Abstract
This research aimed to investigate the effects of resistance exercise on symptoms, physical function, and quality of life (QoL) in gastrointestinal cancer patients undergoing chemotherapy. Patients were quasi-randomly divided into the resistance exercise group and the relaxation control group, and machine-based resistance exercise was performed twice a week for 12 weeks under the guidance of experienced therapists. The QoL of patients was analyzed by EORTC-QLQ-C30. Resistance exercise training significantly reduced the incidences of lack of energy (inter-group P = .011), nausea (inter-group P = .007), acid reflux (inter-group P = .042), and back pain (inter-group P = .0009). Twelve weeks of resistance exercise training significantly elevated the muscular strength of leg press (inter-group P = .021) and leg extension (inter-group P = .041), and the muscular endurance of leg press (inter-group P = .005). The participants’ performance in 6-m fast walk (inter-group P = .008), 6-m backwards walk (inter-group P = .016), and chair rise (inter-group P = .031) were dramatically improved. Fatigue (inter-group P = .024) and appetite loss (inter-group P = .012) in the resistance exercise group were significantly lower than the relaxation control group. In conclusion, the beneficial effects of resistance exercise on symptoms, physical function and QoL in gastrointestinal cancer patients undergoing chemotherapy were demonstrated. Resistance exercise training reduced the incidences of nausea and acid reflux, improved physical function, and alleviated fatigue and appetite loss in gastrointestinal cancer patients undergoing chemotherapy.
Keywords: gastrointestinal cancer, chemotherapy, resistance exercise, quality of life
Introduction
Gastrointestinal cancer is comprised of several different types of cancers, such as gastric, hepatic, pancreatic, and colorectal cancers.1 The pathogenesis of gastrointestinal cancer triggers a variety of severe symptoms, including pain, nausea, diarrhea, fatigue, and sleeping disorders.2,3 Chemotherapy is one of the widely used therapeutic approaches for gastrointestinal cancer. Despite the benefits in the treatment of gastrointestinal cancer, chemotherapy causes several side effects which interfere with the patients’ functional capacity and quality of life (QoL).4 Possible consequences of chemotherapy include nausea, ulcerations, emesis, diarrhea, severe malabsorption, dehydration, and electrolyte disorder.5 Advances in terms of improved survival alongside with better QoL in patients with gastrointestinal cancer are based on the improved treatment of acute toxic effects caused during and after chemotherapy.
Physical exercise is an effective method in improving the physical health of cancer patients.6 During chemotherapy, exercise interventions have both physiological and psychological benefits and improve the QoL in cancer patients.7 Physical exercises include aerobic and resistance training. Most randomized exercise trials focus on the effects of aerobic training.8 Recently, evidence has demonstrated that resistance exercise has larger effect sizes than aerobic exercise in modulating cancer-related fatigue.9 Resistance exercise is a kind of exercise intervention which causes the contraction of muscles against external resistance and improves the muscular mass, strength, and bone density.10 Recent evidence indicates that resistance exercise during neoadjuvant chemoradiation treatment is feasible in rectal cancer patients and promotes physical function.11 In breast cancer patients undergoing adjuvant chemotherapy, resistance exercise alleviates physical fatigue and maintains QoL.12 It has been demonstrated that the symptoms and physical condition of gastrointestinal cancer patients during palliative chemotherapy are enhanced by both resistance and aerobic exercise.13 But the function of resistance exercise is only confirmed in gastrointestinal cancer patients during palliative chemotherapy.
In this research, we aimed to investigate the effects of resistance exercise on symptoms, physical function and QoL in gastrointestinal cancer patients undergoing chemotherapy.
Methods
Patient Eligibility
This study was registered in Chinese Clinical Trial Register (#ChiCTR2000033103). Patients with gastrointestinal cancer undergoing chemotherapy were recruited in this research from 2016 to 2019. Gastrointestinal cancers included rectal, cholangiocellular, colon, gastric, and pancreatic cancer. Patients with tumor–node–metastasis (TNM) stage II, III, or IV tumor were recruited. Patients who received concomitant radiotherapy were not excluded, neither those who underwent tumor resection surgery before their inclusion in the study. Eligibility criteria included age ≥18 years, life expectancy ≥6 months, body mass index (BMI) ≥18 kg/m2, and having capacity and willingness to execute the study protocol. Exclusion criteria included participating in other aerobic or resistance training before this research, having contraindications for resistance training, or having other concurrent malignant diseases. All the participants in this research were recruited from Quanzhou First Hospital Affiliated to Fujian Medical University. This research was approved by the Ethics Committee of Quanzhou First Hospital Affiliated to Fujian Medical University. All patients involved in this research were informed in detail about the process and purpose of the experiment and signed the corresponding informed consent forms.
Grouping and Randomization
In this study, 237 patients were recruited and 33 of them were excluded. The participants were quasi-randomized into 2 groups according to the order they came for evaluation: resistance exercise group and relaxation control group. Researchers involved in the recruitment, data collection and analyses were blind to patient group assignment.
Exercise Intervention
Machine-based resistance exercise was performed twice a week for 12 weeks under the guidance of experienced therapists. A complete resistance exercise needed approximately 60 minutes and was composed of 8 different machine-based progressive resistance exercises. These exercises included leg extension, leg curl, leg press, shoulder internal and external rotation, seated row, latissimus pull down, shoulder flexion and extension, and butterfly and butterfly reverse. Each exercise included 3 sets for 8 to 12 repetitions at a weight of 60% to 80% of one repetition maximum. In 3 consecutive exercise sessions, if 3 sets of an exercise (12 repetitions) were successfully completed, the weight was increased at least by 5% in the next session.
Relaxation control was composed of progressive muscle relaxation without any aerobic or muscle strengthening exercise.14 Relaxation intervention was also performed approximately 60 minutes twice a week for 12 weeks. The exercise and control intervention were all performed at an exercise facility located in the hospital.
Outcome Measures
Demographic and clinical characteristics of the participants in both resistance exercise group and relaxation control group were recorded. Incidences of symptoms, including loss of appetite, lack of energy, nausea, mouth ulcers, acid reflux, cough, back pain, fever, and alopecia, were all calculated at baseline and post-intervention. Adverse event items were evaluated based on the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE). A grade of 0 indicated no adverse events, whereas grade 1 or higher indicated the presence of adverse events.
The primary outcome was defined as physical functions, as described in detail below. One repetition maximum method was used to assess the dynamic muscular strength for leg press, seated row, leg extension, and chest press. One repetition maximum method was the maximal weight which was lifted. At the weight of 70% of 1 repetition maximum, the maximal repetition number for chest press and leg press were employed to evaluate the muscular endurance of the participants. Physical performance tests included 6-m usual walk, 6-m fast walk, 6-m backwards walk, 400-m walk, and chair rise.
The QoL of the patients were analyzed through the administration of European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30). EORTC QLQ-C30 was a widely used tool for cancer patient QoL evaluation with 30 items. The score of each item was from 0 to 100 and 100 represented the best QoL or worst symptom. The change larger than 10 points in the score of items was considered to be clinically significant.
Statistical Analysis
After collecting the questionnaires, SPSS Statistics Version 22.0 software was employed to perform statistical analysis. Sample size was determined using established statistical power analysis. Differences between means of each compared treatment group were divided by the standard deviation to determine the standardized effect size, then using 5% as significance level in Student t-test followed by Mann Whitney test and 90% power, the minimum required sample size was calculated, which was sufficient for our current sample size after consideration of dropouts. Chi squared test was used to compare the occurrence of symptoms between 2 groups. The changes in an item’s average score after treatment were analyzed through paired t test followed by Wilcoxon matched-pairs signed rank test. Statistical analysis was significant when P value <.05.
Results
Patient Characteristics
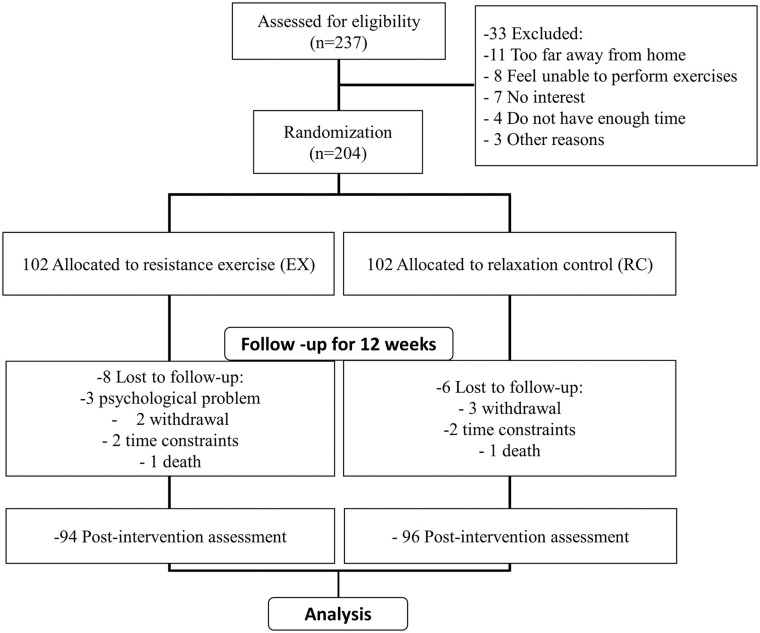
Participant flow in this research is shown in Figure 1. During the evaluation of eligibility, 237 patients met the inclusion criteria and 204 were finally recruited in the research study. The participants were quasi-randomly separated into resistance exercise group (n = 102) and relaxation control group (n = 102). During this research, 8 participants in the resistance exercise group and 6 in the relaxation control group were lost to follow-up. Finally, 94 participants in the resistance exercise group and 96 in the relaxation control group finished the research and their data were recorded and analyzed.
Figure 1.
Research framework of this study.
The baseline demographic and clinical characteristics of participants were summarized in Table 1. The demographic characteristics (age, sex, body weight, body mass index, and tobacco use) showed no significant difference between these 2 groups. Five different cancers were involved in this research, including rectal, cholangiocellular, colon, gastric, and pancreatic cancers.
Table 1.
Demographic and Clinical Characteristics of the Patients Analyzed.
| Characteristics | Study group |
P | |
|---|---|---|---|
| Resistance exercise (n = 94) | Relaxation control (n = 96) | ||
| Age (years) | 55.4 ± 11.6 | 52.3 ± 12.4 | .352 |
| Male gender | 54 (57.4 %) | 51 (53.1 %) | .563 |
| Body weight (kg) | 69.6 ± 14.3 | 71.3 ± 15.4 | .472 |
| BMI | 26.3 ± 4.9 | 25.9 ± 4.4 | .318 |
| Smoking before diagnosis | 30 (31.9 %) | 28 (29.2 %) | .753 |
| Still smoking at baseline | 10 (10.6 %) | 12 (12.5 %) | .821 |
| Tumor diagnosis | |||
| Rectal cancer | 23 (24.5 %) | 20 (20.8 %) | .605 |
| Cholangiocellular cancer | 17 (18.1 %) | 22 (22.9 %) | .474 |
| Colon cancer | 29 (30.8 %) | 24 (25 %) | .419 |
| Gastric cancer | 18 (19.1 %) | 18 (18.8 %) | 1.000 |
| Pancreatic cancer | 7 (7.5 %) | 12 (12.5 %) | .334 |
| Tumor Stage TNM | |||
| II | 19 (20.2 %) | 17 (17.7 %) | .713 |
| III | 49 (52.1 %) | 56 (58.3 %) | .466 |
| IV | 26 (27.7 %) | 23 (24.0 %) | .620 |
| Previous tumor resection | 74 (78.7 %) | 70 (72.9 %) | .399 |
| Time between tumor resection and recruitment (days) | 63.6 ± 18.1 | 55.7 ± 16.3 | .248 |
| Concomitant radiotherapy | 23 (24.5 %) | 15 (15.6 %) | .149 |
| Days since first chemotherapy | 56.3 ±17.6 | 61.4 ± 19.6 | .276 |
| Chemotherapy protocol | |||
| Oxaliplatin + capecitabine | 36 (38.3 %) | 31 (32.3 %) | .448 |
| Fluorouracil + leucovorin | 21 (22.3 %) | 26 (27.1 %) | .503 |
| Oxaliplatin + leucovorin + fluorouracil | 17 (18.1 %) | 23 (23.9 %) | .375 |
| Others | 20 (21.3 %) | 16 (16.7 %) | .462 |
BMI, body mass index.
Values were expressed as n (percentage) or mean ± SD.
Occurrence of Symptoms
Incidences of symptoms in the participants are shown in Table 2. At baseline, there was no difference in the incidences of symptoms between the resistance exercise group and the relaxation control group. After the performance of resistance exercise, the incidences of lack of energy, nausea, acid reflux, and back pain were significantly lower than in the relaxation control group. But resistance exercise did not influence the incidences of loss of appetite, mouth ulcers, cough, fever, and alopecia in the gastrointestinal cancer patients undergoing chemotherapy.
Table 2.
Incidences of Symptoms Reported for Patients Included in The Trial.
| Symptoms | Study group |
P-value | ||
|---|---|---|---|---|
| Resistance exercise (n = 94) | Relaxation control (n = 96) | |||
| Loss of appetite | Baseline | 49 (52.1 %) | 52 (54.2 %) | .885 |
| Post-intervention | 44 (46.8 %) | 56 (58.3 %) | .146 | |
| P-value | 0.559 | 0.663 | ||
| Lack of energy | Baseline | 61 (64.9 %) | 58 (60.4 %) | .551 |
| Post-intervention | 50 (53.2 %) | 69 (71.9 %) | .011 | |
| P-value | 0.138 | 0.127 | ||
| Nausea | Baseline | 54 (57.4 %) | 58 (60.4 %) | .768 |
| Post-intervention | 46 (48.9 %) | 66 (68.8 %) | .007 | |
| P-value | 0.306 | 0.291 | ||
| Mouth ulcers | Baseline | 35 (37.2 %) | 40 (41.7 %) | .556 |
| Post-intervention | 33 (35.1 %) | 46 (47.9 %) | .079 | |
| P-value | 0.879 | 0.468 | ||
| Acid reflux | Baseline | 50 (53.2 %) | 45 (46.9 %) | .468 |
| Post-intervention | 40 (42.6 %) | 56 (58.3 %) | .042 | |
| P-value | 0.189 | 0.148 | ||
| Cough | Baseline | 37 (39.4 %) | 35 (36.5 %) | .765 |
| Post-intervention | 30 (31.9 %) | 42 (43.8 %) | .102 | |
| P-value | 0.361 | 0.377 | ||
| Back pain | Baseline | 32 (34.1 %) | 30 (31.3 %) | .757 |
| Post-intervention | 18 (19.2 %) | 40 (41.7 %) | .0009 | |
| P-value | 0.031 | 0.177 | ||
| Fever | Baseline | 23 (24.5 %) | 26 (27.1 %) | .741 |
| Post-intervention | 18 (19.1 %) | 25 (26.1 %) | .299 | |
| P-value | 0.480 | 0.871 | ||
| Alopecia | Baseline | 34 (36.2 %) | 30 (31.3 %) | .539 |
| Post-intervention | 27 (28.7 %) | 36 (37.5 %) | .219 | |
| P-value | 0.350 | 0.448 | ||
Values were expressed as n (percentage, %).
Chi square test. Bold values indicate P < 0.05.
Muscular Strength, Muscular Endurance, and Physical Performance
Muscular strength, muscular endurance, and physical performance for patients included in the trial are shown in Table 3. For muscular strength, both leg press and leg extension were significantly improved by resistance exercise, while the chest press and seated row were not influenced by resistance exercise, as no difference was observed between these 2 groups. For muscular endurance, only leg press showed a significant progress with the help of resistance exercise, while chest press was not influenced in this process.
Table 3.
Effects of Resistance Exercise (EX) on Physical Performance, Muscular Strength, and Endurance Measures for Patients Included in The Trial.
| Variables | Study group |
P-value | ||
|---|---|---|---|---|
| Resistance exercise (n = 94) | Relaxation control (n = 96) | |||
| Muscular strength (kg) | ||||
| Chest press | Baseline | 35.2 ± 17.2 | 36.1 ± 16.9 | .256 |
| Post-intervention | 39.4 ± 16.3 | 32.6 ± 17.8 | .315 | |
| P-value | 0.417 | 0.126 | ||
| Seated row | Baseline | 65.3 ± 21.5 | 66.8 ± 22.9 | .523 |
| Post-intervention | 67.1 ± 19.6 | 63.9 ± 23.1 | .428 | |
| P-value | 0.371 | 0.442 | ||
| Leg press | Baseline | 118.4 ± 42.3 | 121.6 ± 39.5 | .294 |
| Post-intervention | 158.3 ± 41.7 | 112.8 ± 40.1 | .021 | |
| P-value | 0.034 | 0.086 | ||
| Leg extension | Baseline | 54.8 ± 18.6 | 55.9 ± 19.2 | .548 |
| Post-intervention | 69.8 ± 23.3 | 51.7 ± 24.8 | .041 | |
| P-value | 0.028 | 0.215 | ||
| Muscular endurance (rep) | ||||
| Chest press | Baseline | 9.7 ± 3.8 | 10.5 ± 4.8 | .263 |
| Post-intervention | 12.4 ± 4.2 | 9.5 ± 5.2 | .114 | |
| P-value | 0.174 | 0.632 | ||
| Leg press | Baseline | 11.5 ± 5.1 | 10.2 ± 6.3 | .424 |
| Post-intervention | 22.4 ± 6.8 | 8.6 ± 7.2 | .005 | |
| P-value | 0.016 | 0.173 | ||
| Physical performance (s) | ||||
| 6-m usual walk | Baseline | 4.5 ± 0.7 | 4.2 ± 0.6 | .328 |
| Post-intervention | 3.9 ± 0.5 | 4.6 ± 0.7 | .037 | |
| P-value | 0.264 | 0.327 | ||
| 6-m fast walk | Baseline | 2.9 ± 0.3 | 2.8 ± 0.4 | .137 |
| Post-intervention | 2.6 ± 0.4 | 3.1 ± 0.6 | .008 | |
| P-value | 0.048 | 0.216 | ||
| 6-m backwards walk | Baseline | 14.6 ± 3.2 | 14.4 ± 3.8 | .527 |
| Post-intervention | 11.8 ± 2.6 | 15.2 ± 2.9 | .016 | |
| P-value | 0.031 | 0.365 | ||
| 400-m walk | Baseline | 227.1 ± 24.7 | 231.4 ± 27.1 | .448 |
| Post-intervention | 222.4 ± 28.2 | 238.3 ± 24.9 | .116 | |
| P-value | 0.292 | 0.491 | ||
| Chair rise | Baseline | 10.7 ± 1.3 | 10.3 ± 1.5 | .554 |
| Post-intervention | 9.2 ± 1.6 | 10.9 ± 1.2 | .031 | |
| P-value | 0.023 | 0.117 | ||
Values were expressed as mean ± SD. Bold values indicate P < 0.05.
After the 12-week resistance exercise training, when compared with the participants in relaxation control group, there were significant improvements in the 6-m usual walk, 6-m fast walk, 6-m backwards walk, and chair rise. There was no significant change in the 400-m walk time.
Quality of Life
Before and after the training intervention, the QoL of the participants in both groups were evaluated through EORTC-QLQ-C30 (Table 4). The global QoL score showed no difference between these 2 groups after resistance exercise training. In the relaxation control group, 12-week resistance exercise training did not significantly elevate the score of physical function and role function. But in the relaxation control group, the scores of physical function and role function declined dramatically during this period. So, we observed significantly higher scores of physical function and role function in the resistance exercise group than in the relaxation control group after the training intervention. The same phenomenon was also observed in the score of social function. The scores of fatigue and appetite loss were dramatically lower in the resistance exercise group than in the relaxation control group after the training intervention. It was indicated that resistance exercise training had benefits in alleviating the symptoms generated during chemotherapy.
Table 4.
Effects of Resistance Exercise (EX) on Quality of Life (EORTC-QLQ-C30).
| Outcomes | Study group |
P-value | ||
|---|---|---|---|---|
| Resistance exercise (n = 94) | Relaxation control (n = 96) | |||
| Quality of life-EORTC QLQ30 (scale 0–100) | ||||
| Global QoL | Baseline | 56.8 ± 19.5 | 58.6 ± 20.3 | .486 |
| Post-intervention | 60.3 ± 21.7 | 55.2 ± 22.6 | .116 | |
| P-value | 0.427 | 0.271 | ||
| Physical function | Baseline | 83.6 ± 15.7 | 84.7 ± 18.2 | .724 |
| Post-intervention | 86.3 ± 17.4 | 72.4 ± 15.9 | .035 | |
| P-value | 0.458 | 0.026 | ||
| Emotional function | Baseline | 69.2 ± 22.7 | 67.5 ± 19.9 | .552 |
| Post-intervention | 68.3 ± 18.6 | 68.7 ± 21.6 | .528 | |
| P-value | 0.637 | 0.485 | ||
| Role function | Baseline | 62.3 ± 24.7 | 64.1 ± 21.3 | .52 |
| Post-intervention | 66.7 ± 19.4 | 54.9 ± 17.9 | .041 | |
| P-value | 0.624 | 0.036 | ||
| Cognitive function | Baseline | 79.4 ± 19.5 | 81.7 ± 20.6 | .316 |
| Post-intervention | 74.1 ± 22.7 | 78.3 ± 21.8 | .411 | |
| P-value | 0.226 | 0.396 | ||
| Social function | Baseline | 72.2 ± 24.1 | 68.9 ± 20.3 | .363 |
| Post-intervention | 75.8 ± 23.4 | 60.1 ± 19.2 | .047 | |
| P-value | 0.393 | 0.058 | ||
| Sleep disturbance | Baseline | 29.7 ± 17.8 | 32.2 ± 22.3 | .227 |
| Post-intervention | 27.2 ± 19.3 | 34.5 ± 21.0 | .378 | |
| P-value | 0.496 | 0.525 | ||
| Dyspnea | Baseline | 25.1 ± 16.8 | 22.4 ± 18.5 | .117 |
| Post-intervention | 26.7 ± 17.9 | 31.9 ± 20.3 | .165 | |
| P-value | 0.615 | 0.038 | ||
| Nausea and vomiting | Baseline | 22.5 ± 10.9 | 23.2 ± 9.8 | .341 |
| Post-intervention | 19.5 ± 11.3 | 26.6 ± 12.5 | .287 | |
| P-value | 0.373 | 0.416 | ||
| Appetite loss | Baseline | 18.3 ± 8.9 | 17.6 ± 7.5 | .663 |
| Post-intervention | 12.2 ± 7.1 | 22.6 ± 6.9 | .012 | |
| P-value | 0.195 | 0.274 | ||
| Fatigue | Baseline | 21.4 ± 9.7 | 19.9 ± 8.7 | .438 |
| Post-intervention | 14.1 ± 7.3 | 24.3 ± 10.3 | .024 | |
| P-value | 0.044 | 0.142 | ||
QoL, quality of life.
Values were expressed as mean (SD). Bold values indicate P < 0.05.
Discussion
This quasi-randomized controlled trial in gastrointestinal cancer patients undergoing chemotherapy illustrated the benefits of resistance exercise in the improvement of physical function and QoL, as well as in alleviating symptoms.
It has been reported that the performance of physical activity has association with reduced risk of several different kinds of cancers, such as colon and breast cancers.15 In colon cancer patients, participation in physical activity after diagnosis is confirmed to have benefits in the reduction of mortality and recurrence risks, independent of pathologic, demographic, and prognostic factors.16 Another research study has further demonstrated that the influence of post-diagnosis physical activity on the disease outcomes of colorectal and breast cancer patients is in a dose-dependent manner.17
Despite having effective functions in the therapy of cancer, the administration of chemotherapy and radiation therapy will simultaneously cause some treatment-related side effects.18 The side effects generated by chemotherapy significantly decrease the patients’ functional capacity and QoL. During both chemotherapy and radiation therapy, exercise interventions are illustrated to have a variety of benefits in both physiological and psychological conditions in cancer patients.7,19 Currently, the recommended exercise treatment modality includes aerobic exercise and resistance exercise, and both are found to improve the musculoskeletal and cardiovascular functions of cancer patients.20,21 It is illustrated that patients with advanced gastrointestinal cancer prefer resistance exercise training since resistance exercise training has a higher potential for individualization, variability, and diversification than aerobic exercise training.13 The function of resistance exercise in patients with advanced gastrointestinal cancer has been reported and the effects of resistance exercise on biological parameters, physical performance, and QoL were evaluated.13 But the research is only restricted to the gastrointestinal cancer patients undergoing palliative chemotherapy. We aimed to investigate the effects of resistance exercise on symptoms, physical function, and QoL in gastrointestinal cancer patients undergoing chemotherapy. In our research, the participants were treated by different chemotherapy protocols, including oxaliplatin + capecitabine, fluorouracil + leucovorin, oxaliplatin + leucovorin + fluorouracil, and others. In this research, 5 different kinds of cancer were involved, including rectal, cholangiocellular, colon, gastric, and pancreatic cancer. Since the adherence rate was not influenced by the performance of resistance exercise training, it was suggested that the high potential for individualization, variability, and diversification in resistance exercise made it equally acceptable as relaxation.
Enormous psychological and physiological stress is experienced by cancer patients after the performance of chemotherapy. Chemotherapy impairs the immune system, weakens the resistance of body, and causes several symptoms.22 A research has reported the complex network of toxic effects generated by radiation and chemotherapy in patients with gastrointestinal tumors.5 In this research, we focused on 9 different side effects induced by chemotherapy, including loss of appetite, lack of energy, nausea, mouth ulcers, acid reflux, cough, back pain, fever, and alopecia. Results of this research showed that resistance exercise training could reduce the incidence of symptoms induced by chemotherapy in gastrointestinal cancer patients.
Anorexia, metabolic abnormality, and physical functionality reduction can be generally observed in cancer patients and result in persistent loss of weight and skeletal muscle mass.23 In cancer patients, the reduction of physical performance and QoL can be found during chemotherapy.24,25 Another research also reports that colorectal cancer patients who perform supervised exercise intervention during chemotherapy have greater physical functioning than those in a usual care group.26 In this research, we investigated whether resistance exercise training could improve the physical functions of gastrointestinal cancer patients undergoing chemotherapy. The lower body muscular strength and muscular endurance was significantly enhanced by resistance exercise training. It seemed that resistance exercise had a better effect on improving the lower body muscular strength and muscular endurance. Participants’ performance in 6-m fast walk, 6-m backwards walk, and chair rise were significantly improved compared to the participants in relaxation control group. These results demonstrated the potential benefits of resistance exercise in improving physical function of gastrointestinal cancer patients undergoing chemotherapy.
In breast cancer patients, resistance exercise can alleviate physical fatigue and maintain QoL during chemotherapy.12 In another study, resistance exercise increased the global QoL score in advanced gastrointestinal cancer patients undergoing palliative chemotherapy but had no significant difference when compared with the baseline.13 In our research, the global QoL score was also elevated in the resistance exercise group after the 12-week training but had no significant difference when compared with the relaxation control group. Participants in the resistance exercise group had a relatively stable physical function and role function during chemotherapy. Several studies have illustrated that physical activity has beneficial effects on QoL, physical function, and fatigue levels.27,28 Resistance exercise training also showed benefits in alleviating fatigue and appetite loss in gastrointestinal cancer patients during chemotherapy.
There is no research on the influence of resistance exercise on chemotherapy efficacy in rodent models. However, in carcinoma rodent models, resistance exercise reduces histopathological grade, causes less nuclear pleomorphism and fewer mitotic cells, decreases viable tumor area, and decreases tumor cell proliferation.29 In rats, the performance of resistance exercise is effective in reducing oxidative stress, increasing antioxidant capacity, decreasing protein degradation, and regulating growth factor expression in skeletal muscle.30 The molecular mechanism of resistance exercise in regulating symptoms, physical function, and QoL in cancer patients undergoing chemotherapy is still unknown. Since resistant exercise reduces the toxic effects in gastrointestinal cancer patients caused by chemotherapy, the molecular mechanism during this process is needed to be further investigated.
There were some limitations in the current study. To get a more accurate conclusion, the sample size in this research should be larger. The participants in this research did not include all types of gastrointestinal cancers, and it was possible that the effect of resistance exercise training might vary among different kinds of cancers. Furthermore, the effects of aerobic exercise should also be investigated. The comparison between resistance exercise and aerobic exercise will help us to find the best physical exercise in gastrointestinal cancer patients during chemotherapy. Last, this study was a quasi-randomized trial. Further exploration of the contributions of resistance exercise during chemotherapy for gastrointestinal cancer should be done using well-designed randomized trials.
Conclusion
In conclusion, we demonstrate the effects of resistance exercise on symptoms, physical function and QoL in gastrointestinal cancer patients undergoing chemotherapy in this quasi-randomized trial. Resistance exercise training reduced the incidences of nausea and acid reflux, improves physical functions, and alleviated fatigue and appetite loss in gastrointestinal cancer patients during chemotherapy.
Footnotes
Authors’ Contributions: Yijin Hong, Chunmei Wu and Biyu Wu performed the experiments, analyzed and interpreted the data. Chunmei Wu was the major contributors in writing the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chunmei Wu  https://orcid.org/0000-0003-4108-6042
https://orcid.org/0000-0003-4108-6042
Reference
- 1. Shinoto M, Ebner DK, Yamada S. Particle radiation therapy for gastrointestinal cancers. Curr Oncol Rep. 2016;18:17. [DOI] [PubMed] [Google Scholar]
- 2. Schmiegel W, Pox C, Arnold D, Porschen R, Rödel C, Reinacher-Schick A. Colorectal carcinoma: the management of polyps, (neo)adjuvant therapy, and the treatment of metastases. Dtsch Arztebl Int. 2009;106:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gong D, Hai J, Ma J, et al. ML-SA1, a TRPML1 agonist, induces gastric secretion and gastrointestinal tract inflammation in vivo. STEMedicine. 2020;1:e3. [Google Scholar]
- 4. Javier NS, Montagnini ML. Rehabilitation of the hospice and palliative care patient. J Palliat Med. 2011;14:638-648. [DOI] [PubMed] [Google Scholar]
- 5. Grabenbauer GG, Holger G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2016;30:655-664. [DOI] [PubMed] [Google Scholar]
- 6. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715-727. [DOI] [PubMed] [Google Scholar]
- 7. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396-4404. [DOI] [PubMed] [Google Scholar]
- 8. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123-133. [DOI] [PubMed] [Google Scholar]
- 10. Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142-152. [DOI] [PubMed] [Google Scholar]
- 11. Singh F, Galvao DA, Newton RU, Spry NA, Baker MK, Taaffe DR. Feasibility and preliminary efficacy of a 10-week resistance and aerobic exercise intervention during neoadjuvant chemoradiation treatment in rectal cancer patients. Integr Cancer Ther. 2018;17:952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137:471-480. [DOI] [PubMed] [Google Scholar]
- 13. Jensen W, Baumann FT, Stein A, et al. Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer. 2014;22:1797-1806. [DOI] [PubMed] [Google Scholar]
- 14. Ferguson JM, Marquis JN, Taylor CB. A script for deep muscle relaxation. Dis Nerv Syst. 1977;38:703-708. [PubMed] [Google Scholar]
- 15. Rezende LFM, Sa TH, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826-833. [DOI] [PubMed] [Google Scholar]
- 16. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535-3541. [DOI] [PubMed] [Google Scholar]
- 17. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293-1311. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344-351. [DOI] [PubMed] [Google Scholar]
- 20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243-274. [DOI] [PubMed] [Google Scholar]
- 21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 22. Beaver CC, Magnan MA. Managing chemotherapy side effects: achieving reliable and equitable outcomes. Clin J Oncol Nurs. 2016;20:589-591. [DOI] [PubMed] [Google Scholar]
- 23. Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104. [DOI] [PubMed] [Google Scholar]
- 24. Ragnhammar P, Hafstrom L, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282-308. [DOI] [PubMed] [Google Scholar]
- 25. Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Mohile S. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: a review. Oncol Hematol Rev. 2012;8:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc. 2016;48:767-775. [DOI] [PubMed] [Google Scholar]
- 27. Lowe SS, Watanabe SM, Courneya KS. Physical activity as a supportive care intervention in palliative cancer patients: a systematic review. J Support Oncol. 2009;7:27-34. [PubMed] [Google Scholar]
- 28. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage. 2006;31:421-430. [DOI] [PubMed] [Google Scholar]
- 29. Padilha CS, Testa MT, Marinello PC, et al. Resistance exercise counteracts tumor growth in two carcinoma Rodent models. Med Sci Sports Exerc. 2019;51:2003-2011. [DOI] [PubMed] [Google Scholar]
- 30. Cai M, Wang Q, Liu Z, Jia D, Feng R, Tian Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018;213:40-49. [DOI] [PubMed] [Google Scholar]