Abstract
Silver nanoparticles (AgNPs) are utilized in surgical implants and medical textiles, thus providing access to the circulation. While research was conducted primarily in healthy models, AgNP-induced toxicity evaluations in disease conditions are critical, as many individuals have pre-existing conditions. Specifically over 20% of United States adults suffer from metabolic syndrome (MetS). It was hypothesized that MetS may increase susceptibility to AgNP-mediated toxicity due to induction of differential inflammation and altered biodistribution. Mice were injected with 2mg/kg AgNPs, and organs assessed for inflammatory gene expression (TNF-α, CXCL1, CXCL2, CCL2, TGF-β, HO-1, IL-4, IL-13), and Ag content. AgNPs were determined to induce differential inflammation in healthy and MetS mice. While AgNP exposure increased TNF-α, CXCL1, TGF-β, HO-1, and IL-4 expression within healthy mouse spleen, MetS-treated animals demonstrated decreased CXCL1, IL-4, and IL-13 expression. Healthy and MetS mice livers exhibited similar inflammatory responses to one another. AgNPs localized primarily to the liver and spleen, although Ag was present in all examined organs. In organs of minor AgNP deposition, such as kidney, gene expression was variable. Induction of inflammatory genes did not correspond with biodistribution, suggesting disease-related variations in AgNP-mediated adverse responses. These findings indicate that disease may influence inflammation and biodistribution, impacting AgNP clinical applications.
Keywords: Obesity, Nanomedicine, Nanotoxicity
Introduction
In recent years, the use of nanoparticles has expanded to include a multitude of applications, including manufacturing, consumer products, electronics, and medicine. Silver nanoparticles (AgNPs) are one of the most popular nanomaterials due to their antimicrobial activity, leading to their incorporation into cosmetics, electronics, textiles and food packaging (Zhang et al. 2016a; Tran et al 2013; Echegoyen and Nerín 2013; Kaweeteerawat et al 2017). AgNPs are also utilized in a variety of biomedical applications that result in their direct introduction into the circulatory system, including burn creams, wound dressings, venous catheters, and surgical implants (Chaloupka et al 2010; Ge et al. 2014; Song and Kim 2009). Previously Benn and Cavanagh (2010) demonstrated that medical textiles with AgNP coatings are particularly prone to leaching and releasing AgNPs to the surrounding environment. However, direct introduction may not be strictly necessary due to their small size as inhaled or ingested AgNPs may translocate to the circulation (Kemi 2016). In addition, AgNPs were proposed to be utilized as a vaccine adjuvant and as a component of cancer treatment, which might result in their direct injection into the circulation (Sanchez-Guzman et al. 2019; Chugh et al. 2018, Taha et al. 2019). Despite their widespread potential applications in medicine, the toxicity of AgNPs to humans remains a subject of scientific investigation (Kermanizadeh et al, 2016; Alaraby et al 2016). Investigators reported that exposure to AgNPs produced oxidative stress, cytotoxicity and genotoxicity in human cell lines (macrophages, lung adenocarcinoma, and mesenchymal stem cells), potentially indicating that AgNP exposure may result in impaired immune responses and slower healing (Haase et al. 2011; Hackenberg et al. 2011; Rosario et al 2018). Other studies have more directly examined the use of AgNPs in medicine, examining the effects of AgNPs within commercially available dressings, and found these to be hazardous to keratinocytes and fibroblasts, inducing cytotoxicity (Lam et al. 2004; Paddle-Ledinek et al. 2006; Poon and Burd 2004). While AgNPs are utilized increasingly in biomedicine, it is not known if the benefits of their use outweigh potential toxicity concerns.
Mammalian models have provided further evidence of unintended adverse effects resulting from AgNP exposure. When inhaled or injected subcutaneously, AgNPs subsequently biodistribute throughout the body, depositing primarily in the liver but also reaching the brain, lungs, kidneys, and other organs (Tang et al. 2009; Takenaka et al. 2001). Ingested and injected AgNPs are eliminated from most organs fairly quickly, though these materials are retained for months within the brain (Zande et al. 2012; Dziendzikowska et al. 2012; Bergin et al. 2016). This is particularly concerning, as AgNPs may induce oxidative stress in various tissues (Martins, Jr et al 2017) and alter gene expression in multiple regions of the brain, resulting in apoptosis and neurotoxicity (Rahman et al. 2009). AgNPs were also demonstrated to stimulate an inflammatory response in both in vitro and in vivo models. Various cell types were shown to exhibit increased expression of pro-inflammatory markers following exposure to AgNPs, including macrophages, fibroblasts, and endothelial cells (Trickler et al. 2010; Park et al. 2011). In animal models, AgNP exposure was found to exert a similar effect, eliciting enhanced expression of pro-inflammatory cytokines primarily in liver, a major site of AgNP deposition (Ramadi et al. 2016; Chen et al. 2016). However, while toxicity of AgNPs has been the subject of much scrutiny, there remain gaps in the scientific knowledge regarding susceptible subpopulations that may be at risk for unexpected and exacerbated responses following exposure.
The majority of studies conducted investigating AgNP toxicity were performed in healthy models. This is a major oversight, as medical treatments, including those containing AgNPs, are utilized primarily in individuals who are in some way unhealthy. Examples include the use of AgNPs in the treatment of injuries, such as in burn creams or in surgeries as an antibacterial/antifungal coating on tools and implants (Chaloupka et al 2010; Ge et al. 2014; Song and Kim 2009). Further, many individuals in the United States and worldwide exist with some form of underlying disease state (CDC 2019; Halpin et al. 2010). Metabolic syndrome (MetS), a disease characterized by dyslipidemia, elevated blood pressure, insulin resistance, and increased body weight, affects over one-fifth of the United States population (Beltrán-Sánchez et al. 2013; Aguilar et al. 2015). In addition, the prevalence of MetS in the United States and worldwide is increasing (Desroches and Lamarche 2007; Ford et al. 2004). MetS is an established risk factor for development of many serious chronic diseases, including renal disease, diabetes, and cardiovascular disease (Cirillo et al. 2006; Galassi et al. 2006; Grundy 2006). Individuals with MetS were noted to be more vulnerable to adverse health outcomes such as cardiovascular depression, heart beat variability, and altered cardiac repolarization as a result of exposure to nano-sized particulate matter (Wagner et al. 2014; Devlin et al. 2014; Chen and Schwartz 2008). Evaluations of the NHANES database in conjunction with the U.S. EPA’s Aerometric Information Retrieval system demonstrated an association between PM10 and PM2.5 exposures and circulating white blood cells and elevated levels of C-reactive protein, a cardiovascular disease inflammatory marker, which worsened as severity of MetS rose. This further demonstrates the increased susceptibility of diseased individuals to enhanced inflammation following exposures (Chen and Schwartz 2008; Dabass et al. 2018). Although the mechanisms underlying this increased susceptibility are not fully understood, it is thought that individuals with MetS possess higher levels of baseline inflammation, predisposing them to both acute adverse health outcomes and development of chronic diseases (Esser et al. 2014; Haffner 2006; Isomaa et al. 2001; Paoletti et al. 2006).
It is likely that individuals with MetS may be susceptible not only to particulate matter (PM) inhalation, but also to intravenous exposure to biomedically utilized engineered particles such as AgNPs. The aim of this study was to examine variations in inflammatory response and biodistribution following intravenous exposure to AgNPs through utilization of mouse models representing either healthy or MetS conditions. An understanding of differences in inflammation and biodistribution between healthy and diseased conditions might enable better understanding of the differential exposure responses, and thus susceptibility of vulnerable subpopulations. Ultimately, these results might assist in utilization of in vitro and in vivo studies to service the development of medical devices containing AgNPs and their use in clinical settings.
Materials and Methods
AgNP Characterization.
AgNPs with a diameter of 20 nm suspended in citrate were purchased (Nanocomposix, San Diego, CA). AgNPs were characterized to verify the specifications provided by the manufacturer. Polydispersion index, ζ-potential, and hydrodynamic size were assessed in DI water with AgNPs at a concentration of 25 μg/ml (n=4/AgNP). The number of AgNPs/ml was determined by NP tracking software (Nanosight, Malvern, Westborough, MA) (n=4/AgNP). While many varieties of AgNPs are commercially available, AgNPs utilized in this study display similar properties to certain formulations used in biomedicine (Dunn and Edwards-Jones 2004; Park and Lee 2013).
Induction of MetS and AgNP Exposure.
Healthy male 6-week-old C57BL6 mice (Charles River Laboratories, Wilmington, MA) were provided with water ad libitum and fed either a normal diet (10% kcal from fat) or a high fat Western diet (60% kcal from fat) (Research Diets, New Brunswick, NJ) to induce development of MetS. Mice were assessed for key components of MetS, obesity, dyslipidemia, and insulin resistance to verify development of the disease. While markers of hyperglycemia were not directly measured, it may be extrapolated that animals were likely hyperglycemic based upon previous studies utilizing the same high fat diet-induced model of MetS (Kennedy et al. 2010). Following 14 weeks on the diets, mice were injected via the tail vein with 2 mg/kg of AgNPs or saline as a control. While many previous studies utilized injection doses in excess of 50 mg/kg, toxicological effects were observed at doses of approximately 2 mg/kg (Xue et al. 2012; Tang et al. 2008; 2009; El Mahdy et al. 2014). The selected dose of 2 mg/kg, in addition to being known to induce adverse effects from other studies, is also relevant to real world exposures. Medical textiles were found to leach significant amounts AgNPs (Benn and Cavanagh 2010). The use of these textiles in combination with medical tools or implants which also contain an AgNP coating has the capacity to increase human exposure to and beyond the dose utilized in this study. Further, AgNPs are proposed as a cancer treatment and vaccine adjuvant, both applications which would result in direct introduction of a significant amount of AgNPs into the circulatory system (Sanchez-Guzman et al. 2019; Chugh et al. 2018). Mice were separated into two groups, one of which was utilized for an assessment of inflammation and the other to examine differential biodistribution. Animals were housed in a temperature-controlled, 12 hr light/dark cycle room for the duration of the study, which was conducted in compliance with the regulation and approved by the Animal Care and Use Committee of Purdue University.
Organ Collection and Blood Characterization.
Twenty-four hr following AgNP injection, mice were sacrificed, and liver, spleen, kidneys, intestines, lungs, brain, heart, and aorta extracted. Organs were immediately frozen in liquid nitrogen following removal. Blood was collected via cardiac puncture, and the aorta severed to remove additional blood from the circulation. In accordance with previous investigations of injected NPs, perfusion of the body was not performed (Lee et al. 2016; Javidi et al. 2019; Snyder et al. 2016; Sumner et al. 2016; Yang et al. 2017; Meng et al. 2014). Immediately following collection, an aliquot of blood was set aside for assessment of glycated hemoglobin (HBA1c) using a commercially available assay kit (Crystal Chem, Elk Grove Village, IL). The remaining blood was centrifuged for 10 min at 3,500g and 4°C to isolate serum. Serum was characterized for traditional lipid endpoints via commercially available kits to measure total bound and unbound cholesterol, HDL, and LDL/VLDL (Bioassay Systems, San Francisco, CA). Serum insulin was also measured in serum using a commercially available assay kit (Crystal Chem, Elk Grove Village, IL).
Gene Expression Assessment.
Total RNA was isolated from each organ of the gene expression cohort and converted to cDNA. AgNP-induced variations in the inflammatory response were measured through assessment of alterations in gene expression tumor necrosis factor-α (TNF-α), chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-X-C motif) ligand 2 (CXCL2), interleukin 4 (IL-4), and interleukin 13 (IL-13). In addition, fibrotic marker transforming growth factor-β (TGF-β) and oxidative stress marker heme oxygenase 1 (HO-1) were assessed. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. All genes were analyzed via real-time RT-qPCR (n=7–8 per group).
Metal Quantification.
All organs from the biodistribution cohort were digested with concentrated nitric acid in a MARSXpress microwave-accelerated reaction system. Digested samples were diluted up to 1000- fold in 0.1% nitric acid prior to quantification using an atomic absorption spectrophotometer with a graphite tube atomizer (Agilent, Santa Clara, CA). Sample concentration was normalized to tissue weight of the digested organ for each measurement.
Statistical Analysis.
Significant differences in blood parameters, organ metal content, and gene expression were determined using a two-way ANOVA with disease (healthy or MetS) and exposure (control or AgNP exposure) as the two factors. A Tukey’s post-hoc test was utilized for multi-comparison analysis (p < 0.05). GraphPad Prism 8 software (GraphPad, San Diego, CA) was used to generate graphs and for all statistical assessment. Graphed data are presented as mean ± standard error of the mean (SEM.)
Results
Characterization of Mouse Model and AgNPs.
AgNPs were characterized based upon the parameters of hydrodynamic size, polydispersion index, and ζ-potential and found to match the manufacturer’s specifications (Table 1). MetS mice exhibited elevated body weight, HDL, LDL/VLDL, and total cholesterol compared to healthy animals (Table 2). These results were expected and consistent with previous models of diet-induced MetS (Pettersson et al. 2012; Cao et al. 2014). Further, serum insulin was increased in MetS mice suggesting the presence of insulin resistance, a component of MetS (Beltrán-Sánchez et al. 2013). HBA1c, a marker of diabetes, was unchanged between healthy and MetS models. AgNP exposure did not markedly alter weight or any of the assessed blood parameters.
Table 1.
AgNP Characterization.
| Particle Concentration (NPs/μg × 109) | Hydrodynamic Size (nm) | Polydispersion Index | ζ Potential (mV) | |
|---|---|---|---|---|
| AgNPs | 5.5 ± 0.6 | 27.0 ± 0.2 | 0.09 ± 0.006 | −54.8 ± 0.3 |
Data represent mean ± SEM, n=4/group. AgNPs were characterized via assessment of ζ-potential, hydrodynamic size, and polydispersion index.
Table 2.
Characterization of Healthy and MetS Mice.
| Healthy Control | Healthy AgNPs | MetS Control | MetS AgNPs | |
|---|---|---|---|---|
| Body Weight (g) | 34.3 ± 0.9 | 32.6 ± 1.7 | 50.4 ± 1.1# | 48.75 ± 1.2# |
| HDL (mg/dL) | 132.7 ± 10.1 | 128.7 ± 13.4 | 220.9 ± 10.7# | 244.3 ± 19.18# |
| LDL/VLDL (mg/dL) | 43.1 ± 1.3 | 46.3 ± 2.7 | 56.1 ± 2.7# | 61.3 ± 4.1# |
| Total Cholesterol (mg/dL) | 159.8 ± 12.9 | 157.8 ± 10.3 | 273.5 ± 18.6# | 298.4 ± 16.1# |
| Insulin (ng/mL) | 0.53 ± 0.1 | 0.59 ± 0.1 | 8.26 ± 2.4# | 7.52 ± 2.1# |
| % HBA1c | 4.21 ± 0.1 | 4.45 ± 0.2 | 4.55 ± 0.6 | 4.78 ± 0.2 |
Serum from healthy and MetS mice was characterized for traditional lipid endpoints via commercially available kits to measure total bound and unbound cholesterol, HDL, LDL/VLDL, insulin, and glycated hemoglobin (HBA1c).
denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05). All analyses are reported as mean ± SEM, n= 7–8/group.
AgNP Biodistribution.
AgNPs were determined to primarily biodistribute to the liver, intestines, and spleen with minor amounts in lungs, blood, kidneys, brain, heart, and aorta (Figure 1). A larger proportion of AgNPs was detected in liver of MetS than healthy mice, while a smaller portion was identified in intestines, spleen, and lungs. In each specific organ tested, silver levels were at or below the limit of detection for unexposed control animals (Figure 2). The concentration of Ag present in the spleen 24 hr following injection was the same between healthy and MetS mice. MetS mice possessed a higher Ag concentration in liver, kidneys, and heart. The concentration of Ag in the lungs was higher in healthy than MetS mice. Both MetS and healthy mice had the same concentration of Ag in the brain.
Figure 1.
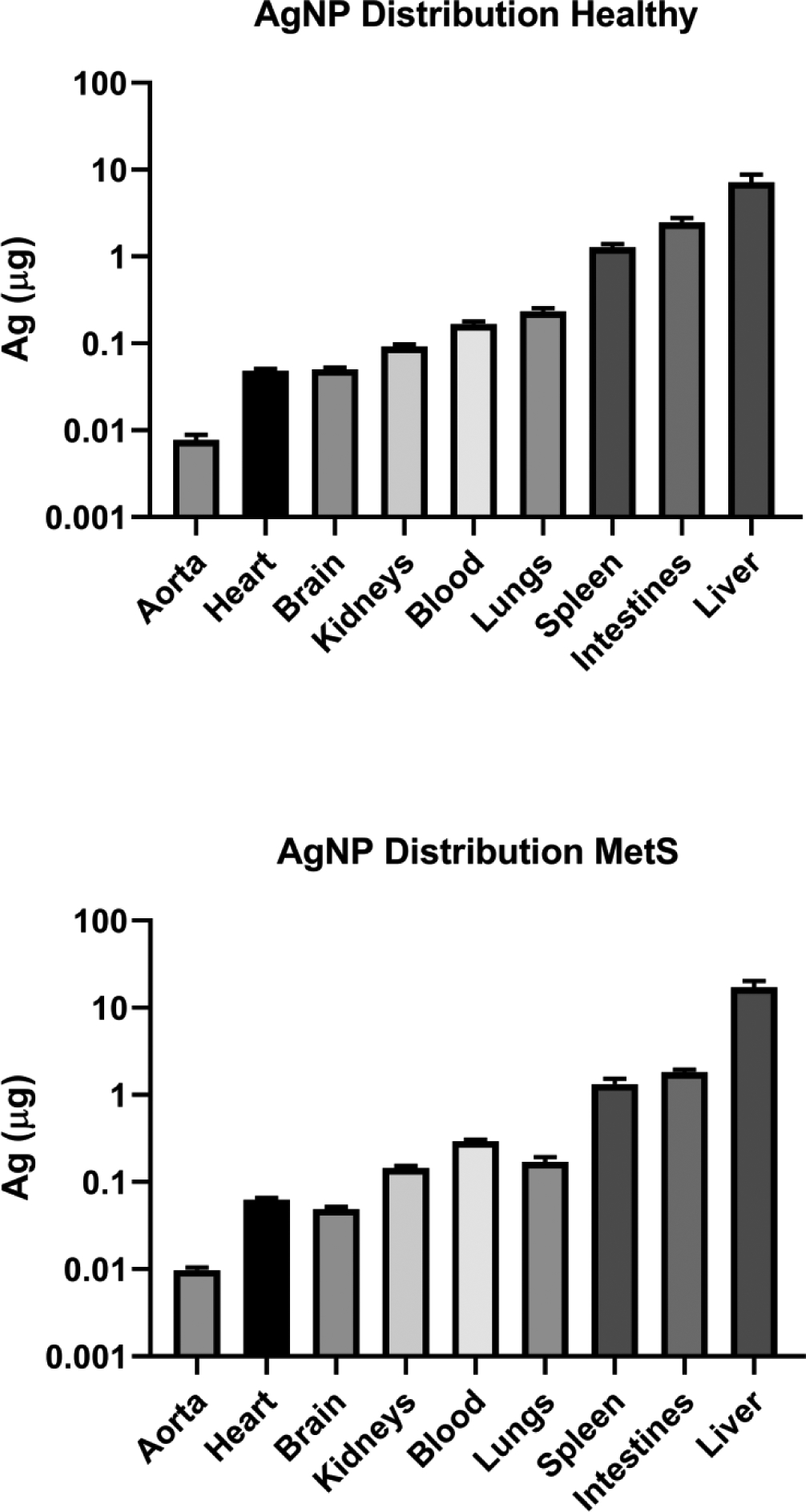
Average Abundance of AgNPs in Each Organ of Healthy and MetS Mice Treated with AgNPs. The Ag content of each organ of mice treated with AgNPs was assessed via atomic absorption spectroscopy.
Figure 2.
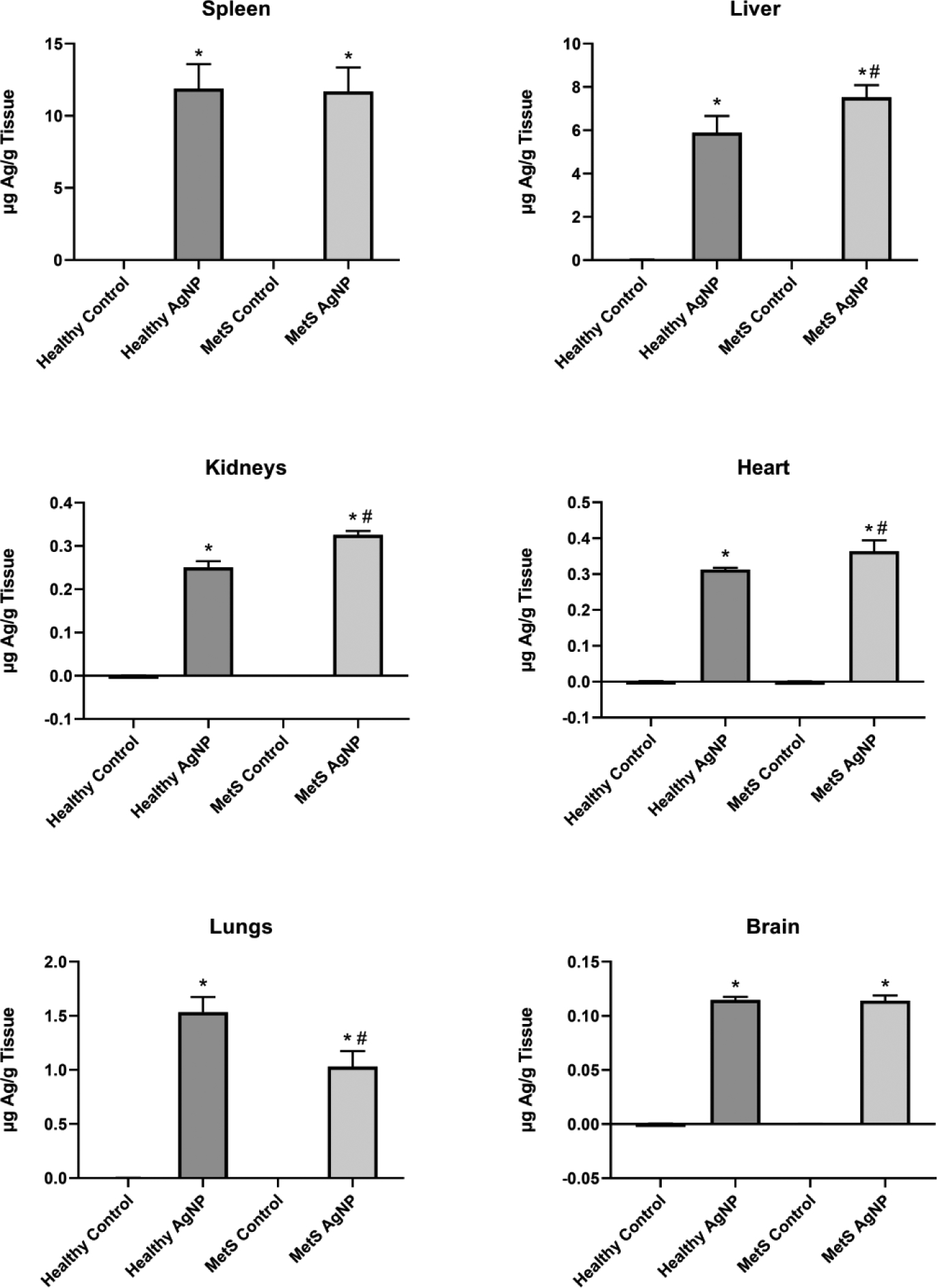
Average Concentration of AgNPs in Selected Organs of Treated and Control Healthy and MetS Mice. The Ag content of each organ was assessed via atomic absorption spectroscopy. The metal content in μg was divided by the weight of the organ in g in order to assess data in terms of Ag concentration. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
Gene Expression.
The expression of genes related to inflammation, fibrosis, and oxidative stress was assessed in a variety of tissues in order to determine differential responses to MetS and AgNP exposure. A full summary of genes assessed and organ-specific responses to AgNP exposure may be found in Table 3. MetS was found to decrease IL-13 and CXCL1 expression in liver compared to healthy control mice (Figure 3). AgNP exposure enhanced expression of Th1 inflammatory response genes (CCL2, TNF- α, CXCL1, CXCL2), TGF- β (a marker of fibrosis), and HO-1 (indicative of oxidative stress) in healthy and MetS mice, with all increases reaching level of statistical significance with the exception of CXCL1 in healthy mice. Liver expression of Th2 inflammatory response genes (IL-4 and IL-13) were unaffected by AgNP exposure, remaining at approximately the same levels as observed in saline treated control mice.
Table 3.
Summary of Changes in Gene Expression Following Exposure to AgNPs.
| Liver | Spleen | Kidney | Heart | Lung | Brain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | MetS | Healthy | MetS | Healthy | MetS | Healthy | MetS | Healthy | MetS | Healthy | MetS | |
| Chemokine (C-C motif) ligand 2 (CCL2) | ↑ | ↑ | - | - | - | ↓ | - | ↑ | ↓ | ↓ | ↑ | - |
| Chemokine (C-X-C motif) ligand 1 (CXCL1) | - | ↑ | ↑ | - | ↑ | ↓ | - | ↑ | ↓ | ↓ | ↓ | ↓ |
| Chemokine (C-X-C motif) ligand 2 (CXCL2) | ↑ | ↑ | - | - | ↑ | - | - | ↑ | - | ↓ | - | - |
| Tumor necrosis factor α (TNF-α) | ↑ | ↑ | ↑ | - | ↓ | - | - | ↑ | ↓ | - | ↓ | - |
| Transforming growth factor β (TGF-β) | ↑ | ↑ | ↑ | - | ↓ | ↑ | - | - | ↓ | ↓ | ↓ | - |
| Heme oxygenase 1 (HO-1) | ↑ | ↑ | ↑ | - | - | ↑ | - | - | ↓ | - | ↓ | ↓ |
| Interleukin 4 (IL-4) | - | - | ↑ | ↓ | ||||||||
| Interleukin 13 (IL-13) | - | - | - | ↓ | ||||||||
Statistically significant increases and decreases in expression as compared to unexposed groups are denoted by “up” and “down” arrows, respectively. Lack of any statistically significant change is indicated by a dash.
Figure 3.
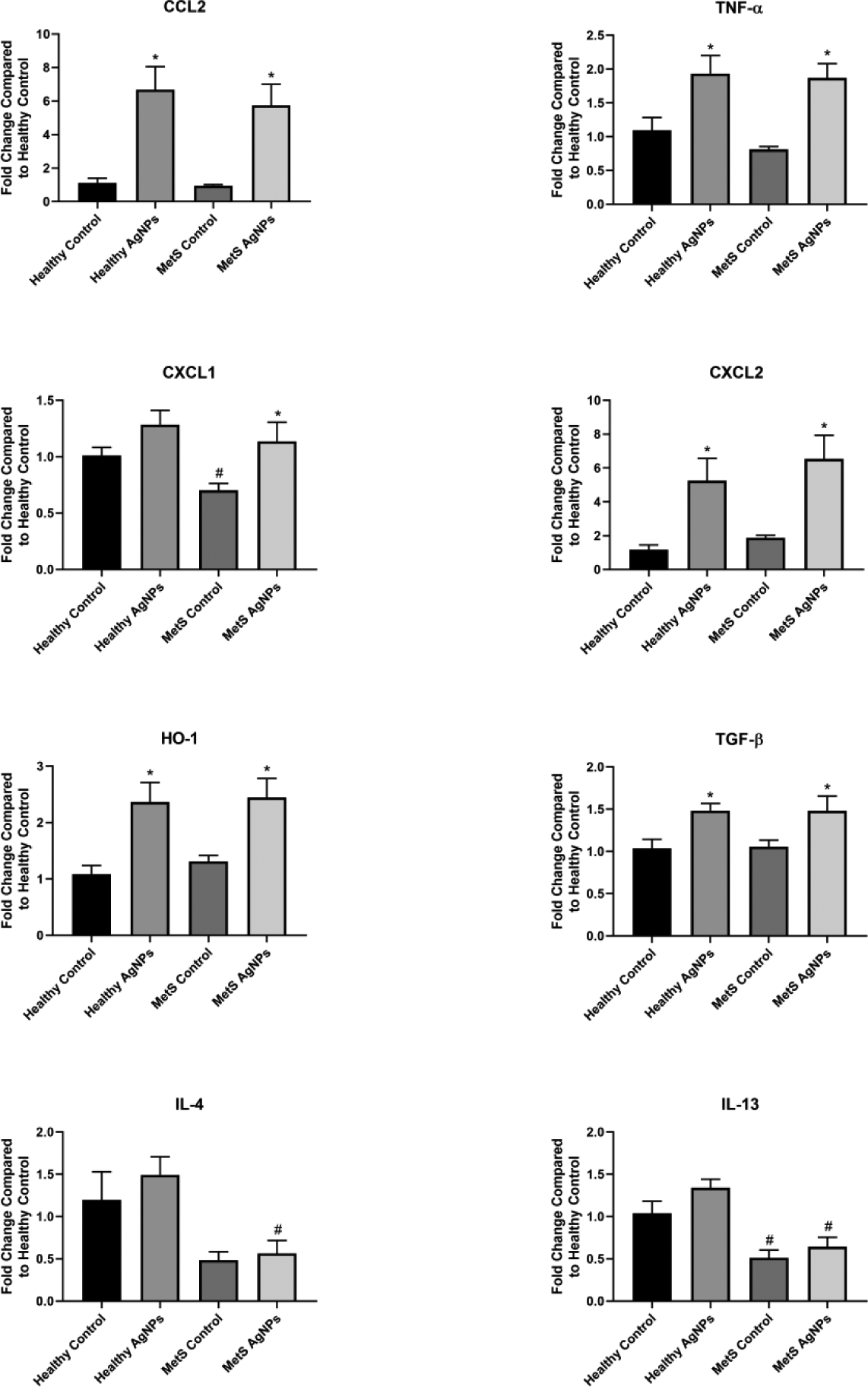
Changes in Gene Expression in the Liver Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
At baseline, MetS mice displayed a higher expression of CCL2, TNF- α, CXCL2, HO-1, TGF- β, and IL-13 in spleen compared to healthy mice (Figure 4). In healthy mice, AgNP exposure resulted in an elevated expression of TNF- α, CXCL1, HO-1, TGF- β, and IL-4 in the spleen. The influence of AgNP exposure on gene expression was less consistent in MetS mice. Both IL-4 and IL-13 expression were significantly decreased compared to control MetS. CXCL2 within the spleen was unaffected by MetS or exposure to AgNPs.
Figure 4.
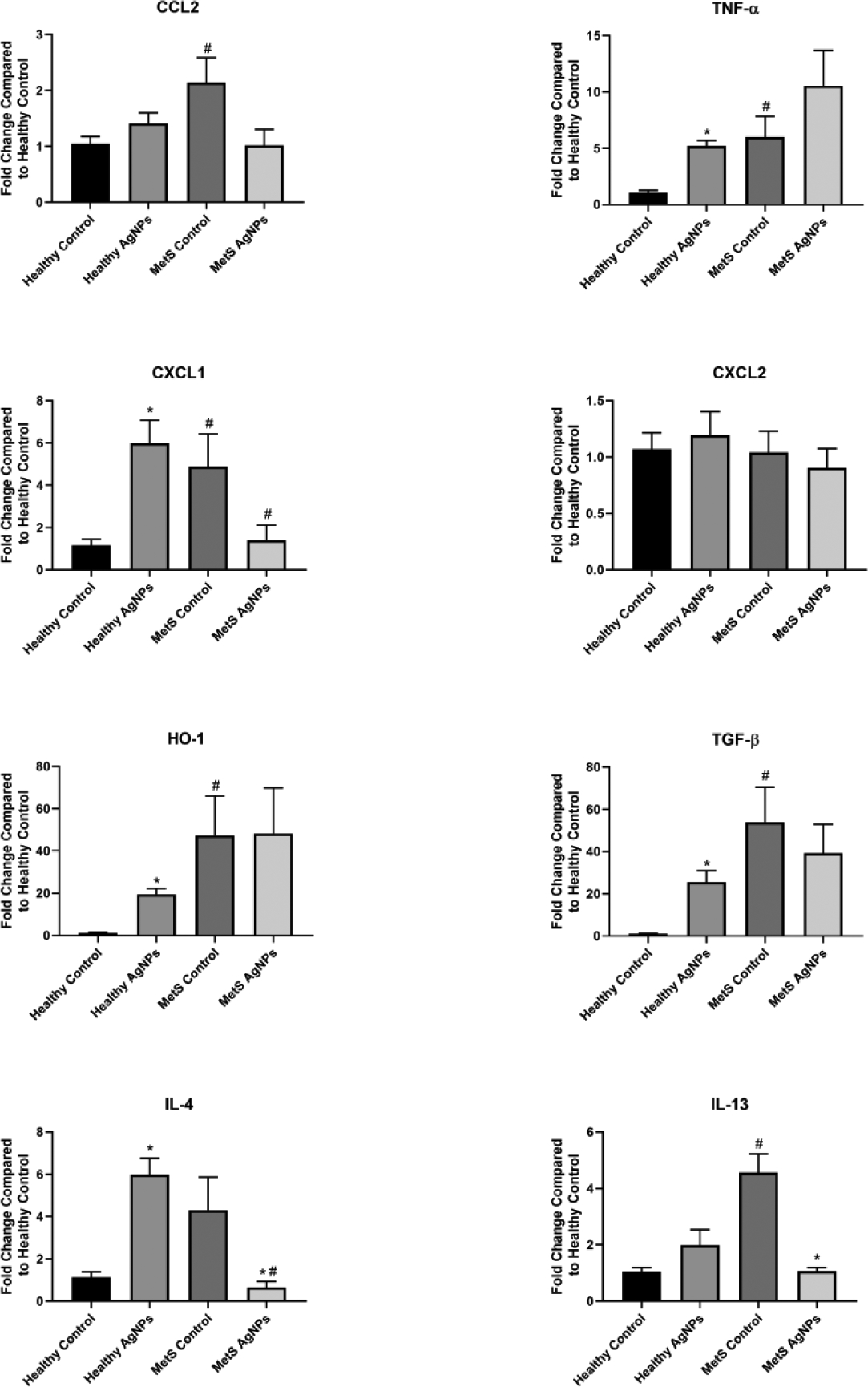
Changes in Gene Expression in the Spleen Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
The reaction of kidney to MetS was variable based upon the gene examined. CCL2, CXCL1, and CXCL2 were all significantly higher at baseline in MetS than healthy mice, while TNF- α and TGF- β were significantly lower (Figure 5). HO-1 was unaffected at baseline by MetS. Alterations in kidney gene expression of mice following AgNP exposure were similarly variable; in healthy mice, CXCL1 and CXCL2 expression was up-regulated by AgNP exposure, TNF- α and TGF- β expression was down-regulated, and CCL2 and HO-1 was unaltered. MetS exhibited differential responses to AgNPs, with CCL2 and CXCL1 down-regulated, HO-1 and TGF- β up-regulated, and CXCL2 unaltered.
Figure 5.
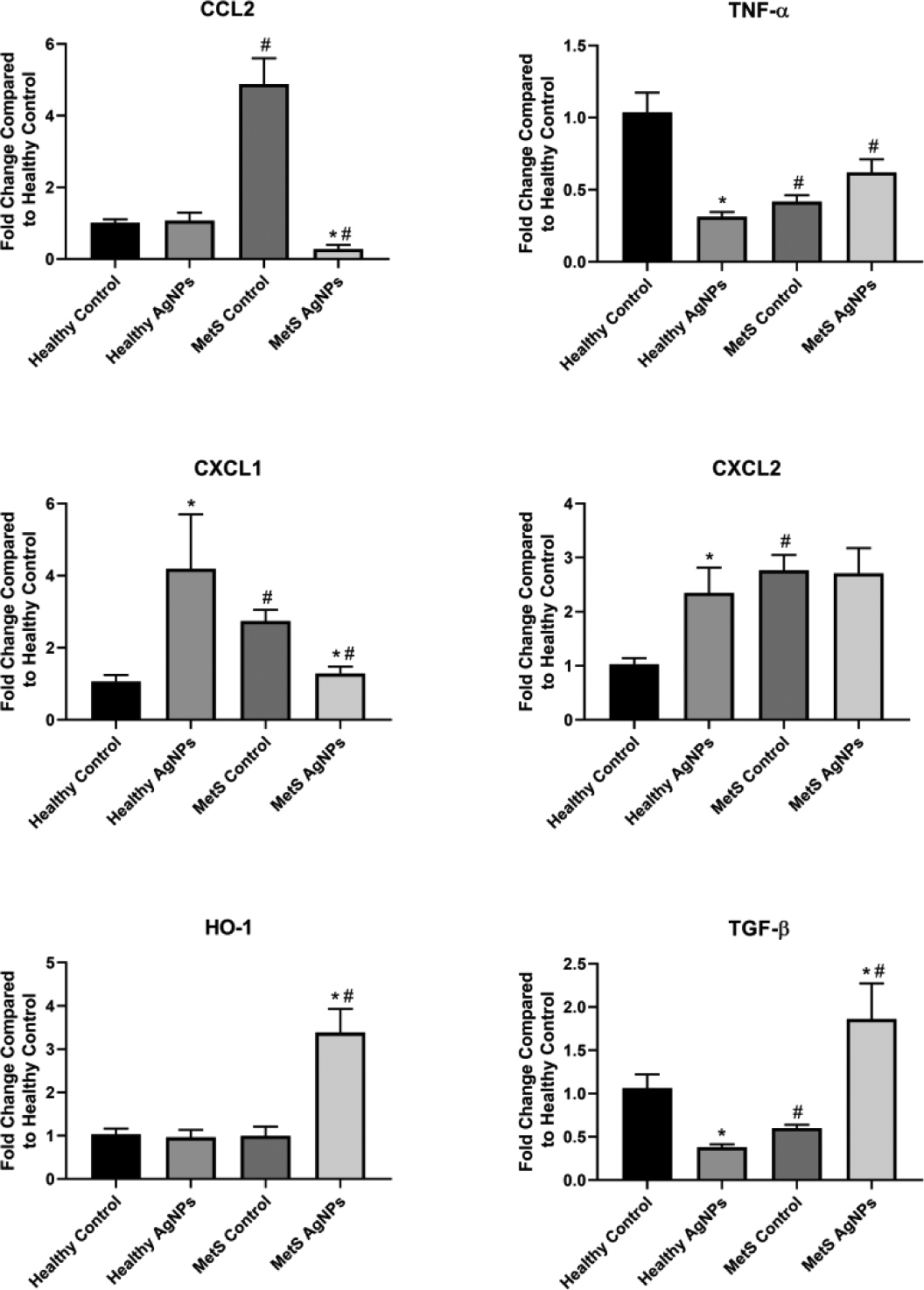
Changes in Gene Expression in the Kidney Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
Within the heart, most cytokines tested were the same between healthy and MetS at baseline, with the exceptions of TNF- α and TGF- β, which were lower in MetS compared to healthy animals (Figure 6). AgNP exposure did not induce any significant alterations in expression of genes evaluated. In contrast, MetS mice exhibited significant elevations in expression of CCL2, TNF- α, CXCL1, and CXCL2 following AgNP exposure. HO-1 and TGF- β expression in the MetS heart were unaffected by AgNP exposure.
Figure 6.
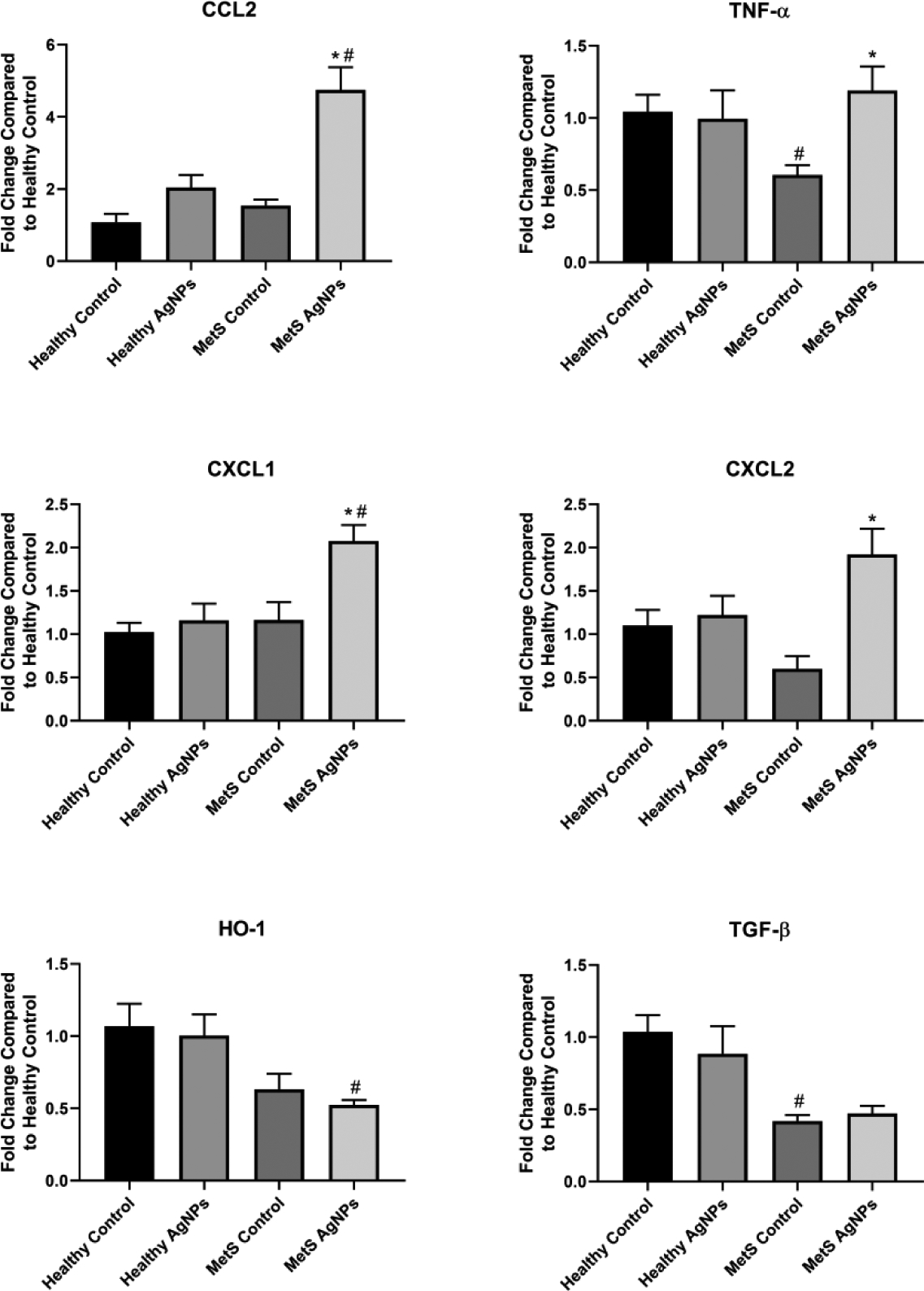
Changes in Gene Expression in the Heart Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
The lung was among the most consistent of the organs tested regarding alterations in gene expression. CCL2, TNF- α, HO-1, and TGF- β gene expression was noted to be down-regulated in unexposed MetS animals compared to unexposed healthy, while unexposed MetS mice displayed higher expression of CXCL1 and CXCL2 than healthy (Figure 7). Upon exposure to AgNPs, gene expression was reduced in both healthy and MetS mice. In healthy mice, this decrease was significant for all genes with exception of CXCL2, and in MetS mice, the fall was significant in CCL2, CXCL1, CXCL2, and TGF- β.
Figure 7.
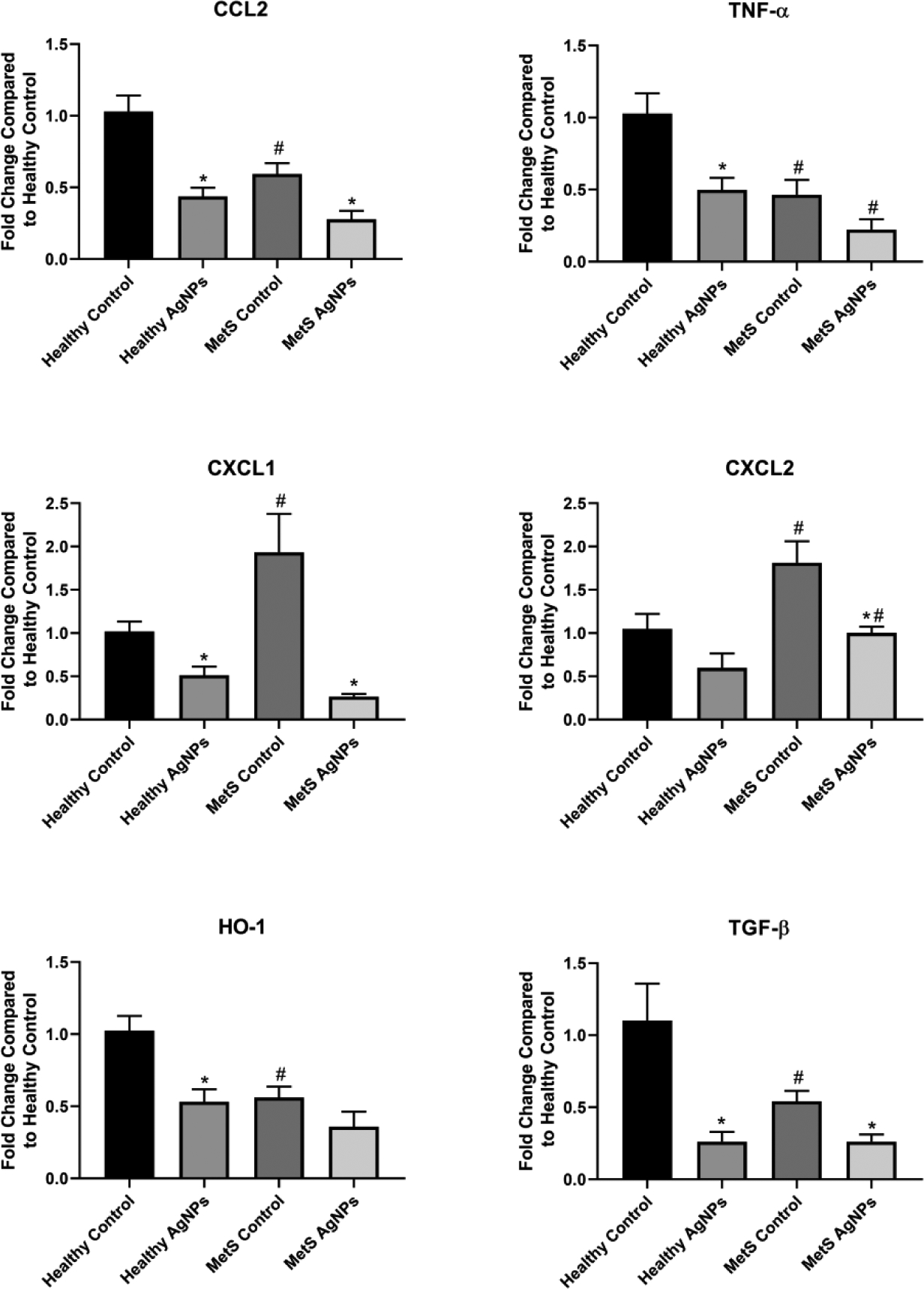
Changes in Gene Expression in the Lung Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
Gene expression within the brain was also found to be altered in MetS; specifically, TNF- α was determined to be significantly lower in MetS mice compared to healthy at baseline (Figure 8). AgNP exposure increased gene expression of CCL2 in the brain of healthy mice while lowering expression of CCL2, TNF- α, CXCL1, HO-1, and TGF- β. Following AgNP exposure, MetS mice reduced gene expression of CXCL1 and HO-1 was noted.
Figure 8.
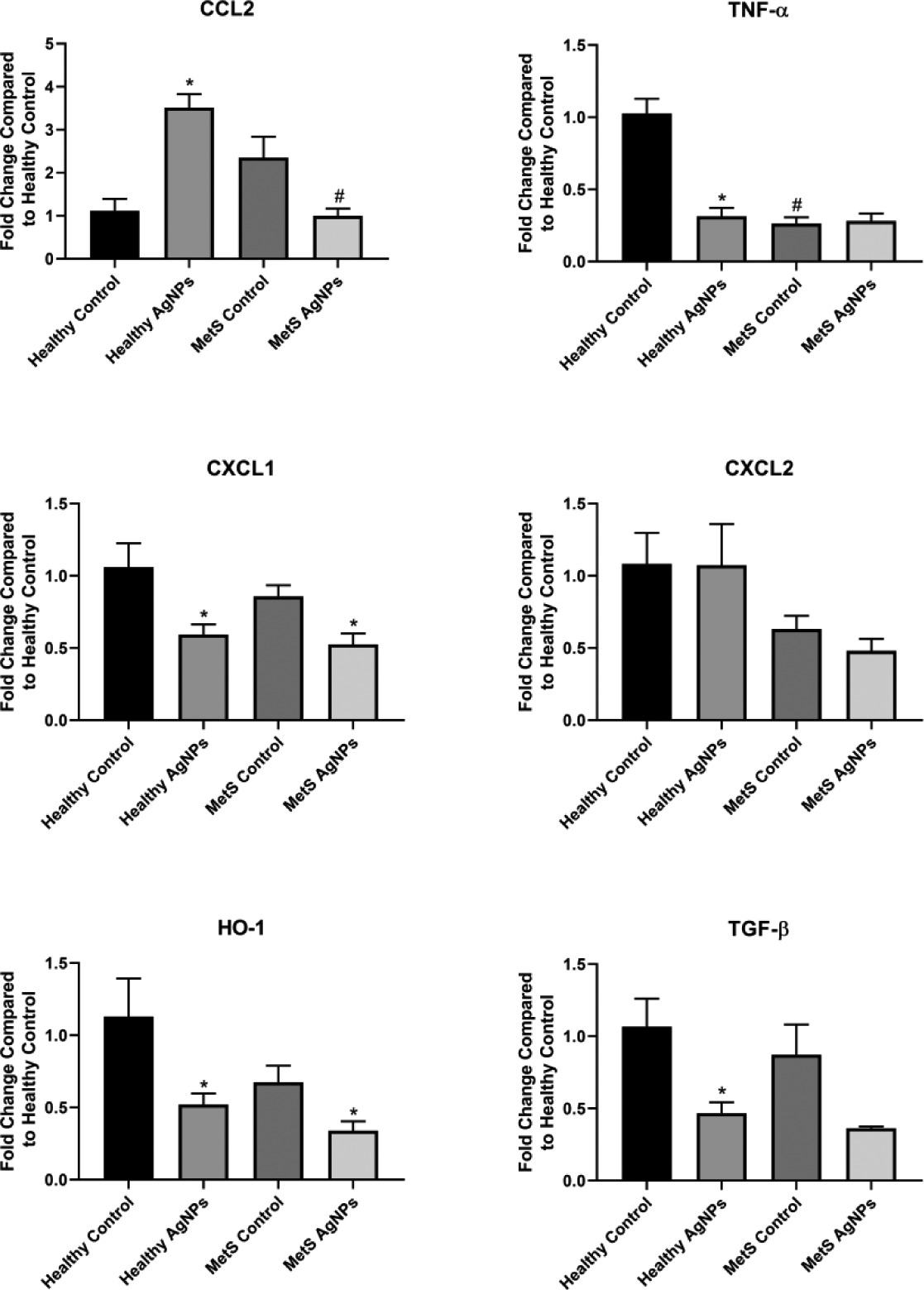
Changes in Gene Expression in the Brain Following Exposure to AgNPs in Healthy or MetS Mice. Gene expression of TNF-α, CCL2, CXCL1, CXCL2, HO-1, and TGF- β and GAPDH (control) was assessed through PCR to evaluate the inflammatory, pro-fibrotic, and oxidative stress responses induced by AgNP exposure. All analyses are reported as mean ± SEM (n=6–8/group). * denotes statistical significance as compared to the corresponding untreated control group, and # denotes statistical significance as compared to the corresponding healthy group. Comparisons were performed by two-way ANOVA with Tukey post hoc analysis; p<0.05).
Discussion
MetS is an increasingly common condition, and individuals with MetS have demonstrated enhanced susceptibility to exposures. Although AgNPs are increasingly incorporated into biomedical applications due to their antibacterial and antifungal properties, more research is needed to fully understand potential toxicological effects. Most studies of NP safety focused on healthy models; however, NP toxicity and biological interactions may be altered by diseases such as MetS. This study utilized healthy and diseased mouse models to examine differential organ-specific inflammatory responses to AgNPs in MetS. Data determined that MetS mice exhibit organ-specific inflammatory responses that are, in some cases, distinct from those of healthy animals, and that these effects are not explained solely by differential biodistribution patterns.
At baseline, prior to the introduction of any AgNPs, MetS mouse livers contained the same or lower levels of inflammatory gene expression than healthy ones. This is seemingly contradictory with many previous studies of the effect of MetS, which showed increased expression of inflammatory markers in the liver, including TNF- α, CCL2, and others (Bieghs and Trautwein 2013; Rector et al. 2008). However, recent investigators reported that chronic inflammatory stimulation, such as that produced by obesity results in a phenomenon known as T cell exhaustion (Wang et al. 2019; Green and Beck 2017; Aguilar and Murphy 2018). T cell exhaustion is characterized by progressive loss of function, decreased proliferation, and overall inactivity (Wherry and Kurachi 2015; Wherry 2011). It is conceivable that our MetS mice, having been fed a high-fat Western diet, generated the necessary conditions to induce hepatic T cell exhaustion. These conditions include dysregulation of immune-active metabolites such as leptin, adiponectin, and glucose, all of which are known to be altered due to increased body weight (Sun and Karin 2012; Aguilar and Murphy 2018; Beyer et al. 2016). Further, there is evidence to support that insulin resistance, which is a component of MetS, may lead to decreased activation of T cells and reductions in inflammatory cytokines (Andersen et al. 2016; Lteif et al. 2005; Roberts et al. 2013). Unlike the liver, T cells within the spleen do not appear to have experienced exhaustion, likely because the spleen is not a major site of ectopic fat deposition (Pou et al. 2009). Baseline expression of the majority of cytokines tested in the spleen was higher in MetS than healthy, possibly due to an elevated number of macrophages and activated T cells producing Th1 and Th2 cytokines. Taken together, our findings demonstrate that MetS in mice, when induced by a high-fat Western diet, results in organ-dependent inflammatory dysregulation.
Following 14 weeks on a healthy or high-fat Western diet, animals were injected with 2 mg/kg of AgNPs. While previous investigators utilized doses at and above 50 mg/kg to elicit an inflammatory response, such a dose is not relevant to real-world exposures (Xue et al. 2012; Tang et al. 2008; 2009; El Mahdy et al. 2014). The dose of 2 mg/kg was selected for (1) therapeutic relevance given AgNP leaching from currently utilized medical textiles and devices, (2) proposed uses in vaccines and cancer treatment, and (3) capacity to induce a toxicological response. Differences in biodistribution were observed between healthy and MetS group that did not appear to be a result of the overall larger dose administered to MetS mice. Specifically, only livers, kidneys, and hearts of MetS mice were observed to exhibit a higher concentration of Ag than healthy ones, while other organs such as the spleen and brain had equivalent Ag levels. Despite receiving a lesser total amount of AgNPs at dosing, lungs from healthy mice contained a higher concentration of Ag compared to MetS mice. Within the liver, a higher % Ag accumulated in MetS mice compared to healthy suggesting differential hepatic deposition. The healthy mice possessed a far larger proportion of total Ag in intestines possibly as a result of elimination in fecal matter, which is the primary route for elimination of NPs which enter the body (Zhang et al. 2016b). Taken together, these data indicate that MetS mice eliminate AgNPs more slowly than healthy animals. While information is lacking regarding the influence of MetS on NP clearance, it is known that liver function is impaired in obesity, and hepatobiliary clearance is the primary method of eliminating NPs from the body (Zhang et al. 2016b; Watanabe et al. 2008; Kim and Younossi 2008). Thus, it is conceivable that MetS-induced reduction in liver function impaired the ability of the liver to remove AgNPs from the bloodstream, resulting in less efficient clearance and a higher proportion of AgNPs retained within the liver.
Although a greater concentration of AgNPs were present in liver of MetS compared to healthy mice, both models demonstrated a similar magnitude of inflammatory gene expression changes. This may be attributed to immune cells in MetS liver being less able to respond to an immune challenge than those of healthy mice, an assertion supported by (Wang et al. 2018) who showed that exhausted T cells maintain their phenotype even after repeated exposure to antigens. The apparent lack of altered expression of Th2 cytokines IL-4 and IL-13 in response to AgNP exposure may be easily understood in MetS mice as those cytokines are produced primarily by T cells, which may be impaired due to disease (Rincón et al. 1997; Rael and Lockey 2011). However, the lack of expression of Th2 cytokines in healthy mice seems counter to previous findings, which found numerical elevation of IL-4 and IL-13 as a result of AgNP exposure (Park et al. 2010; Chang et al. 2013). These seemingly contradictory results may be attributed to kinetics of AgNP elimination; specifically, the half-life of AgNPs in male mice was reported to be approximately 15 hr, indicating that mice in our study were able to go through almost two full half-lives between time of exposure and necropsy (Xue et al. 2012). Further, previous investigations which demonstrated increased expression of Th2 cytokines were conducted in lungs and serum, but not liver. Data indicate that the modest Th2 response which occurred in healthy liver following exposure to AgNPs may have already been resolved by the time of necropsy and organ collection yielding to a more Th1-dominant inflammatory response in the healthy mouse model.
Following exposure to AgNPs expression of all cytokines assessed in the spleen of the healthy mice were increased with the exception of CXCL2. Previous investigators demonstrated that AgNPs, upon entering the spleen, produced tissue damage and a loss of cellularity to the red pulp (Sardari et al. 2012; Genter et al. 2012). Further, intravenously and intranasally administered AgNPs were found to elevate the level of T cells and macrophages within the spleen following exposure (Genter et al. 2012; Jong et al. 2013). Thus, it is conceivable that AgNPs entered the spleen through the circulation and induced damage to the red pulp, resulting in enhanced expression of TNF- α, CXCL1, HO-1, TGF- β, and IL-4. In the MetS group, the reaction of the spleen varied from that of healthy mice. In comparison to the unexposed group, CXCL2 and HO-1 remained unchanged, while IL-4 and IL-13 were reduced. Given the high degree of cytotoxicity initiated by AgNPs to macrophages and T cells observed in in vitro studies, this fall in expression may be primarily attributed to the death of the cells which were previously producing cytokines (Eom and Choi 2010; Pratsinis et al. 2013). In addition, a portion of the immune cells within the spleen may have migrated to other areas of the body following AgNP exposure in an attempt to resolve the insult, further contributing to diminished cytokine expression (Haan and Kraal 2012). Taken together, evidence indicates differential immune responses in the spleen resulting from AgNP exposure are attributed to MetS. The presence of MetS may reduce immune responses from the spleen and impair the ability to manage subsequent immune challenges.
Although the majority of Ag was identified in the spleen and liver of mice 24 hr following exposure, alterations in gene expression were observed in other organs with relatively minor accumulation as in lungs, kidney, heart, and brain. Interestingly, introduction of AgNPs into the healthy kidney altered cytokine expression such that its gene expression resembled that of a MetS kidney. This may be due to AgNPs inducing general inflammatory mechanisms similar to those enhanced due to MetS. Gottipolu et al (2009) noted in other exposures such as specifically diesel exhaust inhalation the resultant transcriptomic profiles in healthy rats resembled models of cardiovascular disease. Further, studies demonstrated that exposures may contribute to development and progression of chronic diseases (Chen et al. 2008; Anto et al. 2001). Several investigators also reported that individuals with pre-existing diseases may be increasingly sensitive to exposures (Chen and Schwartz 2008; Devlin et al. 2014; Wagner et al. 2014). In our current study, MetS resulted in exacerbated induction of inflammatory genes in the heart that were not observed in healthy animals. Data suggest that the cardiovascular system of individuals with MetS may be increasingly susceptible to the adverse responses associated with AgNP exposure. MetS is a risk factor for cardiovascular disease development. This enhanced inflammatory signaling may accelerate the progression of cardiovascular disease.
As expected, the brain demonstrated the least accumulation of Ag 24 hr following injection in both models. However, AgNPs were found to be present within the brain tissue as well as within the brain circulatory system. The presence of AgNPs within brain tissue is based upon a volume of 35–40 μl blood/g brain tissue and the mean measured concentration of Ag within tblood (0.25 μg/g for healthy and 0.39 μg/g for the MetS mice), indicating crossing of the blood-brain barrier (Chugh et al. 2009). Expression of CCL2, a macrophage chemotactic gene, was elevated only in the healthy model. Evidence indicates that MetS may decrease the ability of the brain to respond to challenges and exposures that require an immune response via up-regulation of CCL2 and recruitment of macrophages. Other markers of inflammation and oxidative stress were reduced in the brain in response to AgNP exposure. Gonzalez-Carter et al (2017) demonstrated that AgNPs reduced inflammation in microglia which experienced an immune challenge. While the amount of AgNPs that accumulate in the brain is small, Lee et al (2013) showed that AgNPs remained in the tissue for extended periods of time. This may indicate that exposures that results in AgNP deposition in the brain may initiate greater long-term effects than those observed in other organs.
The reductions in inflammatory gene expression observed in our study may inhibit brain immune responses to subsequent challenges. Interestingly, although less Ag was found to accumulate within the lung in MetS compared to healthy, similar decreases in inflammatory genes were noted following exposure. Inhalation of AgNPs was found to induce an inflammatory response in healthy mouse models, suggesting variations in immune response within organs based upon route of administration. Taken together, data suggest that intravenously delivered AgNPs diminish expression of a number of pro-inflammatory genes within the brain and lung of both healthy and MetS mice. In this study, differential alterations were observed between healthy and MetS mice in the expression of genes involved in inflammation, oxidative stress, and fibrosis, suggesting distinct susceptibility due to underlying disease. Further, it is possible that biodistribution of AgNPs varies due to the presence of MetS.
While this study furthered our understanding of the toxicological impact of MetS on AgNP exposures, there is still much that remains unknown. Our study was not without limitations, as the results are specific to AgNPs within the circulation and may have limited applications to other routes of administration, such as oral or inhalation exposure to AgNPs. In addition, our study examined only a single dose and time point, eliminating the possibility of identifying diseased-induced differences in dose-response or biokinetics of AgNPs. Future studies are needed to assess alternate exposure routes, multiple time points, and a variety of doses following exposure to identify the disease-dependent variations in elimination rates of AgNPs and responses. Further, the effects of AgNPs on immunological cells in each organ require additional evaluation, particularly T cells and macrophages. Research into the toxicological impact of exposures such as AgNPs in diseased individuals is vital, as they represent a susceptible subpopulation.
Acknowledgements
This study was supported by the National Institute of Environmental Health Sciences Grant number ES024392. Dr. Wei Zheng is thanked for the generous use of the atomic absorption spectrophotometer belonging to his lab.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Aguilar EG, and Murphy WJ. 2018. Obesity induced T cell dysfunction and implications for cancer immunotherapy. Current Opinion in Immunology 51: 181–186. 10.1016/j.coi.2018.03.012.Obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar M, Bhuket T, Torres S, Liu B, and Wong RJ. 2015. Prevalence of the metabolic syndrome in the United States, 2003–2012. Journal of the American Medical Association 313: 1973–1974. [DOI] [PubMed] [Google Scholar]
- Alaraby M, B Annangi, Marcos R, and Hernánde A. 2016. Drosophila melanogaster as a suitable in vivo model to determine potential side effects of nanomaterials: A review. Journal of Toxicology and Envirommental Health B 19: 65–104 [DOI] [PubMed] [Google Scholar]
- Andersen CJ, Murphy KE, and Fernandez ML. 2016. Impact of obesity and metabolic syndrome on Iimmunity. Advances in Nutrition 7: 66–75. 10.3945/an.115.010207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anto JM, Vermeire P, Vestbo J, and Sunyer J. 2001. Epidemiology of chronic obstructive pulmonary disease. European Respiratory Journal 17: 982–994. [DOI] [PubMed] [Google Scholar]
- Beltrán-Sánchez H, Harhay MO, Harhay MM, and McElligott S. 2013. Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. Journal of the American College of Cardiology 62: 697–703. 10.1016/j.jacc.2013.05.064.Prevalence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn T, and Cavanagh B. 2010. The release of nanosilver from consumer products used in the home. Journal of Environmental Quality 39: 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin IL, Wilding LA, Morishita M, Walacavage K, Ault AP, Axson JL, Stark DI, et al. 2016. Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. Nanotoxicology 10: 352–360. 10.3109/17435390.2015.1072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, Thabet Y, et al. 2016. Tumor-necrosis factor impairs CD4 + T cell – mediated immunological control in chronic viral infection. Nature Immunology 17: 593–603. 10.1038/ni.3399. [DOI] [PubMed] [Google Scholar]
- Bieghs V, and Trautwein C. 2013. The innate immune response during liver inflammation and metabolic disease. Trends in Immunology 34: 446–452. 10.1016/j.it.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, Li H, et al. 2014. Free radical biology and medicine hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radical Biology and Medicine 67: 396–407. 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- CDC. 2019. “Chronic diseases in america.”
- Chaloupka K, Malam Y, and Seifalian AM. 2010. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends in Biotechnology 28: 580–588. 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Chang H, Hsiao T, Wu C, Chang H, Lee C, Chang C, and Cheng T. 2013. Allergenicity and toxicology of inhaled silver nanoparticles in allergen-provocation mice models. International Journal of Nanomedicine 8: 4495–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Goldberg MS, and Villeneuve PJ. 2008. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Reviews on Environmental Health 23: 243–291. [DOI] [PubMed] [Google Scholar]
- Chen J, and Schwartz J. 2008. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environmental Health Perspectives 116: 612–617. 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhao L, Bai R, Liu Y, Han L, Xu Z, Chen F, Autrup H, Long D, and Chen C. 2016. Silver nanopartilces induced oxidative and endoplasmic reticulum stresses in mouse tissues: Implications for the development of acute toxicity after intravenous administration. Toxicology Research 1: 602–608. 10.1039/c5tx00464k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh BP, Lerch JP, Yu LX, Pienkowski M, Harrison RV, Henkelman RM, and Sled JG. 2009. NeuroImage measurement of cerebral blood volume in mouse brain regions using micro-computed tomography. NeuroImage 47: 1312–1318. 10.1016/j.neuroimage.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Chugh H, Sood D, Chandra I, Tomar V, Dhawan G, and Chandra R. 2018. Role of gold and silver nanoparticles in cancer nano-medicine. Artificial Cells, Nanomedicine, and Biotechnology 46: 1210–1220. 10.1080/21691401.2018.1449118 [DOI] [PubMed] [Google Scholar]
- Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, Nakagawa T, and Johnson RJ. 2006. Uric acid, the metabolic syndrome, and renal disease. Journal of American Society of Nephrology 17: 165–168. 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- Dabass A, Talbott EO, Rager JR, Marsh GM, Venkat A, Holguin F, and Sharma RK. (2018). Systemic inflammatory markers associated with cardiovascular disease and acute and chronic exposure to fine particulate matter air pollution (PM2. 5) among US NHANES adults with metabolic syndrome. Environmental Research, 161: 485–491. [DOI] [PubMed] [Google Scholar]
- Desroches S, and Lamarche B. 2007. The evolving definitions and increasing prevalence of the metabolic syndrome. Applied Physiology, Nutrition, and Metabolism 32: 23–32. 10.1139/H06-095. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, and Graff D. 2014. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicological Sciences 140: 61–72. 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- Dunn K, and Edwards-Jones V. 2004. The role of acticoatTM with nanocrystalline silver in the management of burns. Burns 30: S1–S9. 10.1016/j.burns.2004.06.000. [DOI] [PubMed] [Google Scholar]
- Dziendzikowska K, Gromadzka-ostrowska J, Lankoff A, Oczkowski M, Krawczy A, Chwastowska J, Sadowska-bratek M, Chajduk E, Wojewódzka M, and Du M. 2012. Time-dependent biodistribution and excretion of silver nanoparticles in male wistar rats. Journal of Applied Toxicology 32: 920–928. 10.1002/jat.2758. [DOI] [PubMed] [Google Scholar]
- Echegoyen Y, and Nerín C. 2013. Nanoparticle release from nano-silver antimicrobial food containers. Food and Chemical Toxicology 62: 16–22. 10.1016/j.fct.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Eom H, and Choi J. 2010. P38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in jurkat T cells. Environmental Science & Technology 44: 8337–8342. [DOI] [PubMed] [Google Scholar]
- Esser N, Legrand-poels S, Piette J, Scheen J, and Paquot N. 2014. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Research and Clinical Practice 105: 141–150. 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, and Mokdad AH. 2004. Increasing prevalence of the metabolic sydrome among U.S adults. Diabetes Care 27: 2444–2449. [DOI] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, and He J. 2006. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. American Journal of Medicine 119: 812–819. 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Ge L, Li Q, Wang M, Ouyang J, Li X, and Xing MQM. 2014. Nanosilver particles in medical applications: synthesis, performance, and toxicity. International Journal of Nanomedicine 9: 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genter MB, Newman NC, Howard SG, Ali SF, and Bolton B. 2012. Distribution and systemic effects of intranasally administered 25 nm silver nanoparticles in adult mice. Toxicologic Pathology 40: 1004–1013. 10.1177/0192623312444470. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Carter DA, Leo BF, Ruenraroengsak P, Chen S, Goode AE, Theodorou IG, Chung KF, et al. 2017. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Scientific Reports 7: 1–14. 10.1038/srep42871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiler MC, Ledbetter AD, Krantz T, Linak WP, et al. 2009. One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environmental Health Perspectives 117: 38–46. 10.1289/ehp.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WD, and Beck MA. 2017. Obesity altered T cell metabolism and the response to infection. Current Opinion in Immunology 46: 1–7. 10.1016/j.coi.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM 2006. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. Journal of the American College of Cardiology 47: 1093–1100. 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Haan JMMD, and Kraal G. 2012. Innate immune functions of macrophage subpopulations in the spleen. Journal of Innate Immunity 4: 437–445. 10.1159/000335216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A, Tentschert J, Jungnickel H, Graf P, Mantion A, Draude F, Plandl J, et al. 2011. Toxicity of silver nanoparticles in human macrophages: Uptake, intracellular distribution and cellular responses. Journal of Physics: Conference Series 304: 1–14. 10.1088/1742-6596/304/1/012030. [DOI] [Google Scholar]
- Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, and Kleinsasser N. 2011. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicology Letters 201: 27–33. 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Haffner SM 2006. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. American Journal of Cardiology 97: 2–11. 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Halpin HA, Morales-suárez-varela MM, and Martin-moreno JM. 2010. Chronic disease prevention and the new public health. Public Health Reviews 32: 120–154. [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen M, and Groop L. 2001. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24: 683–689. [DOI] [PubMed] [Google Scholar]
- Javidi J, Haeri A, Nowroozi F, and Dadashzadeh S. 2019. Pharmacokinetics, tissue distribution and excretion of Ag2S quantum dots in mice and rats: The effects of injection dose, particle size and surface charge. Pharmaceutical Research 36: 1–16. [DOI] [PubMed] [Google Scholar]
- Jong WHD, Van Der Ven LTM, Sleijffers A, Park MVDZ, Jansen EHJM, Van Loveren H, and Vandebriel RJ. 2013. Biomaterials systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 34: 8333–8343. 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- Kaweeteerawat C, Ubol PN, Sangmuang S, Aueviriyavit S, and Maniratanachote R. 2017. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. Journal of Toxicology and Envirommental Health A 80: 1276–1289. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Ellacott KLJ, King VL, and Hasty AH. 2010. Mouse models of the metabolic syndrome. Disease Models & Mechanisms 3: 156–166. 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi Swedish Chemicals Agency. 2016. Uptake and Biodistribution of Nanoparticles.
- Kermanizadeh A, Gosens I, MacCalman L, Johnston H, Danielsen PH, Jacobsen NR, Lenz A-G, Frenandes T, Scins R, Cassee FR, wallin H, Kreyling W, Stoeger T, Loft S, Moeller P and Stone V. 2016. A multilaboratory toxicological assessment of a panel of 10 engineered nanomaterials to human health- the highlights, limitations, and current and future challenges. Journal of Toxicology and Envirommental Health B 19: 1–28. [DOI] [PubMed] [Google Scholar]
- Kim CH, and Younossi ZM. 2008. Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome. Cleveland Clinic Journal of Medicine 74: 721–728. [DOI] [PubMed] [Google Scholar]
- Lam PK, Chan ESY, Ho WS, and Liew CT In. 2004. In vitro cytotoxicity testing of a nanocrystalline silver dressing (acticoat) on cultured keratinocytes. British Journal of Biomedical Science 61: 125–127. 10.1080/09674845.2004.11732656. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim YS, Song KS, Ryu HR, Sung JH, Park JD, Park HM, et al. 2013. Biopersistence of silver nanoparticles in tissues from sprague–dawley rats. Particle and Fibre Toxicology 10: 1–14. 10.1186/1743-8977-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CLD, Fashir SB, Castilho ML, Hupman MA, Raniero LJ, Alwayn I, and Hewitt KC. 2016. Epidermal growth factor receptor-specific nanoprobe biodistribution in mouse models. Journal of Pharmaceutical Sciences 105: 25–30. 10.1016/j.xphs.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Lteif AA, Han K, and Mather KJ. 2005. Obesity, insulin resistance, and the metabolic syndrome determinants of endothelial dysfunction in whites and blacks. Circulation 112: 32–38. 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- Mahdy MME, Ahmed TAS, Aly HS, Mohammed FF, and Shaalan MI. 2014. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Experimental and Toxicologic Pathology 67: 21–29. 10.1016/j.etp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Martins Jr A C, Azevedo LF, Rocha CCS, Carneiro MFH, Venancio VP, Almeida MR, Antunes LMG, Hott RC, Rodrigues JL, Ogunjimi AT, Adeyemi JA, Barbosa F. 2017. Evaluation of distribution, redox parameters, and genotoxicity in wistar rats co-exposed to silver and titanium dioxide nanoparticles. Journal of Toxicology and Envirommental Health A 80: 1156–1165. [DOI] [PubMed] [Google Scholar]
- Meng J, Ji Y, Liu J, Cheng X, Guo H, Zhang W, Meng J, et al. 2014. Using gold nanorods core/ silver shell nanostructures as model material to probe biodistribution and toxic effects of silver nanoparticles in mice. Nanotoxicology 8: 686–696. 10.3109/17435390.2013.822593. [DOI] [PubMed] [Google Scholar]
- Paddle-Ledinek JE, Nasa Z, and Cleland HJ. 2006. Effect of different wound dressings on cell viability and proliferation. Plastic and Reconstructive Surgery 117: 110S–118S. 10.1097/01.prs.0000225439.39352.ce. [DOI] [PubMed] [Google Scholar]
- Paoletti R, Bolego C, Poli A, and Cignarella A. 2006. Metabolic syndrome, inflammation and atherosclerosis. Vascular Health and Risk Management 2: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Bae E, Yi J, Kim Y, Choi K, Hee S, Yoon J, Chun B, and Park K. 2010. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environmental Toxicology and Pharmacology 30: 162–168. 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Park K, and Lee Y. 2013. The stability of citrate-capped silver nanoparticles in isotonic glucose solution for intravenous injection. Journal of Toxicology and Environmental Health A 76: 1236–1245. 10.1080/15287394.2013.849215 [DOI] [PubMed] [Google Scholar]
- Park MVDZ, Neigh AM, Vermeulen JP, De LJJ, Verharen HW, Briedé JJ, Van Loveren H, and De Jong WH. 2011. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32: 9810–9817. 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- Pettersson U, Walde TB, Carlsson P, Jansson L, and Phillipson M. 2012. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One 7: 1–10. 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon VKM, and Burd A. 2004. In vitro cytotoxity of silver: implication for clinical wound care. Burns 30: 140–147. 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffman U, Lieb K, Vasan RS, O’Donnell CJ, and Fox CS. 2009. Patterns of abdominal fat distribution. Diabetes Care 32: 481–485. 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratsinis A, Hervella P, Leroux J, and Pratsinis SE. 2013. Toxicity of silver nanoparticles in macrophages. Small 9: 2576–2584. 10.1002/smll.201202120. [DOI] [PubMed] [Google Scholar]
- Rael EL, and Lockey RF. 2011. Interleukin-13 signaling and its role in asthma. World Allergy Organization Journal 4: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Murdock RC, Schlager JJ, Hussain SM, and Ali SF. 2009. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicology Letters 187: 15–21. 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Ramadi KB, Mohamed YA, Al-sbiei A, Almarzooqi S, Bashir G, Al-Dhanhani A, Sarawathiamma D, et al. 2016. Acute systemic exposure to silver-based nanoparticles induces hepatotoxicity and NLRP3-dependent inflammation. Nanotoxicology 10: 1061–1074. 10.3109/17435390.2016.1163743. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Wei Y, and Ibdah JA. 2008. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World Journal of Gastroenterology 14: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón BM, Anguita J, Nakamura T, Fikrig E, and Flavell RA. 1997. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+ T cells. Journal of Experimental Medicine 185: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CK, Hevener AL, and Barnard RJ. 2013. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Comprehensive Physiology 3: 1–58. 10.1002/cphy.c110062.Metabolic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario F Hoet P, Nogueira AJA, Santos C, and Oliveira H. 2018. Differential pulmonary in vitro toxicity of two small-sized polyvinylpyrrolidone-coated silver nanoparticles. Journal of Toxicology and Envirommental Health A 81: 675–690. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guzman D, Le P, Villeret B, Sola N, Le R, Guyard A, Kemmel A, Crestani B, Sallenave J, and Garcia-verdugo I. 2019. Biomaterials silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials 217: 1–14. 10.1016/j.biomaterials.2019.119308 [DOI] [PubMed] [Google Scholar]
- Sardari RRR, Zarchi SR, Talebi A, Nasri S, Imani S, Khoradmehr A, and Sheshde SAR. 2012. Toxicological effects of silver nanoparticles in rats. African Journal of Microbiology Research 6: 5587–5593. 10.5897/AJMR11.1070. [DOI] [Google Scholar]
- Snyder RW, Fennell TR, Wingard CJ, Mortensen NP, Holland NA, Shannahan JH, Pathmasiri W, Lewin AH, and Sumner SCJ. 2016. Distribution and biomarker of carbon-14 labeled fullerene C60 ([14C(U)]C60) in pregnant and lactating rats and their offspring after maternal intravenous exposure. Journal of Applied Toxicology 35: 1438–1451. 10.1002/jat.3177.Distribution [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, and Kim BS. 2009. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess and Biosystems Engineering 32: 79–84. 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- Sumner SCJ, Snyder RW, Wingard C, Mortensen NP, Holland NA, Shannahan JH, Dhungana S, Pathmasiri W, Lewin AH, and Fennell TR. 2016. Distribution and biomarkers of carbon-14 labeled fullerene C60 ([14C(U)]C60) in female rats and mice for up to 30 days after intravenous exposure. Journal of Applied Toxicology 35: 1452–1464. 10.1002/jat.3110.Distribution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, and Karin M. 2012. Obesity, inflammation, and liver cancer. Journal of Hepatology 56: 704–713. 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha E, Djouider F, and Banoqitah E. (2019). Monte Carlo simulation of dose enhancement due to silver nanoparticles implantation in brain tumor brachytherapy using a digital phantom. Radiation Physics and Chemistry 156: 15–21. [Google Scholar]
- Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, and Heyder J. 2001. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environmental Health Perspectives 109: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, Wan Z, and Xi T. 2008. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Applied Surface Science 255: 502–504. 10.1016/j.apsusc.2008.06.058. [DOI] [Google Scholar]
- Tang J, Xiong Ling, Wang Shuo, Wang Jianyu, Liu Li, Li Jiage, Yuan Fuqiang, and Xi Tingfei. 2009. Distribution, translocation and accumulation of silver nanoparticles in rats. Journal of Nanoscience and Nanotechnology 9: 4924–4932. 10.1166/jnn.2009.1269. [DOI] [PubMed] [Google Scholar]
- Tran QH, Nguyen VQ, and Le A. 2013. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences: Nanoscience and Nanotechnology 4: 1–20. [Google Scholar]
- Trickler W, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, et al. 2010. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicological Sciences 118: 160–170. 10.1093/toxsci/kfq244. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Allen K, Yang H, Nan B, Morishita M, and Mukherjee B. 2014. Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: Effects of diet-induced metabolic syndrome. Environmental Health Perspectives 122: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Pan W, Liu Y, Luo J, Zhu D, Lu Y, Feng X, Gigley JP, and Franco MA. 2018. Hepatitis B virus-specific CD8+ T cells maintain functional exhaustion after antigen reexposure in an acute activation immune environment. Frontiers in Immunology 9: 1–14. 10.3389/fimmu.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, et al. 2019. Function during tumor progression and PD-1 checkpoint blockade. Nature Medicine 25: 141–151. 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Yaginuma R, Ikejima Ki, and Miyazaki A. 2008. Liver diseases and metabolic syndrome. Journal of Gastroenterology 43: 509–518. 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. 2011. T cell exhaustion. Nature Immunology 12: 6–13. 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, and Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology 15: 486–499. 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Zhang S, Huang Y, and Zhang T. 2012. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. Journal of Applied Toxicology 32: 890–899. 10.1002/jat.2742. [DOI] [PubMed] [Google Scholar]
- Yang L, Kuang H, Zhang W, Aguilar ZP, Wei H, and Xu H. 2017. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Scientific Reports 7: 1–12. 10.1038/s41598-017-03015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zande MVD, Vandebriel RJ, Doren EV, Kramer E, Rivera ZH, Serrano-rojero CS, Gremmer ER, et al. 2012. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6: 7427–7442. 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu Z, Shen W, and Gurunathan S. 2016a. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. International Journal of Molecular Sciences 17: 1534 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Poon W, Tavares AJ, Mcgilvray ID, and Chan WCW. 2016b. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. Journal of Controlled Release 240: 332–348. 10.1016/j.jconrel.2016.01.02 [DOI] [PubMed] [Google Scholar]


