Abstract
Background
Lung disease is a leading cause of morbidity and mortality. A breach in the lung alveolar-epithelial barrier and impairment in lung function are hallmarks of acute and chronic pulmonary illness. This review is part two of our previous work. In part 1, we demonstrated that CdM is as effective as MSCs in modulating inflammation. Herein, we investigated the effects of mesenchymal stromal cell (MSC)-conditioned media (CdM) on (i) lung architecture/function in animal models mimicking human lung disease, and (ii) performed a head-to-head comparison of CdM to MSCs.
Methods
Adhering to the animal Systematic Review Centre for Laboratory animal Experimentation protocol, we conducted a search of English articles in five medical databases. Two independent investigators collected information regarding lung: alveolarization, vasculogenesis, permeability, histologic injury, compliance, and measures of right ventricular hypertrophy and right pulmonary pressure. Meta-analysis was performed to generate random effect size using standardized mean difference with 95% confidence interval.
Results
A total of 29 studies met inclusion. Lung diseases included bronchopulmonary dysplasia, asthma, pulmonary hypertension, acute respiratory distress syndrome, chronic obstructive pulmonary disease, and pulmonary fibrosis. CdM improved all measures of lung structure and function. Moreover, no statistical difference was observed in any of the lung measures between MSCs and CdM.
Conclusions
In this meta-analysis of animal models recapitulating human lung disease, CdM improved lung structure and function and had an effect size comparable to MSCs.
Keywords: Conditioned media, Mesenchymal stem cell, Lung disease, Animal, Review
Background
Pulmonary illness is a leading cause of morbidity and mortality [1]. In children, acute respiratory exacerbations are a common reason for primary care visits and are often implicated in hospitalizations [2, 3]. Many of these pulmonary conditions result in impairments in lung function that may last into adulthood [4, 5]. Consequently, identifying novel therapies for lung disease is highly warranted.
A unifying theme in many lung diseases includes inflammation [6–8]. While some inflammation is necessary to combat new disease and for proper wound healing, chronic inflammation may result in altered lung structure and function. During an acute illness, current therapies focus on restoring lung function by abating inflammation [9–11]. For instance, glucocorticoids are the mainstay therapy for reducing inflammation during acute exacerbations of asthma [12]. More recently, mesenchymal stromal/stem cells (MSCs) have shown encouraging outcomes in animal models of lung inflammation [13–15].
MSCs are promising agents as they are easily harvested, can be rapidly expanded, and can secrete factors (exosomes, microvesicles, microRNA) known to reduce inflammation [16–18]. The “secretome” or “conditioned media” of MSCs is considered biologically active and can be easily collected from the surrounding fluid of propagating cells [19–21]. Remarkably, preclinical studies suggest MSC conditioned media (CdM) may be as restorative as the MSCs themselves [22, 23]. We supported this observation in a previous systematic review and meta-analysis demonstrating that CdM is as effective as MSCs in modulating inflammation [24].
This review is an extension of our previous work. In this review, we examined the effects of CdM on (i) lung architecture/function in animal models recapitulating lung disease and (ii) compare these findings to MSCs. Given that the therapeutic benefit of MSCs is attributed to a paracrine fashion, we believed CdM would have comparable effects to MSCs.
Methods
Overview and literature search
The methods in our review abide to those outlined by the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) [25]. Our protocol was registered through the Collaborative Approach to Meta-Analysis and Review of Data from Experimental Studies (CAMARADES) [26]. Details are described in our previous publication.
We conducted a literature search in five databases using the following terms: mesenchymal stem cell-conditioned media, lung disease, and animal. The last search was performed on March 17th, 2020. Three independent investigators evaluated titles and abstracts, followed by full-text review.
Inclusion criteria and outcomes of interest
We included studies administering MSC-CdM to animal models of acute lung injury or acute respiratory distress syndrome (ALI/ARDS), asthma, bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), pneumonia, pulmonary fibrosis (PF), and pulmonary hypertension (PH). Refer to Supplementary File 1 for the list of included studies.
Outcomes of interest
Measures of lung structure and/or function were our primary endpoint. Lung architecture and function were assessed under the following categories: alveolarization, vasculogenesis, right ventricular hypertrophy, fibrosis, permeability, pulmonary pressures, compliance, and lung injury. Although the pathogenesis of the included lung diseases are heterogeneous, we combined all processes irrespective of disease. This was conducted to obtain a scoping overview of the impact of CdM on biologic processes implicated in lung disease. Subsequently, we assessed lung structure/function by disease in our subgroup analysis. Excluded studies were those which did not provide data concerning our primary outcome of inflammation.
Data extraction
Three groups of investigators were used (ED and CE; RN and JM; ME, DM, and SM) to collect data. Uniformity of data was assessed by the primary author. This data included general study design, animal model characteristics, conditioned media characteristics, and outcomes of interest.
Data analysis
A random effects model was used to generate forest plots. A minimum of three studies were required for each outcome to proceed with a meta-analysis. The estimated effect size of CdM or MSC on lung architecture/function was determined using standardized mean difference (SMD) with a 95% confidence interval (CI). Statistical heterogeneity between studies was calculated using the I2 metric, and funnel plots were used to examine publication bias. If more than six articles were included per outcome, we conducted a subgroup analysis for disease, animal species, and route and dose of CdM administration. All statistical analyses were performed in R version 3.6.2; packages used included dmetar, metafor, and meta.
Results
Study selection
Our literature search resulted in 245 articles. After removing duplicates and viewing the titles and abstracts, 55 articles underwent full-text review. Twenty-nine articles met inclusion (refer to Supplementary Figure 1).
Study details
Table 1 summarizes the relevant study characteristics. Articles included in the review were published between the years 2009 to 2020. BPD was the most common animal model (n = 8), followed by ALI/ARDS (n = 5) and asthma (n = 5). All of the studies used rodents to induce their lung model.
Table 1.
Detailed summary of information extracted from included studies
| No. | Author (year) | Study design | Animal characteristics | Intervention characteristics | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Disease model | Disease induction | Animal model Gender |
Age | Source; (Origin) | Dose; delivery; timing; frequency | Lung architecture/function | ||
| 1 | Ahmadi (2016) | Asthma | Ovalbumin |
Wistar rats Male |
Adult | Bone marrow | 50 μl; IV; 1-day post sensitization; × 1 | Tracheal reactivity |
| 2 | Ahmadi (2017) | Asthma | Ovalbumin |
Wistar rats Male |
Adult | Bone marrow | 50 μl; IT; 1-day post sensitization; × 1 | Histologic lung injury |
| 3 | Aslam (2009) | BPD | Hyperoxia |
FVB mice Mixed |
Neonate | Bone marrow |
50 μl; IV; postnatal day 4; × 1 |
Alveolarization RVH Vasculogenesis |
| 4 | Chailakhyan (2014) | ALI | LPS from E. coli |
Wistar rats Male |
NR | Bone marrow |
1000 μl; IV; 1 h after LPS injection; × 1 |
Histologic lung injury |
| 5 | Chaubey (2018) | BPD | Hyperoxia |
C57BL/6 mice NR |
Neonate | Human umbilical cord tissue | 100 μl; IP; PN2 and PN4; × 1 |
Alveolarization RVH Pulmonary artery pressure |
| 6 | Cruz (2015) | Asthma | Aspergillus fumigatus sensitization |
C57/BL6 mice Male |
Adult | Bone marrow | 200 μl; IV; 14 days after Aspergillus challenge; × 1 | Histologic lung injury |
| 7 | Curley (2013) |
ALI/ ARDS |
High stretch mechanical ventilation |
Sprague–Dawley rats (pathogen-free) Male |
Adult | Bone marrow | 300 μl; IT; 2.5–3 h post injury initiation; × 1 |
Alveolarization Histologic lung injury Compliance Wet, dry lung weight ratios Blood gas |
| 8 | Felix (2020) | PF | Bleomycin |
Wistar rats NR |
Adult | Adipose tissue | 200 μl; IV; 10 days after induction; × 1 | Histologic lung injury Fibrosis |
| 9 | Gülaşı (2015) | BPD | Hyperoxia |
Wistar rats Mixed |
Neonate | Bone marrow | 25 μl; IT; on the 11th day; at every inspiration; × 1 | Alveolarization |
| 10 | Hansmann (2012) | BPD | Hyperoxia |
FVB mice Mixed |
Adult | Bone marrow | 50 μl; IV postnatal day 14; × 1 |
Alveolarization Fibrosis Compliance/Resistance |
| 11 | Hayes (2015) | VILI/ALI | Ventilator-induced |
Sprague–Dawley rats Male |
Adult | Bone marrow | 500 μl; 1.5 h after injury; × 1 |
Alveolarization Permeability Compliance |
| 12 | Huh (2011) |
Emphysema (COPD) |
Cigarette smoke-induced |
Lewis rats Female |
Adult | Bone marrow | 300 μl; IV; 6 months of age; × 10 |
Alveolarization Vascularization Pulmonary artery pressure |
| 13 | Hwang (2016) | LIRI | Left lung was clamped, re-ventilated, and perfused |
Sprague–Dawley rats Male |
Adult | Bone marrow | 200 μl, IT, 30 min prior to disease induction; × 1 | Permeability |
| 14 | Ionescu (2012) | ARDS | LPS from E. coli |
C57/BL6 mice Male |
Adult | Bone marrow | 30 μl; IT; 4 h post-LPS exposure; × 1 | Permeability Histologic lung injury |
| 15 | Kennelly (2016) | COPD | Receptor knockout |
NOD-SCID IL-2rgnull Mice NR |
NR | Human bone marrow | IN, day 0 + 6 h; × 2 | Alveolarization |
| 16 | Keyhanmanesh (2018) | Asthma | Ovalbumin |
Wistar rats Male |
Adult | Bone marrow | 50 μl; IV, single dose, day 33; repeated dose days 33–35 | Histologic lung injury |
| 17 | Li (2018) | PF | Silica |
Wistar rats Female |
Adult | Bone marrow | 1 mL, IT, days 1 and 4 post-silica; × 2 |
Fibrosis Histologic lung injury |
| 18 | Lu (2015) | ARDS | LPS from E. coli |
C57/BL6 mice Male |
NR | Adipose tissue | 200 μl; IV; 4 h post-LPS exposure; × 1 | Permeability |
| 19 | Pierro (2012) | BPD | Hyperoxia |
Newborn rats Mixed |
Neonate | Human umbilical cord blood | 7 μl/g; IP; postnatal day 4–21 (prevention studies) or from postnatal day 14–28 (regeneration studies); × 18 vs. × 15 |
Alveolarization Vascularization RVH Compliance Exercise capacity |
| 20 | Rahbarghazi (2019) | Asthma | Ovalbumin |
Wistar rats Male |
Adult | Bone marrow | 50 μl; IT; day 33; × 1 | Histologic lung injury |
| 21 | Rathinasabapathy (2016) | PH | Monocrotaline |
Sprague–Dawley rats Male |
Adult | Adipose tissue | 100 μl; IV; 14 days post-MCT exposure; × 1 |
Vasculogenesis RVH Fibrosis |
| 22 | Sadeghi (2019) | SM | CEES |
C57/BL6 mice Male |
6–8 weeks | Adipose tissue | 500 μl; IP; start week 28; × 8 | Fibrosis |
| 23 | Shen (2014) | PF | Bleomycin |
Wistar rats Female |
NR | Bone marrow | 200 μl; IT; at 6 h and on day 3 following disease induction; × 2 | Fibrosis |
| 24 | Su (2019) | ALI | LPS from E. coli |
C57BL/6 mice Male |
8–12 weeks old | NR | 200 μl; IV; 4 h after disease induction; × 1 | Lung injury |
| 25 | Sutsko (2012) | BPD | Hyperoxia |
Sprague–Dawley rats Mixed |
Neonate | Bone marrow | 50 μl; IT; postnatal day 9; × 1 |
Alveolarization Vascularization RVH |
| 26 | Tropea (2012) | BPD | Hyperoxia |
FVB mice NR |
Neonate | Bone marrow | 50 μl; IV; postnatal day 4; × 1 | Alveolarization |
| 27 | Wakayama (2015) | ARDS | Bleomycin |
C57/BL6J mice Female |
Adult | Human exfoliated deciduous teeth | 500 μl; IV; 24 h post-bleomycin exposure; × 1 | Fibrosis |
| 28 | Waszak (2012) | BPD | Hyperoxia |
Sprague–Dawley rats Mixed |
Neonate | Bone marrow | 1 μl/g; IP; postnatal day 0 to postnatal day 21; × 22 |
Alveolarization Vasculogenesis RVH Pulmonary artery pressure |
| 29 | Zhao (2014) | Bronchiolitis obliterans | Transplanted donor trachea |
C57BL/6 mice Male |
Adult | Placenta derived | Volume NR; IT; 3rd day after transplantation; × 1 | Tracheal luminal obliteration |
ALI acute lung injury, ARDS acute respiratory distress syndrome, BPD bronchopulmonary dysplasia, CEES-2 chloroehtyl ethyl sulfide, COPD chronic obstructive pulmonary disease, IP intraperitoneal, IT intratracheal, IV intravenous, LIRI lung ischemia reperfusion injury, LPS lipopolysaccharide, MCT monocrotaline, NR not reported, PF pulmonary fibrosis, RVH right ventricular hypertrophy, SM sulfur mustard chemical lung injury, VILI ventilator-induced lung injury
CdM characteristics
Conditioned media properties are summarized in Supplementary File 3. Stem cells were most isolated from bone marrows and cultured in Dulbecco’s modified Eagle’s medium. Incubation time of the CdM ranged from 24 to 72 h. The volume of CdM administered ranged from 25 μl to 1 ml.
Alveolarization
CdM: improved alveolarization with an SMD of 1.32 (95% CI 0.99, 1.65; 12 studies; Fig. 1a) with moderate heterogeneity (I2 = 67%; p < 0.01).
MSC: improved alveolarization with an SMD of 1.80 (95% CI 1.52, 2.07; 9 studies; Fig. 1b) with mild heterogeneity between groups (I2 = 36%; p = 0.01).
CdM vs. MSC: no significant difference (Supplementary Figure 2).
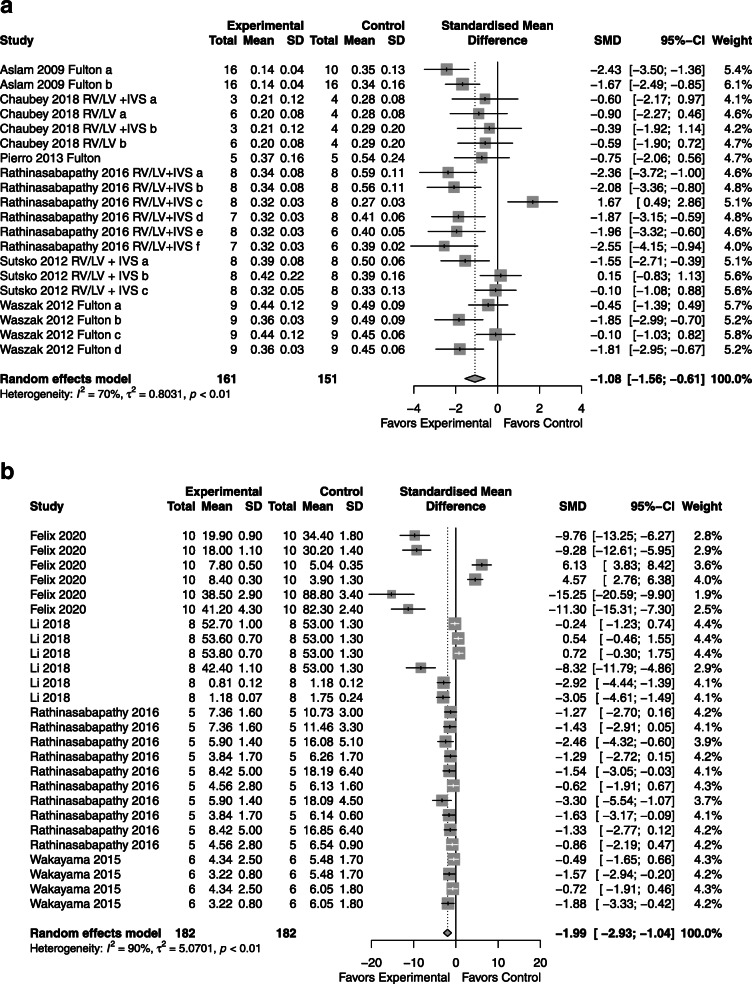
Fig. 1.

Effect size of CdM (a) and MSC (b) on lung alveolarization. Forest plots demonstrate SMD with 95% confidence interval
Right ventricular hypertrophy
CdM: favored CdM over control with an SMD of − 1.08 (95% CI − 1.56, − 0.61); 6 studies; Fig. 2a) with significant heterogeneity (I2 = 70%; p < 0.01).
MSC: favored over the control with an SMD of − 1.05 (95% CI − 1.69, − 0.42; 3 studies, Fig. 2b) with significant heterogeneity between groups (I2 = 71%; p < 0.01).
CdM vs. MSC: no significant difference (SMD − 0.22, 95% CI − 0.36, 0.16; Supplementary Figure 3).
Fig. 2.
Effect size of CdM (a) and MSC (b) on right ventricular hypertrophy. Forest plots demonstrate SMD with 95% confidence interval
Lung fibrosis
CdM: favored CdM over control with an SMD of − 1.08 (95% CI − 1.56, − 0.61; 6 studies; Fig. 3a) with significant heterogeneity (I2 = 70%; p < 0.01).
MSC: favored MSC over the control with an SMD of − 1.99 (95% CI − 2.93, − 1.04; 4 studies; Fig. 3b) with significant heterogeneity between groups (I2 = 90%; p < 0.01).
CdM vs. MSC: the comparison between CdM and MSCs was similar (refer to Supplementary Figure 4).
Fig. 3.
Effect size of CdM (a) and MSC (b) on lung fibrosis. Forest plots demonstrate SMD with 95% confidence interval
Vasculogenesis
CdM: superior to control with an SMD of − 2.46 (95% CI − 3.22, − 1.70; 6 studies; Fig. 4a) with moderate heterogeneity (I2 = 76%; p < 0.01).
MSC: superior to control with an SMD of − 2.29 (95% CI -3.01, − 1.56; 4 studies; Fig. 4b) with mild heterogeneity between groups (I2 = 35%; p = 0.14).
CdM vs. MSC: overall effectiveness between CdM and MSCs again showed no significant difference (Supplementary Figure 5).
Fig. 4.
Effect size of CdM (a) and MSC (b) on lung vascularization. Forest plots demonstrate SMD with 95% confidence interval
Permeability
CdM: permeability assessment favored CdM over control with an SMD of − 0.99 (95% CI − 1.32, − 0.66; 5 studies; Fig. 5a) homogeneity that is non-significant (I2 = 11.0%; p = 0.33).
MSC: in the evaluation of permeability, the MSC was favored over the control with an effect size of − 1.54 (95% CI -2.13, − 0.95; 4 studies; Fig. 5b) with heterogeneity between groups (I2 = 57.0%; p < 0.01).
CdM vs. MSC: equal effectiveness (Supplementary Figure 6).
Fig. 5.
Effect size of CdM (a) and MSC (b) on lung permeability. Forest plots demonstrate SMD with 95% confidence interval
Pulmonary pressures
CdM: improvement in right ventricular pressures compared to control with an SMD of − 0.69 (95% CI − 0.99, − 0.39; 5 studies; Fig. 6a) with moderate heterogeneity (I2 = 51%; p < 0.01).
MSC: superior to control with an SMD of − 1.63 (95% CI − 2.02, − 1.24; 3 studies; Fig. 6b) with moderate heterogeneity (I2 = 63%; p < 0.01).
CdM vs. MSC: comparable (please refer to Supplementary Figure 7).
Fig. 6.
Effect size of CdM (a) and MSC (b) on pulmonary pressures. Forest plots demonstrate SMD with 95% confidence interval
Histologic lung injury
CdM: improvement in histologic lung injury compared to control with an SMD of − 6.05 (95% CI − 8.72, − 3.38; 3 studies; Fig. 7a) with significant heterogeneity (I2 = 87%; p < 0.01).
MSC: superior to control with an SMD of − 2.01 (95% CI -3.41, − 0.60; 3 studies; Fig. 7b) with significant heterogeneity (I2 = 88%; p < 0.01).
CdM vs. MSC: less than 3 studies; comparison not performed.
Fig. 7.
Effect size of CdM (a) and MSC (b) on histologic lung injury. Forest plots demonstrate SMD with 95% confidence interval
Compliance
CdM: improvement in lung compliance compared to control with an SMD of 1.75 (95% CI 0.81, 2.69; 4 studies; Fig. 8a) with significant heterogeneity (I2 = 76%; p < 0.01).
MSC: improvement in lung compliance compared to control with an SMD of 2.33 (95% CI 1.84, 2.82; 3 studies; Fig. 8b) with no heterogeneity (I2 = 0%; p = 0.5).
CdM vs. MSC: not applicable as less than three studies performed a head-to-head comparison.
Fig. 8.
Effect size of CdM (a) and MSC (b) on pulmonary compliance. Forest plots demonstrate SMD with 95% confidence interval
All outcomes for lung structure and function combined
CdM: Supplementary Figure 8A shows the SMD of − 1.38 (with 95% CI of − 1.57, − 1.19) favoring CdM over control.
MSC: Supplementary Figure 8B shows the SMD of − 1.66 (with 95% CI of − 1.91, − 1.41) favoring MSC over control.
CdM vs. MSC: no difference was appreciated between CdM and MSC when all outcomes were combined (Supplementary Figure 8C).
Subgroup analysis
Stratification of data was performed by lung disease, tissue source, dose, and route of delivery of CdM. Evaluation was performed if more than 6 studies had data.
Alveolarization
Supplementary Figure 9A–D demonstrates that CdM had the greatest impact on alveolarization in BPD animal models (SMD 1.67) and when the media was derived from cord blood (SMD 2.89), given at a dose of 7 μl/g (SMD 2.89), and delivered via the intraperitoneal route (SMD 1.56).
RVH
Supplementary Figure 10A–D depicts that CdM significantly improved RVH in BPD animal models (SMD − 0.93) and only when the media was derived from adipose tissue (SMD − 1.05), given at a dose of 100 μl (SMD − 1.14) and delivered intravenously (SMD − 0.86).
Fibrosis
Supplementary Figure 11A–D illustrates that CdM had the greatest impact in animal models of BPD and PH (SMD − 4.1, − 3.4, respectively) and when the media was derived from adipose tissue (SMD − 2.61), given at a dose of 50 μl (SMD − 4.10) and delivered intravenously (SMD − 1.95).
Vascularization
Supplementary Figure 12A–D shows that CdM had the greatest impact in animal models of COPD (SMD − 8.09), when the media was derived from adipose tissue (SMD − 2.61), given at a dose of 300 μl (SMD − 8.09) and delivered intravenously (SMD − 3.65).
Risk of bias
No study was judged as low risk across all ten domains. Eight studies stated that the allocation selection was random. Most studies (n = 25) had similar groups at baseline. Risk of bias was large regarding allocation concealment, whether authors mention random housing of animals, and blinding of caregivers or random selection of outcome. All studies were found to sufficiently report complete data and being free from other bias. Refer to Supplementary File 2 [27].
Publication bias
Supplementary Figures 13, 14, 15, 16, 17, 18, 19, and 20 illustrate publication bias through funnel plots. Overall, publication bias was low in all the outcomes except for lung permeability.
Discussion
Preclinical studies reiterate the ability MSCs have on dampening lung inflammation. This capacity is largely due to the paracrine secretion of MSC factors (microvesicles, exosomes) that provide a basis for future cell-free therapies for human disease [28–31]. This is the first review to directly compare the effects of CdM vs MSCs on lung structure and function in animal models of diverse lung disease. Overall, we found that CdM improved measures of alveolarization, right ventricular hypertrophy, lung fibrosis, vasculogenesis and permeability. Furthermore, CdM reduced pulmonary pressures, ameliorated histologic lung injury, and increased lung compliance. We found that CdM was comparable to MSCs in all lung measures evaluated individually and when combined.
The bioactive factors contained in the CdM of MSCs have been the focus of multiple studies and review articles [32–34]. Congruent with the findings found in this review, Hansmann et al. show that MSC-CdM, compared to CdM from lung fibroblasts, reversed alveolar injury, normalized lung function (airway resistance), and reversed RVH [35]. Additionally, the same group recently demonstrated that MSC exosomes (molecular cargo found within CdM) restored lung architecture, stimulated pulmonary blood vessel formation, and modulated lung inflammation [22]. In an E. coli pneumonia-induced ALI mouse model, MSC microvesicles (also found in MSC-CdM) reduced lung permeability and histologic injury score and were equivalent to MSCs [36]. Together, these findings, and those in recent reviews, substantiate the results found in this review [37, 38].
This year, Augustine et al. published a network meta-analysis comparing stem cell and cell-free therapies in preclinical measures of BPD. MSC-CdM had a similar effect size to MSCs regarding alveolarization (MSC SMD 1.71 vs. CdM SMD1.68), angiogenesis (SMD 2.24 vs. 1.79), and pulmonary remodeling (1.29 vs. 1.22) [39]. Similar to their results, this review showed that CdM had among the largest impact on measures of alveolarization and vasculogenesis, processes critical for appropriate lung healing, development, and function [40]. Although vasculogenesis/angiogenesis is an important process to restore lung function/structure, it can also enhance remodeling and thus worsen outcomes in other lung diseases such as asthma or pulmonary fibrosis [41]. In Supplementary Figure 12A, we demonstrate that this process improved in BPD, pulmonary hypertension, and COPD but was not assessed in asthma/pulmonary fibrosis.
In the study by Hayes et al., they found that MSCs were superior to CdM in a rodent model of ventilator-induced lung injury. However, our review suggests that when you compile the literature, there were no significant benefits of using cells over CdM. We cannot explain why CdM was not comparable in this study; however, an important challenge that remains in the field includes the rigorous testing of key variables (tissue source, dose, route, disease, etc.) that may impact the quality of CdM [42–44]. For instance, we found that the intravenous route provided optimal results. Moreover, multiple administrations of CdM may augment vascular development, as seen in the study by Huh et al (n = 10 intravenous injections). Conversely, the optimal source and dose of CdM is dependent on the variable or the lung disease. This brings to light that it will be incredibly challenging to find a single CdM product that is ideal for all lung diseases. Thus, the idea of “one-size-fits-all” does not hold true for regenerative cells or products. Illustrating this concept, Rathinasabapathy et al. showed greater improvement in measures of RVH compared to other studies measuring right ventricular size. Important differences seen in the study by Rathinasabapathy and colleagues was that they used a different animal model (PH vs. BPD) and age of rodents (adult vs. neonatal) [45].
As investigators, we should attempt to tease out these characteristics in order to have the ideal product(s) for our lung disease of interest. In this way, we may have translational success in future clinical studies. Refining these features will take time but will play a vital role in efficacy. Moreover, pinpointing small and large animal models of lung disease that will recapitulate what occurs at the patient bedside is essential if we want to move the needle in the field [46].
The plausibility of using a cell-free product as a therapeutic agent for lung disease is substantiated by newly registered human clinical trials. For instance, NCT04235296 and NCT04234750 are evaluating safety of MSC-CdM in regulating wound inflammation and promoting wound healing in burn injury. Another Phase I trial (NCT04134676) plans to study the therapeutic potential of umbilical cord tissue-derived stem cell CdM on chronic skin ulcers. Trials valuing the safety of stem cell CdM constituents (exosomes) are also underway for ischemic stroke (NCT3384433) and ocular conditions (NCT04213248, NCT03437759).
There are several limitations to our systematic review and meta-analysis, many of which mirror those published in our previous report. We incorporated multiple animal models of lung disease that have diverse pathologic processes resulting in their etiology. Also, most of the studies lacked methodologic details rendering them with an unclear risk of bias. Moreover, although preclinical models of lung disease have been helpful in identifying targetable mechanisms/processes, they oftentimes lack the intricacies of human disease. Thus, meticulous efficacy studies in large animals may be one approach to mitigate translational failure in human trials.
Conclusion
This review demonstrates that the administration of CdM in animal models of lung disease improves lung architecture and function. When compared to MSCs, CdM is as efficacious and provides a basis that cell-free products are a viable option for future studies. However, mores studies are needed to identify how specific variables (tissue source, route of delivery, concentration, etc.) may impact/strengthen their therapeutic potential.
Supplementary information
Additional file 1: Figure S1. Flow diagram demonstrating study selection process.
Additional file 2: Figure S2. Effect size of CdM vs. MSC on lung alveolarization. . Forest plots demonstrate SMD with 95% confidence interval.
Additional file 3: Figure S3. Effect size of CdM on right ventricular hypertrophy. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 4: Figure S4. Effect size of MSC on lung fibrosis. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 5: Figure S5. Effect size of CdM vs. MSC on pulmonary vasculogenesis. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 6: Figure S6. Effect size of CdM vs. MSC on lung permeability. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 7: Figure S7. Effect size of CdM vs. MSC on pulmonary pressures. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 8: Figure S8. Effect size of CdM (a), MSCs (b), and CdM vs. MSC (c) on all eight outcomes. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 9: Figure S9. Effect size of CdM on lung alveolarization by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 10: Figure S10. Effect size of CdM on right ventricular hypertrophy by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 11: Figure S11. Effect size of CdM on lung fibrosis by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 12: Figure S12. Effect size of CdM on pulmonary vascularization by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 13: Figure S13. Funnel plot assessing for publication bias of CdM on lung alveolarization.
Additional file 14: Figure S14. Funnel plot assessing for publication bias of CdM on right ventricular hypertrophy.
Additional file 15: Figure S15. Funnel plot assessing for publication bias of CdM on lung fibrosis.
Additional file 16: Figure S16. Funnel plot assessing for publication bias of CdM on pulmonary vasculogenesis.
Additional file 17: Figure S17. Funnel plot assessing for publication bias of CdM on lung permeability.
Additional file 18: Figure S18. Funnel plot assessing for publication bias of CdM on pulmonary pressures.
Additional file 19: Figure S19. Funnel plot assessing for publication bias of CdM on histologic lung injury.
Additional file 20: Figure S20. Funnel plot assessing for publication bias of CdM on lung compliance.
Additional file 21: File S1. List of articles included in this review.
Additional file 22: File S2. SYRCLE risk of bias.
Additional file 23: File S3. CdM characteristics.
Acknowledgements
Dr. Robert Cote, Director of the Writing Skills Program at the University of Arizona, Tucson.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- BPD
Bronchopulmonary dysplasia
- CAMARADES
Collaborative Approach to Meta-Analysis and Review of Data from Experimental Studies
- CdM
Conditioned media
- CF
Cystic fibrosis
- CI
Confidence interval
- MSC
Mesenchymal stem/stromal cell
- NCT
National clinical trial
- PH
Pulmonary hypertension
- SMD
Standardized mean difference
- SYRCLE
Systematic Review Centre for Laboratory Animal Experimentation
Authors’ contributions
AM contributed to the design of review, analysis of data, manuscript data, and oversight. RN contributed to the data collection, manuscript writing, and risk of bias. KH contributed to the data collection and manuscript writing. CE contributed to the database search, design of review, assembly of data, and analysis of data. JM contributed to the data collection. AM contributed to the design of review, manuscript writing, and interpretation of data. ED contributed to the database search, design of review, and data collection. SZ contributed to the manuscript writing and interpretation of data. ME contributed to the data collection, risk of bias, and Tables 1 and 2. DM contributed to the data collection and Tables 1 and 2. SM contributed to the data collection and Tables 1 and 2. The author(s) read and approved the final manuscript.
Funding
Supported by NIH NCATS KL2 TR001118, UT Health San Antonio School of Medicine pilot grant, Parker B. Francis Foundation (provided to senior author).
Availability of data and materials
Availability of data and materials will be available through figshare upon publication of the manuscripts.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have looked through the manuscript and approved the submission.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-020-01900-7.
References
- 1.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 2.Winterstein AG, Choi Y, Cody MH. Association of age with risk of hospitalization for respiratory syncytial virus in preterm infants with chronic lung disease. JAMA Pediatr. 2018;172:154–160. doi: 10.1001/jamapediatrics.2017.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxwell BG, Nies MK, Ajuba-Iwuji CC, Coulson JD, Romer LH. Trends in hospitalization for pediatric pulmonary hypertension. Pediatrics. 2015;136:241–250. doi: 10.1542/peds.2014-3834. [DOI] [PubMed] [Google Scholar]
- 4.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 5.Savran O, Ulrik CS. Early life insults as determinants of chronic obstructive pulmonary disease in adult life. Int J Chron Obstruct Pulmon Dis. 2018;13:683–93. 10.2147/COPD.S153555. Published 2018 Feb 26. [DOI] [PMC free article] [PubMed]
- 6.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–50. [DOI] [PubMed]
- 7.Virk H, Arthur G, Bradding P. Mast cells and their activation in lung disease. Transl Res. 2016;174:60–76. [DOI] [PubMed]
- 8.Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). 2017;131(13):1541–58. [DOI] [PubMed]
- 9.Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161–75. [DOI] [PubMed]
- 10.Becerra-Díaz M, Wills-Karp M, Heller NM. New perspectives on the regulation of type II inflammation in asthma. F1000Research. 2017;6:1014. doi: 10.12688/f1000research.11198.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque R Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med. 2016;119:23–8. [DOI] [PubMed]
- 12.Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A Practical Approach to Severe Asthma in Children [published correction appears in Ann Am Thorac Soc. 2018;15(6):767–8. [DOI] [PMC free article] [PubMed]
- 13.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 14.Zhong H, Fan XL, Fang S, Bin LYD, Wen W, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent chronic allergic airway inflammation via TGF-β1-Smad2/Smad3 signaling pathway in mice. Mol Immunol. 2019;109:51–57. doi: 10.1016/j.molimm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Cui P, Xin H, Yao Y, et al. Human amnion-derived mesenchymal stem cells alleviate lung injury induced by white smoke inhalation in rats. Stem Cell Res Ther. 2018;9(1):101. [DOI] [PMC free article] [PubMed]
- 16.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 17.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28841158. [cited 2017 Dec 16]. [DOI] [PMC free article] [PubMed]
- 18.Moreira A, Kahlenberg S, Hornsby P. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol. 2017;59:R109–R120. doi: 10.1530/JME-17-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. [DOI] [PMC free article] [PubMed]
- 20.Cruz FF, PRM R. Hypoxic preconditioning enhances mesenchymal stromal cell lung repair capacity. Stem Cell Res Ther. 2015;6:130. doi: 10.1186/s13287-015-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. [DOI] [PMC free article] [PubMed]
- 22.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emukah C, Dittmar E, Naqvi R, et al. Mesenchymal stromal cell conditioned media for lung disease: a systematic review and meta-analysis of preclinical studies. Respir Res. 2019;20(1):239. [DOI] [PMC free article] [PubMed]
- 25.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and metaanalysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. [DOI] [PubMed]
- 26.Welcome to CAMARADES. Available from: http://www.dcn.ed.ac.uk/camarades/. [cited 2020 Jan 30].
- 27.Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruno S, Kholia S, Deregibus MC, Camussi G. The role of extracellular vesicles as paracrine effectors in stem cell-based therapies. Adv Exp Med Biol. 2019;1201:175–193. doi: 10.1007/978-3-030-31206-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. doi: 10.3390/ijms21030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Wang J, Hu J, Fu B, Mao Z, Zhang H, et al. Extracellular vesicles for acute kidney injury in preclinical rodent models: a meta-analysis. Stem Cell Res Ther. 2020;11:11. doi: 10.1186/s13287-019-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis GR, Mitsialis SA, Kourembanas S. “Good things come in small packages”: application of exosome-based therapeutics in neonatal lung injury. Pediatr Res. 2018;83:298–307. doi: 10.1038/pr.2017.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [DOI] [PMC free article] [PubMed]
- 33.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Augustine S, Avey MT, Harrison B, Locke T, Ghannad M, Moher D, et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: Systematic review and meta-analysis of preclinical studies. Stem Cells Transl Med. 2017;6:2079–2093. doi: 10.1002/sctm.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanyu Z, Feilong H. Emerging role of extracellular vesicles in lung injury and inflammation. Biomed Pharmacother. 2019;113:108748. doi: 10.1016/j.biopha.2019.108748. [DOI] [PubMed] [Google Scholar]
- 38.Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7:355. doi: 10.3390/jcm7100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augustine S, Cheng W, Avey MT, Chan ML, Lingappa SMC, Hutton B, et al. Are all stem cells equal? Systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia. Stem Cells Transl Med. 2020;9:158–168. doi: 10.1002/sctm.19-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvira CM. Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Front Med. 2016;3:21. doi: 10.3389/fmed.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kropski J, Richmond B, Gaskill C, Foronjy R, Majika S. Deregulated angiogenesis in chronic lung diseases: a possible role for lung mesenchymal progenitor cells (2017 Grover Conference Series) Pulm Circ. 2018;8:2045893217739807. doi: 10.1177/2045893217739807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sensebé L, Gadelorge M, Fleury-Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: a review. Stem Cell Res Ther. 2013;4:66. doi: 10.1186/scrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33. [DOI] [PMC free article] [PubMed]
- 44.Lopes-Pacheco M, Robba C, Rocco P, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2020;36:83–102. doi: 10.1007/s10565-019-09493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathinasabapathy A, Bruce E, Espejo A, et al. Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis. Br J Pharmacol. 2016;173(19):2859–2879. doi: 10.1111/bph.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamuleau SAJ, van der Naald M, Climent AM, Kraaijeveld AO, Wever KE, Duncker DJ, et al. Translational research in cardiovascular repair. Circ Res. 2018;122:310–318. doi: 10.1161/CIRCRESAHA.117.311565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Flow diagram demonstrating study selection process.
Additional file 2: Figure S2. Effect size of CdM vs. MSC on lung alveolarization. . Forest plots demonstrate SMD with 95% confidence interval.
Additional file 3: Figure S3. Effect size of CdM on right ventricular hypertrophy. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 4: Figure S4. Effect size of MSC on lung fibrosis. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 5: Figure S5. Effect size of CdM vs. MSC on pulmonary vasculogenesis. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 6: Figure S6. Effect size of CdM vs. MSC on lung permeability. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 7: Figure S7. Effect size of CdM vs. MSC on pulmonary pressures. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 8: Figure S8. Effect size of CdM (a), MSCs (b), and CdM vs. MSC (c) on all eight outcomes. Forest plots demonstrate SMD with 95% confidence interval.
Additional file 9: Figure S9. Effect size of CdM on lung alveolarization by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 10: Figure S10. Effect size of CdM on right ventricular hypertrophy by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 11: Figure S11. Effect size of CdM on lung fibrosis by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 12: Figure S12. Effect size of CdM on pulmonary vascularization by disease (a), source (b), dose (c), and route (d). Forest plots demonstrate SMD with 95% confidence interval.
Additional file 13: Figure S13. Funnel plot assessing for publication bias of CdM on lung alveolarization.
Additional file 14: Figure S14. Funnel plot assessing for publication bias of CdM on right ventricular hypertrophy.
Additional file 15: Figure S15. Funnel plot assessing for publication bias of CdM on lung fibrosis.
Additional file 16: Figure S16. Funnel plot assessing for publication bias of CdM on pulmonary vasculogenesis.
Additional file 17: Figure S17. Funnel plot assessing for publication bias of CdM on lung permeability.
Additional file 18: Figure S18. Funnel plot assessing for publication bias of CdM on pulmonary pressures.
Additional file 19: Figure S19. Funnel plot assessing for publication bias of CdM on histologic lung injury.
Additional file 20: Figure S20. Funnel plot assessing for publication bias of CdM on lung compliance.
Additional file 21: File S1. List of articles included in this review.
Additional file 22: File S2. SYRCLE risk of bias.
Additional file 23: File S3. CdM characteristics.
Data Availability Statement
Availability of data and materials will be available through figshare upon publication of the manuscripts.