Abstract
Background:
Pancreatic cancer is an aggressive type of cancer with poor prognosis, short survival rate, and high mortality. Drug resistance is a major cause of treatment failure in the disease. MiR-331-3p has been reported to play an important role in several cancers. We previously showed that miR-331-3p is upregulated in pancreatic cancer and promotes pancreatic cancer cell proliferation and epithelial-to-mesenchymal transition–mediated metastasis by targeting ST7L. However, it is uncertain whether miR-331-3p is involved in drug resistance.
Methods:
We investigated the relationship between miR-331-3p and pancreatic cancer drug resistance. As part of this, microRNA mimics or inhibitors were transfected into pancreatic cancer cells. Quantitative polymerase chain reaction was used to detect miR-331-3p expression, and flow cytometry was used to detect cell apoptosis. The Cell Counting Kit-8 assay was used to measure the IC50 values of gemcitabine in pancreatic cancer cells. The expression of multidrug resistance protein 1, multidrug resistance-related protein 1, breast cancer resistance protein, β-Catenin, c-Myc, Cyclin D1, Bcl-2, and Caspase-3 was evaluated by Western blotting.
Results:
We confirmed that miR-331-3p is upregulated in gemcitabine-treated pancreatic cancer cells and plasma from chemotherapy patients. We also confirmed that miR-331-3p inhibition decreased drug resistance by regulating cell apoptosis and multidrug resistance protein 1, multidrug resistance-related protein 1, and breast cancer resistance protein expression in pancreatic cancer cells, whereas miR-331-3p overexpression had the opposite effect. We further demonstrated that miR-331-3p effects in drug resistance were partially reversed by ST7L overexpression. In addition, overexpression of miR-331-3p activated Wnt/β-catenin signaling in pancreatic cancer cells, and ST7L overexpression restored activation of Wnt/β-catenin signaling.
Conclusions:
Taken together, our data demonstrate that miR-331-3p contributes to drug resistance by activating Wnt/β-catenin signaling via ST7L in pancreatic cancer cells. These data provide a theoretical basis for new targeted therapies in the future.
Keywords: miR-331-3p, WNT, drug resistance
Introduction
Morbidity and mortality associated with pancreatic cancer (PC) has increased in recent years, making it one of the most malignant and fatal cancers worldwide.1 Because of atypical early symptoms, and a lack of effective early-stage diagnostic techniques, most patients with PC progress rapidly to advanced stages, with the number of potentially operable patients being 10% to 15%.2 In addition to surgery, PC treatment includes radiotherapy, immunotherapy, chemotherapy, and so on. The main chemotherapeutic drug for PC is gemcitabine (GEM), and some clinical trials have found it improves survival rates in patients with PC to some extent. But, unfortunately, drug resistance has also significantly reduced GEM efficacy.3,4 Therefore, drug resistance reversal in PC has become a major therapeutic research focus in recent years.
MicroRNAs (miRNAs) are small noncoding RNA molecules that generally influence gene expression by binding to messenger RNA (mRNA) subsets, with which they share partial complementarity and suppress the stability and/or translation of such mRNAs.5 Through interactions with a wide spectra of mRNAs encoding proteins with diverse functions, miRNAs have emerged as potent posttranscriptional regulators of several cellular processes, including cell survival, death, division, differentiation, and senescence.6 Recently, several miRNAs have been found to play important roles in tumor drug resistance via the regulation of oncogenes or tumor suppressor genes.7 It has been shown that miR-331-3p plays an important role in many tumors, such as colorectal cancer,8 liver cancer,9 lung cancer,10 and gastric cancer.11 Studies have also shown that miR-331-3p is involved in tumor proliferation, apoptosis, metastasis, and epithelial-to-mesenchymal transition (EMT).8-10,12 As cell apoptosis and EMT are closely associated with drug resistance, we inferred that miR-331-3p may also participate in drug resistance. Our previous studies have demonstrated that miR-331-3p is upregulated in PC and promotes PC cell proliferation and EMT-mediated metastasis by targeting ST7L.13 ST7L (ST7-like), also as known as ST7R, is a paralog of the tumor suppressor gene, ST7.14 ST7L is downregulated in some cancers and acts as a tumor suppressor in gastric cancer,15 glioma,16 ovarian cancer,17 and hepatocellular carcinoma.18 However, the significance of ST7L in PC and whether miR-331-3p is involved in drug resistance through ST7L remain to be explored.
β-Catenin-dependent Wnt signaling, also known as canonical Wnt signaling, is a highly conserved signaling pathway closely associated with cell proliferation and differentiation.19 The Wnt/β-catenin pathway is a family of proteins implicated in many vital cellular functions, such as stem cell regeneration and organogenesis. Several intracellular signal transduction pathways are induced by Wnt, notably the Wnt/β-catenin-dependent pathway or canonical pathway and the noncanonical or β-catenin-independent pathway. β-catenin, the major effector of Wnt/β-catenin signaling, translocates to the cell nucleus to form complexes with coregulators of transcription factors, thus promoting the transcription of oncogenes such as Cyclin D and C-myc.20 It is reported that Wnt/β-catenin signaling activation promotes chemoradiotherapy resistance.21 Li et al observed that miR-378 functions as an onco-miRNA by targeting the ST7L/Wnt/β-catenin pathway in cervical cancer cells.22 Some studies have shown that ST7L downregulation inhibits the activation of β-catenin.16,18 However, the role of ST7L/Wnt/β-catenin signaling in PC remains unknown. This study aimed to verify that miR-331-3p was involved in drug resistance and activated Wnt/β-catenin signaling via ST7L in PC.
Materials and Methods
Clinical Specimens
Admission plasma of 15 patients with PC who had received chemotherapy and control plasma samples from 15 patients with PC who had not received chemotherapy were recruited from the Tongren Hospital of Wuhan University and Renmin Hospital of Wuhan University. All peripheral plasma samples were collected in EDTA tubes, centrifuged at 1000g for 15 minutes at 4 °C, and processed within 4 hours. The plasma was then gently transferred to a fresh, RNase/DNase-free, 1.5 mL EP tube (Axygen) and stored at −80 °C. This study was approved by the Ethics and Science Committee of Wuhan University Tongren Hospital (KY2019-004). All human body research was conducted in accordance with the requirements of Helsinki institutions, the state, and the declaration.
Cell Lines and Cell Culture
The human PC cell lines PANC1 and MIAPaCa2 were provided by the Cell Bank of Type Culture Collection of Chinese Academy of Sciences. Drug-resistant cells PANC1/Gem and MIAPaCa2/Gem were donated by the teacher of the Central Laboratory of Renmin Hospital of Wuhan University. They continue to treat PC cells with increasing concentrations of GEM, which makes them resistant to GEM. All cells were cultured in Dulbecco’s modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum (NQBB), 100 mg/mL streptomycin, and 100 IU/mL penicillin (Thermo Fisher Scientific). Cells were incubated in a humidified incubator at 37 °C with 5% CO2.
RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA from cell lines was isolated using the TRIzol reagent (Invitrogen). Plasma miRNA from patients with PC was extracted from a mirVana PARIS kit (Ambion), according to the manufacturer’s instructions. The plasma miRNA and total RNA reverse transcription were performed using the reverse transcription kit (TOYOBO). For quantitative reverse transcription polymerase chain reaction (qRT-PCR), 2 µg total RNA was reverse transcribed with miR-331-3p, U6 RT primers, or oligo-dT with M-MLV reverse transcriptase. The qRT-PCR was performed with kits and produced the following reaction: 2 µL RT products, 5 pmol forward primer, 5 pmol reverse primer, 7.5 µL 2X SYBR-Green buffer, and nuclease-free water to 15 μL. The reverse transcription products were subsequently analyzed using UltraSYBR Mixture (ComWin Biotech) on an ABI StepOne Plus qPCR system (Applied Biosystems). Primers of miR-331-3p and U6 were obtained from RiboBio Corporation. MicroRNA expression levels were normalized with U6 snRNA as a reference and expressed as 2−[CT (miRNA) − CT (U6)], with CT denoting the threshold cycle.
Cell Transfection
Hsa-miR-331-3p mimic (sense: 5′-GCCCCUGGG CCUAUCCUAGAA-3′; antisense: 5′-CUAGGAUAGGCCCAGGGGCUU-3′), hsa-miR-331-3p inhibitor (5′-UUCUAG GAU AGG CCCAGG GGC-3′), hsa-miR mimic negative control (sense: 5′-UUC UCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′), and hsa-miR inhibitor negative control (5′-CAGUACUUUUGUGUAGUA CAA-3′) were synthesized by GenePharma. PANC-1 and MIA paCa-2 cells were transfected with miR-331-3p mimics (40 nM), miR-331-3p inhibitors (80 nM), or their negative control using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. The ST7L expression plasmid was constructed by inserting the ST7L open reading frame sequence into the pCMV6 vector (OriGene). The plasmid was transfected according to the manufacturer’s instructions.
Analysis of Cell Apoptosis
Pancreatic cancer cells were transfected in 6-well plates and 20 µM GEM (Sigma) was added to each well the next day. After incubating for 24 hours, cells were washed by phosphate-buffered saline (PBS) and stained using the Annexin V-PE Detection kit (BD Biosciences), according to the manufacturer’s protocols. All of the samples were analyzed using the FACS Caliber II Sorter and Cell Quest FACS System (BD Biosciences).
Western Blot Analysis
Cells were cleaned with 1× PBS buffer and then lysed in radioimmunoprecipitation assay buffer containing protease inhibitors (Beyotime), and the protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific), according to the manufacturer’s protocols. Mix the soluble lysate with sample buffer and boil for 10 minutes. Equal amounts of proteins were separated by 10% sodiumdodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to polyvinylidene difluoride membranes (Millipore). The membranes were then blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 (TBST) for 2 hours, then followed by incubation with the primary antibodies against multidrug resistance protein 1 (MDR1; 1:1000; BD Biosciences), multidrug resistance-related protein 1 (MRP1; 1:1000; BD), breast cancer resistance protein (BCRP; 1:1000; BD), β-catenin (1:1000; Abcam), c-myc (1:1000; Abcam), cyclin D1 (1:1000; Abcam), Bcl-2 (1:1000; Abcam), Caspase-3 (1:500; Abcam), and β-actin (1:1000; Santa Cruz) overnight at 4 °C. After washing with TBST, the blots were incubated with horseradish peroxidase conjugated secondary antibody (1:5000; A0216; Beyotime Institute of Biotechnology) at 37 °C for 1 hour. Thereafter, the protein level was detected by the enhanced chemiluminescence Western blot analysis system.
Analysis of IC50
Cell Counting Kit-8 (CCK-8) analysis kit (Dojindo) determined the drug resistance. Cells were seeded into a 96-well plate at a density of 2 × 103 cells per well, and 0, 0.2, 1, 5, 25, and 125 μM GEM was added to the 96-well plate the next day. After 72 hours of incubation, 10 µL CCK-8 solution was added to each pore. The cells were incubated at 37 °C for 2 hours, and the absorbance at 450 nm was measured by enzyme-linked immunosorbent assay (Dasit).
Statistical Analysis
All the statistical analysis was carried out by SPSS software, while the illustration data were carried out by graphic flat prism. Student t test or 1-way analysis of variance was used to evaluate statistical significance, which was expressed as mean ± SD (P < .05).
Results
MiR-331-3p Is Upregulated in GEM-Treated PC Cells and Plasma From Chemotherapy Patients
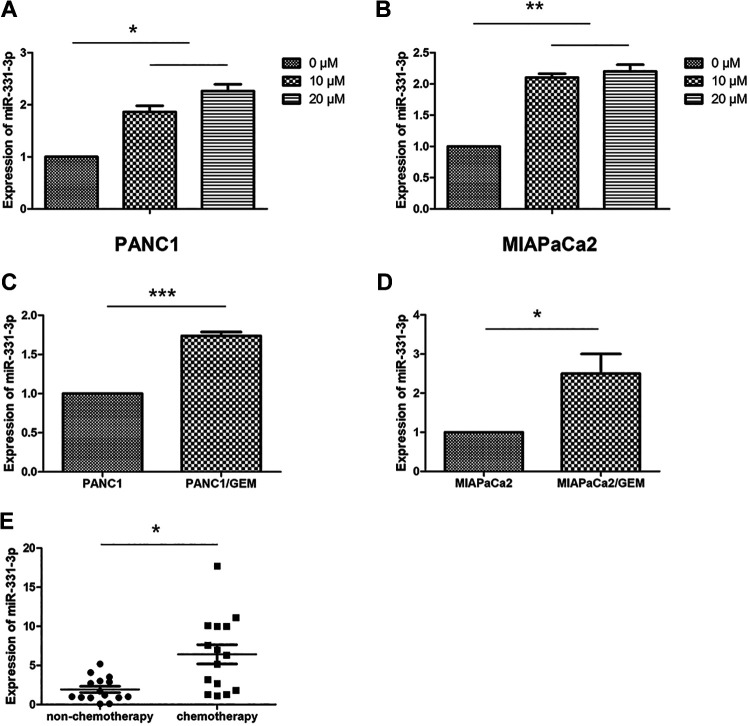
Gemcitabine is now recognized as a drug for the treatment of PC. It is reported that GEM stops DNA strand synthesis, breaks DNA strands, induces apoptosis, and induces apoptosis of cancer cells,3 so we use GEM to study drug resistance in PC cells. To investigate whether miR-331-3p was involved in PC drug resistance, we used qPCR to detect the expression of miR-331-3p in these cells. We observed that miR-331-3p was upregulated in PANC1 and MIAPaCa2 cells treated with GEM (0, 10, and 20 μM) for 6 hours (Figure 1A and B). We then detected the expression of miR-331-3p in the drug-resistant cells, PANC1/Gem, and MIAPaCa2/Gem. Our results showed that mir-331 expression in PANC1/GEM cells was upregulated when compared with PANC1 cells alone and that Mir-331 expression in MIAPaCa2/Gem cells was also upregulated when compared with MIAPaCa2 cells alone (Figure 1C and D). In addition, miR-331-3p was upregulated in plasma fractions from 15 patients with PC receiving chemotherapy, when compared with control plasma fractions from 15 patients with PC who had not received chemotherapy (Figure 1E). These results indicated that miR-331-3p is closely related to the drug resistance of PC.
Figure 1.
MiR-331-3p is upregulated in drug-treated pancreatic cancer (PC) cells and plasma of chemotherapy patients. A and B, Quantitative polymerase chain reaction (qPCR) analysis of relative expression levels of miR-331p in PANC1 and MIAPaCa2 cells treated with gemcitabine (GEM; 0, 10, 20 μM). C and D, The qPCR analysis of relative expression levels of miR-331p in PANC1/Gem and MIAPaCa2/Gem. E, The qPCR analysis of relative expression levels of miR-331-3p in plasma of 15 patients with PC who had received chemotherapy and control plasma samples from 15 patients with PC who had not received chemotherapy. All data are presented as the mean ± SD. *P < .05. **P < .01. ***P < .001.
MiR-331-3p Promotes Drug Resistance in PC Cells
In considering PC apoptosis induced by GEM, we treated PC cells with 10 μM GEM, which were transfected with miR-331-3p mimics or miR-331-3p inhibitors. After incubation for 24 hours, we analyzed apoptosis by flow cytometry and found that miR-331-3p overexpression reduced the numbers of apoptotic cells in PANC1 and MIAPaCa2 cells, while miR-331-3p inhibition increased these numbers (Figure 2A and B). To further explore the effects of miR-331-3p on apoptosis in PC cells treated with 10 μM GEM, we investigated the expression of the apoptosis-related genes, Bcl-2, and Caspase-3 by Western blotting. The results showed that miR-331-3p overexpression decreased Bcl-2 expression and increased Caspase-3 expression, whereas inhibition of miR-331-3p had the opposite effect (Figure 2C and D). Next, we used the CCK-8 kit to measure the IC50 to reflect PC cell sensitivity to GEM. Our results showed that the IC50 of PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimics was significantly increased, while the IC50 of cells transfected with miR-331-3p inhibitors was significantly decreased (Figure 2E and F). Multidrug resistance protein 1, MRP1, and BCRP are believed to be related to drug resistance23-25; therefore, we performed Western blotting to detect the expression of these drug resistance markers in PANC1 and MIAPaCa2 cells. Our results showed that miR-331-3p overexpression increased the expression of MDR1, MRP1, and BCRP. Conversely, miR-331-3p inhibition decreased the expression of MDR1, MRP1, and BCRP (Figure 2G and H). These results supported the hypothesis that miR-331-3p promoted drug resistance in PC cells.
Figure 2.
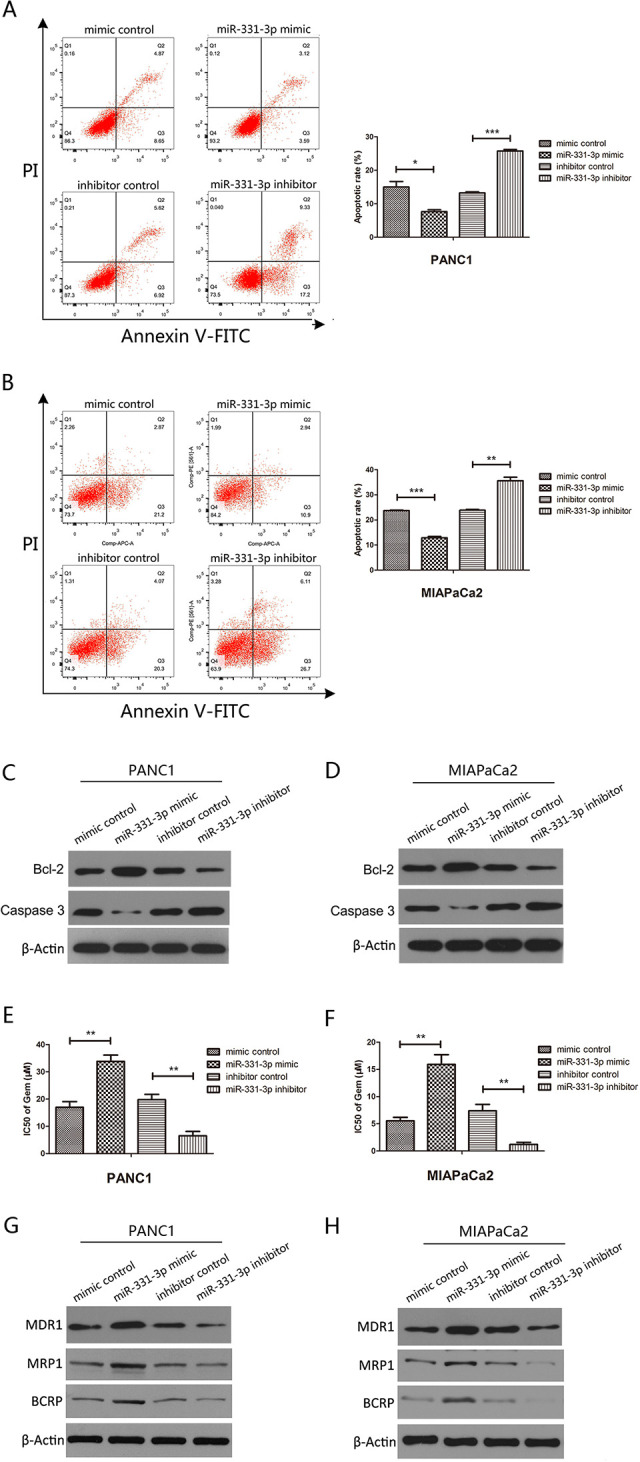
MiR-331-3p increases gemcitabine (GEM) resistance in pancreatic cancer cell lines. A and B, Flow cytometry assessment of apoptosis in PANC1and MIAPaCa2 cells transfected with miR-331-3p mimics or inhibitors and treated with 20 µM GEM for 24 hours. The total events shown in the lower right-hand and upper right-hand quadrants are apoptotic cells. C and D, Western blot analysis of Bcl-2 and Caspase-3 in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimic or inhibitors. E and F, The IC50 values of GEM in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimics or inhibitors for 72 hours using Cell Counting Kit-8 (CCK-8) assay. G and H, Western blot analysis of multidrug resistance protein 1 (MDR1), multidrug resistance-related protein 1 (MRP1), and breast cancer resistance protein (BCRP) in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimic or inhibitors. β-actin is used as loading control. All data are presented as the mean ± SD. *P < .05. **P < .01. ***P < .001.
MiR-331-3p Promotes Drug Resistance by Targeting ST7L in PC Cells
Our previous study confirmed that ST7L was a new downstream target of miR-331-3p,13 but whether miR-331-3p promoted drug resistance by targeting ST7L was still not clear. We transfected miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids into PANC-1 and MIAPaCa2 cells. The results from flow cytometry showed that ST7L overexpression promoted apoptotic effects in PC cells mediated by miR-331-3p (Figure 3A and B). The results from Western blotting further confirmed that ST7L overexpression promoted apoptotic effects in PC cells, mediated by miR-331-3p (Figure 3C and D). In addition, we confirmed that overexpression of miR-331-3p increased the GEM IC50 in PC cells, while ST7L overexpression reversed the promotion of miR-331-3p (Figure 3E and F). Additionally, our Western blotting assays demonstrated that miR-331-3p overexpression upregulated the expression of MDR1, MRP1, and BCRP, but the effects were partially offset by ST7L reintroduction (Figure 3G and H). Collectively, these data demonstrated that ST7L reintroduction partially reversed miR-331-3p-mediated drug resistance in PC cells.
Figure 3.
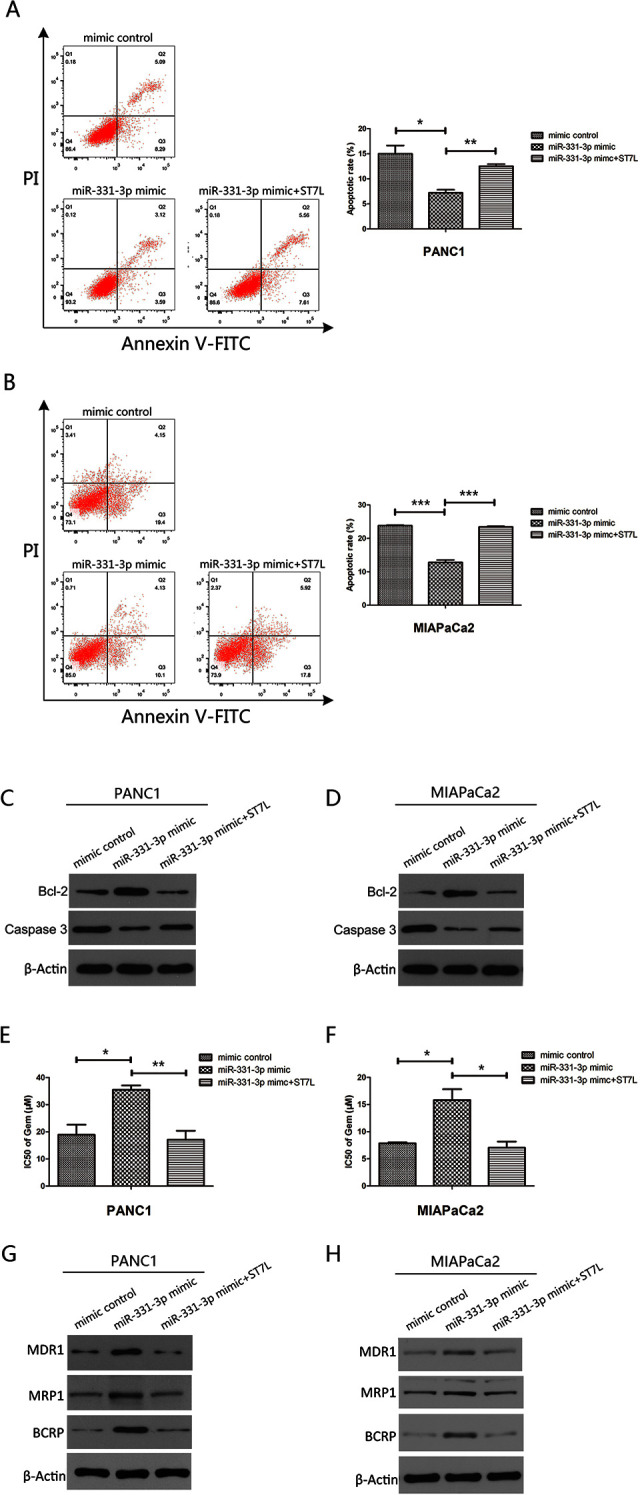
MiR-331-3p participates in drug resistance of pancreatic cancer cells via targeting ST7L. A and B, Flow cytometry assessment of apoptosis in PANC1and MIAPaCa2 cells transfected with miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids and treated with 20 µM gemcitabine (GEM) for 24 hours. The total events shown in the lower right-hand and upper right-hand quadrants are apoptotic cells. C and D, Western blot analysis of Bcl-2 and Caspase-3 in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids and treated with 20 µM GEM. E and F, The IC50 values of GEM in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids for 72 hours using Cell Counting Kit-8 (CCK-8) assay. G and H, Western blot analysis of multidrug resistance protein 1 (MDR1), multidrug resistance-related protein 1 (MRP1), and breast cancer resistance protein (BCRP) in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimic or miR-331-3p mimics plus ST7L expression plasmids. β-actin is used as loading control. All data are presented as the mean ± SD. *P < .05, **P < .01. ***P < .001. β-actin is used as loading control.
MiR-331-3p Activates WNT/β-Catenin Signaling via ST7L
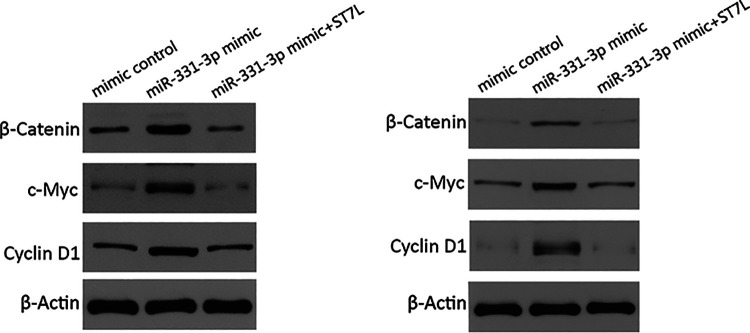
The Wnt/β-catenin signaling pathway is involved in the development and progression of several human cancers.20 β-catenin is the major effector of Wnt/β-catenin signaling, and Cyclin D and C-myc are key downstream genes of this Wnt/β-catenin signaling pathway. When the Wnt signal is activated, the inhibition of β-catenin phosphorylation leads to increased β-catenin in cells, allowing increased β-catenin to enter the nucleus to activate its targets.20 To determine whether miR-331-3p participated in drug resistance by activating Wnt/β-catenin signaling in PC cells, β-catenin, Cyclin D, and C-myc expression were analyzed in PC cells transfected with miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids. Western blotting assays showed that miR-331-3p overexpression increased activated β-catenin, C-myc, and cyclin D1 protein levels, which were rescued by increasing ST7L expression, and there was no significant difference in the expression of total β-catenin between miR-331-3p mimics and miR-331-3p mimics plus ST7L (Figure 4). These results demonstrated that miR-331-3p promoted drug resistance by targeting ST7L and activating the Wnt/β-catenin signaling pathway in PC cells.
Figure 4.
MiR-331-3p actives Wnt/β-catenin signaling via ST7L. Western blot analysis of activated β-catenin, total β-catenin, Cyclin D1, and C-myc in PANC1 and MIAPaCa2 cells transfected with miR-331-3p mimics or miR-331-3p mimics plus ST7L expression plasmids. β-actin is used as loading control.
Discussion
At present, the treatment of PC requires comprehensive treatments with radical surgical resection as the core approach.26 However, because of its rapid progress and insidious onset, many patients lose the opportunity for surgical resection. With the wide use of chemotherapy drugs, the sensitivity of PC to GEM and other chemotherapy drugs has decreased significantly, which makes PC treatment more difficult.3,4 Therefore, the investigation of drug resistance mechanisms in PC is crucial.
MicroRNAs are short RNA molecules of 19 to 25 nucleotides in size that regulate posttranscriptional silencing of target genes.27 A single miRNA can target hundreds of mRNAs and influence the expression of many genes involved in functional interacting pathways.28 Recently, the role of miRNAs in PC drug resistance has been recognized. Several reports have shown that some miRNAs, such as mir-455-3p, miR-141, mir-184, miR-199a, and mir-421, contribute to drug resistance in different tumors.29-33 Mir-331-3p is generally upregulated in several human cancers, including PC.13 Recent studies have shown that mir-331 is involved in the occurrence and development of tumors.8-12 However, whether mir-331-3p is related to drug resistance in PC cells has not been explored.
In this study, we demonstrated that miR-331-3p contributed to drug resistance in PC cells and that miR-331-3p expression in plasma fractions from 15 patients with PC who had received chemotherapy was upregulated when compared with control plasma fractions from 15 patients with PC who had not received chemotherapy. We then explored the possible mechanisms of miR-331-3p-induced GEM resistance in PC cells. We used flow cytometry to identify cell apoptotic effects in PC cells treated with GEM. Our results showed that miR-331-3p inhibited apoptosis in both PANC1 and MIAPaCa2 cells. Furthermore, Western blot analysis demonstrated that miR-331-3p promoted apoptosis in PC cells by regulating the apoptosis-related proteins, Bcl-2, and Caspase-3. IC50 values of GEM in PANC1 and MIAPaCa2 cells indicated that miR-331-3p overexpression significantly increased these IC50 values, whereas miR-331-3p inhibition decreased them. Furthermore, Western blotting demonstrated that miR-331-3p promoted drug resistance in PC cells by regulating the drug resistance-related proteins MDR1, MRP1, and BCRP.
ST7L was confirmed by its similarity to the ST7 tumor suppressor gene in the chromosome 7q31 region and clustering with the WNT2B gene in a tail-to-tail manner in a chromosomal region. WNT2 and the WNT2B isoform 2 (WNT2B2) are positive regulators of the WNT-β-catenin signaling pathway, which plays important roles in carcinogenesis.34 In this study, by transfecting PC cells with miR-331-3p mimics, or miR-331-3p mimics plus ST7L expression plasmids, we confirmed that miR-331-3p participated in drug resistance via ST7L in PC cells. The Wnt/β-catenin pathway has long been associated with tumorigenesis, tumor plasticity, and cancer stem cells, and high expression levels of the Wnt pathway have been associated with poor cancer survival.35 ST7L was reported to inhibit the Wnt/β-catenin signaling pathway and act as a tumor suppressor gene in several cancers.16,18,22 β-catenin is a primary component of Wnt/β-catenin signaling, and Cyclin D and C-myc are major downstream effectors of Wnt/β-catenin signaling.20 When Wnt signal is activated, the inhibition of β-catenin phosphorylation leads to increased β catenin in cells, and increased β-catenin enters the nucleus to activate its targets; therefore, we examined the expression of activated β-catenin, total β-catenin, Cyclin D1, and C-myc in our PC cells. We observed that miR-331-3p overexpression increased activated β-catenin, Cyclin D1, and C-myc expression in PC cells and that ST7L appeared to reverse the promotion of miR-331-3p on the Wnt/β-catenin pathway. These findings suggested that miR-331-3p was linked to drug resistance in PC cells by activating Wnt/β-catenin signaling via ST7L.
In summary, we provided strong evidence that miR-331-3p directly targeted ST7L to promote drug resistance in PC cells. Additionally, our studies elucidated a novel mechanism whereby miR-331-3p was involved in drug resistance by activating the Wnt/β-catenin signaling pathway via ST7L. Our data suggested that miR-331-3p may be a promising target in the treatment of PC.
Abbreviations
- BCRP
breast cancer resistance protein
- CCK-8
Cell Counting Kit-8
- EMT
epithelial-to-mesenchymal transition
- GEM
gemcitabine
- MDR1
multidrug resistance protein 1
- MiRNA
microRNA
- MRP1
multidrug resistance-related protein 1
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PC
pancreatic cancer
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- TBST
Tris-buffered saline with Tween-20
Footnotes
Authors’ Note: T.Z., X.T., and X.H conceived and designed the experiments. T.Z., J.T., M.L., and Q.Z. performed the experiments. T.Z., W.L., W.C., M.L., and X.T. analyzed data. X.H. and T.Z. wrote the manuscript. Ting Zhan and Xiaoli Chen contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported from the Natural Science Foundation of Hubei Province in China 2018CFB338 (T.Z.), the Wuhan Health and Family Planning Commission Medical Research Project WX19Q40 (T.Z.), the Health Commission of Hubei Province scientific research project WJ2019H387 (T.Z.), the Central Guidance Local Science and Technology Development Special of Hubei Province 2019ZYYD067 (X.H.).
ORCID iD: Ting Zhan  https://orcid.org/0000-0003-0622-7890
https://orcid.org/0000-0003-0622-7890
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21(11):3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voutsadakis IA. Molecular predictors of gemcitabine response in pancreatic cancer. World J Gastrointest Oncol. 2011;3(11):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takamori H, Keiichiro K, Masahiko H, et al. Perioperative intra-arterial and systemic chemotherapy for pancreatic cancer. Ann Surg Oncol. 2011;18(4):1110–1115. [DOI] [PubMed] [Google Scholar]
- 5. Kishikawa T, Motoyuki O, Motoko O, Takeshi Y, Akemi T, Kazuhiko K. Circulating RNAs as new biomarkers for detecting pancreatic cancer. World J Gastroenterol. 2015;21(28):8527–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng H, Yalei W, Jiaojiao S, et al. MicroRNA-148a suppresses the proliferation and migration of pancreatic cancer cells by down-regulating ErbB3. Pancreas. 2016;45(9):1263–1271. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Sarkar FH. MicroRNA targeted therapeutic approach for pancreatic cancer. Int J Biol Sci. 2016; 12(3):326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao D, Sui Y Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35(2):1075–1082. [DOI] [PubMed] [Google Scholar]
- 9. Jin W, Ning Z, Lingling W, Jieli Y, Fugen Y, Kunhe Z. MiR-331-3p Inhibition of the hepatocellular carcinoma (HCC) Bel-7402 cell line by down-regulation of E2F1. J Nanosci Nanotechnol. 2019;19(9):5476–5482. [DOI] [PubMed] [Google Scholar]
- 10. Li X, Jiali Z, Yuanqi L, et al. MicroRNA-331-3p inhibits epithelial-mesenchymal transition by targeting ErbB2 and VAV2 through the Rac1/PAK1/beta-catenin axis in non-small-cell lung cancer. Cancer Sci. 2019;110(6):1883–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang L, Xing S, Xin C, et al. Circular RNA Circ CACTIN promotes gastric cancer progression by sponging MiR-331-3p and regulating TGFBR1 expression. Int J Biol Sci. 2019;15(5):1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epis MR, Keith MG, Dianne J.B, et al. MiR-331-3p and aurora kinase inhibitor II co-treatment suppresses prostate cancer tumorigenesis and progression. Oncotarget. 2017;8(33):55116–55134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Hesheng L, Xiaoyi L, et al. MiR-331-3p functions as an oncogene by targeting ST7 L in pancreatic cancer. Carcinogenesis. 2018;39(8):1006–1015. [DOI] [PubMed] [Google Scholar]
- 14. Katoh M. Molecular cloning and characterization of ST7 R (ST7-like, ST7 L) on human chromosome 1p13, a novel gene homologous to tumor suppressor gene ST7 on human chromosome 7q31. Int J Oncol. 2002;20(6):1247–1253. [PubMed] [Google Scholar]
- 15. Kirikoshi H, Katoh M. Expression of ST7 R (ST7-like, ST7 L) in normal tissues and cancer. Int J Oncol. 2002;21(1):193–196. [PubMed] [Google Scholar]
- 16. Chen L, Anling Z, Yongli L, et al. MiR-24 regulates the proliferation and invasion of glioma by ST7 L via beta-catenin/Tcf-4 signaling. Cancer Lett. 2013;329(2):174–180. [DOI] [PubMed] [Google Scholar]
- 17. Yang Z, Xiang-Ling W, Ru B, et al. MiR-23a promotes IKK alpha expression but suppresses ST7 L expression to contribute to the malignancy of epithelial ovarian cancer cells. Br J Cancer. 2016;115(6):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Zhuang L, Xin W, Zusen W, et al. MicroRNA-23b functions as an oncogene and activates AKT/GSK3beta/beta-catenin signaling by targeting ST7 L in hepatocellular carcinoma. Cell Death Dis. 2017;8(5):e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Sahli S, Ying X, Lisheng W, Sheng L. Wnt signaling in cancer metabolism and immunity. Cancers (Basel). 2019;11(7):904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh N, Uday H, Ankita M, et al. The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci. 2019;1443(1):54–74. [DOI] [PubMed] [Google Scholar]
- 21. Emons G, Melanie S, Sebastian R, et al. Chemoradiotherapy resistance in colorectal cancer cells is mediated by Wnt/beta-catenin signaling. Mol Cancer Res. 2017;15(11):1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Fengxia Y, Meiyan W, Wenjun C, Zhen Y. MiR-378 functions as an onco-miRNA by targeting the ST7L/Wnt/beta-catenin pathway in cervical cancer. Int J Mol Med. 2017;40(4):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu-Kreyche P, Hong S, Anthony MM, et al. Lysosomal P-gp-MDR1 confers drug resistance of brentuximab vedotin and its cytotoxic payload monomethyl Auristatin E in tumor cells. Front Pharmacol. 2019;10:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conseil G, Arama-Chayoth M, Yossi T, Cole PCS. Structure-guided probing of the leukotriene C4 binding site in human multidrug resistance protein 1 (MRP1; ABCC1). FASEB J. 2019;33(10):10692–10704. [DOI] [PubMed] [Google Scholar]
- 25. Willis BA, Scott WA, Ayan-Oshodi M, et al. Assessment of transporter polymorphisms as a factor in a BCRP drug interaction study with lanabecestat. J Clin Pharmacol. 2020;60(1):107–116. [DOI] [PubMed] [Google Scholar]
- 26. Shi S, Wantong Y, Jin X, Jiang L, Chen L, Xianjun Y. Combinational therapy: new hope for pancreatic cancer? Cancer Lett. 2012;317(2):127–135. [DOI] [PubMed] [Google Scholar]
- 27. Brunetti O, Antonio R, Aldo S, et al. MicroRNA in pancreatic adenocarcinoma: predictive/prognostic biomarkers or therapeutic targets? Oncotarget. 2015;6(27):23323–23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18(3):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seki N. A commentary on MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J Hum Genet. 2011;56(5):339–340. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Zhou T, Ya L, Gang C, Yunping L, Xing H. Microrna-199a is able to reverse cisplatin resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett. 2013;6(3):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tung MC, Po-Lin L, Ya Wen C, et al. Reduction of microRNA-184 by E6 oncoprotein confers cisplatin resistance in lung cancer via increasing Bcl-2. Oncotarget. 2016;7(22):32362–323S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhan T, Xiaodong H, Xia T, et al. Downregulation of MicroRNA-455-3p links to proliferation and drug resistance of pancreatic cancer cells via targeting TAZ. Mol Ther Nucleic Acids. 2018;10:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ge X, Xinyang L, Fengjuan L, et al. MicroRNA-421 regulated by HIF-1alpha promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7(17):24466–24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saitoh T, Katoh M. Molecular cloning and characterization of mouse St7r (St7-like, St7 l). Int J Mol Med. 2002;10(1):119–123. [PubMed] [Google Scholar]
- 35. Lopez-Knowles E, Sarah JZ, McNeil CM, et al. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19(1):301–309. [DOI] [PubMed] [Google Scholar]