Abstract

Two-dimensional (2D) transition metal nanosheets are promising catalysts because of the enhanced exposure of the active species compared to their 3D counterparts. Here, we report a simple, scalable, and reproducible strategy to prepare 2D phosphate nanosheets by forming a layered structure in situ from phytic acid (PTA) and transition metal precursors. Controlled combustion of the organic groups of PTA results in interlayer carbon, which keeps the layers apart during the formation of phosphate, and the removal of this carbon results in ultrathin nanosheets with controllable layers. Applying this concept to vanadyl phosphate synthesis, we show that the method yields 2D ultrathin nanosheets of the orthorhombic β-form, exposing abundant V4+/V5+ redox sites and oxygen vacancies. We demonstrate the high catalytic activity of this material in the vapor-phase aerobic oxidation of ethyl lactate to ethyl pyruvate. Importantly, these β-VOPO4 compounds do not get hydrated, thereby reducing the competing hydrolysis reaction by water byproducts. The result has superior selectivity to ethyl pyruvate compared to analogous vanadyl phosphates. The catalysts are highly stable, maintaining a steady-state conversion of ∼90% (with >80% selectivity) for at least 80 h on stream. This “self-exfoliated” synthesis protocol opens opportunities for preparing structurally diverse metal phosphates for catalysis and other applications.
Keywords: metal phosphate, β-phase, ultrathin nanosheets, heterogeneous catalysis, aerobic oxidation, lactic acid
Introduction
Ultrathin two-dimensional (2D) materials are attracting increased attention for several applications, thanks to their distinctive electronic, optical, semiconducting, and catalytic properties.1,2 Single- or few-layer 2D sheets expose more interior atoms than their bulk counterparts, with abundant surface-active sites and vacancy defects.3,4 The 2D confinement effect also can shorten mass and heat diffusion pathways.5 This makes them promising candidates for designing efficient heterogeneous catalysts.6
The main state-of-the-art methods for preparing few-layer nanosheets are gas/liquid exfoliation, ion intercalation, or mechanical cleavage.7 These top-down approaches are suitable for stacked materials with interplanar van der Waals interactions, such as graphene, boron nitride, and carbon nitride.8 Synthesizing 2D nanosheets from nonlayered materials is much more difficult.9 It requires harsh conditions and gives varied thicknesses and low yields.10 Alternatively, 2D nonlayered nanosheets can be produced through template-assisted synthesis,11,12 surfactant self-assembly,13,14 oriented attachment growth,15 and inorganic–organic lamellar hybrid intermediates.16 Still, making high-quality ultrathin nanosheets of nonlayered inorganic materials remains a challenge.17,18
For example, vanadium phosphates (VPOs) are composed of alternating vanadium octahedra (VO6) and phosphate tetrahedra (PO4).19 Several crystal structures in different oxidation states are known, such as V5+ vanadyl phosphate (i.e., αI-, αII-, β-, ω-, δ-, ε-, and γ-VVOPO4) and V4+ vanadyl pyrophosphate [(VIVO)2P2O7].20−24 The β-phase is thermodynamically the most stable.25 However, because this compact structure has fewer accessible active sites, its catalytic activity is low.26 We hypothesized that this problem could be avoided by structuring β-VOPO4 as thin nanosheets, thus exposing more surface V4+/V5+ redox couples.27 VOPO4 nanosheets are currently prepared by intercalation–exfoliation of bulk α-VOPO4·2H2O, exploiting the weak hydrogen bonds between layers.28 However, unlike layered α-VOPO4, the 3D network of the nonlayered β-phase is unsuitable for this method, giving no control over the number of layers.
Here, we report a new template-free and scalable method for preparing 2D β-VOPO4 ultrathin nanosheets with controlled layers. These sheets are made by self-assembly of vanadyl sulfate (VOSO4) and phytic acid (PTA) precursors, which are abundant and inexpensive; vanadyl sulfate is a byproduct of crude oil refining, and PTA is a renewable plant-based acid. The PTA molecules are the key to this synthesis: (i) they react with VOSO4 as strong chelating agents, suppressing the agglomeration of vanadium species, and (ii) they form carbon layers between the vanadium–phosphate complexes from the cyclohexane rings during the hydrothermal process. Subsequent pyrolysis removes the PTA, creating more accessible surface and increasing the number of V4+/V5+ redox sites and oxygen vacancies. This self-exfoliating concept is also general, giving access to various thin transition metal phosphate sheets. We used this method to make active and selective catalysts from inactive forms of phosphates by structuring them as thin nanosheets, thereby exposing more surface active species. The resulting 2D β-VOPO4 ultrathin sheets are excellent catalysts for the vapor-phase air oxidation of ethyl lactate to ethyl pyruvate (Figure S1, see the Supporting Information for full experimental details).
Results and Discussion
Synthesis and Characterization of β-VOPO4 Nanosheets
Figure 1a illustrates the synthesis strategy for 2D β-VOPO4 ultrathin sheets. In the first step of this simple two-step process, PTA and VOSO4 are dissolved in aqueous solutions, separately. After mixing the two solutions, the vanadium–PTA coordination precursors are formed by the self-assembly of vanadyl ions and PTA. Subsequently, ammonia was added to the mixture, adjusting the pH to ∼6. Adding ammonia accelerates the complexation and helps to form cross-linked networks by surrounding the vanadium–PTA micelles. The amorphous mixture is then subjected to a hydrothermal treatment (Figure S2). Then, the cyclohexane part of PTA is carbonized, forming VOP@C hybrids. Note that the growth of carbon was restricted between the interlayers of VOP–PTA hybrids, forming extended 2D carbon layers. In the second step, the in situ-formed carbon templates were removed by heat treatment at 550 °C, yielding 2D β-VOPO4 nanosheets.
Figure 1.
(a) Schematic summary of the synthesis procedure for 2D β-VOPO4 nanosheets. (b) X-ray diffraction pattern of 2D β-VOPO4 nanosheets (the inset shows the model of layered β-VOPO4); (c,d) SEM images of β-VOPO4 flakes stacked by the layered structure; (e) AFM image of few-layer β-VOPO4 nanosheets (inset: the corresponding 3D demonstration); (f) thickness of nanosheets derived from AFM measurement; (g) representative TEM images of β-VOPO4 nanosheets; and (h–j) magnified HRTEM images of β-VOPO4 nanosheets taken along [101], [102], and [110] directions.
Powder X-ray diffraction analysis confirmed the formation of the pure orthorhombic β-VOPO4 phase (Figure 1b, cf. PDF#71-0859). Scanning electron microscopy (SEM) images show the stacked 2D plates with a smooth surface, shaped edges and corners, indicating a typical lamellar layered morphology (Figure 1c,d). Analysis of the sample by atomic force microscopy (AFM) (Figures 1e and S3) and the corresponding AFM height profile (Figure 1f) indicate that the VOPO4 samples comprise three stacks (see the inset in Figure 1e), each with the same average thickness of ∼6 nm. This confirms that we successfully synthesized the β-VOPO4 nanosheets with a controlled thickness of about 7–8 atomic monolayers. The ultrathin and nearly transparent features of VOPO4 nanosheets are also shown by transmission electron microscopy (TEM) (Figure 1g), upholding the AFM results. Moreover, the high-resolution TEM (HRTEM) images show the clear lattice fringes with interplanar distances of 0.53, 0.32, and 0.40 nm (Figure 1h–j), which can be assigned to the (101), (102), and (110) planes of the β-VOPO4 structure, respectively.
We hypothesized that PTA plays a key role in the formation of 2D β-VOPO4 nanosheets. To test this, we ran a control experiment, where instead of PTA, we used phosphoric acid (H3PO4) as the P precursor (all other conditions were identical). The resulting VOPO4 material is denoted as PA–VOPO4. As shown in Figure 2a, the XRD pattern of PA–VOPO4 is almost identical to that of the PTA-derived VOPO4 nanosheets (denoted as PTA–VOPO4), yielding a typical β structure. Unlike layered α-VOPO4 with 2D anisotropic growth (the adjacent layers are connected with weak van der Waals force),28 diffraction peaks do not shift in the β-phase.29 However, the ratios of both (011)/(101) and (002)/(201) planes in PTA–VOPO4 are higher than those of PA–VOPO4 (Figures 2a and S4), probably because of the lamellar layered structure of PTA–VOPO4.
Figure 2.
(a) Comparison of the X-ray diffraction patterns of two β-VOPO4 catalysts: phytic acid-derived VOPO4 nanosheets (PTA–VOPO4) and the corresponding phosphoric acid-derived VOPO4 (PA–VOPO4); (b) magnified HRTEM images of PA–VOPO4, and the inset shows the V2O5 nanoparticles with the (200) lattice space. (c) Schematic of the self-assembly process of VOSO4 and P precursors: PTA and phosphoric acid (PA).
However, an additional peak appeared at 20.3° in PA–VOPO4, which belongs to crystalline V2O5. This indicates that some of the vanadyl species are aggregated into V2O5. Indeed, a representative HRTEM image of PA–VOPO4 (Figure 2b) confirmed that the V2O5 nanocrystallites are dispersed on the VOPO4 matrix. The inset clearly shows lattice fringes of 0.218 nm, corresponding to the (200) lattice space of crystalline V2O5. The chemical mapping by energy-dispersive X-ray (EDX) spectroscopy showed that the mean atomic V/P ratio of surface PTA–VOPO4 is 0.9 (Table S1), much lower than that of PA–VOPO4 (1.41, see Table S2), indicating the aggregation of vanadia species on the surface of PA–VOPO4.
Both PTA and phosphoric acid are strong chelating agents and coordinate to VOSO4. As shown in Figure 2c, PTA comprises six phosphoric acids attached to a cyclohexane ring. Unlike plain phosphoric acid, the steric hindrance of PTA prevents aggregation during the co-assembly process. Moreover, during the hydrothermal treatment, the cyclohexane segments of PTAs are carbonized into a carbon framework, suppressing the agglomeration of vanadium species to V2O5. Indeed, after the hydrothermal treatment, the nitrogen sorption isotherm of PTA-derived VOP (PTA–VOPHT) showed increased N2 uptake in the low relative pressure (P/P0 < 0.1) and a hysteresis loop in the region of P/P0 > 0.4, suggesting the formation of a carbon rich in micropores and mesopores (Figure S5a). In contrast, the PA–VOPHT gave negligible N2 adsorption. Subsequent calcination removes the carbon, giving PTA–VOPO4, showing a steep rise in the range of P/P0 > 0.9 (Figure S5b), which can be assigned to the interlayer voids. We also prepared PA–VOPO4 samples with different VOSO4/PA molar ratios (0.5 and 0.8) under otherwise identical conditions, trying to avoid the formation of crystalline V2O5. As is evident from the XRD patterns in Figure S6 in the Supporting Information, crystalline V2O5 was present in these samples, further verifying the vital role of the PTA precursor. We conclude that owing to the confined carbonization of PTA, the formed carbon acts as an in situ template, giving the desired few-layer nanosheets. Control experiments wherein the vanadium–PTA complex was directly calcined without any hydrothermal treatment led to the formation of amorphous VPO (Figure S7), upholding our conclusion.
We then studied the catalytic performance of these β-VOPO4 materials in the vapor-phase oxidative dehydrogenation of ethyl lactate with air to give ethyl pyruvate in a fixed-bed reactor (eq 1). Lactates are biomass-derived “platform molecules,”30 and direct aerobic oxidation of lactate is a sustainable route to biobased pyruvate, an important intermediate in the food, cosmetics, pharmaceutical, and agrochemical sectors.31,32 Previously, we showed that aerobic oxidation of ethyl lactate requires relatively high temperatures. However, the pyruvate is easily overoxidized on the catalyst surface at such high temperatures, lowering the product selectivity.33 A series of control experiments confirmed that the reaction is in the kinetic regime, with no mass-transfer limitations. Then, we measured the selectivity to ethyl pyruvate against ethyl lactate conversion over phytic acid-derived VOPO4 nanosheets (PTA–VOPO4) and phosphoric acid-derived 3D VOPO4 nanoparticles (PA–VOPO4) (Figure 3a). PTA–VOPO4 outperformed PA–VOPO4 under identical reaction conditions. At the same conversion of ethyl lactate, PTA–VOPO4 is more selective for ethyl pyruvate than PA–VOPO4. The ethyl pyruvate yield at different reaction temperatures was much higher over PTA–VOPO4 nanosheets (Figure 3b), confirming the enhanced catalytic activity. Previously, we demonstrated that this reaction is kinetically favored in the presence of isolated amorphous vanadium oxide sites, while crystalline V2O5 can catalyze this reaction but not selective.34,35 PA–VOPO4 features well-dispersed V2O5 nanocrystals on the surface, yet its catalytic activity and selectively are lower compared to that of PTA–VOPO4. This is probably because the surface V4+/V5+ active sites and oxygen vacancies of PA–VOPO4 are less accessible than those in PTA–VOPO4. We then reasoned that the reactivity enhancement on PTA–VOPO4 is due to the exposed V4+/V5+ redox sites and oxygen vacancies.
 |
1 |
Figure 3.

Vapor-phase oxidative dehydrogenation of ethyl lactate with air to give ethyl pyruvate over various β-VOPO4 catalysts: (a) Selectivity to ethyl pyruvate plotted against conversion over PTA–VOPO4 and PA–VOPO4. Reaction conditions: ethyl lactate WHSV = 6.25 h–1, air carrier flow rate = 2.25 L/h; (b) corresponding temperature-resolved yield profile of ethyl pyruvate. All data were taken after 2 h on stream.
Factors Governing Activity and Selectivity
We further characterized the β-VOPO4 sheets to understand this enhanced activity. The full X-ray photoelectron spectroscopy (XPS) survey spectra showed V, P, and O in all samples (Figure S8), in accordance with elemental mapping from high-angle annular dark-field scanning TEM (HAADF–STEM) analysis (Figures 4a and S9). As shown in Figure 4b, the spectra of V 2p can be deconvoluted into two peaks centered at 517.2 and 518.0 eV, which are associated with V4+ and V5+ species, respectively. The PTA–VOPO4 nanosheets gave a much higher V4+/(V4+ + V5+) ratio of 40%, in comparison to 30% in the bulk PA–VOPO4 material. Thus, ultrathin nanosheets expose more accessible surface sites, thereby increasing the number of surface V4+ species. The enhanced catalytic activity can be assigned to the increased V4+/V5+ redox active sites of β-VOPO4 nanosheets.36 From redox perspectives, introducing V4+ into β-VOPO4 nanosheets increases the number of defects and oxygen vacancies. Elsewhere, we have reported that the oxidation of ethyl lactate follows a Mars–van Krevelen mechanism: ethyl lactate adsorbed on the catalytic surface is oxidized by the lattice oxygen, and then, the resultant oxygen vacancies are replenished by gas-phase oxygen during the oxidation reaction.33 Thus, both surface lattice oxygens and oxygen vacancies play key roles in aerobic oxidation of ethyl lactate to ethyl pyruvate. These two species can be roughly estimated from the O 1s XPS spectrum (Figure 4c): the peak at ∼532.5 eV can be attributed to the lattice oxygen (OI) and the peak at ∼531.0 eV can be attributed to the adsorbed oxygen species at the vacancy sites (OII).4 The OI peak has a larger area for the PTA–VOPO4 nanosheets than PA–VOPO4, indicating that the former exposes more lattice oxygen atoms. Additionally, OII oxygens can enhance the mobility of oxygen species; they are more easily reduced and favorable for the oxidation reaction. PTA–VOPO4 has a higher OII/(OI + OII) ratio, indicating abundant structural defects and oxygen vacancies. Moreover, as the structural defects and oxygen vacancies decrease the electron charge density around phosphorus of PTA–VOPO4, the P 2p1/2 and P 2p3/2 peaks shift slightly toward a higher binding energy in comparison with PA–VOPO4 (Figure 4d).37
Figure 4.
(a) STEM image and corresponding elemental mappings of V, P, and O of PTA–VOPO4 nanosheets. XPS studies showing high-resolution V 2p spectra (b), high-resolution O 1s spectra (c), and high-resolution P 2p spectra (d) of PA–VOPO4 and PTA–VOPO4 nanosheets. (e) PALS spectra of PTA–VOPO4 nanosheets (the inset shows the positron annihilation lifetimes and the corresponding intensities in PA–VOPO4 and PTA–VOPO4 nanosheets, respectively). (f) Schematic diagram of defects and vacancies, originating from the removal of carbon layers, in PTA–VOPO4 nanosheets.
Further information on the structural defects was obtained from positron annihilation lifetime spectroscopy (PALS). Figure 4e shows a typical PALS spectrum of PTA–VOPO4. All the PALS spectra could be fitted to two positron lifetime components with a reasonable variance of fit (1.0–1.1). The first positron lifetime (τ1) in the range of ∼208–240 ps is attributed to positron annihilation in the bulk of the materials (see the inset in Figure 4e). The longer lifetime (τ2) in the range of ∼422–478 ps indicates the presence of larger-size defects, that is, vacancy clusters present either in the bulk or at the grain boundaries of the samples. The τ1 values of PTA–VOPO4 and PA–VOPO4 are nearly the same, showing the identical lattice structure. The intensity corresponding to larger components (I2) is higher (40%) for nanosheets compared to that of bulk PA–VOPO4 (36%), suggesting more vacancy defects for PTA–VOPO4 nanosheets. Positrons trapped at the defect sites predominantly annihilate with the surrounding elements and hence provide information about the chemical surrounding. They are efficiently trapped either at negatively charged or neutral open volume defects such as vacancy clusters. According to the crystal structure of the samples, cation vacancy defects (e.g., V or P based vacancy defects) are surrounded by oxygen atoms. Figure S10 shows the ratio curves of these samples with respect to a reference Si, in which the peak at PL ≈ 10 × 10–3moc indicates the annihilation with the surrounding oxygen atoms at the defect sites. The corresponding peak intensity of PTA–VOPO4 is higher than that of PA–VOPO4, indicating that the defects present in the nanosheets have more O atoms in the surrounding lattice sites. Thus, our ultrathin VOPO4 nanosheets expose more lattice oxygen and oxygen vacancies (Figure 4f), which can explain their high catalytic activity.
Self-Exfoliated Synthesis of Other Transition Metal Phosphate Nanolayers for Catalytic Aerobic Oxidation of Ethyl Lactate to Ethyl Pyruvate
Based on this “self-exfoliated” synthesis protocol, we successfully made V-, Ni-, Co-, and Fe-based phosphates (see the experimental section in the Supporting Information for details). These desired few-layer nanosheets with thicknesses of 2–6 nm were confirmed by HRTEM and AFM analyses (Figures 5a–c and S11). The combination of XRD, SEM, and EDX analyses showed that these phosphates have lamellar layered morphologies with high crystallinity, purity, and uniformity (Figures S12 and S13 and Tables S3–S5, in the Supporting Information). Thus, our synthesis method is general, facile, and scalable via a two-step process (hydrothermal and calcination treatment).
Figure 5.
Morphological and microstructural characterization of various PTA-derived metal-phosphate nanosheets (a–c); NiPO4 nanosheets: (a1–a3) TEM images and (a4) AFM image; CoPO4 nanosheets: (b1–b3) TEM images and (b4) AFM image; FePO4 nanosheets: (c1–c3) TEM images and (c4) AFM image. Note: the corresponding height profiles of phosphate nanosheets are shown in Figure S11, which were derived from AFM measurement.
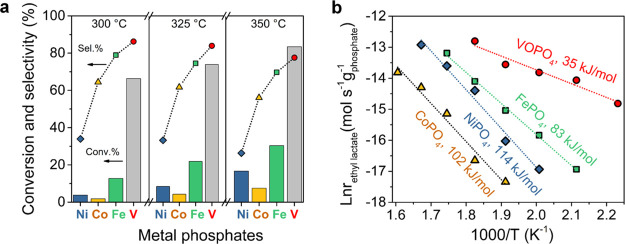
All the PTA-derived phosphate nanosheets were then tested in the vapor-phase aerobic oxidation of ethyl lactate with air at different reaction temperatures (300, 325, and 350 °C). As shown in Figure 6a, control experiments confirmed that Ni and Co showed some conversion but selectivity to ethyl pyruvate was low, owing to the hydrolysis of ethyl pyruvate on Co- and Ni-based phosphate.36 Intriguingly, VOPO4 nanosheets exhibited the best catalytic performance among all the phosphate catalysts tested in this study, giving a remarkably high activity and selectivity. To better understand this, the apparent activation energies (Ea) were calculated based on Arrhenius plots (Figure 6b), from the data collected below 15% ethyl lactate conversion. The corresponding Ea value for VOPO4 (35 kJ/mol) is much smaller than that for other phosphates: FePO4 (83 kJ/mol), CoPO4 (102 kJ/mol), and NiPO4 (114 kJ/mol). This result confirmed that VOPO4 is intrinsically more active for ethyl lactate oxidation.
Figure 6.
(a) Comparisons of ethyl lactate conversion and ethyl pyruvate selectivity over various PTA-derived metal phosphate nanosheets: VOPO4, FePO4, CoPO4, and NiPO4. Reaction conditions: ethyl lactate WHSV = 8 h–1, air flow rate = 2.25 L/h, and reaction temperature: 300, 325, and 350 °C. (b) Arrhenius plots for steady-state ethyl lactate consumption rate over various phosphate catalysts, and the apparent activation energy (Ea) was measured at a series of temperatures below 15% ethyl lactate conversion.
Comparisons of Catalytic Efficiency of Various Vanadium Phosphorus Oxides for Aerobic Oxidation to Ethyl Lactate
Olier et al. reported that all vanadium phosphorus oxides (VPO) can be hydrated except for β-VOPO4, owing to its highly stable structure.38 This is consistent with our XPS measurements (Figure 4c), where no surface-chemisorbed water was detected (∼533 eV). As a result, the competing hydrolysis is suppressed, which may explain why the β-VOPO4 nanosheets gave such high selectivity to pyruvate compared with other metal phosphate catalysts.
To test this, we prepared a series of bulk VPO catalysts for comparison with our nanosheets: vanadyl pyrophosphate [(VO)2P2O7], vanadyl phosphate dihydrate (VOPO4·2H2O), and vanadyl hydrogen phosphate hemihydrate (VOHPO4·0.5H2O, see the Supporting Information for full experimental details). Their crystalline structures were confirmed by XRD and Raman spectroscopy (Figures S14 and S15).39,40Figure 7a shows the selectivity–conversion plots. All the VPO catalysts were active in lactate-to-pyruvate reaction. Interestingly, β-VOPO4 nanosheets showed the highest ethyl pyruvate selectivity, reaching over 90% at an ethyl lactate conversion of ∼25%. Even at a high ethyl lactate conversion of ∼80%, the selectivity is as high as 80% compared with ∼60% for (VO)2P2O7. Control experiments were performed at a steady-state conversion of ∼6% for all the tested catalysts (the carbon balances were >98%) to better differentiate the influence of the VPO phases on product selectivity. As shown in Figure 7b, β-VOPO4 nanosheets gave over 99% selectivity to ethyl pyruvate, while a series of byproducts were detected on other three VPO catalysts, such as acetaldehyde, ethanol, acetic acid, ethyl acetate, and COx. This indicates that except for the β-phase, the VPO catalysts undergo the competing overoxidation, hydrolysis, decarbonylation, and decarboxylation (see Figure 7c). The byproduct distribution is different among different catalysts. VOHPO4·0.5H2O and VOPO4·2H2O gave higher selectivity to ethanol than (VO)2P2O7, owing to the hydrolysis of ester on their hydrated surfaces.
Figure 7.
(a) Selectivity to ethyl pyruvate plotted against conversion for different VPO catalysts: 2D β-VOPO4 nanosheets, (VO)2P2O7, VOHPO4·0.5 H2O, and VOPO4·2H2O. (b) Comparison of the selectivity of various products at an ethyl lactate conversion of ∼6% (β-VOPO4 nanosheets: 6.0%, (VO)2P2O7: 6.2%, VOHPO4·0.5 H2O: 5.7%, and VOPO4·2H2O: 6.4%). The carbon balances were >98%. (c) Reaction pathway for the aerobic oxidation of ethyl lactate on the VPO catalysts. (d) Mass-specific activity for pyruvate formation over VPO catalysts in the temperature range 250–325 °C. (e) Comparisons of the area-specific production rate of pyruvate over 2D β-VOPO4 nanosheets and (VO)2P2O7. (f) Stability test of the 2D β-VOPO4 nanosheets under optimized conditions (WHSV = 3 h–1 and T = 300 °C).
We also compared the mass-specific activity (calculated as grams of pyruvate produced per gram of the catalyst per hour) over VPO catalysts at different temperatures (Figure 7d). Our β-VOPO4 catalyst outperformed the classical phosphates, especially at high reaction temperatures over 300 °C. Moreover, layered β-VOPO4 and (VO)2P2O7 gave similar specific Brunauer–Emmett–Teller areas of 33 and 25 m2/g, respectively, much higher than VOHPO4·0.5H2O (16 m2/g), VOPO4·2H2O (9 m2/g). We then plotted the area-specific catalytic rates for pyruvate production over β-VOPO4 and (VO)2P2O7 catalysts. As shown in Figure 7e, the area-specific activity for β-VOPO4 nanosheets is almost twice higher than that for (VO)2P2O7 at 300 °C. The stability and regenerability are key factors for a heterogenous catalyst in its practical application. We tested the stability of our β-VOPO4 nanosheets under optimized conditions (WHSV = 3 h–1, T = 300 °C, see Table S6). As shown in Figure 7f, this catalyst is highly stable, with a steady-state conversion of ∼90% (over 80% selectivity) for at least 80 h without significant loss of activity. We also used the same catalyst bed for a series of testing studies, and for this, the catalyst was cleaned and regenerated by simply passing air at 500 °C for 2 h and switching off the ethyl lactate feed. The XRD, TEM, and XPS analyses further confirmed that the structure was well preserved after multiple regenerations (Figures S16–S18 in the Supporting Information). Most of the 2D nanosheets are still far from commercialization because their cost is a problem to scale-up. Our 2D β-VOPO4 nanosheets are promising in this regard because they can be readily achieved from inexpensive starting materials such as VOSO4 and PTA. VOSO4 is a byproduct of crude oil refining (ca. 2000–5000 $/ton), and PTA is a renewable inexpensive plant-based acid (ca. 6500 $/ton).41 The metal salts are also cheap; therefore, these nanosheets are industrially viable catalysts cost-wise as well.
Conclusions
We report the synthesis of 2D ultrathin phosphate nanosheets by a new template-free “self-exfoliated” strategy using renewable PTA. PTA acts as a strong chelating agent, but can also be carbonized in situ into carbon templates, which are responsible for the precisely controlled few-layer nanosheets. Application of this method to VPO produces β-VOPO4 ultrathin nanosheets, which expose abundant V4+/V5+ redox sites and oxygen vacancies. Importantly, β-VOPO4 does not get hydrated, thereby reducing the competing hydrolysis by water byproducts. These features result in a superior catalytic activity and selectivity in the aerobic oxidation of ethyl lactate to ethyl pyruvate compared to the classical VPO. The inexpensive β-VOPO4 nanosheets show good long-term stability and facile recovery. These nanosheets are not only among the best heterogeneous catalysts for the vapor-phase oxidation of lactate to pyruvate, they also show for the first time that the “inert” β-VOPO4 phase can be an efficient oxidation catalyst under the right conditions. Note that this is a general synthesis method, giving access to various metal phosphate nanosheets, such as Ni, Co, and Fe. Therefore, this work opens a new avenue for the synthesis of new transition metal phosphate nanosheets for catalysis and other applications.
Acknowledgments
W.Z. thanks the China Scholarship Council (201506140058) for a PhD fellowship. P.O. thanks the Spanish Ministry of Economy and Competitiveness (MINECO) (Project CTM2015-63864-R) and the European Union (FEDER) for funding. P.O. also thanks the access and technical assistance from the Scientific-Technical Services of the University of Oviedo. This work is part of the Research Priority Area Sustainable Chemistry of the University of Amsterdam, http://suschem.uva.nl.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.9b04452.
Experimental procedures; additional characterization data of vanadyl phosphate catalysts including XRD patterns, nitrogen adsorption–desorption isotherms, SEM/TEM/AFM images, EDX spectra, XPS spectra, PALS spectra, and Raman spectra; and results of reaction parameters on aerobic oxidation of ethyl lactate with air (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chhowalla M.; Shin H. S.; Eda G.; Li L.-J.; Loh K. P.; Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- Ling T.; Wang J.-J.; Zhang H.; Song S.-T.; Zhou Y.-Z.; Zhao J.; Du X.-W. Freestanding Ultrathin Metallic Nanosheets: Materials, Synthesis, and Applications. Adv. Mater. 2015, 27, 5396–5402. 10.1002/adma.201501403. [DOI] [PubMed] [Google Scholar]; Gitis V.; Chung S.-H.; Shiju N. R. Conversion of furfuryl alcohol into butyl levulinate with graphite oxide and reduced graphite oxide. FlatChem 2018, 10, 39. 10.1016/j.flatc.2018.08.002. [DOI] [Google Scholar]
- Hong J.; Jin C.; Yuan J.; Zhang Z. Atomic Defects in Two-Dimensional Materials: From Single-Atom Spectroscopy to Functionalities in Opto-/Electronics, Nanomagnetism, and Catalysis. Adv. Mater. 2017, 29, 1606434. 10.1002/adma.201606434. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Wang L.; Zhang Y.; Ding Y.; Bi Y. Ultrathin FeOOH Nanolayers with Rich Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem., Int. Ed. 2018, 57, 2248–2252. 10.1002/anie.201712499. [DOI] [PubMed] [Google Scholar]
- Xiong P.; Ma R.; Sakai N.; Sasaki T. Genuine Unilamellar Metal Oxide Nanosheets Confined in a Superlattice-like Structure for Superior Energy Storage. ACS Nano 2018, 12, 1768–1777. 10.1021/acsnano.7b08522. [DOI] [PubMed] [Google Scholar]
- Ng W. H. K.; Gnanakumar E. S.; Batyrev E.; Sharma S. K.; Pujari P. K.; Greer H. F.; Zhou W.; Sakidja R.; Rothenberg G.; Barsoum M. W.; Shiju N. R. The Ti3 AlC2 MAX Phase as an Efficient Catalyst for Oxidative Dehydrogenation of n-Butane. Angew. Chem., Int. Ed. 2018, 57, 1485–1490. 10.1002/anie.201702196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.; Zhang X.; Xie Y. Advances and challenges in chemistry of two-dimensional nanosheets. Nano Today 2016, 11, 793–816. 10.1016/j.nantod.2016.10.004. [DOI] [Google Scholar]
- Geim A. K.; Grigorieva I. V. Van der Waals heterostructures. Nature 2013, 499, 419. 10.1038/nature12385. [DOI] [PubMed] [Google Scholar]
- Di J.; Xia J.; Li H.; Liu Z. Freestanding atomically-thin two-dimensional materials beyond graphene meeting photocatalysis: Opportunities and challenges. Nano Energy 2017, 35, 79–91. 10.1016/j.nanoen.2017.03.030. [DOI] [Google Scholar]
- Zhu W.; Gao X.; Li Q.; Li H.; Chao Y.; Li M.; Mahurin S. M.; Li H.; Zhu H.; Dai S. Controlled Gas Exfoliation of Boron Nitride into Few-Layered Nanosheets. Angew. Chem., Int. Ed. 2016, 55, 10766–10770. 10.1002/anie.201605515. [DOI] [PubMed] [Google Scholar]
- Huang X.; Li S.; Huang Y.; Wu S.; Zhou X.; Li S.; Gan C. L.; Boey F.; Mirkin C. A.; Zhang H. Synthesis of hexagonal close-packed gold nanostructures. Nat. Commun. 2011, 2, 292. 10.1038/ncomms1291. [DOI] [PubMed] [Google Scholar]
- Cheng W.; He J.; Yao T.; Sun Z.; Jiang Y.; Liu Q.; Jiang S.; Hu F.; Xie Z.; He B.; Yan W.; Wei S. Half-Unit-Cell α-Fe2O3 Semiconductor Nanosheets with Intrinsic and Robust Ferromagnetism. J. Am. Chem. Soc. 2014, 136, 10393–10398. 10.1021/ja504088n. [DOI] [PubMed] [Google Scholar]
- Wang J.; Xu Y.; Ding B.; Chang Z.; Zhang X.; Yamauchi Y.; Wu K. C.-W. Confined Self-Assembly in Two-Dimensional Interlayer Space: Monolayered Mesoporous Carbon Nanosheets with In-Plane Orderly Arranged Mesopores and High Graphitized Framework. Angew. Chem., Int. Ed. 2018, 57, 2894–2898. 10.1002/anie.201712959. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Liao T.; Dou Y.; Hwang S. M.; Park M.-S.; Jiang L.; Kim J. H.; Dou S. X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813. 10.1038/ncomms4813. [DOI] [PubMed] [Google Scholar]
- Schliehe C.; Juarez B. H.; Pelletier M.; Jander S.; Greshnykh D.; Nagel M.; Meyer A.; Foerster S.; Kornowski A.; Klinke C.; Weller H. Ultrathin PbS Sheets by Two-Dimensional Oriented Attachment. Science 2010, 329, 550–553. 10.1126/science.1188035. [DOI] [PubMed] [Google Scholar]
- Son J. S.; Wen X.-D.; Joo J.; Chae J.; Baek S.-i.; Park K.; Kim J. H.; An K.; Yu J. H.; Kwon S. G.; Choi S.-H.; Wang Z.; Kim Y.-W.; Kuk Y.; Hoffmann R.; Hyeon T. Large-Scale Soft Colloidal Template Synthesis of 1.4 nm Thick CdSe Nanosheets. Angew. Chem., Int. Ed. 2009, 48, 6861–6864. 10.1002/anie.200902791. [DOI] [PubMed] [Google Scholar]
- Wang F.; Wang Z.; Shifa T. A.; Wen Y.; Wang F.; Zhan X.; Wang Q.; Xu K.; Huang Y.; Yin L.; Jiang C.; He J. Two-Dimensional Non-Layered Materials: Synthesis, Properties and Applications. Adv. Funct. Mater. 2017, 27, 1603254. 10.1002/adfm.201603254. [DOI] [Google Scholar]
- Dou Y.; Zhang L.; Xu X.; Sun Z.; Liao T.; Dou S. X. Atomically thin non-layered nanomaterials for energy storage and conversion. Chem. Soc. Rev. 2017, 46, 7338–7373. 10.1039/c7cs00418d. [DOI] [PubMed] [Google Scholar]
- Centi G.; Trifiro F.; Ebner J. R.; Franchetti V. M. Mechanistic aspects of maleic anhydride synthesis from C4 hydrocarbons over phosphorus vanadium oxide. Chem. Rev. 1988, 88, 55–80. 10.1021/cr00083a003. [DOI] [Google Scholar]
- Weng W.; Al Otaibi R.; Alhumaimess M.; Conte M.; Bartley J. K.; Dummer N. F.; Hutchings G. J.; Kiely C. J. Controlling vanadium phosphate catalyst precursor morphology by adding alkane solvents in the reduction step of VOPO4·2H2O to VOHPO4·0.5H2O. J. Mater. Chem. 2011, 21, 16136–16146. 10.1039/c1jm12456k. [DOI] [Google Scholar]
- Centi G. Vanadyl Pyrophosphate - A Critical Overview. Catal. Today 1993, 16, 5–26. 10.1016/0920-5861(93)85002-h. [DOI] [Google Scholar]
- Coulston G. W.; Bare S. R.; Kung H.; Birkeland K.; Bethke G. K.; Harlow R.; Herron N.; Lee P. L. The Kinetic Significance of V5+ in n-Butane Oxidation Catalyzed by Vanadium Phosphates. Science 1997, 275, 191–193. 10.1126/science.275.5297.191. [DOI] [PubMed] [Google Scholar]
- Hutchings G. J.; Desmartin-Chomel A.; Olier R.; Volta J.-C. Role of the product in the transformation of a catalyst to its active state. Nature 1994, 368, 41. 10.1038/368041a0. [DOI] [Google Scholar]
- Lashier M.; Schrader G. L. Reactive lattice oxygen sites for C4 hydrocarbon selective oxidation over β-VOPO4. J. Catal. 1991, 128, 113–125. 10.1016/0021-9517(91)90071-b. [DOI] [Google Scholar]
- Gopal R.; Calvo C. Crystal structure of β VPO5. J. Solid State Chem. 1972, 5, 432–435. 10.1016/0022-4596(72)90089-8. [DOI] [Google Scholar]
- Willinger M. G.; Su D. S.; Schlögl R. Electronic structure of β-VOPO4. Phys. Rev. B: Condens. Matter Mater. Phys. 2005, 71, 155118. 10.1103/physrevb.71.155118. [DOI] [Google Scholar]
- Eichelbaum M.; Hävecker M.; Heine C.; Karpov A.; Dobner C.-K.; Rosowski F.; Trunschke A.; Schlögl R. The Intimate Relationship between Bulk Electronic Conductivity and Selectivity in the Catalytic Oxidation of n-Butane. Angew. Chem., Int. Ed. 2012, 51, 6246–6250. 10.1002/anie.201201866. [DOI] [PubMed] [Google Scholar]
- Wu C.; Lu X.; Peng L.; Xu K.; Peng X.; Huang J.; Yu G.; Xie Y. Two-dimensional vanadyl phosphate ultrathin nanosheets for high energy density and flexible pseudocapacitors. Nat. Commun. 2013, 4, 2431. 10.1038/ncomms3431. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang X.; Ling Y.; Li F.; Bond A. M.; Zhang J. Controllable synthesis of few-layer bismuth subcarbonate by electrochemical exfoliation for enhanced CO2 reduction performance. Angew. Chem., Int. Ed. 2018, 57, 13283–13287. 10.1002/anie.201807466. [DOI] [PubMed] [Google Scholar]
- Beerthuis R.; Rothenberg G.; Shiju N. R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. 10.1039/c4gc02076f. [DOI] [Google Scholar]
- Xu P.; Qiu J.; Gao C.; Ma C. Biotechnological routes to pyruvate production. J. Biosci. Bioeng. 2008, 105, 169–175. 10.1263/jbb.105.169. [DOI] [PubMed] [Google Scholar]
- Sugiyama S.; Kikumoto T.; Tanaka H.; Nakagawa K.; Sotowa K.-I.; Maehara K.; Himeno Y.; Ninomiya W. Enhancement of Catalytic Activity on Pd/C and Te–Pd/C During the Oxidative Dehydrogenation of Sodium Lactate to Pyruvate in an Aqueous Phase Under Pressurized Oxygen. Catal. Lett. 2009, 131, 129–134. 10.1007/s10562-009-9920-3. [DOI] [Google Scholar]
- Zhang W.; Innocenti G.; Ferbinteanu M.; Ramos-Fernandez E. V.; Sepulveda-Escribano A.; Wu H.; Cavani F.; Rothenberg G.; Shiju N. R. Understanding the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over vanadia/titania catalysts. Catal. Sci. Technol. 2018, 8, 3737–3747. 10.1039/c7cy02309j. [DOI] [Google Scholar]
- Zhang W.; Ensing B.; Rothenberg G.; Raveendran Shiju N. Designing effective solid catalysts for biomass conversion: Aerobic oxidation of ethyl lactate to ethyl pyruvate. Green Chem. 2018, 20, 1866–1873. 10.1039/c8gc00032h. [DOI] [Google Scholar]
- Zhang W.; Oulego P.; Slot T. K.; Rothenberg G.; Shiju N. R. Selective Aerobic Oxidation of Lactate to Pyruvate Catalyzed by Vanadium-Nitrogen-Doped Carbon Nanosheets. ChemCatChem 2019, 11, 3381–3387. 10.1002/cctc.201900819. [DOI] [Google Scholar]
- Zhang W.; Innocenti G.; Oulego P.; Gitis V.; Wu H.; Ensing B.; Cavani F.; Rothenberg G.; Shiju N. R. Highly selective oxidation of ethyl lactate to ethyl pyruvate catalysed by mesoporous vanadia–titania. ACS Catal. 2018, 8, 2365–2374. 10.1021/acscatal.7b03843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majjane A.; Chahine A.; Et-tabirou M.; Echchahed B.; Do T.-O.; Breen P. M. X-ray photoelectron spectroscopy (XPS) and FTIR studies of vanadium barium phosphate glasses. Mater. Chem. Phys. 2014, 143, 779–787. 10.1016/j.matchemphys.2013.10.013. [DOI] [Google Scholar]
- Benabdelouahab G. F.; Volta J. C.; Olier R. New Insights into VOPO4 Phases Through Their Hydration. J. Catal. 1994, 148, 334–340. 10.1006/jcat.1994.1214. [DOI] [Google Scholar]
- Li X.; Ko J.; Zhang Y. Highly Efficient Gas-Phase Oxidation of Renewable Furfural to Maleic Anhydride over Plate Vanadium Phosphorus Oxide Catalyst. ChemSusChem 2018, 11, 612–618. 10.1002/cssc.201701866. [DOI] [PubMed] [Google Scholar]
- Wang F.; Dubois J.-L.; Ueda W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J. Catal. 2009, 268, 260–267. 10.1016/j.jcat.2009.09.024. [DOI] [Google Scholar]
- Samotus B.; Schwimmer S. Phytic Acid as a Phosphorus Reservoir in the Developing Potato Tuber. Nature 1962, 194, 578. 10.1038/194578b0.13911680 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.