Abstract
Cell size is partly determined through coordination between cell growth and division. How this coordination is achieved mechanistically remains mostly unknown. However, quantitative experiments together with computational modelling have reinvigorated the field and are elucidating underlying molecular processes. Size homeostasis may be achieved through different modes of regulation, including “sizers”, “adders” and “timers.” For sizer regulation, the cell division cycle does not proceed until a minimal size has been reached, requiring that the cell monitors its own size. Here, we highlight progress in defining sizer mechanisms in fission and budding yeasts showing how accumulation or dilution of key molecules can be used to monitor cell size during growth. We also discuss a potential role for sizers in bacterial size control.
Keywords: Cell size homeostasis, Sizer mechanism, Cell cycle
Highlights
-
•
Sizer mechanisms where cells sense their own size play a crucial role in size control.
-
•
Activator accumulation model proposed for a sizer mechanism in fission yeast.
-
•
Inhibitor dilution model proposed for a sizer mechanism in budding yeast.
-
•
Sizer mechanisms may play a hidden role in bacterial size control.
Introduction
The fundamental process of how cell size is determined remains one of the key questions in cell biology. In actively proliferating cells, cell size is achieved through coordination of cell growth with cell division. For symmetrically dividing cells, the cell roughly doubles in size before it divides. To maintain a given size, cells exhibit size homeostasis mechanisms in which cells of abnormal size can return to normal size within one or more cell cycles.
Most progress in dissecting size control has been made in simple model organisms such as bacteria and yeast. Studying these unicellular organisms has numerous advantages over more complex cell types, including genetic tractability, ease of cell cycle analyses, and simple shapes that facilitate quantitative size measurements. Recent advances in microscopy, high throughput image analyses [1] and microfluidics [2] have led to new quantitative studies that are revealing multiple modes of size control.
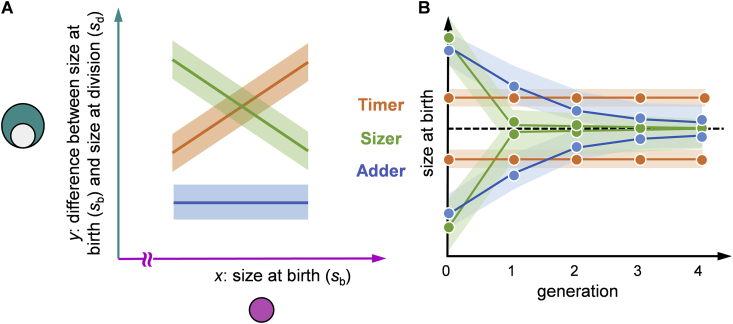
Three general categories of size homeostasis behaviour have been identified: (i) “sizer” behaviour, where cells monitor their own size and, for example, divide at a fixed target size; (ii) “adder” behaviour, where cells add a fixed size increment; (iii) “timer” behaviour, where cells grow for a fixed time duration. Distinguishing between these modes is facilitated by measuring the cell size at the time of birth and at the time of the next division. As shown in Figure 1A, data obtained from experiments when plotting size increase from birth to division vs size at birth, shows a typical trend specific for each of these three behaviours: the linear regression slope is ideally −1 for a sizer, 0 for an adder, and +1 for a timer (assuming exponential growth in time). Although each behaviour shows a specific value of the slope, it is important to remember that similar values can also be obtained from more complex modes of size control, including those that utilize a combination of different mechanisms.
Figure 1.
Three reference behaviours for size control. (A) Size control behaviours of growing cells are assessed by measurements of size at birth and division. The graph shows a plot of size grown over the cell cycle (y = sd − sb) vs size at birth (x = sb). A sizer shows a slope of −1 (since sd is constant, then y = sd − x). An adder has a slope close to 0 (since sd − sb = Δ is constant, then y = Δ). A timer has a slope of +1 if cells exhibit exponential growth (for growth rate g and division time tc, we have , giving ; for homeostatic size control we require tc = (ln 2)/g, giving y = x). (B) The effect of size homeostasis behaviours on correction of long and short cell sizes across several subsequent generations. Dashed line indicates the target cell size.
In terms of the number of generations needed to adjust to an incorrect size at birth, the sizer behaviour is the most efficient, requiring only one generation (Figure 1B). In contrast, the adder behaviour requires multiple generations for full size correction, while for a timer, the flat curve across the generations in Figure 1B reveals only weak size homeostasis.
The dissection of size control requires a sophisticated approach: identification of core molecular components and quantitative measurements of key parameters, all in the framework of computational modelling [3]. In this review, we describe recent interdisciplinary work in this direction, for yeast and bacteria. We will focus primarily on sizer regulation, addressing the central question of how cells can sense how big they are. Alternative modes, particularly the adder, have been the subject of recent comprehensive reviews 4, 5, and so will be discussed in less detail here.
Activator accumulation as a sizer mechanism in fission yeast
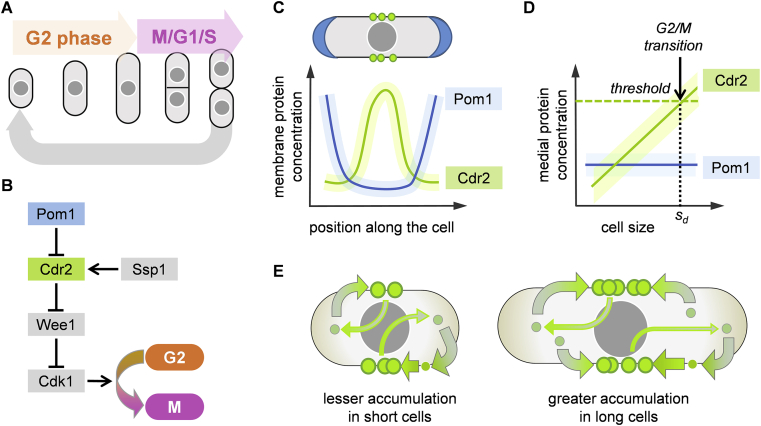
Fission yeast is a rod-shaped single-celled eukaryote whose length increases with time during the G2 phase of cell cycle, while its width does not change significantly. During mitosis and cytokinesis, cell elongation stops and the cell subsequently divides into two (almost) identical halves (Figure 2A). The precision of the length at which division occurs (about 14 μm) is high, with the variance being only 7%. Early research showed evidence for sizer homeostatic behaviour: cells that were born with a shorter (longer) length elongated more (less) before entering mitosis, such that a constant cell length at division is preserved [6]. Hence, the slope in a plot as in Figure 1A is close to −1 6, 7. However, more recent analysis of cells of different widths reveals that cells enter mitosis at a specific surface area, rather than volume or length, suggesting that they may be sensing surface area for this regulation [8].
Figure 2.
Sizer mechanism in fission yeast. (A) Cell cycle with elongation during G2 phase. (B) Cascade of kinases upstream of Wee1 that regulates G2/M transition. (C) Localization of Pom1 and Cdr2 along the cortical membrane. (D) Proposed activator accumulation mechanism. Medial membrane concentration of Pom1 and Cdr2 during cell elongation: Cdr2 levels increase up to a threshold, then the cell enters mitosis. (E) Sketch of the molecular implementation of an accumulation sizer mechanism: cytoplasmic Cdr2 moves and forms clusters only on the medial cortex.
Attempts to decipher the molecular mechanism responsible for fission yeast size control started decades ago 9, 10, 11, 12, 13, 14. Mutants exhibiting altered cell sizes led to the identification of a cascade of cell cycle regulatory factors that, by reciprocal inhibition or activation, regulate entry to mitosis. The cyclin dependent kinase Cdk1, the central mitotic regulator, is regulated directly by the Wee1 kinase, the Cdc25 phosphatase and the cyclin B Cdc13. The prevailing consensus is that regulators upstream of Cdk1 may be used to sense cell size and promote mitotic entry when cells have grown to a minimal cell size.
More specifically, a system of protein kinases upstream of Wee1 has been proposed to contribute to sizer regulation (Figure 2B). These include (but are not limited to) Cdr2, Pom1 and Ssp1 ∗∗8, 15, 16. Localization patterns of these factors have suggested how the cell may query its geometry and sense its size. During interphase, Cdr2 localizes to 80–160 “nodes” [17], clusters of peripheral membrane proteins which form a medial band encircling the nucleus. Ssp1 is a cytoplasmic protein that activates the kinase activity of Cdr2 [15], whereas Pom1 is a membrane kinase that inhibits Cdr2 activity and membrane localization. Pom1 localises on the membrane in the form of concentration gradients emanating from the cell tips 18, 19, 20, 21 (Figure 2C). As the cell elongates by growth during the cell cycle, the distance between cell tips and cell centre gradually increases. One prominent hypothesis proposed that the Pom1 levels on the plasma membrane in the medial region of the cell gradually reduce as the cell grows and, when diminished below a certain threshold, could trigger mitosis 22, 23. However, more recent data has shown that this Pom1 level at midcell is constant regardless of the cell length ∗∗8, 16 (Figure 2D) and that pom1 mutants still exhibit size homeostasis (data analogous to Figure 1A giving −0.8 as a slope) [7]. The Pom1 gradient hypothesis is therefore not currently favoured.
Recent work has instead proposed an “activator accumulation” model for Cdr2 nodes as part of a sizer mechanism [8]. The nodes include proteins involved in cell cycle regulation and cytokinesis, including the anillin-like protein Mid1 24, 25, 26, 27. The total amount of Cdr2 in the cell increases proportionally with cell size (as is the case for many proteins), and interestingly, the number of nodes also correlates with cell size ∗∗8, 17. Since most cellular Cdr2 resides in the nodes (about 50–70% of the total ∗∗8, 17), and since the area of the cortical region occupied by the nodes is kept roughly constant through interphase, the local Cdr2 concentration in this region is proportional to cell area (Figure 2D–E). As confirmed by quantitative modelling, the local Cdr2 cluster density then has the properties of a size indicator revealing global information about cell size. When this density reaches a threshold, it triggers the cell to enter mitosis through downstream regulation of Wee1 and Cdk1 12, 28. As discussed above, accumulation of Cdr2 within a cortical band of fixed area is critical for size sensing in this mechanism. If the size of the region of accumulation also scales with overall cell size, then size sensing is lost. How the area of the Cdr2 accumulation region is controlled and kept fixed is still unclear. Potential candidates involved in this regulation include Mid1 (as a positive signal emanating from the nucleus), the size of the underlying nucleus, the endoplasmic reticulum, although other limiting factors could also play a role 29, 30, 31.
While Cdr2 is an attractive candidate for a sizer molecule, additional molecules could also be sizers, including other nodal proteins, as well as more downstream elements. For example, recent work has shown that Cdc25, the Cdk1 phosphatase that counters the activity of Wee1, may also contribute to a sizer mechanism [32]. While most cell cycle proteins are maintained at approximately constant concentrations during the cell cycle (including Wee1, Cdr2 and Cdk1), Cdc25 and Cyclin B Cdc13 protein concentrations increase as cells grow. This property suggests an activator accumulation mechanism. It is therefore possible that size control is a product of multiple, redundant mechanisms. Nevertheless, our existing knowledge about fission yeast clearly supports the presence of robust size control via a sizer mechanism.
Inhibitor dilution as a sizer mechanism in budding yeast
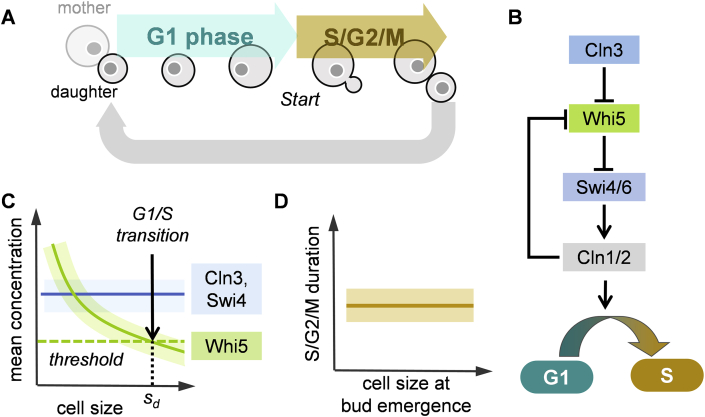
In the budding yeast Saccharomyces cerevisiae, a round mother cell grows and then begins to form a small daughter bud (Figure 3A). A sizer mechanism has been suggested for the G1/S transition at Start, in which the unbudded cell reaches a minimal size before committing to the rest of the cell cycle. Commonly, the mother from the previous division is already large enough and thus more rapidly passes Start. In contrast, daughter buds that are recently born are smaller and grow exponentially with time during G1 to obtain the proper size before passing Start. After Start, cells go on to replicate their DNA, form a bud, grow further and then divide. This asymmetry in size at division is also coupled with an asymmetric localization of key proteins (such as the transcription factors Ace2 and Ash1 in the daughter cell) resulting in different Start regulation for the daughter vs mother cells [33].
Figure 3.
Sizer mechanism in budding yeast. (A) Cell cycle for the daughter cell. Note that the cell achieves a minimal size in G1 phase before committing to the rest of the cell cycle at Start. (B) Positive feedback in the signalling pathways that regulate the G1/S transition. (C) Proposed inhibitor dilution mechanism: the number of Whi5 molecules does not increase in G1, so when the cell grows large enough, the Whi5 concentration falls below a threshold, allowing for activation of Start and budding. (D) The S/G2/M component of the cell cycle may follow a timer behaviour.
Budding yeast may exhibit a sizer mechanism during G1, and a timer behaviour for subsequent phases of the cell cycle. A plot of time during G1 multiplied by the growth rate g vs logarithm of the cell size at birth shows a steep trend at small cell volumes, with a slope of about −0.7, close to that expected for a sizer. However, for large daughter cells, a slope of around −0.3 is observed [34], indicating that a pure sizer mechanism may be an over-simplification, with potential adder-like control also contributing [35]. Nevertheless, recent advances have illuminated a molecular mechanism for possible size control during G1. Figure 3B illustrates the signalling cascade that regulates the G1/S transition. The G1 cyclin Cln3 inhibits the activity of Whi5 which inhibits Start by counteracting the transcription factors SBF (Swi4/Swi6) 34, 36. Unlike Cln3, which shows a constant concentration during G1, the Whi5 concentration decreases as the cell grows [37] (Figure 3C). Since Whi5 is a stable protein that is synthesized mostly during S/G2/M, the reduction in its concentration during G1 is due to dilution caused by an increase in cell size. When cell volume is sufficiently large, Whi5 concentration drops below a given threshold to trigger the G1/S transition allowing subsequent DNA replication and budding (Figure 3C). Measurement of the Whi5 concentration showed that this “inhibitor dilution” principle is valid in both haploid and diploid cases with different copy numbers of the gene [37]. In mutant cells lacking Whi5, the slope discussed above drops to −0.2, indicating that the sizer mechanism is substantially compromised [34]. Overall, these findings offer support for a sizer mechanism in budding yeast that relies on a size-dependent drop in Whi5 concentration through dilution.
One key assumption for this model regards the initial concentration of Whi5 in daughter cells. The dilution mechanism requires that the Whi5 concentration reduces with increasing cell size (Figure 3C), with the Whi5 concentration being lower for the bigger mother cell but higher for the smaller daughter cell. As a result, Whi5 molecules must be appropriately redistributed between the two compartments before septum formation. Experiments have indeed shown such a difference in the two Whi5 concentrations [37], but a mechanistic explanation for how this is achieved is still lacking.
The second part of the cell cycle (S/G2/M) is believed to have rather different regulation. Because of the exponential growth of the bud with time (with rate g for the bud, whereas the mother's growth stops almost entirely after G1), the size at division (mother sM plus daughter sD) is , where t2 is the duration of S/G2/M. This analysis leads to a constant size ratio of daughter to mother of . This ratio is about 0.5–0.8 depending on the growth medium, but is independent of cell size, supporting timer behaviour (Figure 3D) [35], as also found in Ref. [37]. So far, no molecular mechanism has been suggested for how this second checkpoint might function. However, an effective timer could arise from a minimal time needed to perform certain cell cycle tasks, such as DNA replication and mitosis.
Evidence for composite size control behaviour in bacteria
Size homeostasis analysis demonstrates that many bacteria, including Escherichia coli, Bacillus subtilis and Caulobacter crescentus, show phenomenology consistent with adder regulation. However, the underlying molecular basis remains unclear ∗38, ∗39, 40. Furthermore, recent studies in E. coli show how such adder behaviour may also be explained by composite control that incorporates properties of adders, sizers and timers.
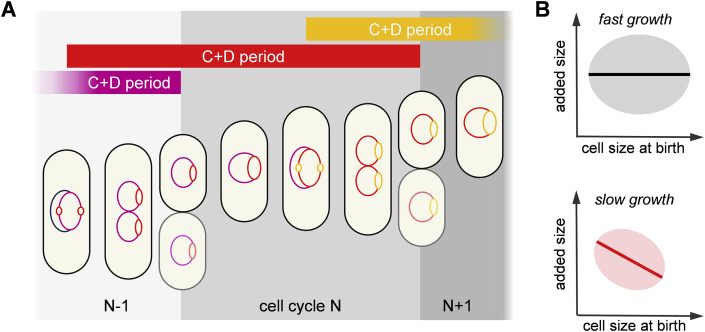
In the case of E. coli in a rich nutrient growing medium, the time between successive cell divisions is shorter than the chromosome replication time. Cells overcome this problem through multiple origins of DNA replication initiation, which does however create a more complicated pattern of coordination between DNA replication and growth. However, building on work from Donachie [41], and from Cooper and Helmstetter [42], stable cell cycles can still be assured through a combination of sizer and timer control 43, ∗∗44, 45. In this scenario, size sensing triggers DNA replication (at a fixed cell volume per origin of replication), followed by a fixed time duration up to division (the C + D period).
This scenario has been tested experimentally by measuring the timing of initiation of chromosome replication relative to cell division in individual E. coli cells [44]. In normal/fast growing conditions, the time between successive divisions in E. coli is shorter than the C + D period (Figure 4A). Immediately after division, DNA replication has therefore already started and only a fraction of the constant C + D period remains. Since cell division subsequently occurs after this residual C + D period, regardless of the cell size at birth, the associated cell size increase will be uncorrelated to the cell size at birth. The authors of Ref. [44] suggest that this process will reveal an adder-like behaviour (Figure 4B, top). However, at low growth rates, when the sizer and timer act on the same cell cycle, the increase in cell size shows a negative correlation with respect to the size at birth, typical of a sizer [44] (Figure 4B, bottom). Furthermore, a subsequent investigation has shown that cell size at replication initiation per origin is invariant under a wide set of perturbations [46]. These results offer support for combined sizer-timer behaviour, and moreover show that interpreting size homeostasis plots like those in Figures 1A and 4B can be difficult. Behaviours that superficially appear to belong to one of the three simple size controls may in fact turn out to be more nuanced on further analysis.
Figure 4.
Possible mixed sizer plus timer behaviour in E. coli. (A) In fast growing conditions, the C + D period is longer than the time between successive divisions: multiple DNA replication initiations therefore take place, with division occurring when the C + D period initiated during a previous division cycle ends. (B) Fast growing conditions can mask the sizer effect by showing a lack of correlation between added size and size at birth (upper panel). This correlation reappears at slower growth rates (lower panel).
Currently, no firm molecular basis exists for a mixed sizer-timer or for an adder mechanism. For the latter a theoretical autorepressor model has been proposed [47], and it has been speculated that dynamics of the DNA replication initiator DnaA may be involved ∗35, 48, 49. However, other hypotheses are emerging, including an accumulation mechanism based on the relative rates of cell volume and cell surface synthesis in C. crescentus and E. coli [50]. Assuming that the instantaneous rate of surface area growth is a function of cell volume, leads to a model where the cell surface area to volume ratio exhibits homeostasis. Moreover, the natural periodicity of this ratio over the cell cycle could lead to an accumulation of membrane building blocks that might be used to trigger cell division, potentially leading to adder-like control. Overall, more work is needed, but at present a role for “hidden” sizers even in bacterial cell size control cannot be excluded.
Conclusions
In this review, we have described the current understanding of cell size control, focussing on sizer mechanisms. There is still considerable debate in the field over which cells use sizers vs timers vs adders, or in some combination. What is particularly exciting is that molecular mechanisms that can plausibly explain cell size regulation are now being revealed. The mechanisms of accumulating activators, such as Cdr2 in fission yeast, and inhibitor dilution, such as Whi5 in budding yeast, provide paradigms for understanding how cells may sense their own size ∗∗8, ∗∗37. It is likely that similar principles will be operating in other cell types. The general ability of sizers to rapidly correct for errors in size control within one generation could be a significant advantage for cells implementing this mechanism.
Both accumulation and dilution mechanisms rely on the regulation of the concentration of a specific protein, i.e. copy number divided by size. For most of the proteins, such a ratio may seem unsuitable as a size monitor, even for relative size changes. Intriguingly, yeasts may have solved this limitation by molecular mechanisms that carefully control one of the two quantities in the ratio. In fission yeast, the Cdr2 protein localizes in a cortical region of fixed area, so that the relevant size in the density ratio is not the cell size but the size of the cortical region, while in budding yeast, it is the Whi5 copy number that remains constant during G1.
Overall, these studies demonstrate the exciting prospects and challenges of studying cell size regulation. Although the concepts of sizers, adders and timers serve as useful terms to define and guide our thinking, it is likely that detailed mechanistic studies will ultimately reveal more nuanced and complex modes of regulation. In the future, quantitative interdisciplinary studies will be critical to dissect out the multiple layers of control responsible for this universal cellular process.
Acknowledgements
We would like to thank Benjamin Knapp and Rea Antoniou-Kourounioti for a critical reading of the manuscript. F.C. and M.H. acknowledge financial support from Bilateral NSF-BBSRC grant NSF-MCB1638195 and BB/M023796/1.
This review comes from a themed issue on Development and differentiation (2017)
Edited by Philip Greulich and Ben MacArthur
Contributor Information
Fred Chang, Email: fred.chang@ucsf.edu.
Martin Howard, Email: martin.howard@jic.ac.uk.
References
- 1.Nketia T., Sailem H., Rohde G., Machiraju R., Rittscher J. Analysis of live cell images: methods, tools and opportunities. Methods. 2017;115:65–79. doi: 10.1016/j.ymeth.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Wang P., Robert L., Pelletier J., Dang W.L., Taddei F., Wright A., Jun S. Robust growth of Escherichia coli. Curr Biol. 2010;20:1099–1103. doi: 10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantes P.A., Grant W., Pritchard R., Sudbery P., Wheals A. The regulation of cell size and the control of mitosis. J Theor Biol. 1975;50:213–244. doi: 10.1016/0022-5193(75)90034-x. [DOI] [PubMed] [Google Scholar]
- 4.Jun S., Taheri-Araghi S. Cell-size maintenance: universal strategy revealed. Trends Microbiol. 2015;23:4–6. doi: 10.1016/j.tim.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Sauls J.T., Li D., Jun S. Adder and a coarse-grained approach to cell size homeostasis in bacteria. Curr Opin Cell Biol. 2016;38:38–44. doi: 10.1016/j.ceb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantes P. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Wood E., Nurse P. Pom1 and cell size homeostasis in fission yeast. Cell Cycle. 2013;12:3417–3425. doi: 10.4161/cc.26462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K.Z., Saunders T.E., Flor-Parra I., Howard M., Chang F. Cortical regulation of cell size by a sizer cdr2p. Elife. 2014;3:e02040. doi: 10.7554/eLife.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study proposes Cdr2 as a molecule responsible for size sensing in S. pombe and proposes the Cdr2 accumulation model as a molecular mechanism. Results also show that fission yeast divides at a specific membrane surface area, consistent with the molecular model.
- 9.Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- 10.Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol General Genet MGG. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- 11.Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 12.Breeding C.S., Hudson J., Balasubramanian M.K., Hemmingsen S.M., Young P.G., Gould K.L. The cdr2+ gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:3399–3415. doi: 10.1091/mbc.9.12.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- 14.Gould K.L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng L., Baldissard S., Kettenbach A.N., Gerber S.A., Moseley J.B. Dueling kinases regulate cell size at division through the SAD kinase Cdr2. Curr Biol. 2014;24:428–433. doi: 10.1016/j.cub.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia P., Hachet O., Hersch M., Rincon S., Berthelot-Grosjean M., Dalessi S., Basterra L., Bergmann S., Paoletti A., Martin S.G. Distinct levels in Pom1 gradients limit Cdr2 activity and localization to time and position division. Cell Cycle. 2014;13:538–552. doi: 10.4161/cc.27411. [DOI] [PubMed] [Google Scholar]
- 17.Akamatsu M., Lin Y., Bewersdorf J., Pollard T.D. Analysis of interphase node proteins in fission yeast by quantitative and super resolution fluorescence microscopy. Mol Biol Cell. 2017;10.1091/mbc.E16-07-0522 doi: 10.1091/mbc.E16-07-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders T.E., Pan K.Z., Angel A., Guan Y., Shah J.V., Howard M., Chang F. Noise reduction in the intracellular pom1p gradient by a dynamic clustering mechanism. Dev Cell. 2012;22:558–572. doi: 10.1016/j.devcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersch M., Hachet O., Dalessi S., Ullal P., Bhatia P., Bergmann S., Martin S.G. Pom1 gradient buffering through intermolecular auto-phosphorylation. Mol Syst Biol. 2015;11:818. doi: 10.15252/msb.20145996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachet O., Berthelot-Grosjean M., Kokkoris K., Vincenzetti V., Moosbrugger J., Martin S.G. A phosphorylation cycle shapes gradients of the DYRK family kinase Pom1 at the plasma membrane. Cell. 2011;145:1116–1128. doi: 10.1016/j.cell.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Bähler J., Pringle J.R. Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev. 1998;12:1356–1370. doi: 10.1101/gad.12.9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moseley J.B., Mayeux A., Paoletti A., Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–860. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]
- 23.Martin S.G., Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–856. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- 24.Almonacid M., Moseley J.B., Janvore J., Mayeux A., Fraisier V., Nurse P., Paoletti A. Spatial control of cytokinesis by Cdr2 kinase and Mid1/anillin nuclear export. Curr Biol. 2009;19:961–966. doi: 10.1016/j.cub.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Celton-Morizur S., Bordes N., Fraisier V., Tran P.T., Paoletti A. C-terminal anchoring of mid1p to membranes stabilizes cytokinetic ring position in early mitosis in fission yeast. Mol Cell Biol. 2004;24:10621–10635. doi: 10.1128/MCB.24.24.10621-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laplante C., Huang F., Tebbs I.R., Bewersdorf J., Pollard T.D. Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. Proc Natl Acad Sci. 2016;113:E5876–E5885. doi: 10.1073/pnas.1608252113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vavylonis D., Wu J.-Q., Hao S., O'shaughnessy B., Pollard T.D. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 2008;319:97–100. doi: 10.1126/science.1151086. [DOI] [PubMed] [Google Scholar]
- 28.Kanoh J., Russell P. The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast. Mol Biol Cell. 1998;9:3321–3334. doi: 10.1091/mbc.9.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rincon S.A., Paoletti A. Seminars in cell & developmental biology. Elsevier; 2016. Molecular control of fission yeast cytokinesis; pp. 28–38. [DOI] [PubMed] [Google Scholar]
- 30.Schmoller K.M., Skotheim J.M. The biosynthetic basis of cell size control. Trends Cell Biol. 2015;25:793–802. doi: 10.1016/j.tcb.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D., Vjestica A., Oliferenko S. Plasma membrane tethering of the cortical ER necessitates its finely reticulated architecture. Curr Biol. 2012;22:2048–2052. doi: 10.1016/j.cub.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Keifenheim D., Sun X.-M., D'Souza E., Ohira M.J., Magner M., Mayhew M.B., Marguerat S., Rhind N. Size-dependent expression of the mitotic activator Cdc25 suggests a mechanism of size control in fission yeast. Curr Biol. 2017;10:1491–1497. doi: 10.1016/j.cub.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Talia S., Wang H., Skotheim J.M., Rosebrock A.P., Futcher B., Cross F.R. Daughter-specific transcription factors regulate cell size control in budding yeast. PLoS Biol. 2009;7:e1000221. doi: 10.1371/journal.pbio.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Talia S., Skotheim J.M., Bean J.M., Siggia E.D., Cross F.R. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature. 2007;448:947–951. doi: 10.1038/nature06072. [DOI] [PubMed] [Google Scholar]
- Soifer I., Robert L., Amir A. Single-cell analysis of growth in budding yeast and bacteria reveals a common size regulation strategy. Curr Biol. 2016;26:356–361. doi: 10.1016/j.cub.2015.11.067. [DOI] [PubMed] [Google Scholar]; In this paper, an adder hypothesis is proposed for S. cerevisiae and supported by single cell measurements of cell size in different growing conditions.
- 36.Palumbo P., Vanoni M., Cusimano V., Busti S., Marano F., Manes C., Alberghina L. Whi5 phosphorylation embedded in the G1/S network dynamically controls critical cell size and cell fate. Nat Commun. 2016;7 doi: 10.1038/ncomms11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoller K.M., Turner J., Kõivomägi M., Skotheim J.M. Dilution of the cell cycle inhibitor Whi5 controls budding-yeast cell size. Nature. 2015;526:268–272. doi: 10.1038/nature14908. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper presents a molecular mechanism for sizer control in S. cerevisiae based on dilution of the Whi5 protein.
- Campos M., Surovtsev I.V., Kato S., Paintdakhi A., Beltran B., Ebmeier S.E., Jacobs-Wagner C. A constant size extension drives bacterial cell size homeostasis. Cell. 2014;159:1433–1446. doi: 10.1016/j.cell.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; In these two papers, data on cell size at birth and at division for E. coli, B. subtilis and C. crescentus is presented, showing that a constant size is added between these times. These results suggest an adder behaviour for size control in these bacteria.
- Taheri-Araghi S., Bradde S., Sauls J.T., Hill N.S., Levin P.A., Paulsson J., Vergassola M., Jun S. Cell-size control and homeostasis in bacteria. Curr Biol. 2015;25:385–391. doi: 10.1016/j.cub.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; In these two papers, data on cell size at birth and at division for E. coli, B. subtilis and C. crescentus is presented, showing that a constant size is added between these times. These results suggest an adder behaviour for size control in these bacteria.
- 40.Willis L., Huang K.C. Sizing up the bacterial cell cycle. Nat Rev Microbiol. 2017 doi: 10.1038/nrmicro.2017.79. [DOI] [PubMed] [Google Scholar]
- 41.Donachie W.D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968;219:1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- 42.Cooper S., Helmstetter C.E. Chromosome replication and the division cycle of Escherichia coli. Br. J Mol Biol. 1968;31:519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- 43.Osella M., Nugent E., Lagomarsino M.C. Concerted control of Escherichia coli cell division. Proc Natl Acad Sci. 2014;111:3431–3435. doi: 10.1073/pnas.1313715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallden M., Fange D., Lundius E.G., Baltekin Ö., Elf J. The synchronization of replication and division cycles in individual E. coli cells. Cell. 2016;166:729–739. doi: 10.1016/j.cell.2016.06.052. [DOI] [PubMed] [Google Scholar]; This paper explores the hypothesis of a mixed mechanism that combines a sizer and timer in E. coli. Single cell measurements of cell size, replication timing and growth rate in different growing conditions demonstrate how a sizer component can be masked to generate adder-like behaviour.
- 45.Zheng H., Ho P.-Y., Jiang M., Tang B., Liu W., Li D., Yu X., Kleckner N.E., Amir A., Liu C. Interrogating the Escherichia coli cell cycle by cell dimension perturbations. Proc Natl Acad Sci. 2016;113:15000–15005. doi: 10.1073/pnas.1617932114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Si F., Li D., Cox S.E., Sauls J.T., Azizi O., Sou C., Schwartz A.B., Erickstad M.J., Jun Y., Li X., Jun S. Invariance of initiation mass and predictability of cell size in Escherichia coli. Curr Biol. 2017;27:1278–1287. doi: 10.1016/j.cub.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sompayrac L., Maaløe O. Autorepressor model for control of DNA replication. Nature. 1973;241:133–135. doi: 10.1038/newbio241133a0. [DOI] [PubMed] [Google Scholar]
- 48.Braun R.E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985;40:159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- 49.Skarstad K., Katayama T. Regulating DNA replication in bacteria. Cold Spring Harb Perspect Biol. 2013;5:a012922. doi: 10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L.K., Theriot J.A. Relative rates of surface and volume synthesis set bacterial cell size. Cell. 2016;165:1479–1492. doi: 10.1016/j.cell.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Surface area to volume homeostasis is proposed to be a consequence of both cell volume and cell surface synthesis rates scaling with cell volume. Such a mechanism leads to an accumulation of membrane building blocks that might be used by the cell to trigger division.