Abstract
Ecological studies need experimentation to test concepts and to disentangle causality in community dynamics. While simple models have given substantial insights into population and community dynamics, recent ecological concepts become increasingly complex. The globally important pelagic food web dynamics are well suited to test complex ecological concepts. For instance, trophic switches of individual organisms within pelagic food webs can elongate food webs or shift the balance between autotroph and heterotroph carbon fluxes. Here, we summarize results from mesocosm experiments demonstrating how environmental drivers result in trophic switches of marine phytoplankton and zooplankton communities. Such mesocosm experiments are useful to develop and test complex ecological concepts going beyond trophic level–based analyses, including diversity, individual behavior, and environmental stochasticity.
Highlights
-
•
Ecological experiments need to integrate complexity.
-
•
Planktonic systems offer complexity but still allow experimental manipulation and replication.
-
•
Planktonic systems are key components of global biogeochemical cycles.
-
•
Trophic switches of plankton organisms can have far-reaching consequences on such cycles.
-
•
Mesocosm experiments allow identifications of mechanisms underlying trophic switches.
Introduction
Ecological systems biology studies interactions within and between species to understand the dynamics of communities and their properties such as diversity, productivity, and stability. For this purpose, it is necessary to integrate theory and experiments in systems allowing directed and systematic experimental manipulations to rigorously test ecological principles. Most of the advances in this field have been achieved using microbial systems including bacterial or yeast populations [1]. To date, most studies have focused on ecological systems including just a few populations cultured in so-called microcosms, small-volume laboratory cultivation systems 2, 3. However, it is also important to confront ecological principles with experimental systems closer to the complexity of natural systems in terms of diversity and trophic interactions. Such a necessary increase in complexity usually calls for an increase in experimental scale allowing for larger and more diverse assemblages. Complex ecological systems usually include multiple trophic levels consisting of prokaryote and eukaryote unicellular and multicellular species. Pelagic systems of open-water columns are examples of complex systems that are well suited for experimental analyses as their structural habitat complexity (compared to terrestrial systems) is low and can be easily established in field and laboratory experimental systems. Moreover, pelagic systems are based on microbial primary producers, showing fast numerical responses (short generation time of days to weeks) to manipulations, and pelagic herbivore and even carnivore trophic levels (zooplankton) have short life histories compared to most terrestrial herbivores/carnivores. Therefore, it is possible to experimentally investigate replicated and complex ecological systems with multiple trophic levels, all potentially showing numerical or measurable somatic growth responses to manipulations within short time (days to weeks). Such short experimental durations are ecologically relevant as natural pelagic system dynamics often show comparable response times to changing environmental conditions [4]. However, natural pelagic communities are difficult to follow in space and time, and experimental manipulations are usually immediately diluted and hard to replicate, even if done in naturally semi–self-contained systems such as gyres [5]. One solution to overcome this problem is a so-called mesocosm system. Mesocosms, also called enclosures or limnocorrals, enclose a water column (with or without a sediment compartment), reduce complexity of the system in terms of physical and chemical connectivity, and allow reducing trophic interactions by excluding higher trophic levels such as fish and other larger predators [6]. Therefore, it is possible to investigate pelagic communities within a defined spatial, chemical, and physical environment. Directed experimental manipulations can be performed, and the responses of plankton systems to such experimental manipulations allow causal conclusions, including the replication needed for statistical tests.
On a global scale, open-water pelagic ecosystems represent the most prominent food webs in terms of their abundance, their extremely long evolutionary history, and their importance for global biogeochemical cycles 7, 8. Most anthropogenic stressors such as uncontrolled nutrient enrichment (eutrophication), warming, or elevated CO2 concentrations act directly via plankton on global system responses to these stressors [9]. A detailed and conceptual understanding of pelagic systems, based on mechanistic insights, is therefore mandatory to achieve the desired goal of maintaining a safe operating space for humanity 10, 11.
Pelagic systems are highly size structured; with few exceptions, the larger eats the smaller [12]. The size of pelagic primary producers will therefore strongly influence food chain length and dynamics by size-structured trophic relationships [12]. Each prolongation step within lower food web compartments results in a substantial reduction of production at higher trophic levels, often occupied by economically relevant species. The relative contribution of autotroph and heterotroph processes will determine whether pelagic systems are net heterotroph or autotroph, a point of critical importance for estimations of global carbon cycles [13]. In addition, food web length determines the importance of bottom-up versus top-down control for individual trophic levels [14].
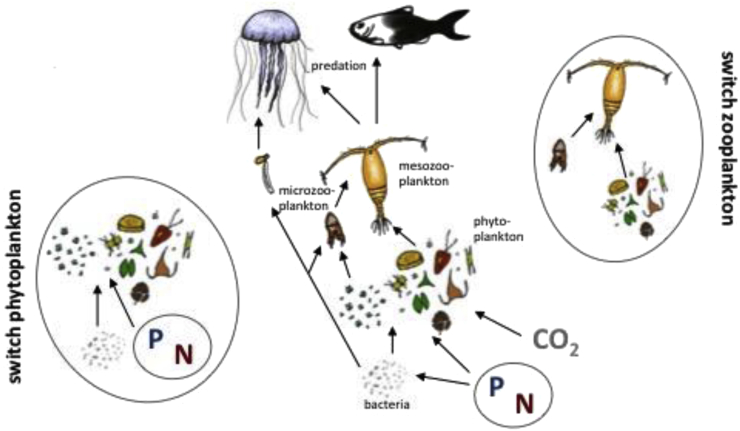
Mechanisms that could change food web length or the relative proportion of autotrophic and heterotrophic food web compartments could therefore be seen as critical ecological system processes with far-reaching consequences (Figure 1). One of the mechanisms that can result in both changes in the length of food chains and changes in the contribution of autotrophic and heterotrophic pathways within food webs are trophic switches of consumers. We define in this article trophic switches not as ontogenetic diet shifts but as environmentally driven shifts in resource uptake of consumers that may have direct and substantial effects on system processes.
Figure 1.
General simplified scheme of a marine plankton food web indicating important trophic switches.
Phytoplankton trophic switches
Phytoplankton is a taxonomically highly diverse group of suspended photosynthetic organisms that are mostly unicellular and responsible for about 50% of global primary production. In contrast to most terrestrial plants, many phytoplankton taxa are mixotrophic. Mixotrophy in the phytoplankton — defined as the combination of photosynthesis and phagotrophic uptake of prey — is linked to the evolution of photosynthesis in protists. The first protists were heterotrophs. The engulfment of oxygenic photosynthetic cyanobacteria without digesting their prey resulted in development of the chloroplasts [15]. Today, this process can still be observed in a number of ciliated protists and dinoflagellates ‘kleptoplastidy’ [16]. At the phylogenetic level, mixotrophy seems to be the most common nutritional mode for pigmented protists, whereas pure autotrophy is restricted to diatoms and coccoid green algae within eukaryote algae and prokaryote cyanobacteria [17]. However, considering abundances in the ecosystems, diatoms and cyanobacteria are major and regionally even dominant contributors to marine primary production [18].
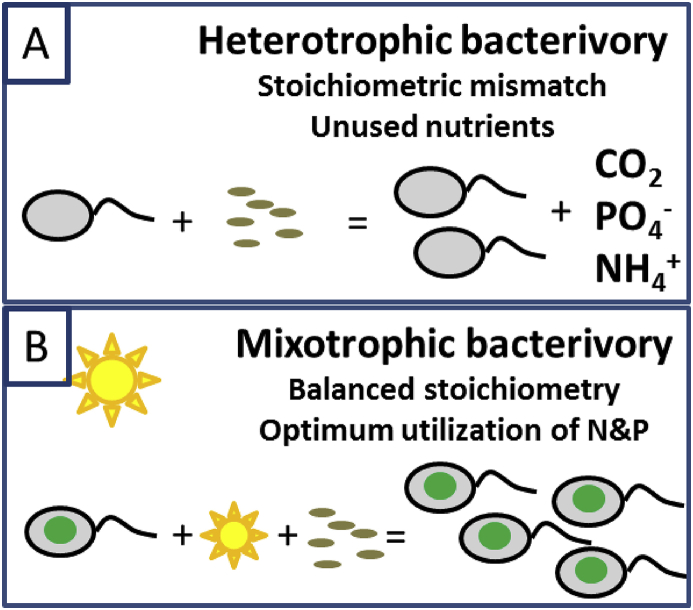
Recent studies revealed that bacterivorous phytoflagellates are key consumers of bacteria, especially in the near-surface waters of oligotrophic seas [19]. Their quantitative importance challenges the classical concept of the microbial loop 20, ∗∗21. Owing to the low carbon (C) content of bacteria, heterotrophic bacterivores face a stoichiometric mismatch with their prey, shunting >50% of the nitrogen (N) and phosphorus (P) ingested with prey to the phytoplankton 20, 22, Figure 2A. Mixotrophic bacterivores, on the other hand, may supplement the missing carbon by photosynthesis, allowing them to utilize all the nutrients ingested with their prey [23] Figure 2B. Their dual mode of nutrition effectively allows mixotrophs to reduce their prey to very low levels ∗24, 25, 26 Figure 2B. The switch from heterotrophic to mixotrophic bacterivory thus comes along with alterations in the abundances of bacteria and picophytoplankton and will influence carbon cycles, bacterial population dynamics, microbial recycling loops, and the net heterotrophy of pelagic systems 27, 28.
Figure 2.
Differences between bacterial consumers for carbon and nutrient fluxes. (a) Heterotrophs. (b) Mixotrophs.
The importance of light as a key factor triggering mixotrophic bacterivory was evidenced in a recent mesocosm study. The importance of mixotrophic bacterivores increased monotonously with increasing light, whereas bacterial densities showed the opposite pattern [24]. Mixotrophy thus represents a light-dependent shortcut in the microbial food web — the availability of light decides whether bacterial production is directly channeled into primary production or not [24].
The consequences of these alterations in the microbial food web for the carbon metabolism and productivity at higher trophic levels are far from clear. Some authors have argued that mixotrophy may enhance trophic transfer 29, 30. It was recently shown that the dual mode of nutrition constrains the elemental stoichiometry of mixotrophs relative to photoautotrophs — cellular C:N and C:P ratios are less variable in mixotrophs, with consequences to the community elemental composition 31, 32. However, for fully understanding the role of mixotrophy for the C-cycling and productivity, a better understanding of the C-metabolism of mixotrophs is required [21]. In addition, the occurrence of harmful metabolites in algae seems to have a functional link to mixotrophy — often species that result in harmful algal blooms (HABs) are mixotrophic flagellates. Especially under high-nutrient conditions, mixotrophs may have detrimental effects on system productivity.
Zooplankton trophic switches
-
1)
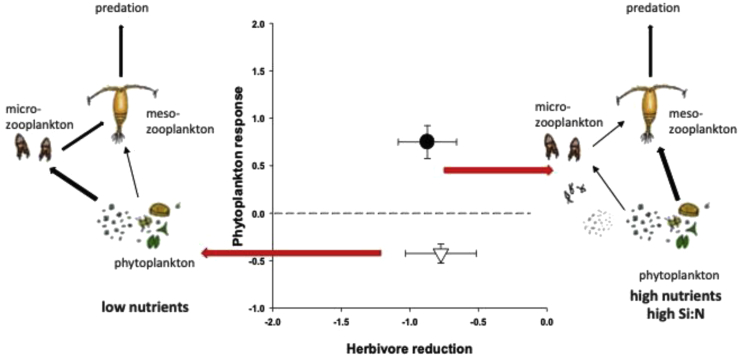
Nutrient-dependent trophic switches: Such switches can be expected when size-selective grazers are confronted with nutrient-dependent shifts in food size. One example for such a system that is well investigated in mesocosm studies and in food web model approaches is marine pelagic communities containing both ciliates and copepods as major phytoplankton grazers. Both groups can substantially contribute to the zooplankton in varying proportions from polar to tropical areas. Copepods prefer larger phytoplankton size classes when having food choices; ciliates usually prefer smaller phytoplankton size classes and/or bacteria. Copepods in addition also feed on ciliates, resulting in intraguild predation food web modules. At small phytoplankton size that is observed during low nutrient availability, copepods prefer to prey on ciliates that feed efficiently on small phytoplankton; copepods are therefore near the third trophic level in the food chain, typically in the North Atlantic 32, 33. With increasing nutrients, phytoplankton increases in size, and copepods may feed directly on phytoplankton. Copepods then have a position near the second trophic level, typically in Eastern upwelling zones, such as off Peru [34]. Such a shortening of food chains by increasing nutrient availability was visible in the outcome of mesocosm experiments studying trophic cascades [19]. It was possible to shorten natural food chains by approximately one step by adding nutrients, keeping all other environmental parameters similar (Figure 3). Such a trophic shift could also result from imbalanced nutrient supply, namely a reduction in silicate (Si) availability in relation to N and P. Manipulating dissolved Si:N ratios in mesocosms resulted in phytoplankton community shifts; high ratios promote large-sized diatoms [35]. These effects were transferred into higher trophic levels as large diatoms are mainly grazed by copepods. Algae grown under low Si:N ratios were mostly grazed by ciliates [35]. However, at least under extremely nutrient-limited conditions such as in the Eastern Mediterranean, it is possible that short nutrient pulses (typically caused by e.g. Saharan dust depositions) may not be visible in a phytoplankton increase. After rapid uptake of the nutrient pulse, stoichiometrically augmented protists are immediately consumed by herbivores, before any significant growth takes place (‘tunneling effect’). In a recent mesocosm experiment, such nutrient pulses resulted in a very effective and rapid transfer (2–4 days) from inorganic nutrients to copepod reproduction concomitant with a decrease in phytoplankton biomass [36].
-
2)
Temperature-induced trophic switches: Similar size changes of phytoplankton as described previously can be induced by temperature. Phytoplankton are bigger in colder parts of the world oceans with picoplankton contributing close to 100% to total biomass in the warmest parts and <1% in the coldest parts of the North Atlantic Ocean [37]. However, the global negative correlation between sea surface temperature and nutrient availability (except for areas of coastal eutrophication) made it difficult to ascribe causality either to temperature or to nutrients, although Maraňon et al. [38] and Maraňon [39] showed by a very comprehensive analysis that a resource index comprising nutrients and light explained most of the global size structure. However, the controversy is still open [40].
Figure 3.
Trophic switches by copepods resulting in differences in food web length. Shown are phytoplankton growth responses after a reduction of herbivores (copepods) by predators. Differences were due to a trophic switch of copepods from predominately ciliates to predominately large phytoplankton as indicated by the opposite direction of trophic cascades. Reduction of copepods by top predators resulted in an increase in phytoplankton in nutrient-enriched mesocosm systems with large phytoplankton (filled circle) but a reduction in phytoplankton in mesocosm systems with small phytoplankton (open triangle). Error bars are 95% confidence intervals. Figure adapted from Ref. [27].
In a series of indoor mesocosms experiments studying the impact of warming on the phytoplankton spring bloom in the Baltic Sea, conspicuous warming effects on phytoplankton cell size were found, in spite of the fact that all mesocosms had the same nutrient inventory [summarized in [41]]. A subsequent experiment crossing the factors of warming with copepod density supported the hypothesis that enhanced grazing and, therefore, removal of larger algae by overwintering copepods could at least in parts explain the size decrease under warming [41]. In a subsequent series of experiments, it was shown that not only under copepod but also under protistan, grazing phytoplankton cell size decreased with warming, although to a lesser extent [42], indicating a temperature effect beyond size-selective grazing. Combining warming with a different extent and nature of nutrient limitation showed a stronger negative temperature effect under more stringent nutrient limitation, being strongest under N-limitation, followed by P-limitation, and then by a balanced supply of N and P (N:P = 16) 43, 44. A review of all mesocosm experiments studying the size response of phytoplankton to warming indicated that within species, size trends had the same direction as community-level ones [45]. Species responses to increasing temperatures were weaker than community-level trends but often stronger than the phenotypic 2.5% shrinkage per °C reported from clonal cultures [46]. Mesocosm experiments and a global circulation model showed such clear effects of warming on phytoplankton size structure, resulting in a trophic switch in the diet of copepods from algae to ciliates, similar to that observed by nutrient manipulations described previously. An increase in temperature effectively resulted in an elongation of pelagic food chains [47].
Besides resulting in trophic switches described previously, size shifts in phytoplankton may also stimulate alternative trophic pathways in zooplankton. Appendicularians, an often abundant member of gelatinous mesozooplankton, are well known to be able to feed on much smaller algae than copepods by their specialized feeding apparatus, largely overlapping with the usual food size range of ciliates/microzooplankton. Environmental shifts resulting in smaller phytoplankton size might therefore also promote appendicularian population development. Recent mesocosm experiments indeed brought evidence for such a temperature-related shift in trophic pathways; increasing temperature resulted in increased appendicularian zooplankton [48]. This shift in trophic pathways toward gelatinous zooplankton has large consequences for carbon cycling and food web functioning.
Conclusion & outlook
Aquatic food web studies often investigate effects on selected trophic levels; much less focus is given to interactions and the dynamic structure of these interactions among trophic levels [49]. On a global scale, estimations of carbon fluxes are still sensitive to large uncertainties in trophic structure [50]. Hence, aforementioned examples may illustrate the importance to identify key processes such as trophic switches and their drivers with large effects on trophic structures and thus ecological system dynamics. Small environmental changes resulted in modifications of the trophic structure and thus substantial carbon flux shifts. Most of the described trophic switches of zooplankton are based on prey body size as a key driver, whereas recent studies 51, 52 point toward important links between body size, temperature, and stoichiometry. Future mesocosm experiments and analyses may be helpful to reveal to which degree the trait body size itself or size-related traits are ultimate reasons behind the observed relationships.
Trophic switches are affected by specific traits of individual organisms. Such switches therefore represent one experimentally investigable mechanism of how specific traits of individual organisms — shaped by their specific life histories — can affect global biogeochemical cycles. Trophic switches are mechanistic and highly dynamic links between behavior and carbon fluxes. They allow a system to respond to an environmental change almost instantaneously with far-reaching consequences for nutrient cycling and trophic transfer but without invoking changes at the level of species composition. Hence, a more detailed understanding of trophic switches may help to include individual behavior and related stochasticity into models of ecosystem dynamics [8].
Mesocosm research has resulted in important mechanistic insights into pelagic systems by directed experimental manipulations of complex natural communities. Hence, community dynamics observed in such experiments depend not only on the enclosed system and the experimental manipulation but also on the initial state of the communities at the start of experiments. Future research concepts based on mesocosm experimentation can benefit from replicating experimental manipulations at different sites, with different systems and different initial stages to separate general effects from system- and site-specific responses.
Ongoing anthropogenic activities affecting global temperatures and nutrient availability may result in seriously changing pelagic species performance and trophic dynamics with hitherto unknown effect sizes of global ecosystem processes. Identifying key processes of trophic dynamics and gaining a mechanistic understanding of their drivers by concept-based experimentation is a promising aspect of how mesocosm experiments can further contribute to a causal understanding of the role of pelagic system dynamics for earth system processes.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors acknowledge funding by AQUACOSM EU Horizon 2020 INFRAIA-project No 731065.
This review comes from a themed issue on Systems ecology and evolution
Edited by Eörs Szathmáry and Ferenc Jordán
References
- 1.Jessup C.M., Kassen R., Forde S.E., Kerr B., Buckling A., Rainey P.B., Bohannan B.J.M. Big questions, small worlds: microbial model systems in ecology. Trends Ecol Evol. 2004;19:189–197. doi: 10.1016/j.tree.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Altermatt F., Fronhofer E.A., Garnier A., Giometto A., Hammes F., Klecka J., Legrand D., Mächler E., Massie T.M., Pennekamp F. Big answers from small worlds: a user's guide for protest microcosms as a model system in ecology and evolution. Methods Ecol Evol. 2015;6:218–231. [Google Scholar]
- 3.Frederickson J.K. Ecological communities by design. Science. 2015;348:1425–1427. doi: 10.1126/science.aab0946. [DOI] [PubMed] [Google Scholar]
- 4.Sommer U., Adrian R., De Senerpont Domis L., Elser J.R., Gaedke U., Ibelings B., Jeppesen E., Lürling M., Molinero J.C., Mooij W.M., Winder M., van Donk E., Winder M. Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst. 2012;43:429–448. [Google Scholar]
- 5.Thingstad T.F., Krom M.D., Mantoura R.F.C., Flaten G.A.F., Groom S., Herut B., Kress N., Law C., Pasternak A., Pitta P., Psarra S., Rassoulzadegan F., Tanaka T., Tselipides A., Wassmann P., Woodward E.M.S., Wexels Riser C., Zodiatis G., Zohary T. Nature of P limitation in the ultraoligotrophic eastern mediterranean. Science. 2005;309:1068–1071. doi: 10.1126/science.1112632. [DOI] [PubMed] [Google Scholar]
- 6.Riebesell U., Lee K., Nejstgaard J.C. Pelagic mesocosms. In: Riebesell U., Fabry V., Hansson L., Gattuso J.-P., editors. Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union; Luxembourg: 2010. [Google Scholar]
- 7.Litchman E., Tezanos Pinto P., Edwards K.F., Klausmeier C.A., Kremer C.T., Thomas M.K. Global biogeochemical impacts of phytoplankton: a trait-based perspective. J Ecol. 2015;103:1384–1396. [Google Scholar]
- 8.Menden-Deurer S., Kiorboe T. Small bugs with a big impact: linking plankton ecology with ecosystem processes. J Plankton Res. 2016;38:1036–1043. [Google Scholar]
- 9.Gruber N. Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Phil Trans Math Phys Eng Sci. 2011;369:1980–1996. doi: 10.1098/rsta.2011.0003. [DOI] [PubMed] [Google Scholar]
- 10.Rockström J., Steffen W., Noone K., Persson Å., Chapin F.S., III, Lambin E.F. A safe operating space for humanity. Nature. 2009;461:472. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 11.Steffen W., Richardson K., Rockström J., Cornell S.E., Fetzer I., Bennett E.M. Planetary boundaries: guiding human development on a changing planet. Science. 2015;347:1259855. doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 12.Sommer U., Charalampous E., Scotti M., Moustaka-Gouni M. Big fish eat small fish: implications for food chain length? Community Ecol. 2018;19:107–115. [Google Scholar]
- 13.Duarte C.M., Regaudie-de-Gioux A., Arrieta J.M., Delgado-Huertas A., Agusti S. The oligotrophic ocean is heterotrophic. Annu Rev Mar Sci. 2013;5:551–569. doi: 10.1146/annurev-marine-121211-172337. [DOI] [PubMed] [Google Scholar]
- 14.Wollrab S., Diehl S., Roos A.M. Simple rules describe bottom-up and top-down control in food webs with alternative energy pathways. Ecol Lett. 2012;15:935–946. doi: 10.1111/j.1461-0248.2012.01823.x. [DOI] [PubMed] [Google Scholar]
- 15.Rockwell N.C., Lagarias J.C., Bhattacharya D. Primary endosymbiosis and the evolution of light and oxygen sensing in photosynthetic eukaryotes. Front Ecol Evol. 2014;2:66. doi: 10.3389/fevo.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoecker D.K., Johnson M.D., de Vargas C., Not F. Acquired phototrophy in aquatic protists. Aquat Microb Ecol. 2009;57:279–310. [Google Scholar]
- 17.Flynn K.J., Stoecker D.K., Mitra A., Raven J.A., Glibert P.M., Hansen P.J. Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J Plankton Res. 2012;35:3–11. [Google Scholar]
- 18.Falkowski P.G., Barber R.T., Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann M., Grob C., Tarran G.A., Martin A.P., Burkill P.H., Scanlan D.J., Zubkov M.V. Mixotrophic basis of Atlantic oligotrophic ecosystems. Proc Natl Acad Sci Unit States Am. 2012;109:5756–5760. doi: 10.1073/pnas.1118179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azam F., Fenchel T., Field J.G., Gray J.S., Meyerreil L.A., Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 1983;10:257–263. [Google Scholar]
- Mitra A., Flynn K.J., Burkholder J.M., Berge T., Calbet A., Raven J.A. The role of mixotrophic protists in the biological carbon pump. Biogeosciences. 2014;11:995–1005. [Google Scholar]; The authors describe the importance of mixotroph nutrition pathways for pelagic carbon cycles. Inclusion of mixotrophs results in shortened and more efficient food chains by nutrient regeneration to primary producers
- 22.Fenchel T. Ecology of heterotrophic microflagellates. 11. Bioenergetics and growth. Mar Ecol Prog Ser. 1982;8:225–323. [Google Scholar]
- 23.Rothhaupt K.O. Nutrient turnover by freshwater bacterivorous flagellates: differences between a heterotrophic and a mixotrophic chrysophyte. Aquat Microb Ecol. 1997;12:65–70. [Google Scholar]
- Ptacnik R., Gomes A., Royer S.-J., Berger S.A., Calbet A., Nejstgaard J.C., Gasol J.M., Isari S., Moorthi S.D., Ptacnikova R., Striebel M., Sazhin A.F., Tsagaraki T.M., Zervoudaki S., Altoja K., Dimitriou P.D., Laas P., Gazihan A., Martínez R.A., Schabhüttl S., Santi I., Sousoni D., Pitta P. A light-induced shortcut in the planktonic microbial loop. Sci Rep. 2016;6:29286. doi: 10.1038/srep29286. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrated a trophc switch towards mixotrophy at high light conditions in mesocosm experiments in an ultra oligotrophic marine system. Light availability had therefore large consequences for microbial predator–prey cycles, bacterial dynamics and nutrient cycles
- 25.Fischer R., Giebel H.A., Hillebrand H., Ptacnik R. Importance of mixotrophic bacterivory can be predicted by light and loss rates. Oikos. 2016;126:713–722. [Google Scholar]
- 26.Ptacnik R., Sommer U., Hansen T., Martens V. Effects of microzooplankton and mixotrophy in an experimental planktonic food web. Limnol Oceanogr. 2004;49(4part2):1435–1445. [Google Scholar]
- 27.Stibor H., Vadstein O., Diehl S., Gelzleichter A., Hansen T., Katechakis A., Lippert B., Løseth K., Peters C., Roederer W., Sandow M., Sundt-Hansen L., Olsen Y. Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecol Lett. 2004;7:321–328. [Google Scholar]
- 28.Wilken S., Soares M., Urrutia-Cordero P., Ratcovich J., Ekvall M.K., Van Donk E., Hansson L.A. Primary producers or consumers? Increasing phytoplankton bacterivory along a gradient of lake warming and browning. Limnol Oceanogr. 2018;63:142–155. [Google Scholar]
- 29.Hammer A.C., Pitchford J.W. The role of mixotrophy in plankton bloom dynamics, and the consequences for productivity. ICES J Mar Sci. 2005;62:833–840. [Google Scholar]
- 30.Katechakis A., Haseneder T., Kling R., Stibor H. Mixotrophic vs. photoautotrophic specialist algae as food for zooplankton: the light:nutrient hypothesis might not hold for mixotrophs. Limnol Oceanogr. 2005;50:1290–1299. [Google Scholar]
- 31.Moorthi S.D., Ptacnik R., Sanders R.W., Fischer R., Busch M., Hillebrand H. The functional role of planktonic mixotrophs in altering seston stoichiometry. Aquat Microb Ecol. 2017;79:235–245. [Google Scholar]
- 32.Nejstgaard J.C., Gismervik I., Solberg P.T. Feeding and reproduction by Calanus finmarchicus, and microzooplankton grazing during mesocosm blooms of diatoms and the coccolithophore Emiliania huxleyi. Mar Ecol Prog Ser. 1997;147:197–217. [Google Scholar]
- 33.Nejstgaard J.C., Hygum B.H., Naustvoll L.-J., Båmstedt U. Zooplankton growth, diet and reproductive success compared in simultaneous diatom- and flagellate-microzooplankton-dominated plankton blooms. Mar Ecol Prog Ser. 2001;221:77–91. [Google Scholar]
- 34.Benavente-Valdés J.R., Méndez-Zavala A., Morales-Oyervides L., Chisti Y., Montañez J. Effects of shear rate, photoautotrophy and photoheterotrophy on production of biomass and pigments by Chlorella vulgaris. J Chem Technol Biotechnol. 2017;92:2453–2459. [Google Scholar]
- 35.Sommer U., Hansen T., Blum O., Holzner N., Vadstein O., Stibor H. Copepod and microzooplankton grazing in mesocosms fertilised with different Si:N ratios: no overlap between food spectra and Si:N influence on zooplankton trophic level. Oecologia. 2005;142:274–283. doi: 10.1007/s00442-004-1708-y. [DOI] [PubMed] [Google Scholar]
- Pitta P., Nejstgaard J.C., Tsagaraki T.M., Zervoudaki S., Egge J.K., Frangoulis C., Lagaria A., Magiopoulos I., Psarra S., Sandaa R.-A., Skjoldal E.F., Tanaka T., Thyrhaug R., Thingstad T.F. Confirming the “Rapid phosphorus transfer from microorganisms to mesozooplankton in the Eastern Mediterranean Sea” scenario through a mesocosm experiment. J Plankton Res. 2016;38:502–521. [Google Scholar]; The authors investigated trophic switches in terms of instantaneous changes in stoichiometry in primary producers rather than biomass increases. This triggers consumers to increased feeding on the nutritious phytoplankton resulting in higher reproductive capacity
- 37.Morán X.A.G., López-Urrutia A., Calvo-Díaz A., Li W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Global Change Biol. 2016;16:1137–1144. [Google Scholar]
- 38.Marañón E., Cermeño P., Latasa M., Tadonléké R.M. Temperature, resources, and phytoplankton size structure in the ocean. Limnol Oceanogr. 2012;57:1266–1278. [Google Scholar]
- 39.Marañón E. Cell size as a key determinant of phytoplankton metabolism and community structure. Annu Rev Mar Sci. 2015;7:241–264. doi: 10.1146/annurev-marine-010814-015955. [DOI] [PubMed] [Google Scholar]
- 40.López-Urrutia A., Morán X.A.G. Temperature affects size-structure of phytoplankton communities in the ocean. Limnol Oceanogr. 2015;60:733–738. [Google Scholar]
- 41.Sommer U., Lewandowska A. Climate change and the phytoplankton spring bloom: warming and overwintering zooplankton have similar effects on phytoplankton. Global Change Biol. 2011;17:154–162. [Google Scholar]
- 42.Peter K.H., Sommer U. Phytoplankton cell size, inter- and intraspecific effects of warming and grazing. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peter K.H., Sommer U. Phytoplankton cell size reduction in response to warming mediated by nutrient limitation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter K.H., Sommer U. Interactive effect of warming, nitrogen and phosphorus limitation on phytoplankton cell size. Ecol Evol. 2015;5:1011–1024. doi: 10.1002/ece3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer U., Peter K.H., Genitsaris S., Moustaka-Gouni M. Do marine phytoplankton follow Bergmann's rule sensu lato? Biol Rev. 2017;92:1011–1026. doi: 10.1111/brv.12266. [DOI] [PubMed] [Google Scholar]; The authors investigated biogeographic evidence from clonal cultures, micro-, and mesocosm experiments regarding the effect of temperature on phytoplankton size. Phytoplankton size become smaller in warmer waters with far-reaching consequences for nutrient uptake, grazing patterns, food web dynamics and sedimentation
- 46.Atkinson D., Ciotti B., Montagnes D.I.S. Protists decrease in size linearly with temperature: ca. 2.5% °C−1. Proc R Soc Lond B Biol Sci. 2003;270:2605–2611. doi: 10.1098/rspb.2003.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska A.M., Boyce D.G., Hofmann M., Matthiessen B., Sommer U., Worm B. Effects of sea surface warming on marine plankton. Ecol Lett. 2017;17:614–623. doi: 10.1111/ele.12265. [DOI] [PubMed] [Google Scholar]; The authors implemented projections from a global circulation model in the mesocosm experiment to examine warming effects on a multi-trophic plankton community. Copepods switched from grazing on phytoplankton to ciliates under warming conditions, thereby elongating food chains
- 48.Winder M., Bouquet J.-M., Rafael Bermúdez J., Berger S.A., Hansen T., Brandes J., Sazhin A.F., Nejstgaard J.C., Båmstedt U., Jakobsen H.H., Dutz J., Frischer M.E., Troedsson C., Thompson E.M. Increased appendicularian zooplankton alter carbon cycling under warmer more acidified ocean conditions. Limnol Oceanogr. 2017;62:1541–1551. [Google Scholar]
- 49.Verity P.G., Smetacek V. Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar Ecol Prog Ser. 1996;130:277–293. [Google Scholar]
- Steinberg D.K., Landry M.R. Zooplankton and the ocean carbon cycle. Annu Rev Mar Sci. 2017;9:413–444. doi: 10.1146/annurev-marine-010814-015924. [DOI] [PubMed] [Google Scholar]; A review about the importance of zooplankton for carbon dynamics, ranging from zooplankton ecophysiology to global marine carbon budgets. Long-term and future changes in the importance of zooplankton for carbon cycling are evaluated
- 51.Daines S.J., Clark J.R., Lenton T.M. Multiple environmental controls on phytoplankton growth strategies determine adaptive responses of the N: P ratio. Ecol Lett. 2014;17:414–425. doi: 10.1111/ele.12239. [DOI] [PubMed] [Google Scholar]
- 52.Yvon-Durocher G., Dossena M., Trimmer M., Woodward G., Allen A.P. Temperature and the biogeography of algal stoichiometry. Global Ecol Biogeogr. 2015;24:562–570. [Google Scholar]