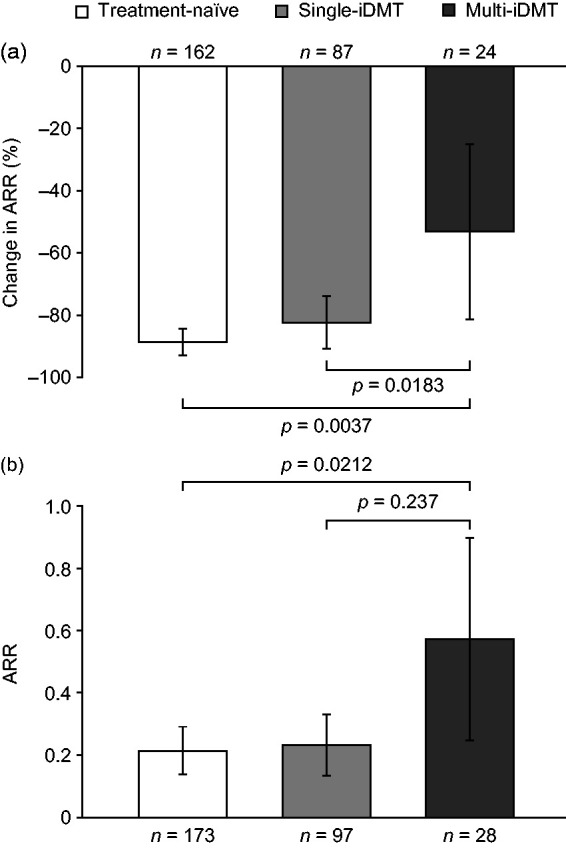
Comparison of ARR in treatment-naïve patients and previously treated patients by subgroup. (a) reduction in ARR on study compared with the year before enrolment and (b) ARR on study. Data are mean (95% confidence intervals).
Comparisons across three groups by Kruskal–Wallis test; comparison between single-iDMT and multi-iDMT subgroups by Mann–Whitney–Wilcoxon test.
Noting insufficient statistical power to assess the primary endpoint (28% power to detect a between-group difference in ARR of −0.1), no difference in ARR was observed between treatment-naïve (n = 173) and previously treated (n = 128) patients (mean ARR [95% CI], 0.21 [0.14, 0.29] vs 0.30 [0.20, 0.41]; p = 0.1668; Table 2), nor was any difference detected in the PP population (treatment naïve [n = 139], 0.22 [0.13, 0.30] vs previously treated [n = 100], 0.27 [0.16, 0.38]; p = 0.2914). ARR was highest in the multi-iDMT group (treatment-naïve, 0.21 [0.14, 0.29]; single-iDMT [n = 97], 0.23 [0.13, 0.33]; multi-iDMT [n = 28], 0.57 [0.25, 0.90]; p = 0.0212), even when compared only with the single-iDMT subgroup (p = 0.0237; Figure 2(b)).
Table 2.
Primary and secondary efficacy endpoints (ITT population).
| Parameter | Treatment-naïve patients (n=185) | Previously treated patients (n=135) | Total(n=320) | p-valuea |
|---|---|---|---|---|
| Primary endpoint | ||||
| ARR | 0.21 (0.14, 0.29) (n=173) | 0.30 (0.20, 0.41) (n=128) | 0.1668 | |
| Clinical endpoints | ||||
| Mean (SE) time to first relapse, months | 9.8 (0.22) | 10.2 (0.32) | 10.4 (0.19) | 0.3217 |
| Maximum relapse severity (among relapsing patients only), n (%) | 0.3917b | |||
| Mild | 11 (33.3) | 11 (35.5) | 22 (34.4) | |
| Moderate | 22 (66.7) | 18 (58.1) | 40 (62.5) | |
| Severe | 0 | 2 (6.5) | 2 (3.1) | |
| Total duration of relapses, days | 63.8 (27.3, 100.3) (n=33) | 42.4 (17.9, 66.9) (n=31) | 53.5 (31.6, 75.3) (n=64) | 0.4973 |
| Patients with relapses requiring hospitalization, n (%) | 3 (9.1) | 2 (6.5) | 5 (7.8) | 1.0000b |
| Patients with no relapses, n (%) | 152 (82.2) | 104 (77.0) | 256 (80.0) | 0.2577c |
| Change in EDSS score from baseline | −0.06 (−0.18, 0.05) (n=179) | −0.05 (−0.20, 0.09) (n=131) | −0.06 (−0.15, 0.03) (n=310) | NR |
| Categorical change in EDSS score, n (%) | 0.7567c | |||
| Improved | 30 (16.8) | 23 (17.6) | 53 (17.1) | |
| Stable | 134 (74.9) | 94 (71.8) | 228 (73.6) | |
| Worsening | 15 (8.4) | 14 (10.7) | 29 (9.4) | |
| MRI endpoints | ||||
| T2 lesions at week 48, n | 2.0 (1.5, 2.5) (n=163) | 1.6 (1.0, 2.1) (n=106) | 1.8 (1.5, 2.2) (n=269) | 0.1091 |
| PBVC from baseline, %d | −0.60 (−0.76, −0.43) (n=92) | −0.39 (−0.58, −0.19) (n=43) | −0.53 (−0.66, −0.40) (n=135) | 0.2312 |
| Disease activity | ||||
| Free from clinical disease activity, n (%) | 133 (71.9) | 90 (66.7) | 223 (69.7) | 0.2166 |
| Missing values, n (%) | 3 (1.6) | 0 | 3 (0.9) | |
| No active T2 lesions, n (%) | 70 (37.8) | 59 (43.7) | 129 (40.3) | 0.0414 |
| Missing values, n (%) | 22 (11.9) | 29 (21.5) | 51 (15.9) |
Note: Data are mean (95% confidence interval) unless specified otherwise.
ARR: annualized relapse rate; EDSS: Expanded Disability Status Scale; ITT: intention-to-treat; NR: not reported; PBVC: percentage brain volume change; SE: standard error.
aTreatment-naïve patients vs previously treated patients determined using the Mann–Whitney–Wilcoxon test unless noted otherwise.
bFisher’s exact test.
cχ2-test.
dLow n was attributable to scans missing or unanalysable.
No significant differences were observed between the treatment-naïve and previously treated groups for secondary endpoints of time to first relapse, relapse severity, relapse duration, the percentage of patients needing hospitalization, or the percentage of relapse-free patients on study (Table 2). Among these parameters, the only significant difference between the single-iDMT and multi-iDMT subgroups was time to first relapse (10.5 months vs 6.1 months; p = 0.0429). Based on 95% CIs, there was no mean change in EDSS score in any group. Consistent with small numerical decreases in mean EDSS score observed in all groups and subgroups, categorical analysis of EDSS score showed that disability was stable or improved among approximately 90% of patients in each group (Table 2).
The mean (95% CI) number of active T2 lesions at week 48 was similar in the treatment-naive (2.0 [1.5, 2.5]) and previously treated groups (1.6 [1.0, 2.1]) (p = 0.1091; Table 2), across the treatment-naïve group, single-iDMT (1.6 [1.0, 2.4], n = 80) and multi-iDMT subgroups (1.4 [0.4, 2.4], n = 24) (p = 0.2720), and between the iDMT subgroups (p = 0.8483). Average PBVC from baseline was −0.53% and no significant differences were seen in any of the between-group or between-subgroup comparisons (Table 2).
There was no difference among treatment-naïve and previously treated groups in the proportion of patients free from clinical disease activity (133 [71.9%] vs 90 [66.7%]; p = 0.2166, Table 2). Although a smaller proportion of treatment-naïve than previously treated patients were free from active T2 lesions at the end of the study (70 [37.8%] vs 59 [43.7%]; p = 0.0414), data were missing for a larger proportion of previously treated patients (21.48%) than treatment-naïve patients (11.89%; Table 2). In the total population, mean PRIMUS scores at baseline and at week 48 were: symptoms, 5.9 vs 5.9; activity limitation, 26.9 vs 26.9; and QoL, 17.1 vs 17.3. No changes in PRIMUS subscale scores for treatment satisfaction were seen in any groups or subgroups.
There were 42 AEs and six SAEs in the 24 hours following fingolimod initiation; the proportions of patients in the two groups experiencing AEs (treatment naïve, 19 [9.5%]; previously treated, 17 [11.6%]; p = 0.5331) or SAEs (treatment naïve, 4 [2.0%]; previously treated, 2 [1.4%]; p = 0.6516) were similar (Table 3). The most frequent AEs were nervous system disorders (11 [3.2%], mostly dizziness or headache), cardiac disorders (9 [2.6%], either atrioventricular block [including one case of second-degree block] or bradycardia) and gastrointestinal disorders (7 [2.0%]); SAEs were bradycardia/sinus bradycardia (n = 3), atrioventricular block (n = 2) and drug hypersensitivity (n = 1). One case (0.3%) of mild macular oedema was reported.
Table 3.
Adverse events (safety population).a
| Treatment-naïve patients (n=200) | Previously treated patients (n=147) | Total (n=347) | |
|---|---|---|---|
| Events during the first 24 hours n (%) | |||
| Patients with AEs | 19 (9.5) | 17 (11.6) | 36 (10.4) |
| AEs by SOC and PT (> 1 patient in either group) n (%) | |||
| Cardiac disorders | 7 (3.5) | 2 (1.4) | 9 (2.6) |
| Atrioventricular block, first-degree or unclassified | 3 (1.5) | 0 | 3 (0.9) |
| Bradycardia or sinus bradycardia | 3 (1.5) | 2 (1.4) | 5 (1.4) |
| Eye disorders | 2 (1.0) | 0 | 2 (0.6) |
| Gastrointestinal disorders | 4 (2.0) | 3 (2.0) | 7 (2.0) |
| Nervous system disorders | 3 (1.5) | 8 (5.4) | 11 (3.2) |
| Dizziness | 0 | 3 (2.0) | 3 (0.9) |
| Headache | 2 (1.0) | 3 (2.0) | 5 (1.4) |
| Psychiatric disorders | 1 (0.5) | 3 (2.0) | 4 (1.2) |
| Anxiety | 1 (0.5) | 2 (1.4) | 3 (0.9) |
| Skin and subcutaneous tissue disorders | 2 (1.0) | 2 (1.4) | 4 (1.2) |
| Alopecia | 1 (0.5) | 2 (1.4) | 3 (0.9) |
| Patients with AEs related to study drug | 13 (6.5) | 9 (6.1) | 22 (6.3) |
| Patients with SAEs | 4 (2.0) | 2 (1.4) | 6 (1.7) |
| Events after the first 24 hours n (%) | |||
| Patients with AEs | 139 (69.5) | 111 (75.5) | 250 (72.0) |
| AEs by SOC (> 5 patients in either group) n (%) | |||
| Blood and lymphatic system disorders | 12 (6.0) | 9 (6.1) | 21 (6.1) |
| Lymphopenia | 9 (4.5) | 7 (4.8) | 16 (4.6) |
| Eye disorders | 17 (8.5) | 9 (6.1) | 26 (7.5) |
| Gastrointestinal disorders | 27 (13.5) | 23 (15.7) | 50 (14.4) |
| Diarrhoea | 4 (2.0) | 9 (6.1) | 13 (3.8) |
| Nausea | 6 (3.0) | 4 (2.7) | 10 (2.9) |
| General disorders and administration site conditions | 17 (8.5) | 18 (12.2) | 35 (10.1) |
| Fatigue | 11 (5.5) | 9 (6.1) | 20 (5.8) |
| Hepatobiliary disorders | 7 (3.5) | 3 (2.0) | 10 (2.9) |
| Infections | 75 (37.5) | 50 (34.0) | 125 (36.0) |
| Gastroenteritis | 8 (4.0) | 6 (4.1) | 14 (4.0) |
| Nasopharyngitis | 15 (7.5) | 8 (5.4) | 23 (6.6) |
| Oral herpes | 7 (3.5) | 5 (3.4) | 12 (3.5) |
| Pharyngitis | 7 (3.5) | 4 (2.7) | 11 (3.2) |
| Tonsillitis | 6 (3.0) | 3 (2.0) | 9 (2.6) |
| Upper respiratory tract infection | 13 (6.5) | 10 (6.8) | 23 (6.6) |
| Urinary tract infection | 15 (7.5) | 14 (9.5) | 29 (8.4) |
| Injury, poisoning and procedural complications | 9 (4.5) | 7 (4.8) | 16 (4.6) |
| Investigations | 5 (2.5) | 15 (10.2) | 20 (5.8) |
| Increased aminotransferase levelsb | 2 (1.0) | 10 (6.8) | 12 (3.5) |
| Metabolism and nutrition disorders | 3 (1.5) | 9 (6.1) | 12 (3.5) |
| Musculoskeletal and connective tissue disorders | 20 (10.0) | 18 (12.2) | 38 (11.0) |
| Back pain | 9 (4.5) | 3 (2.0) | 12 (3.5) |
| Neck pain | 5 (2.5) | 6 (4.1) | 11 (3.2) |
| Nervous system disorders | 46 (23.0) | 39 (26.5) | 85 (24.5) |
| Headache | 15 (7.5) | 14 (9.5) | 29 (8.4) |
| Migraine | 6 (3.0) | 6 (4.1) | 12 (3.5) |
| Psychiatric disorders | 27 (13.5) | 19 (12.9) | 46 (13.3) |
| Anxiety/anxiety disorderc | 12 (6.0) | 5 (3.4) | 17 (4.9) |
| Depression/depressed moodc | 9 (4.5) | 7 (4.8) | 16 (4.6) |
| Reproductive system and breast disorders | 8 (4.0) | 3 (2.0) | 11 (3.2) |
| Skin and subcutaneous disorders | 20 (10.0) | 14 (9.5) | 34 (9.8) |
| Patients with AEs related to study drug | 63 (31.5) | 49 (33.3) | 112 (32.3) |
| Patients with SAEs | 7 (3.5) | 2 (1.4) | 9 (2.6) |
AE: adverse event; PT: preferred term; SAE: serious adverse event; SOC: system organ class.
aAdverse events were coded according to the Medical Dictionary for Regulatory Activities.
bValues for alanine aminotransferase and aspartate aminotransferase combined.
cAE terms combined.
After the initial 24-hour observation period, there were 819 AEs and 12 SAEs on study; similar proportions of patients experienced AEs (treatment naïve, 139 [69.5%]; previously treated, 111 [75.5%]; p = 0.2177) or SAEs (treatment naïve, 7 [3.5%]; previously treated, 2 [1.4%]; p = 0.2154; Table 3). The most frequent AEs were infections (125 [36.0%], primarily urinary tract or upper respiratory tract infection, or nasopharyngitis), nervous system disorders (85 [24.5%], mainly headache or migraine) and gastrointestinal disorders (50 [14.4%], mostly diarrhoea or nausea); there was one case of superficial spreading melanoma; SAEs were MS relapse (n = 3), cholelithiasis (n = 2), and single cases of acute hepatitis, B-cell lymphoma, brain oedema, epilepsy, lower respiratory tract infection, ovarian cyst, and partial seizure. No deaths were reported.
Discussion
In EARLIMS, both treatment-naïve and previously treated patients at an early stage of MS experienced substantial and similar on-fingolimod reductions in ARR compared with rates seen before enrolment. Longitudinal reductions in ARR were similar to those seen with fingolimod in a recent real-world study,17 and were greater than those reported in pivotal fingolimod trials.12,13
Treatment-naïve patients in EARLIMS had more frequent and severe relapses before enrolment than did those previously treated with iDMTs. After 48 weeks of fingolimod treatment, there was no difference in ARR between the groups. Subgroup analyses revealed no difference in ARR between treatment-naïve patients and those treated with one iDMT, although patients previously treated with multiple iDMTs had higher ARR. In terms of disease burden pre-enrolment, relapse rates, EDSS score and normalized brain volume at baseline were all similar in the single-iDMT and multi-iDMT groups. However, patients in the multi-iDMT group are likely to have experienced disease breakthrough on several therapies, and relapse experiences on multiple treatments could indicate a poorer prognosis.26 Consistent with this hypothesis, this subgroup had the lowest longitudinal reduction in ARR and the shortest time to first relapse on study.
Treatment history was not associated with differences in relapse severity, relapse-related hospitalization, EDSS score, rate of brain volume loss, T2 lesion count, or freedom from clinical disease activity, which was proportionally high in all groups. Although a smaller proportion of treatment-naïve than previously treated patients were free from active T2 lesions, this may simply relate to greater disease activity in the treatment-naïve group before enrolment.
Patient eligibility was a key difference between EARLIMS and the phase 3 trials of fingolimod;12,13,27 EARLIMS recruited younger, less disabled patients within 5 years of diagnosis. However, post hoc analysis of FREEDOMS14 found that, compared with placebo, fingolimod reduced ARR proportionally more in treatment-naïve patients than in patients previously treated with iDMTs. An observational study also found greater longitudinal reductions in ARR among patients receiving fingolimod first line than those receiving it second line to iDMTs. Notably, patients in a real-world setting with an EDSS score <3 or with an ARR ≥2 had the greatest reductions in ARR with fingolimod.17 In terms of efficacy in young patients at earlier stages of MS, results from the PARADIGMS study demonstrated a significantly greater reduction of ARR with fingolimod than with interferon beta-1a in patients below 18 years of age.28
Consistent with studies such as PREFERMS,29 adherence to fingolimod was excellent irrespective of treatment history, and reinforces the notion that patients are more likely to adhere to an oral drug than to an iDMT.30,31 There were no unexpected safety signals in EARLIMS, with no new safety findings. The profile of AEs during first-dose observation and during the study were consistent with the phase 3 trials.12,13,27 Owing to the relatively small sample size (n = 347) and study duration (48 weeks), it is not possible to draw conclusions on the risk of rare SAEs, such as opportunistic infections, associated with fingolimod treatment. Although rare, cases of progressive multifocal leukoencephalopathy and cryptococcal infections, including cryptococcal meningitis, have been reported in the post-marketing setting after 2–3 years of treatment with fingolimod.10,32–34
The main limitation of EARLIMS was that it was underpowered, difficulties with recruitment likely relating to the timing of fingolimod’s second-line approval in Europe (see Appendix for further information). Consequently, the primary endpoint could not be tested, but data are presented with 95% CIs to improve the likelihood of detecting clinically relevant between-group differences.35 The small number of patients in the multi-iDMT group may have biased comparisons, and imaging findings must be interpreted with caution because of missing values.
In EARLIMS, both treatment-naïve patients and those previously treated with iDMTs experienced substantial reductions in ARR when treated with fingolimod. Treatment-naïve patients also saw as much benefit as patients previously treated with one iDMT in all outcomes examined. Accordingly, and unless benefit–risk considerations dictate otherwise, there seems little merit in using low-efficacy iDMTs early in MS when high-efficacy therapy is an option.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_2055217320957358 for Comparison of first-line and second-line use of fingolimod in relapsing MS: The open-label EARLIMS study by Oscar Fernández, Guillermo Izquierdo, Eduardo Aguera, Cristina Ramo, Miguel Hernandez, Diego Silva, Rob Walker, Helmut Butzkueven, Chenyu Wang, Michael Barnett and on behalf of the EARLIMS investigators in Multiple Sclerosis Journal-Experimental, Translational and Clinical
Acknowledgements
The authors wish to thank Eli Garcia (Novartis Farmacéutica S.A., Barcelona, Spain) for her contributions towards the development of the analyses and manuscript, and Dr Ian M. Williams from Oxford PharmaGenesis for editorial and medical writing support.
Conflicting of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: OF reports receiving honoraria as a consultant in advisory boards, as chair/lecturer in meetings, from participation in clinical trials and other research projects promoted by Actelion, Allergan, Almirall, Bayer-Schering, Biogen, Merck Serono, Novartis, Sanofi Genzyme and Teva, and research support from the Hospital Foundation FIMABIS; he has also served as editor of the Revista Española de Esclerosis Múltiple. GI has received speaking and/or advisory board honoraria from Almirall, Bayer, Biogen-Idec, Merck Serono, Novartis, Roche, Sanofi and Teva. EA has received institutional support for research, speaking and/or participation in advisory boards for Biogen, Merck, Novartis and Sanofi Genzyme. CR has received research funding, compensation for travel or speaker honoraria from Almirall, Biogen, Novartis and Sanofi Genzyme. MH has received honoraria from Biogen, Merck, Novartis, Roche and Teva. Diego Silva was an employee of Novartis Pharma AG at the time of writing. RW is an employee of Novartis Pharmaceuticals Australia. CW is an employee of Sydney Neuroimaging Analysis Centre, and has received compensation for conference travel and speaker honoraria from Novartis, Biogen and Roche. HB has served on scientific advisory boards for Biogen, Merck, Novartis, Roche and Sanofi and has received conference travel support from Biogen, Novartis and Teva. He serves on steering committees for trials and studies conducted by Biogen, Merck, Novartis and Roche, and his institutions have received research support from Biogen, Merck and Novartis. MB has received institutional support for research, speaking and/or participation in advisory boards for Biogen, Merck, Novartis, Roche and Sanofi Genzyme.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The EARLIMS study and medical writing support was funded by Novartis Pharma AG.
ORCID iD
Michael Barnett https://orcid.org/0000-0002-2156-8864
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta-1b trial. Neurology 2012; 78: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebers GC, Traboulsee A, Li D, et al. ; Investigators of the 16-year Long-Term Follow-Up Study. Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J Neurol Neurosurg Psychiatry 2010; 81: 907–912. [DOI] [PubMed] [Google Scholar]
- 3.Soelberg Sorensen P. Safety concerns and risk management of multiple sclerosis therapies. Acta Neurol Scand 2017; 136: 168–186. [DOI] [PubMed] [Google Scholar]
- 4.Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev 2017; 16: 658–665. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez O. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord 2017; 17: 75–83. [DOI] [PubMed] [Google Scholar]
- 6.De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizak N, Lugaresi A, Alroughani R, et al. Highly active immunomodulatory therapy ameliorates accumulation of disability in moderately advanced and advanced multiple sclerosis. J Neurol Neurosurg Psychiatry 2017; 88: 196–203. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Gilenya: EPAR product information, www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf (2019, accessed 21 July 2020).
- 11.Australian Therapeutic Goods Administration. Australian public assessment report for fingolimod, https://www.tga.gov.au/auspar/auspar-fingolimod (2011, accessed 21 July 2020).
- 12.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 14.Kremenchutzky M, O'Connor P, Hohlfeld R, et al. Impact of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod: Subgroup analyses of the fingolimod research evaluating effects of daily oral therapy in multiple sclerosis (FREEDOMS) study. Mult Scler Relat Disord 2014; 3: 341–349. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JA, Barkhof F, Comi G, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 2013; 260: 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 2012; 11: 420–428. [DOI] [PubMed] [Google Scholar]
- 17.Izquierdo G, Damas F, Paramo MD, et al. The real-world effectiveness and safety of fingolimod in relapsing-remitting multiple sclerosis patients: an observational study. PLoS One 2017; 12: e0176174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Declaration of Helsinki: ethical principles for medical research involving human subjects. Ferney-Voltaire, France: World Medical Association, 2013, www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 21 July 2020).
- 19.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline: Guideline for Good Clinical Practice E6(R2), https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4.pdf (accessed 21 July 2020).
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) Advisory committee on clinical trials of new agents in multiple sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 23.Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Mult Scler 2009; 15: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002; 17: 479–489. [DOI] [PubMed] [Google Scholar]
- 25.McKenna SP, Doward LC, Twiss J, et al. International development of the patient-reported outcome indices for multiple sclerosis (PRIMUS). Value Health 2010; 13: 946–951. [DOI] [PubMed] [Google Scholar]
- 26.Jokubaitis VG, Spelman T, Kalincik T, MSBase Study Group et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol 2016; 80: 89–100. [DOI] [PubMed] [Google Scholar]
- 27.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 28.Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 2018; 379: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 29.Cree BAC, Wynn D, Cascione M, et al. Key results from PREFERMS: real-world patient retention and outcomes on fingolimod versus platform injectable disease-modifying therapies in early relapsing–remitting multiple sclerosis. Neurology 2016; 78: P3–115. [Google Scholar]
- 30.Warrender-Sparkes M, Spelman T, Izquierdo G, et al. ; on behalf of the MSBase study group. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler 2016; 22: 520–532. [DOI] [PubMed] [Google Scholar]
- 31.Hansen K, Schussel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One 2015; 10: e0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Australian Therapeutic Goods Administration. Australian Product Information – GILENYA® (fingolimod), www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-01890-3&d=202007211016933 (2020, accessed 21 July 2020).
- 33.Berger JR, Cree BA, Greenberg B, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018; 90: e1815–e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord 2016; 9: 158–162. [DOI] [PubMed] [Google Scholar]
- 35.Du Prel JB, Hommel G, Rohrig B, et al. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2009; 106: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_2055217320957358 for Comparison of first-line and second-line use of fingolimod in relapsing MS: The open-label EARLIMS study by Oscar Fernández, Guillermo Izquierdo, Eduardo Aguera, Cristina Ramo, Miguel Hernandez, Diego Silva, Rob Walker, Helmut Butzkueven, Chenyu Wang, Michael Barnett and on behalf of the EARLIMS investigators in Multiple Sclerosis Journal-Experimental, Translational and Clinical