Abstract

The immobilization of biomolecules onto polymeric surfaces employed in the fabrication of biomedical and biosensing devices is generally a challenging issue, as the absence of functional groups in such materials does not allow the use of common surface chemistries. Here we report the use of modified poly-l-lysine (PLL) as an effective method for the selective modification of polymeric materials with biomolecules. Cyclic olefin polymer (COP), Ormostamp, and polydimethylsiloxane (PDMS) surfaces were patterned with modified PLLs displaying either biotin or maleimide functional groups. Different patterning techniques were found to provide faithful microscale pattern formation, including micromolding in capillaries (MIMIC) and a hydrogel-based stamping device with micropores. The surface modification and pattern stability were tested with fluorescence microscopy, contact angle and X-ray photoelectron spectroscopy (XPS), showing an effective functionalization of substrates stable for over 20 days. By exploiting the strong biotin–streptavidin interaction or the thiol–maleimide coupling, DNA and PNA probes were displayed successfully on the surface of the materials, and these probes maintained the capability to specifically recognize complementary DNA sequences from solution. The printing of three different PNA-thiol probe molecules in a microarray fashion allowed selective DNA detection from a mixture of DNA analytes, demonstrating that the modified PLL methodology can potentially be used for multiplexed detection of DNA sequences.
Keywords: polymer substrates, surface patterning, poly-l-lysine, biomolecule adhesion, PNA, microarrays, DNA recognition
Introduction
The interest in polymer materials for microfabrication and biomedical applications has rapidly increased owing to their moldability, optical clarity, and high solvent resistance.1,2 Especially in biotechnology and biosensing applications, the use of plastic substrates has been intensively fueled by the need for reliable, cost-effective ways of producing simple-to-use devices, such as microfluidic and optical chips, and lab-on-a-chip and disposable biosensing devices.3−5
Multiple examples of polymeric materials have been described for biomedical and biosensing purposes. Polycarbonate (PC) substrates have been used for the detection of DNA3 and in microarrays, poly(dimethylsiloxane) (PDMS) has been used in microfluidic chips for digital PCR6 and DNA detection in nanochannels,7 and cyclic olefin (co)polymer (COP/COC) platforms have been used for sandwich immunoassays for antibodies5 and microelectromechanical systems (MEMS).8 In particular, COP substrates are gaining interest as materials in biotechnology and biosensing owing to their ideal properties such as optical transparency, good chemical resistance and low water absorption.9 Similarly, Ormostamp has been successfully investigated as a material for biosensing applications owing to its excellent imprinting capabilities.10,11 However, in general, all plastic materials present a major drawback of lacking chemically reactive groups to allow for stable, covalent surface functionalization. The absence of functional groups therefore prohibits the direct use of commonly applied surface functionalization protocols, such as thiol adsorption on gold12,13 or silanization on SiO2 substrates.14,15
Alternative functionalization methods, comprising photografting,16,17 photochemical patterning,1 and APTES silanization after oxidative treatment,5,18 have been exploited to anchor biomolecules to the initially unreactive polymeric substrates. Likewise, the use of positively charged polymers on negatively charged surfaces has been demonstrated to be an effective method to modify both metallic and metal oxide substrates, introducing at the same time specific functional moieties and antifouling properties.19−23 Particularly poly-l-lysine (PLL) and modified versions thereof, which are biocompatible and positively charged at physiological pH, have been proven to adsorb strongly onto negatively charged substrates such as inorganic (SiO2,24 TiO2,25 Nb2O5,26) and polymeric ones, for example, polydimethylsiloxane (PDMS),27,28 poly(methyl methacrylate) (PMMA),28 and polystyrene (PS).29 Stable electrostatic interactions are achieved upon activation of these surfaces, retaining high stability and reliability over time.
Owing to the easy functionalization and fast self-assembly of PLL, grafted antifouling poly(ethylene glycol) (PEG) groups30,31 and binding moieties, such as biotin,32,28 nitrilotriacetic acid (NTA),33,34 catechol,35 and functional RGD peptides36 or fluorescein,37 were anchored onto fully covered oxide or polymer substrates. At the same time, micropatterns and microarrays containing PLL-PEG or PLL-PEG bearing secondary functionalities for selective immobilization were formed on multiple metal oxide surfaces in combination with stamping,37−40 molecular self-assembly41,42 and electrochemical43 patterning techniques. Saravia et al. used PLL grafted with PEG (PLL-g-PEG) without additional functional groups for the patterning of proteins on silicon oxide surfaces.38 Falconnet and co-workers used PLL-PEG end-functionalized with biotin or RGD peptide (used to promote cell binding) to form microarrays for cell–surface interactions studies.41 Duan et al. reported silicon nano-BioFET biosensors covered by a uniform layer of PLL cografted with short oligo(ethylene glycol) (OEG4) and OEG4-biotin moieties, retaining the antifouling properties and strong surface adhesion.44 Recently, we have demonstrated that PLL polymers with customizable fractions of OEG and maleimide moieties can be easily adsorbed onto both gold and silicon dioxide surfaces, while controlling the type and the density of probe molecules at the interface in the preceding synthetic step.45
Here, we report two approaches to functionalize different types of plastic surfaces commonly used in biosensing applications, such as COP, Ormostamp, and PDMS, by exploiting the adsorption of PLL grafted with OEG4-biotin or OEG4-maleimide for fast, efficient, and selective immobilization of biomolecules. The aim of this work is to demonstrate the versatility and stability of modified PLL polymers for the binding of biomolecular sensing probes to polymeric surfaces, in combination with different patterning techniques to form microstructures at these surfaces. By exploiting the electrostatic interactions between the negatively charged polymer surface and the positively charged modified PLL, a self-assembled monolayer is formed on the surface, bestowing the possibility of bio-orthogonally anchoring a wide range of molecules of biological interest. In particular, COP and Ormostamp surfaces were patterned by micromolding in capillaries (MIMIC) with modified PLL, while microarrays were made on PDMS substrates employing a stamping device. Modified PLLs bearing biotin or maleimide as reactive moieties were employed as adhesion layers, to which biomolecules can be anchored specifically and selectively by different chemical coupling reactions. Engineered DNA and peptide nucleic acid (PNA) probes, for the detection of complementary DNA (cDNA) sequences in solution, were immobilized on the substrates as a proof-of-concept of successful substrate functionalization. Further sensing method development, e.g., aimed at defining the limit of detection or the combination with amplification strategies, is not addressed here.
Results and Discussion
Soft Lithography Strategies Based on Modified PLL
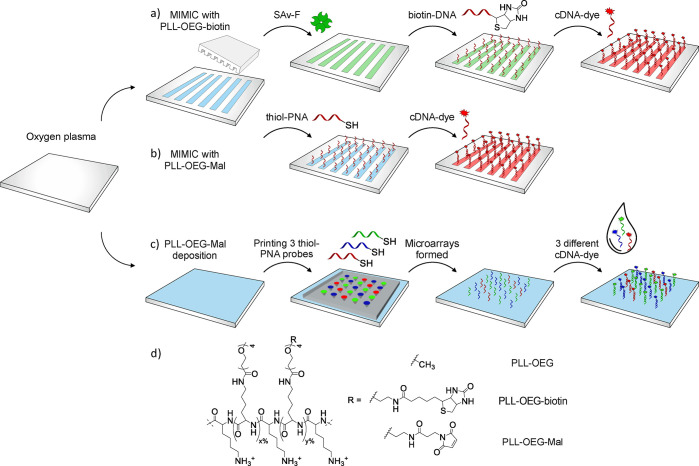
Figure 1 shows a schematic overview of the two soft lithography strategies used to pattern the surface of polymer substrates (of COP, Ormostamp (as a thin layer on poly(ethylene terephthalate)), PET, and PDMS) with modified PLL, and their proof-of-principle application to DNA detection. The formation of bioresponsive patterns was achieved by MIMIC (Figure 1a,b). Upon oxygen plasma activation, COP and Ormostamp substrates were functionalized with biotin or maleimide-modified PLL.
Figure 1.
Soft lithography methods used to pattern polymeric substrates with PLL. After oxygen plasma treatment, MIMIC with either (a) PLL-OEG-biotin or (b) PLL-OEG-Mal defines modified PLL lines on COP or Ormostamp substrates, which are used to orthogonally anchor specific probes as (a) biotinylated DNA or (b) thiol-PNA for cDNA detection. Alternatively, (c) a printing device loaded with three different thiol-PNA probes is used to form a microarray on an activated PDMS substrate, which is used to detect selectively the specific cDNA from a mixture of the three complementary sequences. (d) Structures of the PLL polymers, modified with OEG, OEG-biotin, and OEG-Mal, employed for the surface functionalization. x and y indicate the relative fractions of the modified lysine subunits. Structures of the dyes with linkers and oligonucleotide sequences are shown in Figure S2 and Table S2, respectively.
PLL polymers (15–30 kDa) were grafted with oligo(ethylene glycol) (OEG) spacers and either biotin or maleimide (Mal) moieties, following reported procedures.44,45 All the modified PLLs synthesized, namely, PLL-OEG, PLL-OEG-biotin, and PLL-OEG-Mal, present the OEG functionality to enhance the antifouling properties of the substrate, providing concomitantly a secondary functional group for further selective bio-orthogonal modification, such as biotin or maleimide (Figure 1d). The total degree of functionalization of the PLL polymers has been determined by 1H NMR (see Figure S1 and Table S1), and has been intentionally kept between 20% and 40%, to maintain a balance between a stable surface adhesion and antifouling properties.44−46
Subsequently, DNA probes were anchored on the surface in order to selectively recognize complementary DNA sequences in solution. PLL-OEG-biotin and PLL-OEG-Mal were used either to anchor streptavidin conjugated with fluorescein isothiocyanate (SAv-F) and biotinylated DNA (biotin-DNA1) by exploiting the noncovalent SAv-biotin interaction, or to covalently bind PNA-thiol probes on the substrate by using the specific Michael-type thiol–ene reaction, respectively (structure of dyes and probe sequences are given in Figure S2 and Table S2). Fluorescently labeled cDNA was used to visualize the resulting patterns using fluorescence microscopy.
Alternatively, as shown in Figure 1c, modified PLL was exploited in a hydrogel-filled stamping device with micropores underneath each well.47 The device was loaded with three different thiol-PNA probes (PNA2, PNA3, and PNA4; Table S2), to print proof-of-concept PNA microarrays onto PLL-OEG-Mal-modified polymer substrates. The choice of these specific PNA molecules has been endorsed by their pivotal role in the early detection and monitoring of cancer (Table S2). PNA was used as a probe because of its higher affinity for cDNA sequences, leading to an improved specificity compared to DNA probes.48 Moreover, the higher stability of the PNA/DNA duplex, with respect to DNA/DNA,49 and the resistance of PNA toward enzymes such as nucleases and peptidases50 are properties widely used to increase the sensitivity of DNA biosensing devices.
After binding the probes to the substrates using the stamping device, the DNA recognition and multiplexing capabilities of the patterned substrates were studied by fluorescence microscopy upon incubation of the microarrays with a solution mixture of three cDNA sequences (complementary to the PNA sequences used) labeled with a blue, green, and red fluorescent dye.
Surface Patterning by MIMIC
In order to investigate the applicability of modified PLLs on different polymeric materials, we used the thermoplastic COP and the thermosetting Ormostamp as substrates. After activation by oxygen plasma, a PDMS mold (containing channels 100 μm wide and spaced 100 μm) was used to pattern PLL-OEG-biotin (0.1 mg/mL in PBS, pH 7.4) by MIMIC. After removal of the stamp and rinsing the substrate, fluorescently labeled SAv-F (PBS, pH 7.4) was adsorbed.
Figure 2a and c shows clear fluorescent lines obtained after the functionalization of the substrates, owing to the successful adsorption of SAv-F onto both patterned surfaces. The empty areas in between the lines indicates that the SAv bound only to the areas where PLL-biotin was deposited. The absence of clear fluorescent lines in the control experiments (Figure 2b,d), where patterns were made with only PLL-OEG, i.e., without biotin moieties prior to the SAv-F binding, confirms the absence of nonspecific adsorption of SAv. Therefore, also the antifouling behavior of the locally self-assembled PLL on the plastic surface was proven. The line edge irregularities along the fluorescent lines on the Ormostamp surface (Figure 2c) are likely due to imperfections and roughness of the material itself. Overall, PLL can be successfully adsorbed on both materials owing to the electrostatic interactions between the positively charged amino groups at the lysine side chains at physiological pH25,51 and the negatively charged surfaces after oxygen plasma treatment, and the subsequent molecular recognition is specific.
Figure 2.
Fluorescence microscopy images of SAv-F on (a,b) COP (1500 ms, ISO 200) and (c,d) Ormostamp (2000 ms, ISO 400) surfaces, upon patterning (a,c) PLL-OEG-biotin or (b,d) PLL-OEG (antifouling) by MIMIC (scheme of Figure 1a). (e) Normalized fluorescence intensity profiles (average) of the COP sample patterned with PLL-OEG-biotin and SAv-F monitored after incubation in PBS solution with 1.0 mg/mL BSA, at day 0 (black), 1 (red), 4 (blue), 8 (pink), and 20 (green). All experiments were performed using 0.1 mg/mL of modified PLL polymers during MIMIC, and 0.1 mg/mL SAv-F in PBS at pH 7.4 during subsequent incubation.
To further confirm the attachment of the PLL polymers to the substrates, contact angle goniometry was performed on COP and Ormostamp substrates fully covered with PLL-OEG. The measurements (Table S3) indicate for both substrates a similar value of approximately 31.5°, proving the adsorption of the modified PLL. This value was measured homogeneously all over the surface. In addition, X-ray photoelectron spectroscopy (XPS) was performed on fully functionalized COP surfaces. Figure S3a and b reports the N 1s and S 2p spectra, respectively, for bare, PLL-OEG, and PLL-OEG-biotin-functionalized surfaces. Nitrogen peaks were visible only on substrates functionalized with PLL (both PLL-OEG and PLL-OEG-biotin), while the presence of the S 2p peak of the sulfur was observed only for the PLL-OEG-biotin modification, confirming the desired functionalization of the substrates. All atom percentages are given in Table S4. The carbon data show a decrease upon adsorption of PLL, caused by the larger amount of heteroatoms in PLL compared to the polymer substrate. The highly comparable carbon decrease and concomitant N and O increase observed for PLL-OEG and PLL-OEG-biotin compared to the unmodified substrate indicate adsorption of the PLL variants with comparable coverage.
Surface functionalization with modified PLL requires stability for a considerable period of time in order to be used in biosensing or biomedical applications. Contact angle measurements showed only slightly increased contact angles for PLL-modified surfaces over 10 days when leaving such functionalized substrates in Milli-Q (Table S3), which confirmed long-term coverage of the substrates with PLL. Additionally, the stability of a MIMIC-patterned substrate in solution was tested both over time (Figures 2e and S4) and upon ultrasonication (Figure S5). Figure 2e shows the normalized intensity of fluorescent SAv-F-patterned lines on PLL-biotin-covered COP, monitored during 0, 1, 4, 8, and 20 days in PBS (pH 7.4) with 1.0 mg/mL bovine serum albumin (BSA) to mimic biological samples. The fluorescence intensity slowly decreased over time with a total loss of 40% after 20 days (Figure 2e). The same effect was observed by 10 min sonication with an intensity loss of around 7% (Figure S5). Some pattern inhomogeneities were observed as well, in particular after 20 days (Figure S4j). These results contrast to some extent the contact angle data described above. Possibly, the loss of fluorescence over time is caused by photobleaching of the dye as well as detachment of SAv (by denaturation), but some partial desorption of PLL cannot be excluded.
DNA Recognition at MIMIC-Patterned Substrates
The ability of easily customizing patterned surfaces upon self-assembly of modified PLL is highly appealing for the detection of biomolecules such as proteins and DNA. Therefore, we tested capability of two differently modified PLL polymers, PLL-OEG-biotin and PLL-OEG-Mal, to detect cDNA sequences from solution both on COP and Ormostamp biochip surfaces (Figure 3). As described above, we used MIMIC with either PLL-OEG-biotin or PLL-OEG-Mal followed by the anchoring of the probes (Figure 1a,b). In the case of PLL-OEG-biotin, the consecutive immobilization of SAv-F (0.1 mg/mL) and biotin-DNA1 (1 μM) formed the biorecognition pattern on the surface of COP. In the case of PLL-OEG-Mal, the thiolated probe PNA2 (activated by TCEP treatment just before coupling)45 was reacted onto the Ormostamp surface.
Figure 3.
Fluorescence microscopy images of COP (a–d) and Ormostamp (e,f) substrates. COP substrates were patterned (using MIMIC) with PLL-OEG-biotin, followed by the consecutive deposition of SAv-F and biotin-DNA1. Ormostamp substrates, instead, were functionalized with PLL-OEG-Mal and reacted with thiol-PNA2. Subsequently, COP substrates were incubated with cDNA1-RRED (a,b) or ncDNA1-RRED (c,d), while Ormostamp substrates were incubated with cDNA2-RRED (e) or ncDNA2-RRED (f). Samples were imaged in the green (a,c, SAv-F) and red (b,d–f, RRED) channels, where the image pairs a/b and c/d were measured at the same area of the same samples. All experiments were performed using 0.1 mg/mL solutions of modified PLL polymers, 0.1 mg/mL SAv-F, and 1 μM DNA/PNA probes, cDNA and ncDNA in PBS at pH 7.4. Fluorescence parameters used: (a,c) 1500 ms, ISO 200; (b,d) 2000 ms, ISO 200; (e,f) 2500 ms, ISO 200.
As a proof-of-concept, upon immobilization of the probes (biotin-DNA1 or PNA2) onto the surfaces, solutions of corresponding fluorescent dye-functionalized cDNA-Rhodamine Red (RRED) sequences were put onto the substrates. Figure 3b and e shows the fluorescence images of the COP and Ormostamp substrates after the cDNA-RRED additions. In the case of the COP substrates, functionalized with PLL-OEG-biotin and fluorescent SAv, the presence of clear and bright red patterns shows that the hybridization of the cDNA with the DNA probe had occurred successfully. Moreover, the colocalization of the green lines (Figure 3a), indicating the presence of SAv, with the red ones (Figure 3b), due to the presence of cDNA1-RRED, confirms the selective hybridization of the cDNA to the areas covered with the biotin-DNA1 probe, indicating the absence of nonspecific interactions. When, in a similar experiment, the same type of biorecognition surface was treated with a noncomplementary DNA grafted with the RRED dye (ncDNA1-RRED), the concomitant presence of green (Figure 3c) and the absence of red fluorescence (Figure 3d) confirmed the binding selectivity of cDNA. Moreover, the absence of fluorescence in the case of ncDNA confirmed the beneficial antifouling effect of OEG chains anchored to the PLL backbone, which prevented also the purely electrostatic, nonspecific adsorption of negatively charged DNA strains onto the surface-adsorbed, positively charged PLL.
Analogously, the PNA2 molecule grafted to the PLL-OEG-Mal polymer on Ormostamp exhibited a similarly selective and specific response as evidenced by the comparison between Figure 3e and f, employing the complementary and noncomplementary DNA sequences, respectively. These results show not only the similar behavior and applicability for both types of materials surfaces but also the possibility of using different engineered probes (DNA and PNA) on the polymer surface. Moreover, the use of PLL-OEG-Mal presents the additional advantage of functionalizing substrates with a probe in a single step, avoiding the extra SAv addition used for the functionalization with PLL-OEG-biotin.
To test the stability of the PNA2/cDNA2-RRED-patterned Ormostamp, the substrate was sonicated for 10 min. Despite a loss of fluorescence intensity of about 10% after the treatment (Figure S6), the line width and homogeneity were maintained, indicating the strong, spatioselective adhesion of PLL on Ormostamp and the subsequent formation of the cDNA/PNA complex.
The DNA biorecognition of a probe-modified PLL-OEG-Mal layer on a polymer substrate was also tested in continuous flow by quartz crystal microbalance with dissipation (QCM-D). A SiO2 chip, spin-coated and cured with a thin film of Ormostamp, was activated by oxygen plasma and then subjected to a solution of either PLL-OEG-Mal or PLL-OEG (as a control), followed by anchoring of the deprotected thiol-PNA2. Figure S7 shows the QCM-D time traces (frequency shifts, Δf, in blue) of the binding process for cDNA2-RRED and ncDNA2-RRED sequences on the PLL-OEG-Mal and antifouling PLL-OEG layers. A detectable frequency shift was only observed for cDNA2 adsorbed on a PNA2-bound PLL-OEG-Mal layer, confirming the successful adhesion of PLL, subsequent probe binding, and specific hybridization, as well as its antifouling properties.
Multiplexed DNA Detection
Probe-modified PLL can be also exploited to create bioresponsive microarrays that allow multiplexed DNA detection. We used, as a proof-of-concept, a hydrogel-filled stamping device (Figure S8)47 with an array of 5 × 5 wells that were individually loaded with different PNA-thiol sequences to create the microarrays, yielding dot-patterned fields of 12 × 12 dots, with each dot being 5 μm wide. Each field was addressed by a single well of the stamp, and fields were separated by 350 μm. PDMS was chosen as the substrate material because of its conformal contact with the stamping device. After oxygen plasma activation, the PDMS surface was covered with PLL-OEG-Mal, and then the inked stamp was put in contact with the substrate for approximately 10 min to allow the probe attachment to occur (Figure 1c). Three different PNA thiol sequences were used, here indicated as PNA2, PNA3 and PNA4 (Table S2). Subsequently, the DNA recognition ability of the PNA array was tested by depositing a solution containing one or three complementary DNA probes, each equipped with a different fluorescent dye (cDNA2-DY415, cDNA3-F, and cDNA4-RRED).
Figure 4 shows the fluorescence microscopy images after incubation of a printed dot array with a mixture containing all three cDNA sequences, taken at the same substrate area using blue (Figure 4a), green (Figure 4b), and red (Figure 4c) filters, while Figure 4d shows the composite image. From Figure 4, several aspects can be noted. First of all, each field of dots is visualized only with the proper filter, and each color occurs at a different position, confirming that the cDNA sequences were selectively and orthogonally assembled from the mixture. No cross contamination is observed on the array, demonstrating the excellent selectivity of the PNA probes to recognize their corresponding cDNA sequences. In addition, the absence of fluorescence in the nonprinted areas confirms the antifouling properties provided by the OEG groups bound to the PLL backbone.
Figure 4.
Fluorescence microscopy images, all of the same area of a PNA-functionalized microarray on PDMS, after incubation with a mixture of three complementary DNA sequences each functionalized with a different fluorescent dye, using (a) blue (1500 ms ISO 400), (b) green (2000 ms, ISO 400), and (c) red (2000 ms, ISO 400) filters; panel (d) shows the composite image. The PDMS substrate was first oxidized and then functionalized with 0.1 mg/mL PLL-OEG-Mal. The microarray stamping device was inked with 1 μM solutions of the PNA-thiol sequences and then put in contact with the substrate for 30 s. After stamp removal, the substrate was rinsed and incubated with a mixture containing 0.33 μM of each of the corresponding cDNA sequences.
The selectivity of the PNA microarrays for a specific cDNA sequence was further investigated by hybridization of the same PNA arrays with a solution containing only one fluorescent cDNA sequence at a time. Figures S9–S11 reveal the fluorescent dot arrays corresponding solely to the hybridized cDNA-dye used, retaining the antifouling behavior outside the printed areas. These results demonstrate that the modified PLL methodology is compatible with microarray printing techniques and multiplexed DNA analysis.
Conclusions
In summary, we have demonstrated the versatile formation of micropatterns on three different polymeric materials (COP, Ormostamp, PDMS) by exploiting modified poly-l-lysine polymers for orthogonal adhesion and selective recognition of biomolecules. As a proof-of-concept, DNA and PNA probes were anchored onto the polymeric substrates, and the formed biorecognition surfaces showed excellent selectivity for complementary DNA sequences. We further extended the patterning and recognition to a multiplexed analysis by printing three different PNA-thiol molecules, demonstrating that the modified PLL methodology is compatible with the delivery of engineered probes in microarray fashion.
All in all, these results underline the versatility of modified PLL in combination with patterning techniques for biorecognition and future biosensing applications on a large variety of substrate materials. The strategy outlined here to attach modified PLL with customized appending groups is promising for the specific and stable anchoring of biomolecules onto virtually any polymeric substrate that presents negative charges. This work may contribute to the development of customized responsive (bio)interfaces in medical devices, environment-friendly biosensors, and versatile coatings.
Experimental Section
Materials
Poly-l-lysine hydrobromide (MW = 15–30 kDa by viscosity), EDC, NHS, tablets for 10 mM PBS solution (pH 7.4), 3-trichlorosilylpropyl methacrylate, 2-hydroxyethyl methacrylate (HEMA), ethylene glycoldimethacrylate (EGDMA), tridecafluoro-(1,1,2,2)-tetrahydrooctyl-trichlorosilane, bovine serum albumin, ammonium persulfate (APS), and NaCl salt were purchased from Sigma-Aldrich. Methyl-OEG4-NHS ester, biotin-OEG4-NHS ester, Mal-OEG4-NHS ester, the Zeba spin desalting columns (7 kDa MWCO, 5 mL), the immobilized TCEP disulfide reducing gel (tris[2-carboxyethyl] phospine hydrochloride immobilized onto 4%cross-linked beaded agarose), and the streptavidin-Fluorescein conjugate were obtained from ThermoFisher Scientific. Oligonucleotides were purchased from Eurofins Genomics and used as received. SiO2 QCM chips (with fundamental frequency of 5 MHz) were purchased from Biolin Scientific. Poly(dimethylsiloxane) Sylgard 184 and curing agent were used as received from Dow Corning. Ormostamp material and MA-T1050 were purchased from Microresist. The PNA probes were synthesized using the materials and the procedure previously described for PNA2,52 as briefly reported in the Supporting Information together with the characterization of the newly synthesized PNA3 and PNA4.
Synthesis of Modified PLLs
All modified PLLs were synthesized according to previously reported procedures.44,451H NMR of PLL-OEG(26.1)-biotin(5.7) (400 MHz D2O) δ [ppm] = 1.26–1.58 (lysine γ CH2), 1.63 1.85 (lysine β,δ CH2), 2.25 (biotin linker, CH2–C(=O)–NH−), 2.49 (ethylene glycol CH2 from both OEG and biotin coupled, CH2–C(=O)–NH), 2.75 (biotin, −S–CH2−), 2.98 (free lysine, H2N–CH2−), 3.15 (ethylene glycol CH2 of coupled lysine from both OEG and biotin, C(=O)–NH–CH2), 3.35 (OEG methoxy, O–CH3), 3.53–3.78 (oligo ethylene glycol from both OEG and biotin, CH2–O−), 4.26 (lysine backbone, NH–CH–C(O)−), 4.40 (biotin, −CH–NH–C(=O)–NH−), 4.59 (biotin, −CH–NH–C(=O)–NH−).
1H NMR of PLL-OEG(20.3)-Mal(4.5) (400 MHz D2O) δ [ppm] = 1.26–1.56 (lysine γ CH2), 1.61 1.82 (lysine β,δ CH2), 2.49 (ethylene glycol CH2 from both OEG and Mal coupled, CH2 C(=O) NH), 2.99 (free lysine, H2N CH2), 3.16 (ethylene glycol CH2 of coupled lysine from both OEG and Mal, C(=O)–NH–CH2), 3.35 (OEG methoxy, −O–CH3), 3.58–3.79 (oligo ethylene glycol from both OEG and Mal, CH2–O), 4.29 (lysine backbone, NH–CH–C(O)−), 6.85 (maleimide from coupled Mal, −C(=O)–CH–CH–C(=O)−).
Preparation of PDMS Substrates
Poly(dimethylsiloxane) (PDMS) substrates (for use of the stamping device) and molds for MIMIC were prepared as reported previously.47,53,54 In short, a 10:1 (v/v) mixture of PDMS and curing agent Sylgard 184 was casted against either a flat Petri dish or a silicon master with etched structures (100 × 100 μm2) prepared by lithography. After overnight curing at 60 °C, the PDMS in the Petri dish was stored as is, while the PDMS on the silicon master was cut in small MIMIC molds, and their edges were opened with a scalpel before storing.
MIMIC on COP/Ormostamp
MIMIC was performed following previously reported procedures.47,54 Both PDMS mold and either COP or Ormostamp substrates (approximately 1.5 × 1 cm2) were cleaned by sonication in a mixture of Milli-Q water and EtOH (1:1), dried by a stream of nitrogen, and activated by oxygen plasma for 1 min (Plasma Prep II, SPI Supplies; 200–230 mTorr, 40 mA). Thereafter, the mold was placed on top of the activated polymeric material to form the network of channels, owing to the conformal contact. A drop (10–20 μL) of desired modified PLL solution (0.1 mg/mL in PBS, pH 7.4) was placed at the open edge of the PDMS mold and the liquid filled the channels as a result of capillary forces. The mold was peeled off, and the patterned PLL lines were rinsed copiously with Milli-Q water.
Functionalization of Substrates Patterned by MIMIC
PLL-OEG-biotin patterned substrates were functionalized by consecutively depositing 80 μL of 0.1 mg/mL SAv-F solution in PBS (pH 7.4) for 5 min and 1 μM of biotin-DNA in the same buffer for 10 min. The substrates patterned with PLL-OEG-Mal were covered for 10 min with an 80 μL solution of PNA-thiol probe (1 μM in PBS, pH 7.4), freshly deprotected from the disulfide group (SPDP = 3-(2-pyridyldithio)propionyl) using the TCEP reducing gel following the procedure described in our previous work.45 Hybridization experiments were performed by placing on top of both patterned recognition surfaces (COP and Ormostmap) 80–100 μL of corresponding ncDNA-RRED or cDNA-RRED solutions (1 μM in PBS, pH 7.4) for 10 min. After every deposition step, substrates were rinsed with Milli-Q water and blown dry in a stream of nitrogen.
Fully Covered COP/Ormostamp Substrates for Contact Angle and XPS Measurements
COP and Ormostamp substrates (1.5 × 1.0 cm) were activated as previously described, immersed in a solution of PLL-OEG (0.1 mg/mL in PBS, pH 7.4) for 30 min and then rinsed with Milli-Q water. Control samples were immersed in PBS (pH 7.4) solution, without PLL. Contact angle measurements were recorded before and after the activation, and after the functionalization. Thereafter, the substrates were stored in Milli-Q water, and the contact angle was monitored for 10 days. COP substrates for XPS were prepared following the same procedure, and were fully dipped in the solutions of pure PBS, without or with PLL-OEG or PLL-OEG-biotin (0.1 mg/mL, pH 7.4).
Spin-Coating of Ormostamp on SiO2 QCM and PET Substrates
For all regular Ormostamp substrates described above, using an adapted procedure previously reported,55 a drop of Ormostamp solution was manually dispensed on a PET substrate. By using a laboratory customized imprinting setup, a flat glass wafer previously coated with the antiadhesion coating tridecafluoro-(1,1,2,2)-tetrahydrooctyl-trichlorosilane was pressed against uncured Ormostamp. UV-light from an OmniCure LX400 with 365 nm LED source was exposed through the glass wafer acting as an imprint tool. After being released from the mold, a flat layer of approximately 30 μm of cured Ormostamp was formed on the PET substrate.
Prior to coating, the QCM chips were treated by oxygen plasma (Pico, Diener Electronic GmbH) for 30 s. The Ormostamp material was diluted in Ma-T1050 (1:10,v/v) and spin-coated at 3000 rpm for 60 s. After the spinning, the Ma-T1050 thinner was evaporated out at 130 °C for 10 min on a hot plate. To UV-cure the Ormostamp layer, QCM chips were placed in a vacuum chamber (Pico, Diener Electronic GmbH) and UV-light from OmniCure LX400 with a 365 nm LED source was exposed through a window, forming a 200 nm thin layer of Ormostamp.
Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
Silica-coated (50 nm, QSX303) QCM-D sensors from LOT-Quantum were spin-coated with Ormostamp, and then washed with Milli-Q water and EtOH, sonicated in EtOH for 5 min, dried in a stream of nitrogen and finally oxidized in oxygen plasma (Plasma Prep II, SPI Supplies; 200–230 mTorr, 40 mA) for 1 min. Activated chips were fully immersed in a solution of either PLL-OEG or PLL-OEG-Mal (0.1 mg/mL in PBS, pH 7.4) for 30 min, followed by incubation in 1 μM of activated PNA2 thiol solution (PBS, pH 7.4) for 30 min. After each modification step, the Ormostamp-coated chips were gently rinsed with Milli-Q water and dried in a stream of nitrogen. QCM-D measurements were performed using a Q-Sense E4 4-channel quartz crystal microbalance with a peristaltic pump (Biolin Scientific), monitoring the fifth fundamental overtone. All experiments were performed in PBS buffer (pH 7.4) with a flow rate of 80 μL/min at 22 °C.
X-ray Photoelectron Spectroscopy (XPS)
XPS measurements were performed using a Quantera SXM machine (scanning XPS microprobe) from Physical Electronics equipped with a monochromatic Al Kα X-ray source (1486.6 eV). During the analyses, the filament current was kept at 2.6 mA and the power was 50 W. The working chamber pressure was maintained at 3 × 10–8 Torr. The size of the X-ray beam used in the analysis was 200 μm.
Scanning Electron Microcopy (SEM)
High resolution SEM (JEOL Field Emission JSM-6330F, JEOL Benelux, The Netherlands) with 3 keV electron acceleration was used to image the printing device before and after the hydrogel formation (see below). The hydrogel-filled device was dried overnight at 60 °C prior mounting in the SEM chamber.
Stamping Device Preparation, Loading and Printing on PLL-OEG-Mal Covered Surfaces
The stamping device with hydrogel wells was fabricated according to a previously reported procedure.47 The hydrogel-filled printing device was removed from the Milli-Q water storage solution and gently tapped on both reservoir and printing sides with clean room paper to remove the excess of water. Zooming on the well array (reservoir side) with the help of an optical microscope, each well was individually filled with 0.25–0.5 μL of the desired 1 μM PNA-thiol solution (PNA2, PNA3, and PNA4, freshly deprotected using the TCEP reducing gel)45 using a syringe and allowed to ink for 30 min. The flat PDMS substrate (cut with a scalpel to approximately 1.5 × 1 cm2) was activated following the same procedure as COP and Ormostamp (see the section Fully Covered COP/Ormostamp Substrates for Contact Angle and XPS Measurements) and then functionalized with 80–100 μL of PLL-OEG-Mal solution (0.1 mg/mL in PBS, pH7.4) for 5 min, followed by washing with Milli-Q water and blow drying in a stream of nitrogen. Thereafter, the inked stamping device was gently pressed on top of the PLL-OEG-Mal-functionalized PDMS substrate to obtain conformal contact. A first print on a dummy piece of cleaned PDMS was performed to allow the ink to reach the printing side. After 10 min, the stamping device was demolded from the substrate, which was gently rinsed with Milli-Q water and dried with nitrogen. Then 80–100 μL of solution containing a mixture of correspondent cDNA-dye sequences (cDNA2-DY415, cDNA3-F, and cDNA4-RRED) or only one of them at 0.33 μM (per DNA molecule) in PBS (pH 7.4) was placed on top for 30 min. The excess of DNA solution was washed away with Milli-Q water, and the PDMS substrate was immediately dried under nitrogen flow before the analysis at the fluorescence microscope.
Fluorescence Microscopy
Fluorescence microscopy images were taken in air using an Olympus inverted research microscope IX71 equipped with a mercury burner U-RFL-T as light source and a digital Olympus DP70 camera. A combination of DAPI and Olympus cubes were used to have blue (λex = 430 nm; λem = 470 nm), green (460 nm ≤ λex ≤ 490 nm; λem = 525 nm), and red (510 nm ≤ λex ≤ 550 nm; λem ≥ 590 nm) filters. The linear (average) and 3D intensity profiles were obtained by taking a rectangular selection over the whole picture, elaborated with ImageJ software.
Acknowledgments
The Horizon 2020 Health Project “ULTRAPLACAD” (no. 633937) and Marie Curie Innovative Training Network MULTI-APP (no. 642793) are acknowledged for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsapm.9b00814.
1H NMR spectra of PLL-OEG-biotin and PLL-OEG-Mal; quantification of PLL modification; structures and sequence of PNA and DNA molecules; contact angle goniometry measurements; XPS measurements; fluorescence pictures of patterned substrates; QCM measurements; SEM pictures of printing device; fluorescence pictures of DNA recognition array control experiments; PNA synthesis and characterization (PDF)
Author Contributions
J.M. and D.D. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Kim J.; Hong D.; Jeong S.; Kong B.; Kang S. M.; Kim Y. G.; Choi I. S. Aryl Azide Based, Photochemical Patterning of Cyclic Olefin Copolymer Surfaces with Non-Biofouling Poly[(3-(Methacryloylamino)Propyl)Dimethyl(3- Sulfopropyl)Ammonium Hydroxide]. Chem. - Asian J. 2011, 6, 363–366. 10.1002/asia.201000569. [DOI] [PubMed] [Google Scholar]
- Hochrein M. B.; Reich C.; Krause B.; Rädler J. O.; Nickel B. Structure and Mobility of Lipid Membranes on a Thermoplastic Substrate. Langmuir 2006, 22, 538–545. 10.1021/la051820y. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang Z.; Ou L. M. L.; Yu H. Z. DNA Detection on Plastic: Surface Activation Protocol to Convert Polycarbonate Substrates to Biochip Platforms. Anal. Chem. 2007, 79, 426–433. 10.1021/ac061134j. [DOI] [PubMed] [Google Scholar]
- Becker H.; Gärtner C. Polymer Microfabrication Technologies for Microfluidic Systems. Anal. Bioanal. Chem. 2008, 390, 89–111. 10.1007/s00216-007-1692-2. [DOI] [PubMed] [Google Scholar]
- Raj J.; Herzog G.; Manning M.; Volcke C.; MacCraith B. D.; Ballantyne S.; Thompson M.; Arrigan D. W. M. Surface Immobilisation of Antibody on Cyclic Olefin Copolymer for Sandwich Immunoassay. Biosens. Bioelectron. 2009, 24, 2654–2658. 10.1016/j.bios.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Zhou H.; Jia C.; Jing F.; Jin Q.; Zhao J.; Li G. A Microfluidic Chip Based on Surfactant-Doped Polydimethylsiloxane (PDMS) in a Sandwich Configuration for Low-Cost and Robust Digital PCR. Sens. Actuators, B 2017, 245, 414–422. 10.1016/j.snb.2017.01.161. [DOI] [Google Scholar]
- Peng R.; Li D. Detection and Sizing of Nanoparticles and DNA on PDMS Nanofluidic Chips Based on Differential Resistive Pulse Sensing. Nanoscale 2017, 9, 5964–5974. 10.1039/C7NR00488E. [DOI] [PubMed] [Google Scholar]
- Ma K.-S.; Reza F.; Saaem I.; Tian J. Versatile Surface Functionalization of Cyclic Olefin Copolymer (COC) with Sputtered SiO2 Thin Film for Potential BioMEMS Applications. J. Mater. Chem. 2009, 19, 7914. 10.1039/b904663a. [DOI] [Google Scholar]
- Laib S.; MacCraith B. D. Immobilization of Biomolecules on Cycloolefin Polymer Supports. Anal. Chem. 2007, 79, 6264–6270. 10.1021/ac062420y. [DOI] [PubMed] [Google Scholar]
- Klukowska A.; Kolander A.; Bergmair I.; Muhlberger M.; Leichtfried H.; Reuther F.; Grutzner G.; Schoftner R. Novel Transparent Hybrid Polymer Working Stamp for UV-Imprinting. Microelectron. Eng. 2009, 86, 697–699. 10.1016/j.mee.2008.12.088. [DOI] [Google Scholar]
- Hiltunen M.; Hiltunen J.; Stenberg P.; Aikio S.; Kurki L.; Vahimaa P.; Karioja P. Polymeric Slot Waveguide Interferometer for Sensor Applications. Opt. Express 2014, 22, 7229. 10.1364/OE.22.007229. [DOI] [PubMed] [Google Scholar]
- Bain C. D.; Troughton E. B.; Tao Y. T.; Evall J.; Whitesides G. M.; Nuzzo R. G. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989, 111, 321–335. 10.1021/ja00183a049. [DOI] [Google Scholar]
- Bain C. D.; Evall J.; Whitesides G. M. Formation of Monolayers by the Coadsorption of Thiols on Gold: Variation in the Head Group, Tail Group, and Solvent. J. Am. Chem. Soc. 1989, 111, 7155–7164. 10.1021/ja00200a039. [DOI] [Google Scholar]
- Luderer F.; Walschus U. Immobilization of Oligonucleotides for Biochemical Sensing by Self-Assembled Monolayers: Thiol-Organic Bonding on Gold and Silanization on Silica Surfaces. Top. Curr. Chem. 2005, 260, 37–56. 10.1007/128_003. [DOI] [Google Scholar]
- Briand E.; Humblot V.; Landoulsi J.; Petronis S.; Pradier C. M.; Kasemo B.; Svedhem S. Chemical Modifications of Au/SiO2 Template Substrates for Patterned Biofunctional Surfaces. Langmuir 2011, 27, 678–685. 10.1021/la101858y. [DOI] [PubMed] [Google Scholar]
- Yang W. T.; Ranby B. Bulk Surface Photografting Process and Its Applications.1. Reactions and Kinetics. J. Appl. Polym. Sci. 1996, 62, 533–543. . [DOI] [Google Scholar]
- Rohr T.; Ogletree D. F.; Svec F.; Fréchet J. M. J. Surface Functionalization of Thermoplastic Polymers for the Fabrication of Microfluidic Devices by Photoinitiated Grafting. Adv. Funct. Mater. 2003, 13, 264–270. 10.1002/adfm.200304229. [DOI] [Google Scholar]
- Gandhiraman R. P.; Gubala V.; O’Mahony C. C.; Cummins T.; Raj J.; Eltayeb A.; Doyle C.; James B.; Daniels S.; Williams D. E. PECVD Coatings for Functionalization of Point-of-Care Biosensor Surfaces. Vacuum 2012, 86, 547–555. 10.1016/j.vacuum.2011.08.014. [DOI] [Google Scholar]
- Brink C.; Österberg E.; Holmberg K.; Tiberg F. Using Poly(Ethylene Imine) to Graft Poly(Ethylene Glycol) or Polysaccharide to Polystyrene. Colloids Surf. 1992, 66, 149–156. 10.1016/0166-6622(92)80131-K. [DOI] [Google Scholar]
- Konradi R.; Pidhatika B.; Mühlebach A.; Textor M. Poly-2-Methyl-2-Oxazoline: A Peptide-like Polymer for Protein-Repellent Surfaces. Langmuir 2008, 24, 613–616. 10.1021/la702917z. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Kopecek J.; Andrade J. D. Protein-Resistant Surfaces Prepared by PEO-Containing Block Copolymer Surfactant. J. Biomed. Mater. Res. 1989, 23, 351–368. 10.1002/jbm.820230306. [DOI] [PubMed] [Google Scholar]
- Feller L. M.; Cerritelli S.; Textor M.; Hubbell J. A.; Tosatti S. G. P. Influence of Poly(Propylene Sulfide-Block-Ethylene Glycol) Di- And Triblock Copolymer Architecture on the Formation of Molecular Adlayers on Gold Surfaces and Their Effect on Protein Resistance: A Candidate for Surface Modification in Biosensor Research. Macromolecules 2005, 38, 10503–10510. 10.1021/ma051424m. [DOI] [Google Scholar]
- Pidhatika B.; Möller J.; Vogel V.; Konradi R. Nonfouling Surface Coatings Based on Poly(2-Methyl-2-Oxazoline). Chimia 2008, 62, 264–269. 10.2533/chimia.2008.264. [DOI] [Google Scholar]
- Ruiz-Taylor L. a.; Martin T. L.; Zaugg F. G.; Witte K.; Indermuhle P.; Nock S.; Wagner P. Monolayers of Derivatized Poly(L-Lysine)-Grafted Poly(Ethylene Glycol) on Metal Oxides as a Class of Biomolecular Interfaces. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 852–857. 10.1073/pnas.98.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenausis G. L.; Vörös J.; Elbert D. L.; Huang N.; Hofer R.; Ruiz-Taylor L.; Textor M.; Hubbell J. A.; Spencer N. D. Poly(L-Lysine)-g-Poly(Ethylene Glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption †. J. Phys. Chem. B 2000, 104, 3298–3309. 10.1021/jp993359m. [DOI] [Google Scholar]
- Pasche S.; De Paul S. M.; Vörös J.; Spencer N. D.; Textor M. Poly(L-Lysine)-Graft-Poly(Ethylene Glycol) Assembled Monolayers on Niobium Oxide Surfaces: A Quantitative Study of the Influence of Polymer Interfacial Architecture on Resistance to Protein Adsorption by ToF-SIMS and in Situ OWLS. Langmuir 2003, 19, 9216–9225. 10.1021/la034111y. [DOI] [Google Scholar]
- Lee S.; Vörös J. An Aqueous-Based Surface Modification of Poly(Dimethylsiloxane) with Poly(Ethylene Glycol) to Prevent Biofouling. Langmuir 2005, 21, 11957–11962. 10.1021/la051932p. [DOI] [PubMed] [Google Scholar]
- Marie R.; Beech J. P.; Vörös J.; Tegenfeldt J. O.; Höök F. Use of PLL-g-PEG in Micro-Fluidic Devices for Localizing Selective and Specific Protein Binding. Langmuir 2006, 22, 10103–10108. 10.1021/la060198m. [DOI] [PubMed] [Google Scholar]
- Sun K.; Xie Y.; Ye D.; Zhao Y.; Cui Y.; Long F.; Zhang W.; Jiang X. Mussel-Inspired Anchoring for Patterning Cells Using Polydopamine. Langmuir 2012, 28, 2131–2136. 10.1021/la2041967. [DOI] [PubMed] [Google Scholar]
- Elbert D. L.; Hubbell J. A. Reduction of Fibrous Adhesion Formation by a Copolymer Possessing an Affinity for Anionic Surfaces. J. Biomed. Mater. Res. 1998, 42, 55–65. . [DOI] [PubMed] [Google Scholar]
- Lussi J. W.; Falconnet D.; Hubbell J. A.; Textor M.; Csucs G. Pattern Stability under Cell Culture Conditions— A Comparative Study of Patterning Methods Based on PLL-g-PEG Background Passivation. Biomaterials 2006, 27, 2534–2541. 10.1016/j.biomaterials.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Huang N.; Vörös J.; De Paul S. M.; Textor M.; Spencer N. D. Biotin-Derivatized Poly(L-Lysine)-g-Poly(Ethylene Glycol): A Novel Polymeric Interface for Bioaffinity Sensing. Langmuir 2002, 18, 220–230. 10.1021/la010913m. [DOI] [Google Scholar]
- Zhen G.; Falconnet D.; Kuennemann E.; Vörös J.; Spencer N. D.; Textor M.; Zürcher S. Nitrilotriacetic Acid Functionalized Graft Copolymers: A Polymeric Interface for Selective and Reversible Binding of Histidine-Tagged Proteins. Adv. Funct. Mater. 2006, 16, 243–251. 10.1002/adfm.200500232. [DOI] [Google Scholar]
- Guoliang Z.; Zurcher S.; Falconnet D.; Fei X.; Kuennemann E.; Textor M. NTA-Functionalized Poly(L-Lysine)-g-Poly(Ethylene Glycol): A Polymeric Interface for Binding and Studying 6 His-Tagged Proteins. 2005 IEEE Eng. Med. Biol. 27th Annu. Conf. 2006, 1036–1038. 10.1109/IEMBS.2005.1616595. [DOI] [PubMed] [Google Scholar]
- Saxer S.; Portmann C.; Tosatti S.; Gademann K.; Zürcher S.; Textor M. Surface Assembly of Catechol-Functionalized Poly(L-Lysine)- Graftpoly(Ethylene Glycol) Copolymer on Titanium Exploiting Combined Electrostatically Driven Self-Organization and Biomimetic Strong Adhesion. Macromolecules 2010, 43, 1050–1060. 10.1021/ma9020664. [DOI] [Google Scholar]
- VandeVondele S.; Vörös J.; Hubbell J. A. RGD-Grafted Poly-L-Lysine-Graft-(Polyethylene Glycol) Copolymers Block Non-Specific Protein Adsorption While Promoting Cell Adhesion. Biotechnol. Bioeng. 2003, 82, 784–790. 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- Csucs G.; Michel R.; Lussi J. W.; Textor M.; Danuser G. Microcontact Printing of Novel Co-Polymers in Combination with Proteins for Cell-Biological Applications. Biomaterials 2003, 24, 1713–1720. 10.1016/S0142-9612(02)00568-9. [DOI] [PubMed] [Google Scholar]
- Saravia V.; Küpcü S.; Nolte M.; Huber C.; Pum D.; Fery A.; Sleytr U. B.; Toca-Herrera J. L. Bacterial Protein Patterning by Micro-Contact Printing of PLL-g-PEG. J. Biotechnol. 2007, 130, 247–252. 10.1016/j.jbiotec.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Ricoult S. G.; Goldman J. S.; Stellwagen D.; Juncker D.; Kennedy T. E. Generation of Microisland Cultures Using Microcontact Printing to Pattern Protein Substrates. J. Neurosci. Methods 2012, 208, 10–17. 10.1016/j.jneumeth.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Ricoult S. G.; Pla-Roca M.; Safavieh R.; Lopez-Ayon G. M.; Grütter P.; Kennedy T. E.; Juncker D. Large Dynamic Range Digital Nanodot Gradients of Biomolecules Made by Low-Cost Nanocontact Printing for Cell Haptotaxis. Small 2013, 9, 3186. 10.1002/smll.201370113. [DOI] [PubMed] [Google Scholar]
- Falconnet D.; Koenig A.; Assi F.; Textor M. A Combined Photolithographic and Molecular-Assembly Approach to Produce Functional Micropatterns for Applications in the Biosciences. Adv. Funct. Mater. 2004, 14, 749–756. 10.1002/adfm.200305182. [DOI] [Google Scholar]
- Michel R.; Lussi J. W.; Csucs G.; Reviakine I.; Ketterer B.; Danuser G.; Hubbell J. A.; Textor M.; Spencer N. D. Selective Molecular Assembly Patterning: A New Approach to Micro- and Nanochemical Patterning of Surfaces for Biological Applications. Langmuir 2002, 18, 3281–3287. 10.1021/la011715y. [DOI] [Google Scholar]
- Tang C. S.; Petronis S.; Schmutz P.; Vörös J.; Textor M.; Keller B. Locally Addressable Electrochemical Patterning Technique (LAEPT) Applied to Poly(L-Lysine)-Graft-Poly(Ethylene Glycol) Adlayers on Titanium and Silicon Oxide Surfaces. Biotechnol. Bioeng. 2005, 91, 285–295. 10.1002/bit.20395. [DOI] [PubMed] [Google Scholar]
- Duan X.; Mu L.; Sawtelle S. D.; Rajan N. K.; Han Z.; Wang Y.; Qu H.; Reed M. A. Functionalized Polyelectrolytes Assembling on Nano-BioFETs for Biosensing Applications. Adv. Funct. Mater. 2015, 25, 2279–2286. 10.1002/adfm.201500002. [DOI] [Google Scholar]
- Movilli J.; Rozzi A.; Ricciardi R.; Corradini R.; Huskens J. Control of Probe Density at DNA Biosensor Surfaces Using Poly-L -Lysine with Appended Reactive Groups. Bioconjugate Chem. 2018, 29, 4110–4118. 10.1021/acs.bioconjchem.8b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z.; Wang Y.; Duan X. Biofunctional Polyelectrolytes Assembling on Biosensors - A Versatile Surface Coating Method for Protein Detections. Anal. Chim. Acta 2017, 964, 170–177. 10.1016/j.aca.2017.01.051. [DOI] [PubMed] [Google Scholar]
- Moonen P. F.; Bat E.; Voorthuijzen W. P.; Huskens J. Soft-Lithographic Patterning of Room Temperature-Sintering Ag Nanoparticles on Foil. RSC Adv. 2013, 3, 18498–18505. 10.1039/c3ra43926g. [DOI] [Google Scholar]
- Egholm M.; Buchardt O.; Christensen L.; Behrens C.; Freier S. M.; Driver D. A.; Berg R. H.; Kim S. K.; Norden B.; Nielsen P. E. PNA Hybridizes To Complementary Oligonucleotides Obeying the Watson-Crick Hydrogen-Bonding Rules. Nature 1993, 365, 566–568. 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M.; Schwarz F. P. Thermal Stability of PNA/DNA and DNA/DNA Duplexes by Differential Scanning Calorimetry. Nucleic Acids Res. 1999, 27, 4801–4806. 10.1093/nar/27.24.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidov V. V.; Potaman V. N.; Frank-Kamenetskil M. D.; Egholm M.; Buchard O.; Sönnichsen S. H.; Nielsen P. E. Stability of Peptide Nucleic Acids in Human Serum and Cellular Extracts. Biochem. Pharmacol. 1994, 48, 1310–1313. 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Choksakulnimitr S.; Masuda S.; Tokuda H.; Takakura Y.; Hashida M. In Vitro Cytotoxicity of Macromolecules in Different Cell Culture Systems. J. Controlled Release 1995, 34, 233–241. 10.1016/0168-3659(95)00007-U. [DOI] [Google Scholar]
- Veerbeek J.; Steen R.; Vijselaar W.; Rurup W. F.; Korom S.; Rozzi A.; Corradini R.; Segerink L.; Huskens J. Selective Functionalization with PNA of Silicon Nanowires on Silicon Oxide Substrates. Langmuir 2018, 34, 11395–11404. 10.1021/acs.langmuir.8b02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta A.; Wasserberg D.; Posthuma-Trumpie G.; Subramaniam V.; van Amerongen A.; Corradini R.; Tedeschi T.; Sforza S.; Reinhoudt D. N.; Marchelli R.; Huskens J.; Jonkheijm P. Patterning of Peptide Nucleic Acids Using Reactive Microcontact Printing. Langmuir 2011, 27, 1536–1542. 10.1021/la102756k. [DOI] [PubMed] [Google Scholar]
- Kim E.; Xia Y.; Whitesides G. M. Micromolding in Capillaries: Applications in Materials Science. J. Am. Chem. Soc. 1996, 118, 5722–5731. 10.1021/ja960151v. [DOI] [Google Scholar]
- Wang M.; Hiltunen J.; Uusitalo S.; Puustinen J.; Lappalainen J.; Karioja P.; Myllylä R. Fabrication of Optical Inverted-Rib Waveguides Using UV-Imprinting. Microelectron. Eng. 2011, 88, 175–178. 10.1016/j.mee.2010.10.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.