Abstract
Neurilemmoma, also known as schwannoma or neurinoma, is a tumor that originates from neural sheath Schwann cells. Giant neurilemmomas derived from the retroperitoneum have rarely been reported. We herein describe a woman with a giant retroperitoneal neurilemmoma that was initially incorrectly diagnosed as an inflammatory abdominal mass. The tumor extended from the patient’s hypogastrium to her pelvic cavity and measured 20 × 15 × 10 cm. The tumor was excised via laparotomy and diagnosed as a retroperitoneal neurilemmoma through histological and immunohistochemical examination. Although rare, particularly in the giant form, neurilemmoma should be considered as an important differential diagnosis in patients with a retroperitoneal tumor or inflammatory abdominal mass. Complete excision should be considered for the potential cure of giant retroperitoneal neurilemmomas.
Keywords: Giant neurilemmoma, retroperitoneal tumor, laparotomy, treatment, Schwann cells, histology, immunohistochemistry
Introduction
Neurilemmoma is a type of peripheral nerve sheath soft tissue tumor that occurs as a solitary, encapsulated mass connected to adjacent tissues and organs. The most common sites are the head, neck, and flexor surfaces of the extremities.1–3 Abdominal neurilemmomas are most commonly located in the presacral region and paravertebral space.4–6 They occur in patients of all age groups but are most commonly found in women aged 20 to 50 years.7,8
Neurilemmomas have rarely been found in the retroperitoneal space.9,10 When neurilemmomas present in this location, patients are typically asymptomatic or experience only mild gastrointestinal and/or urinary symptoms.11,12 Therefore, retroperitoneal neurilemmomas can cause a diagnostic dilemma because of both their rarity and occult clinical symptoms. We herein report a rare case involving a woman with a giant asymptomatic retroperitoneal neurilemmoma that was initially misdiagnosed as an abdominal inflammatory mass.
Case history
A 69-year-old woman was admitted to our hospital with a giant mass in her abdomen. She had experienced intermittent abdominal discomfort and constipation for several months. The patient had no family history of neurilemmoma, schwannomatosis, or other benign or malignant tumors. Physical examination revealed a nontender lower abdominal mass. There were no typical clinical manifestations associated with neurofibromatosis type 1, such as café-au-lait macules, axillary freckling, or Lisch nodules. A biopsy had been previously performed during an exploratory laparotomy when she was admitted to another hospital with similar symptoms 3 years prior. The pathological result of the biopsy at that time suggested an inflammatory abdominal mass.
Further laboratory examination at our institution showed that the hemoglobin level, white blood cell counts, and tumor marker levels were within the reference ranges. Abdominal ultrasonography showed a 19- × 15- × 13-cm cystic mass with a regular general morphology and round to oval shape located within the hypogastrium and pelvic cavity. An abdominal computed tomography scan confirmed a capsulated cystic mass (18 × 14.5 × 14 cm) that encompassed the hypogastric region and pelvis (Figure 1(a), (b)). The patient also underwent intravenous pyelography (Figure 2), which showed left-sided hydronephrosis. Fortunately, her serum creatinine level remained within the reference range.
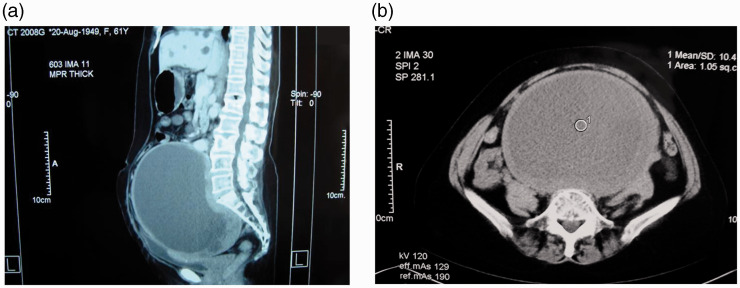
Figure 1.
Abdominal computed tomography revealed a capsulated cystic mass (18 × 14.5 × 14 cm) that encompassed the hypogastric region and pelvis. (a) Median sagittal section. (b) Coronal section. The mass exhibited a close relationship to the bladder, ureter, and rectum. No obvious bony destruction, defect, or resorption was seen.
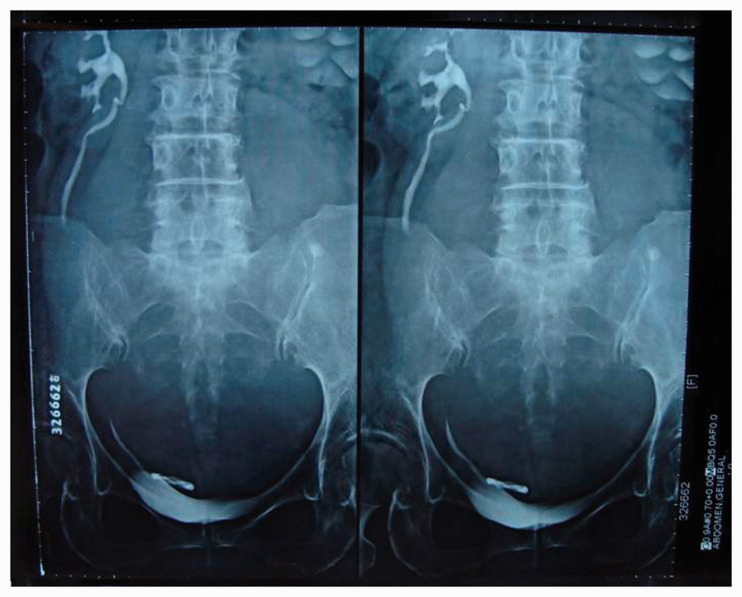
Figure 2.
Intravenous pyelography showed left hydronephrosis due to the compression of the bladder and left ureter by the giant retroperitoneal mass.
A scheduled laparotomy was performed after preoperative assessment. Intraoperatively, we found a giant encapsulated tumor (20 × 15 × 10 cm) tightly bound to the sigmoid colon, mesocolon, and sacrum. The tumor received an abundant blood supply and compressed both the iliac vessels and ureters. After the lesion was separated from adjacent tissues and completely resected, we discovered that the giant tumor was close to the presacral, femoral, and genitofemoral nerves.
Macroscopically, the resected tumor contained both cystic and solid bright-yellow components. Microscopic examination revealed a neurilemmoma with hemorrhage and cystic degeneration. Postoperative gross examination of the tumor specimen revealed a smooth, capsulated mass measuring 20 × 15 × 10 cm. Hematoxylin–eosin staining showed that the solid mass consisted of a proliferation of fusiform cells that formed a palisade or turbinate pattern (Antoni A area) (Figure 3(a), (c)) in some regions. Other regions were composed of myxoid and degenerated tissue with fewer cells and a gelatinous substance (Antoni B area) (Figure 3(b), (d)). Moreover, the Ki-67 index, a cellular proliferation marker, was 15%. Immunohistologically, staining for the S-100 protein in the cytoplasm of tumor cells was positive (Figure 3(e), (f)), while smooth muscle actin, desmin, Dog-1, CD34, CD117, and neuron-specific enolase were negative. Based on these results, the patient was diagnosed with a retroperitoneal neurilemmoma.
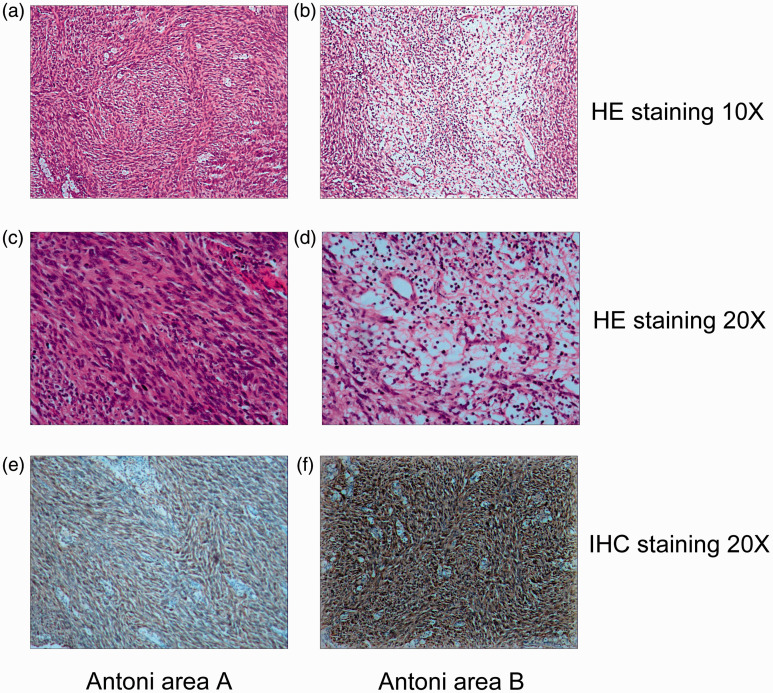
Figure 3.
Histopathology of retroperitoneal neurilemmoma. The mass consisted of a proliferation of fusiform cells that formed a palisade or turbinate pattern ((a) ×10, (c) ×20) and of myxoid and degenerated tissue with cells and gelatinous substance ((b) ×10, (d) ×20). (e, f) S-100-positive cells (×20).
The patient’s postoperative treatment and recovery were uneventful. Abdominal ultrasound and laboratory tests revealed no recurrence after the operation. She was discharged 1 month postoperatively and remained asymptomatic for several months with no reported discomfort or evidence of tumor recurrence.
Discussion
Neurilemmoma, also referred to as schwannoma, neurinoma, and perineural fibroblastoma, originates from Schwann cells derived from the neuroectoderm.11,12 Most neurilemmomas occur in women and are found in peripheral nerve fibers in the flexor surfaces of the limbs, head, and neck.8,13,14 Approximately 0.3% to 3.0% are found in the retroperitoneum, accounting for 0.5% to 1.2% of all retroperitoneal tumors.15–18
The diagnosis of a retroperitoneal neurilemmoma or schwannoma is difficult because these tumors typically become very large before causing clinical symptoms.15,19 The symptoms of neurilemmoma are nonspecific and include vague abdominal pain, dull localized pain, and discomfort,20 which are often difficult to diagnose accurately. Our patient presented with atypical symptoms (discomfort and constipation); therefore, imaging techniques were needed to achieve a rapid diagnosis.
Radiological findings are fundamental in the diagnostic evaluation of retroperitoneal tumors.6,7,21,22 Computed tomography in our case showed a well-defined low or mixed attenuated lesion with cystic necrotic central areas. Findings of cystic change are common in retroperitoneal neurilemmomas (incidence of up to 66%).7,20
A definitive diagnosis requires histopathological and immunohistochemical staining of conventional surgically resected specimens. Although some reports have described the use of endoscopic ultrasound-guided fine-needle aspiration biopsy to diagnose retroperitoneal neurilemmomas or schwannomas,23 this practice is controversial because of its low diagnostic accuracy and potential complications such as damage to adjacent organs and vessels. Thus, neurilemmomas in the retroperitoneal position are often misdiagnosed as neurofibromas, ganglioneuromas, paragangliomas, tumors of mesodermal origin, and retroperitoneal malignant lesions such as malignant fibrous histiocytomas, lymphoma, and liposarcomas.20 Neurilemmomas consist of two histological components: small cellular lesions (Antoni type A tissues) and loose, hypocellular myxoid lesions with microcystic spaces (Antoni type B tissues). The hallmark feature of neurilemmoma is a composition of these Antoni A and B areas, with strong diffuse positivity for S-100 protein in the cytoplasm of the tumor cells (the distinct immunohistochemical feature).24 As in most schwannomas, negative CD34 immunohistochemistry staining in the cytoplasm also confirmed the diagnosis in our case.
The giant neurilemmoma in our patient did not provide much adjacent space in the retroperitoneum. Consequently, the patient underwent another invasive procedure because of the unclear primary pathologic diagnosis. A well-planned preoperative examination was necessary to eliminate the risk of inadequate excision and/or damage to adjacent structures. With respect to the surgical approach, surgeons should have a thorough understanding of the anatomical structures of adjacent organs and accompanying vessels.25,26 Considering the heterogeneous appearance on imaging and risk of recurrence, en bloc radical excision of the mass is usually recommended.27 Although complete excision of a giant neoplasm is challenging and has a risk of complications, it is vital to prevent tumor recurrence or malignant transformation after complete tumor resection by laparoscopic or open surgery.
Conclusion
Retroperitoneal giant neurilemmomas are rare. These lesions can be misdiagnosed as other cystic or solid neoplasms. Complete excision should be considered as the primary treatment. Because of numerous diagnostic and therapeutic issues, the successful treatment of neurilemmomas requires detailed preoperative planning and a multidisciplinary approach. Therefore, an initial diagnosis of retroperitoneal neurilemmoma is critical for a favorable prognosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent
Our hospital ethics committee authorized the publication of this case report (ethical approval number: IRB2019-WZ-132), and informed consent was received from the patient and her family. This case did not involve the use of human subjects or non-routine procedures. All imaging examinations and preoperative and post-operative treatments were in compliance with ethical requirements.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
References
- 1.Sato Y, Imanishi Y, Tomita T, et al. Clinical diagnosis and treatment outcomes for parapharyngeal space schwannomas: a single-institution review of 21 cases. Head Neck 2018; 40: 569–576. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Lu X, Xie S, et al. Intraparotid facial nerve schwannoma: a 17-year, single-institution experience of diagnosis and management. Acta Otolaryngol 2019; 139: 444–450. [DOI] [PubMed] [Google Scholar]
- 3.Fini G, Leonardi A, Mici E, et al. Schwannoma of the parotid gland. Case report. Ann Ital Chir 2015; 86: 1–4. [PubMed] [Google Scholar]
- 4.Kalaycı M, Akyüz U, Demirağ A, et al. Retroperitoneal schwannoma: a rare case. Case Rep Gastrointest Med 2011; 2011: 465062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hori T, Yamagiwa K, Yagi S, et al. Noradrenalin-secreting retroperitoneal schwannoma resected by hand-assisted laparoscopic surgery: report of a case. Surg Today 2006; 36: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 6.Goh BKP, Tan Y, Chung YA, et al. Retroperitoneal schwannoma. Am J Surg 2006; 192: 14–18. [DOI] [PubMed] [Google Scholar]
- 7.Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic-pathologic correlation. Radiographics 2004; 24: 1477–1481. [DOI] [PubMed] [Google Scholar]
- 8.Radojkovic M, Mihailovic D, Stojanovic M, et al. Large retroperitoneal schwannoma: a rare cause of chronic back pain. J Int Med Res 2018; 46: 3404–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshmand S, Youssefzadeh D, Chamie K, et al. Benign retroperitoneal schwannoma: a case series and review of the literature. Urology 2003; 62: 993–997. [DOI] [PubMed] [Google Scholar]
- 10.Fass G, Hossey D, Nyst M, et al. Benign retroperitoneal schwannoma presenting as colitis: a case report. World J Gastroenterol 2007; 13: 5521–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guz BV, Wood DP, Montie JE, et al. Retroperitoneal neural sheath tumors: Cleveland Clinic experience. J Urol 1989; 142: 1434–1437. [DOI] [PubMed] [Google Scholar]
- 12.Vijayan SKL, Shetty S, Bhat SR, et al. Retroperitoneal schwannoma: an atypical presentation. J Clin Diagn Res 2014; 8: ND22–ND23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee NJ, Hruban RH, Fishman EK. Abdominal schwannomas: review of imaging findings and pathology. Abdom Radiol (NY) 2017; 42: 1864–1870. [DOI] [PubMed] [Google Scholar]
- 14.Arshi A, Tajudeen BA, St John M. Malignant peripheral nerve sheath tumors of the head and neck: demographics, clinicopathologic features, management, and treatment outcomes. Oral Oncol 2015; 51: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Gao C, Juzi JT, et al. Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg 2007; 77: 237–240. [DOI] [PubMed] [Google Scholar]
- 16.Kasperlik-Zaluska AA, Roslonowska E, Slowinska-Srzednicka J, et al. 1,111 Patients with adrenal incidentalomas observed at a single endocrinological center. Ann N Y Acad Sci 2006; 1073: 38–46. [DOI] [PubMed] [Google Scholar]
- 17.Chang KL, Crabtree GS, Lim-Tan SK, et al. Primary extrauterine endometrial stromal neoplasms: a clinicopathologic study of 20 cases and a review of the literature. Int J Gynecol Pathol 1993; 12: 282–296. [PubMed] [Google Scholar]
- 18.Pinson CW, ReMine SG, Fletcher WS, et al. Long-term results with primary retroperitoneal tumors. JAMA Surg 1989; 124: 1168–1173. [DOI] [PubMed] [Google Scholar]
- 19.Song JY, Kim SY, Park EG, et al. Schwannoma in the retroperitoneum. J Obstet Gynaecol Res 2007; 33: 371–375. [DOI] [PubMed] [Google Scholar]
- 20.Theodosopoulos T, Stafyla VK, Tsiantoula P, et al. Special problems encountering surgical management of large retroperitoneal schwannomas. World J Surg Oncol 2008; 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes MJ, Thomas JM, Fisher C, et al. Imaging features of retroperitoneal and pelvic schwannomas. Clin Radiol 2005; 60: 886–893. [DOI] [PubMed] [Google Scholar]
- 22.Strauss DC, Hayes AJ, Thomas JM. Retroperitoneal tumours: review of management. Ann R Coll Surg Engl 2011; 93: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo T, Kawakami H, Kuwatani M, et al. Three cases of retroperitoneal schwannoma diagnosed by EUS-FNA. World J Gastroenterol 2011; 17: 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 1983; 49: 299–308. [PubMed] [Google Scholar]
- 25.Abe T, Sazawa A, Harabayashi T, et al. Laparoscopic resection of paraaortic/paracaval neurogenic tumors: surgical outcomes and technical tips. Surg Endosc 2016; 30: 4640–4645. [DOI] [PubMed] [Google Scholar]
- 26.Son S, Woo CG. Schwannoma originating from the common iliac artery: a case report. J Int Med Res. Epub ahead of print 27 May 2019. DOI: 10.1177/0300060519849792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollin DA, Sivarajan G, Shukla P, et al. Juxta-adrenal ancient schwannoma: a rare retroperitoneal tumor. Rev Urol 2015; 17: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]