Abstract
Objectives:
To study the bone changes and curative effect of infliximab in patients with ankylosing spondylitis (AS).
Methods:
AS patients diagnosed and treated in Wuwei People’s Hospital from January 2017 to March 2018 were collected as the study subjects of this study, and the patients were divided into INF group (n=40) and MTX group (n=40) according to the random number table. The expression levels of TNF-α and IL-33 before and after treatment were detected by enzyme-linked immunosorbent assay (ELISA), and bone changes before and after treatment were compared between the two groups. The ROC curves of TNF-α and IL-33 for efficacy prediction of AS were drawn and analyzed.
Results:
After treatment, the expression levels of serum TNF-α and IL-33 in patients in INF group were significantly lower than those in MTX group (P<0.001), and the improvement of bone erosion and tendon thickening in INF group was markedly higher than that in MTX group (P<0.001). The receiver operating characteristic (ROC) curve revealed that the area under the curve (AUC) of TNF-α for predicting efficacy was 0.939, and that of IL-33 was 0.853.
Conclusions:
Infliximab can significantly improve the bone status and has a positive effect in patients with AS, and TNF-α and IL-33 are expected to be used as efficacy predictors of AS.
Keywords: Ankylosing Spondylitis, Efficacy Prediction, IL-33, Infliximab
Introduction
Ankylosing spondylitis (AS) is a chronic immune-mediated inflammatory disease that is characterized by new bone formation in the axial bones, often resulting in progressive, irreversible structural damage and disability[1,2]. The onset of AS commonly occurs in people younger than 45[3], and the prevalence is between 0.1% and 1.4% globally4. It is a common rheumatic disease in China, and more than 90% of Chinese patients are predominantly HLA-B*2704 subtype and HLA-B27 positive, among which the incidence of the latter accounts for 4% to 8% in the general Chinese population[5]. In a study in Taiwan, the incidence of male AS was lower in people under 19 years old, peaked rapidly between 20 and 24 years old, and then gradually declined[6]. AS will severely affect the spinal range of motion and physical function, leading to a low quality of life and posing a direct threat to the physical and mental health of young people. Therefore, the development of safe and effective treatments is essential.
Non-steroidal anti-inflammatory drugs (NSAIDs) are the mainstay of treatment for AS. Among them, indomethacin combined with methotrexate is commonly used for the treatment of AS; however clinical observation over the recent years has revealed that its efficacy is moderate, and it has no significant effect on improving the prognosis[7]. At the same time, conventional treatment will slow down the radiological progress. Tumor necrosis factor (TNF-α) inhibitors are found to be able to make up the shortage of NSAIDs and improve patients’ quality of life[8]. TNF-α is a cytokine produced by activated macrophages that can inhibit osteoblasts and stimulate osteoclasts, which can directly kill tumor cells without significant toxicity to normal cells. TNF-α and IL-33 are overexpressed in patients with AS and are involved in the development of the disease, which may regulate the immune or inflammatory process of AS[9,10]. Infliximab, as one of TNF-α inhibitors, can effectively reduce the level of TNF-α. Studies have confirmed its safety and effectiveness in long-term treatment[11], as well as its significant effect on improving bone density[12]. As the commonly used drugs in the clinical treatment of AS in recent years, TNF-α inhibitors have shown considerable clinical efficacy, but studies on the predictive value of TNF-α and IL-33 in clinical efficacy are still scarce.
In this study, conventional drugs and infliximab were applied to treat AS patients to study their effects on bone, clinical efficacy as well as the predictive value in AS.
Methods and materials
Clinical data collection
Patients with AS who were diagnosed and treated in Wuwei People’s Hospital from January 2017 to March 2018 were collected and randomly divided into two groups: INF group (n=40) and MTX group (n=40). There were 29 males and 11 females in the INF group, with an average age of 29.1±7.6 years, while in MTX group, there were 28 males and 12 females with an average age of 28.8±8.1 years. The study was approved by the Medical Ethics Committee of Wuwei People’s Hospital.
Inclusion and exclusion criteria
AS patients diagnosed and treated in our hospital, and the diagnostic criteria were referred to the 1984 modified New York Criteria of Evaluation of Diagnostic Criteria for Ankylosing Spondylitis[13]. The inclusion criteria were the following: Low back pain and stiffness for more than 3 months, which would improve only during exercise, but not at rest; Limited movement of lumbar sagittal and coronal; Thoracic activity lower than healthy people of similar age and gender; Bilateral sacral arthritis reached or exceeded grade ΙΙ or unilateral sacral arthritis grade ΙΙΙ-ΙV.
The exclusion criteria were: Patients with high methotrexate allergy, allergy to mouse protein, renal insufficiency, active gastric and duodenal ulcers, moderate and severe heart failure, infection, low blood volume, coagulation, or mental disorder.
Treatment plan
Patients in the INF group received an intravenous injection of infliximab (Cilag AG, Switzerland, registration number: S20171001). Patients were given at the first dose of 5 mg/kg, followed by the same dose at 2 and 6 weeks after the first administration and every 6 weeks after that. While patients in the MTX group were treated with conventional drug treatment: Indomethacin sustained-release capsules (Minghe Pharmaceutical Co., Ltd., Hubei, China, State Drugs Administration License No.: H20030063) was orally administrated at 75 mg/time and 1d/time. Also, another oral administration of methotrexate tablets (Sine Pharmaceutical Laboratories Co., Ltd., Shanghai, China, State Drugs Administration License No.: H31020644) was taken at 5 mg/time, once daily. Patients in both groups were treated continuously for 30 weeks.
Sample collection and testing
On the morning of the second day after admission and 30-week treatment, 3 ml of blood was taken from the veins on an empty stomach from each enrolled AS patient, and meanwhile, the same amount of blood samples were drawn from the control group. All the blood samples were left at room temperature for 30 min and centrifuged at 3000 rpm/min for 10 min. Then the supernatant was collected and stored in the refrigerator at -80oC for centralized detection.
ELISA was adopted to measure the serum expression levels of TNF-α (human TNF alpha ELISA kit, Abcam (Shanghai) Trading Co., Ltd., ab181421) and IL-33 (human The expression of IL-33 ELISA kit, Abcam (Shanghai) Trading Co., Ltd., ab223865). Blank wells, standard wells, and sample wells to be tested were set. Blank wells were added with SO with 0 concentration, while standard wells with standard samples, and sample wells with the sample to be tested. In addition to the sample wells, all other wells were added with sample dilutions and horseradish peroxidase (HRP)-labeled antibodies. Then, unbound biotinylated antibodies were removed thorough washing, and HRP-labeled avidin was added. After washing again, TMB substrate was added for color development. TMB turned blue under catalysis and yellow under the action of acid. Finally, the absorbance (OD value) was measured with a microplate reader at a wavelength of 450 nm, and the corresponding concentration was converted from the standard curve.
Outcome measures
Main outcome measures: Bone changes and the expression levels of TNF-α and IL-33 before and after treatment in the two groups were recorded and compared. In addition, Physician global assessment (PGA) score was applied to evaluate the clinical efficacy of the two groups after 30 weeks of treatment (curative effect evaluation standard are shown in Table 1). Serum TNF-α and IL-33 expressions of the two groups of patients before and after treatment were observed. What’s more, according to post-treatment conditions, patients who were basically cured and showed markedly effective were divided into good curative effect group, while those who were improved and ineffective were divided into the poor curative effect group. The expression levels of TNF-α and IL-33 in the two groups before treatment were observed, and ROC curve was drawn.
Table 1.
Evaluation criteria for clinical efficacy.
| Efficacy level | Evaluation criteria |
|---|---|
| Basically cured | PGA score ≥9 |
| PGA score ≥9 | PGA score: 6-9 |
| Improved | PGA score: 2.5-5.9 |
| Ineffective | PGA score < 2.5 |
Secondary outcome measures: Changes in clinical outcome measures before and after treatment in the two groups were observed (Table 1).
Statistical analysis
The collected data were statistical analyzed by SPSS20.0 (Cabit Information Technology Co., Ltd., Shanghai, China), and the required images were plotted using Prism 7 (Softhead Technology Co., Ltd., Shenzhen, China). The counting data were expressed in the form of percentage (%), and compared using Chi-square (expressed as X2). The measurement data were expressed as (Mean±SD), and the comparison between the two groups of normal distribution data was performed using the independent sample t test, which was expressed as P<0.05 indicated that there was a statistical difference between the two groups.
Results
Comparison of clinical data between the two groups
There were no significant differences in age, gender, BMI, smoking history, family history, place of residence or other laboratory indicators in the two groups of patients (P>0.05) (Table 2).
Table 2.
Baseline data of patients.
| Factors | INF group (n=40) | MTX group (n=40) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 29.1±7.6 | 28.8±8.1 | 0.171 | 0.865 |
| Gender | ||||
| Male | 29 (72.50) | 28 (70.00) | 0.061 | 0.804 |
| Female | 11 (27.50) | 12 (30.00) | ||
| BMI (kg/m2) | 22.4±1.5 | 22.8±1.3 | 1.275 | 0.206 |
| Smoking history | ||||
| Yes | 12 (30.00) | 13 (32.50) | 0.058 | 0.809 |
| No | 28 (70.00) | 27 (67.50) | ||
| Family medical history | ||||
| Yes | 5 (12.50) | 6 (15.00) | 0.105 | 0.745 |
| No | 35 (87.50) | 34 (85.00) | ||
| Residence | ||||
| Rural | 22 (55.00) | 24 (60.00) | 0.205 | 0.651 |
| Urban | 18 (45.00) | 16 (40.00) | ||
| CRP (mg/L) | 11.92±7.63 | 12.16±7.42 | 0.143 | 0.887 |
| ESR (mm) | 21.34±8.27 | 20.87±9.12 | 0.241 | 0.810 |
Changes in serum TNF-α and IL-33 expression levels before and after treatment in the two groups
The comparison of serum TNF-α and IL-33 expression levels of patients in the two groups before and after treatment showed no significant difference in serum TNF-α (28.427±7.138) (27.892±6.427) and IL-33 (261.152±28.443) (256.763±26.976) levels between INF group and MTX group before treatment. After treatment, the expression levels of serum TNF-α (12.146±5.752) (18.248±13.426) and IL-33 (121.274±13.426) (137.582±16.223) decreased significantly in both groups (P<0.001), and the serum TNF-α and IL-33 in patients in INF group were substantially lower than those in MTX group (P<0.001) (Figure 1).
Figure 1.
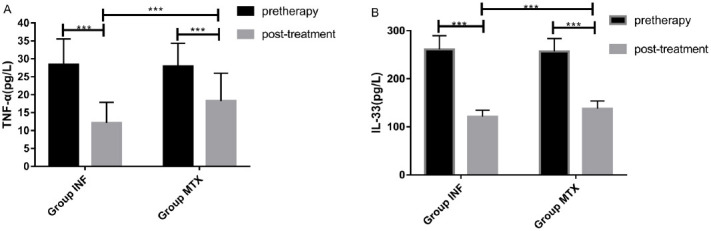
Changes in serum TNF-α and IL-33 expression levels before and after treatment in the two groups of patients. A) Changes in serum TNF-α expression level before and after treatment in the two groups. There was no significant difference in serum TNF-α level between INF group (28.427±7.138) and MTX group (27.892±6.427) before treatment. While it decreased significantly after treatment in the two groups, and the serum TNF-α level in INF group (12.146±5.752) was significantly lower than that in MTX group (18.248 ±13.426). B) Changes in serum IL-33 expression level before and after treatment in the two groups. No significant difference was found in serum IL-33 level between INF group (261.152±28.443) and MTX group (256.763±26.976) before treatment. While the level of serum IL-33 dropped notably in the two groups after treatment, and the serum IL-33 level in INF group (121.274±13.426) was remarkably lower than that in MTX group (137.582±16.223) (P<0.001). *** indicated P<0.001.
Bone changes in the two groups before and after treatment
Of all the 40 patients in the INF group, 31 cases of bone erosion, 2 cases of osteophyte, 17 cases of tendon thickening, and 2 cases of tendon calcification were found before treatment. While there were 33 cases of bone erosion, 3 cases of osteophyte, 18 cases of tendon thickening, and 1 case of tendon calcification in the MTX group before treatment. There was no significant difference in the number of cases of bone lesions between the two groups before treatment (P>0.05). After treatment, there were 8 cases of bone erosion, 1 case of osteophyte, 4 cases of tendon thickening and 2 cases of tendon calcification in the INF group, while in MTX group, there were 17 cases of bone erosion, 2 cases of osteophyte, 10 cases of tendon thickening and 1 case of tendon calcification. The results, as mentioned above, indicated that the degree of bone improvement in the INF group was better than that in the MTX group (P<0.001) (Table 3).
Table 3.
Bone changes in the two groups before and after treatment.
| Indexes | Bone erosion | Osteophyte | Tendon thickening | Tendon calcification | ||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| INF Group (n=40) | 31 (77.50) | 8 (20.00) | 2(5.00) | 1 (2.50) | 17 (42.50) | 4 (10.00) | 2 (5.00) | 2 (5.00) |
| MTX Group (n=40) | 33 (82.50) | 17 (42.5) | 3 (7.50) | 2 (5.00) | 18 (45.00) | 10 (25.00) | 1 (2.50) | 1 (2.50) |
| Χ2 value | 0.313 | 4.713 | 0.213 | 0.346 | 0.503 | 4.021 | 0.346 | 0.346 |
| P value | 0.576 | 0.030 | 0.644 | 0.556 | 0.478 | 0.045 | 0.556 | 0.556 |
Changes in clinical efficacy indexes before and after treatment in the two groups
There was no significant difference in the indexes of morning stiffness time (74±32) (76±29), Bath Ankylosing Spondylitis Functional Index (BASFI) (4.56±2.64) (4.62±2.49), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (5.86±1.64) (5.78±1.81), PGA (4.22±1.63) (4.17±1.78) between INF group and MTX group before treatment (P>0.05). While after treatment, the improvement of morning stiffness time (13±8) (18±7), BASFI (1.98±1.56) (2.99±1.34), BASDAI (2.03±1.22) (3.14±1.42) and PGA (8.03±1.12) (7.11±1.34) in INF group was better than that in MTX group (P<0.05), as shown in Table 4. The clinical efficacy of the patients was evaluated according to the PGA score after treatment. The results showed that 15 patients in INF group were cured, 14 were markedly effective, 9 were improved, and 2 were ineffective, with a total effective rate of 95% [overall effective rate (%) = n (basically cured + markedly effective + improved) / n (ineffective)]. While in the MTX group, 9 cases were cured, 13 cases were considerably effective, 12 cases were improved, and 6 cases were ineffective, with a total effective rate of 85%. The overall effective rate in the INF group was significantly higher than that in the MTX group (Table 5).
Table 4.
Changes in clinical efficacy indexes before and after treatment in the two groups.
| Indexes | Morning stiffness time (min) | BASFI | BASDAI | PGA | ||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| INF group (n=40) | 74±32 | 13±8 | 4.56±2.64 | 1.98±1.56 | 5.86±1.64 | 2.03±1.22 | 4.22±1.63 | 8.03±1.12 |
| MTX group (n=40) | 76±29 | 18±7 | 4.62±2.49 | 2.99±1.34 | 5.78±1.81 | 3.14±1.42 | 4.17±1.78 | 7.11±1.34 |
| T value | 0.248 | 2.975 | 0.105 | 3.106 | 0.207 | 2.804 | 0.131 | 3.332 |
| P value | 0.805 | 0.004 | 0.917 | 0.003 | 0.836 | 0.006 | 0.896 | 0.001 |
Table 5.
Comparison of clinical efficacy between the two groups.
| Groups | Basically cured | Markedly effective | Improved | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| INF group (n=40) | 15.00 (37.50) | 14.00 (35.00) | 9.00 (22.50) | 2.00 (5.00) | 5 (95.00) |
| MTX group (n=40) | 9.00 (22.50) | 13.00 (32.50) | 12.00 (30.00) | 6.00 (15.00) | 35(85.00) |
| T value | 0.018 | ||||
| P value | 5.556 |
Predictive value of TNF-α and IL-33 in patients with AS
The patients in the INF group were grouped according to their clinical efficacy after treatment. The patients who were cured and those with marked effectiveness were divided into good curative effect group (n=51), and the improved and ineffective patients were divided into poor curative effect group (n=29). It was observed that the expression levels of serum TNF-α and IL-33 in the good curative effect group were markedly lower than those of the poor curative effect group, showing a significant difference (P<0.001), as shown in Figure 2. Subsequently, ROC curves were drawn based on the expression levels of TNF-α and IL-33 before treatment in the two groups. The results showed that the AUC of TNF-α in predicting efficacy was 0.939, 95CI%: 0.886-0.992, sensitivity: 93.33%, specificity: 88.00%, and Youden index: 81.33%. While the AUC of IL-33 in predicting efficacy was 0.853, 95CI%: 0.754-0.952, sensitivity: 93.33%, specificity: 88.00%, and Youden index: 81.33%, as shown in Figure 3.
Figure 2.
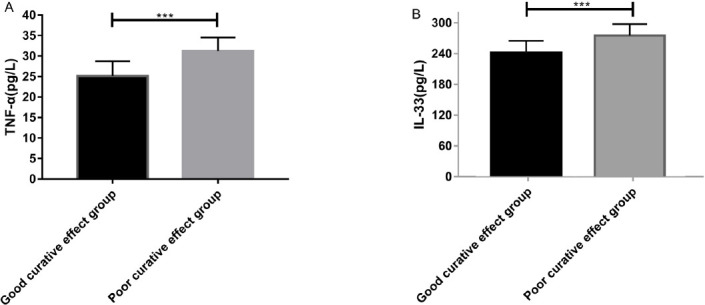
Expression of TNF-α and IL-33 in serum of patients with good and poor curative effect before treatment. A) The serum TNF-α level in the good curative effect group (25.162±3.583) before treatment was notably lower than that of the poor curative effect group (3 1.238±3.274). B) The serum IL-33 level in the good curative effect group (242.748±23.582) was significantly lower than that of the poor curative effect group (276.342±22.465) (P<0.001).
Figure 3.
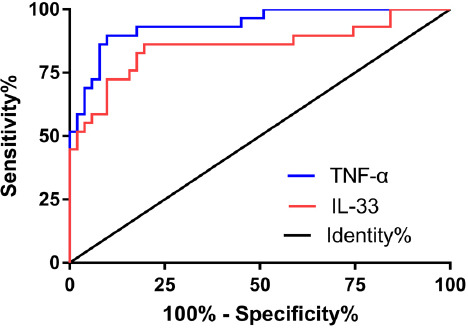
ROC curve for predicting the efficacy of TNF-α and IL-33 in AS patients. The red line was the ROC curve of IL-33 in efficacy prediction of AS, and the AUC was 0.853, 95CI%: 0.754-0.952, sensitivity: 93.33%, specificity: 88.00%, and Youden index: 81.33%. The blue line was the ROC curve of TNF-α in efficacy prediction of AS, the AUC was 0.939, 95CI%: 0.886-0.992, the sensitivity was 93.33%, the specificity was 88.00%, and the Youden index was 81.33%.
Discussion
AS has common clinical manifestations and genetic susceptibility[14], with higher mortality and disability. The pathogenesis of AS remains unclear. The development of ossification in early AS patients is related to inflammation[15], as reported by Walter et al[16], inflammation promoted osteophyte formation. Studies have shown that the inflammatory factor IL-33 is highly expressed in AS patients, which can improve the role of TNF-α and participate in the development of AS[17]. There is also evidence that TNF-α inhibitors can lead to bone renewal and balance, which is beneficial to bone formation[18]. As a commonly used TNF-α inhibitor in clinical practice, INF is comparable to methotrexate in terms of serious adverse events. At the same time, with better efficacy, so infliximab is more advantageous in treatment[19]. In this study, conventional drugs and infliximab were used to treat patients with AS, and their effects on TNF-α and IL-33 expression levels, bone changes and clinical efficacy were studied.
In the present study, the included AS patients were divided into INF group and MTX group according to different treatment methods. By detecting the changes of serum TNF-α and IL-33 expression levels before and after treatment, it was found that there was no significant difference in them between the two groups before treatment. After treatment, the serum TNF-α and IL-33 expression levels decreased significantly in both groups, and the expression levels of TNF-α and IL-33 in serum of patients in INF group were markedly lower than those of patients in MTX group, indicating that TNF-α and IL-33 may be implicated in the progression of AS, suggesting that the two may be used as predictors of curative effect of this disease. It also shows that infliximab could significantly reduce inflammation and improve the condition compared with traditional drugs. In the study of Gong et al[20], IL-33 and IL-33 mRNAs were significantly elevated in patients with AS, suggesting that they played an important role in the development of AS, which was basically consistent with our research. There are studies showing that the expression level of TNF-α in patients with AS is a promising serological indicator involved in AS[21]. We then examined the bone changes before and after treatment in the two groups of patients and found that infliximab significantly improved bone erosion and tendon thickening compared with methotrexate, but had litter effect on improving osteophytes and tendon calcification, indicating that infliximab significantly improved the bone quality of patients with AS with better treatment effect. According to previous research, infliximab is ineffective in 10% of patients, suggesting that lowering the dose may improve the efficacy[22]. In order to further confirm the clinical efficacy of infliximab in AS patients, we further analyzed the morning stiffness time, BASFI, BASDAI, and PGA scores of patients before and after treatment. The improvement of various indexes in INF group was significantly higher than that in MTX group after treatment, suggesting that infliximab remarkably improved the clinical symptoms of patients with AS. Studies by Vicente et al[23] also verified that infliximab was a TNF-α inhibitor with high application frequency and considerable efficacy.
TNF-α and IL-33 are involved in the development of AS. According to the PGA score after treatment, we divided the patients into two groups: good curative effect group and poor curative effect group. It was found that the expression levels of serum TNF-α and IL-33 in the good curative effect group were significantly lower than those in the poor curative effect group, suggesting that TNF-α and IL-33 can sever as predictors of the curative effect of AS. Further plotting the ROC revealed that, the AUC of IL-33 in efficacy prediction of AS was 0.853, 95CI%: 0.754-0.992, the sensitivity was 93.33%, the specificity was 88.00%, and the Youden index was 81.33%. While the AUC of TNF-α in efficacy prediction of AS was 0.939, 95CI%: 0.886-0.992, the sensitivity was 93.33%, the specificity was 88.00%, and the Youden index was 81.33%. All these indicated that TNF-α and IL-33 had high predictive value in patients with AS.
The therapeutic effect of infliximab is widely acknowledged clinically, but the relapse rate after discontinuation is high, and the onset time is short, suggesting that patients with severe active AS must continue treatment[24]. Studies have shown that patients receiving long-term infliximab therapy still maintain stable body function and spinal activity[25]. The treatment time of this study is relatively conventional, but in order to further confirm the clinical efficacy of infliximab, we hope to change the treatment dose and continue to conduct in-depth research on its long-term efficacy to provide valuable references for clinical practice.
In summary, infliximab can significantly improve bone status and has positive effect in AS patients. TNF-α and IL-33 are expected to be used as predictive indicators of AS efficacy.
Authors’ contributions
LL wrote the manuscript, interpreted and analyzed the data. BC and GW designed the study and performed the experiment. HZ was responsible for the analysis and discussion of the data. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 2.Jang JH, Ward MM, Rucker AN, Reveille JD, Davis JC Jr, Weisman MH, Learch TJ. Ankylosing spondylitis:patterns of radiographic involvement-a re-examination of accepted principles in a cohort of 769 patients. Radiology. 2011;258:192–198. doi: 10.1148/radiol.10100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–657. doi: 10.1093/rheumatology/ket387. [DOI] [PubMed] [Google Scholar]
- 4.Akkoc N. Are spondyloarthropathies as common as rheumatoid arthritis worldwide? Curr Rheumatol Rep. 2008;10:371–378. doi: 10.1007/s11926-008-0060-3. [DOI] [PubMed] [Google Scholar]
- 5.Ho HH, Chen JY. Ankylosing spondylitis:Chinese perspective, clinical phenotypes, and associated extra-articular systemic features. Curr Rheumatol Rep. 2013;15:344. doi: 10.1007/s11926-013-0344-0. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh MY, Kuo CF. FRI0428 Epidemiology of Ankylosing Spondylitis in Taiwan:A Nationwide Population Study. Ann Rheum Dis. 2016;2016:590–591. [Google Scholar]
- 7.Li YH, Zhang XY, Li QH, Zheng DH, Dai L. Andersson lesion in ankylosing spondylitis:a clinical study of 14 cases. Zhonghua Yi Xue Za Zhi. 2017;97:517–521. doi: 10.3760/cma.j.issn.0376-2491.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Golder V, Schachna L. Ankylosing spondylitis:an update. Aust Fam Physician. 2013;42:780–784. [PubMed] [Google Scholar]
- 9.Liu Z, Gao L, Wang P, Xie Z, Cen S, Li Y, Wu X, Wang L, Su H, Deng W, et al. TNF-αinduced the enhanced apoptosis of mesenchymal stem cells in ankylosing spondylitis by overexpressing TRAIL-R2. Stem ells nt. 2017;2017:4521324. doi: 10.1155/2017/4521324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XL, Lin TT, Qi CY, Yuan L, Xia LP, Shen H, Lu J. Elevated serum level of IL-33 and sST2 in patients with ankylosing spondylitis:associated with disease activity and vascular endothelial growth factor. J Investig Med. 2013;61:848–851. doi: 10.2310/JIM.0b013e31828deed2. [DOI] [PubMed] [Google Scholar]
- 11.Elalouf O, Elkayam O. Long-term safety and efficacy of infliximab for the treatment of ankylosing spondylitis. Ther Clin Risk Manag. 2015;11:1719. doi: 10.2147/TCRM.S55928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis:a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:155–161. doi: 10.1016/j.semarthrit.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden S, Valkenburg HA, Cats A. Valkenburg, and Arnold Cats. “Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoudi M, Aslani S, Nicknam MH, Karami J, Jamshidi AR. New insights toward the pathogenesis of ankylosing spondylitis;genetic variations and epigenetic modifications. Mod Rheumatol. 2017;27:198–209. doi: 10.1080/14397595.2016.1206174. [DOI] [PubMed] [Google Scholar]
- 15.Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, Zeidler H, Braun J, Sieper J. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:1369–1374. doi: 10.1136/ard.2010.145995. [DOI] [PubMed] [Google Scholar]
- 16.Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis:evidance of a relationship between in flammation and new bone formation. Arthritis Rheum. 2009;60:93–102. doi: 10.1002/art.24132. [DOI] [PubMed] [Google Scholar]
- 17.Han GW, Zeng LW, Liang CX, Cheng BL, Yu BS, Li HM, Zeng FF, Liu SY. Serum levels of IL-33 is increased in patients with ankylosing spondylitis. Clin Rheumatol. 2011;30:1583–1588. doi: 10.1007/s10067-011-1843-x. [DOI] [PubMed] [Google Scholar]
- 18.Arends S, Spoorenberg A, Houtman PM, Leijsma MK, Bos R, Kallenberg CG, Groen H, Brouwer E, van der Veer E. The effect of three years of TNFαblocking therapy on markers of bone turnover and their predictive value for treatment discontinuation in patients with ankylosing spondylitis:a prospective longitudinal observational cohort study. Arthritis Res Ther. 2012;14:R98. doi: 10.1186/ar3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibofsky A, Palmer W, Keystone E, et al. Safety profiles of disease-modifying anti-rheumatic drugs and biologics in patients with rheumatoid arthritis:observations from the RADIUS registry. Arthritis Rheum. 2009;60:1593. [Google Scholar]
- 20.Gong SH, Shi XB, Bao CL. Expression and significance of IL-33 and IL-33 mRNA in peripheral blood of patients with ankylosing spondylitis. Chinese Journal of Primary Medicine and Pharmacy. 2017;24:45–48. [Google Scholar]
- 21.Baraliakos X, Brandt J, Listing J, Haibel H, Sörensen H, Rudwaleit M, Sieper J, Braun J. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept:clinical and magnetic resonance imagingdata. Arthritis Rheum. 2005;53:8568–63. doi: 10.1002/art.21588. [DOI] [PubMed] [Google Scholar]
- 22.Moreno M, Gratacós J, Torrente-Segarra V, Sanmarti R, Morlà R, Pontes C, Llop M, Juanola X. REMINEA tudy roup:Withdrawal of infliximab therapy in ankylosing spondylitis in persistent clinical remission, results from the REMINEA study. Arthritis Res Ther. 2019;21:88. doi: 10.1186/s13075-019-1873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudero-Vilaplana V, Ramírez-Herráiz E, Alañón-Plaza E, Trovato-López N, García-Vicuña R, Carreño-Pérez L, Morell-Baladrón A, Sanjurjo-Sáez M. Efficiency of adalimumab, etanercept and infliximab in ankylosing spondylitis in clinical practice. Int J Clin Pharm. 2015;37:808–814. doi: 10.1007/s11096-015-0124-1. [DOI] [PubMed] [Google Scholar]
- 24.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, et al. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis. 2005;64:229–234. doi: 10.1136/ard.2004.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poddubnyy D, Fedorova A, Listing J, Haibel H, Baraliakos X, Braun J, Sieper J. Physical function and spinal mobility remain stable despite radiographic spinal progression in patients with ankylosing spondylitis treated with TNF-αinhibitors for up to 10 ayears. J Rheumatol. 2016;43:2142–2148. doi: 10.3899/jrheum.160594. [DOI] [PubMed] [Google Scholar]


