Abstract
Objective:
To investigate the expression of micro ribonucleic acid (miR)-214 in the bone tissue and blood of patients with fragility fracture.
Methods:
The expression of miR-214 was detected via quantitative reverse transcription-polymerase chain reaction. The effect of miR-214 on proliferation and apoptosis of osteoblasts were detected via methyl thiazolyl tetrazolium assay and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining.
Results:
The expression of miR-214 in the bone tissue and blood of patients with fragility fracture significantly declined. miR-214 could promote the proliferation of osteoblasts and inhibited the apoptosis of osteoblasts. miR-214 is involved in fracture healing through inhibiting Sox4 and promoting phosphorylation of PI3K/AKT pathway. The expression of BSP in cells treated with miR-214 mimics was significantly increased to 2.5-fold (p=0.0168), while the expression of BSP in cells treated with miR-214 AMO was significantly decreased, reduced to 0.3 times (p=0.0397). The expression of BMP2 in cells treated with miR-214 mimics was significantly increased to 2.5-fold (p=0.003), while the expression of BMP2 was significantly decreased in cells treated with miR-214 AMO, reduced to 0.3 times (p=0.0002). miR-214 can regulate the expression of Sox2, PI3K and AKT proteins.
Conclusion:
MiR-214 regulates the proliferation, apoptosis, bone formation of osteoblasts and participate in the fracture healing process by inhibiting the expression of Sox4, which provided new ideas for clinical treatment of fracture healing.
Keywords: Apoptosis, miR-214, Osteoblasts, Proliferation, Sox4
Introduction
Osteoporosis (OP) is a systemic metabolic disease characterized by decline in bone mineral density, impairment of bone microstructure, increase in bone fragility and decrease in bone mass, which is commonly seen in the elderly, especially in postmenopausal women[1,2]. The incidence rate of fragility fracture is also increased in OP patients due to the increased bone fragility, and fragility fracture is the most severe clinical symptom of OP, seriously affecting the patients’ health and quality of life[3,4]. Fragility fracture refers to any type of fracture caused by the non-traumatic or minimal wound (the severity of wound does not exceed that of fall injury when standing or walking on a level road), and it frequently occurs in the vertebral body, hip, proximal humerus and distal radius[5-7]. At present, the research on fragility fracture mainly focuses on the physical factors, but there are few studies on the relevant indexes of patients[8,9].
Micro ribonucleic acid (miRNA) is a type of non-coding endogenous small RNA existing in eukaryotes, which consists of about 20-25 nucleotides[10], which degrades the target messenger RNA (mRNA) or inhibits its translation through the specific binding to the 3’ untranslated region of target mRNA, thereby regulating a variety of biological functions, such as cell cycle, proliferation, differentiation and apoptosis, organ function and other pathophysiological functions[11]. In recent years, studies have found that miRNAs are closely related to the renewal, growth and metabolism of bone tissues, and the occurrence of bone diseases[12,13]. However, the correlation between miRNAs and fragility fracture is less studied, and its mechanism remains unclear. In the preliminary study, it was found that the expression of miR-214 may be related to the occurrence and development of fracture. In this study, it was found that the expression of miR-214 in the bone tissue and blood of patients with fragility fracture was significantly decreased. The further experiments revealed that miR-214 could regulate fracture healing through inhibiting Sox4 and activating phosphatidylinositol 3-hydroxy kinase/protein kinase B (PI3K/AKT) pathway, which provides a basis and new ideas for the clinical diagnosis and treatment of fragility fracture.
General data and methods
Materials
The human osteoblast cell line HCO was purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. α-MEM culture solution and fetal bovine serum (FBS) were purchased from Hyclone, USA. Trypsin, penicillin-streptomycin and phosphate buffered saline (PBS) were purchased from Solarbio, China. Cell culture flask and other consumables were purchased from NEST, USA. Serum-free Opti medium and TRIzol reagent were purchased from Invitrogen, USA. Methyl thiazolyl tetrazolium (MTT) powder was purchased from Biosharp, China. Organic reagents, such as dimethyl sulfoxide (DMSO), were purchased from Shanghai Rhawn Technology Development Co., Ltd. The reverse transcription kit, transfection reagent X-treme, RNA Keeper and SYBR Green reagent were purchased from Vazyme, China. MiR-214 mimics, miR-214 AMO and its negative control (NC) and PCR primers were designed and synthesized by Shanghai Generay Biotechnology Co., Ltd. The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kit was bought from Roche, Switzerland. 4% paraformaldehyde, protein lysis buffer, Hoechst staining solution, BCA protein concentration assay kit and protein loading buffer were bought from Beyotime, China.
Methods
Collection of clinical samples
A total of 35 patients with fragility fracture treated in The Third Affiliated Hospital of Qiqihar Medical University from January 2016 to March 2017 were randomly selected, including 15 cases of hip fracture, 10 cases of proximal humeral fracture and 10 cases of distal radius fracture. The age ranged from 25 to 70 years old, and the average age was (56.5±18.5) years old, including 17 male patients and 18 female patients, all patients suffered a fragile fracture linked to osteoporosis. Another 35 patients with traumatic fracture were collected as controls. The general information of the two groups is shown in Table 1.
Table 1.
General information of two groups.
| Traumatic fracture group | fragility fracture group | |
|---|---|---|
| Number | 35 | 35 |
| Male | 19 | 17 |
| Female | 16 | 18 |
| Age range (year) | 25-70 | 25-70 |
| Average age (year) | 53.0±20.5 | 56.5±18.5 |
Inclusion criteria in fragility fracture group were: 1) patients with hip fracture, proximal humeral fracture, and distal radius fracture, 2) the injury mechanism was the fragility fracture (falling, slipping, sprain on flat ground, etc.), and 3) patients with fragility fracture for the first time. Inclusion criteria in traumatic fracture group were: 1) patients with hip fracture, proximal humeral fracture, and distal radius fracture, and 2) patients with pathological fracture caused by severe violence, such as traffic accident, crush injury, and falling accident. Exclusion criteria were: 1) patients without history of tumor, tuberculosis, metastatic tumor or chemoradiotherapy, 2) patients complicated with chronic liver or kidney disease, endocrine disease, bone metabolism-related diseases, tumor or blood system diseases, 3) patients who took diphosphonate, calcitonin, estrogen, steroid hormones or other drugs affecting the bone metabolism for a long time, 4) patients who had hysterectomy or oophorectomy, or premature menopause, 5) patients without severe sequelae caused by cerebral arterial vascular diseases, 6) patients with gastric ulcer, inflammatory bowel disease, chronic dysentery, etc. in the previous 2 years, 7) patients who were vegetarians or bedridden for a long time, or who could not take care of themselves, 8) patients with a coagulation abnormality or 9) patients with a mild fracture with no need for surgery.
The bone tissue at the fracture site was collected from patients, immediately treated with RNA Keeper and stored in an ultra-low temperature refrigerator at -80°C or liquid nitrogen tank. At the same time, the blood samples (6 mL fasting blood in the morning) were collected from both groups within 24 h after admission and at 7 d, 14 d and 21 d after standardized fixation treatment. The clinical samples of patients of the same age range caused by trauma in the same period as the disease group was used as the control group, and the control group patients had no osteoporosis. The blood samples were stored in an ultra-low temperature refrigerator at -80 °C or immediately sent for inspection.
The study was approved by the Ethics Committee of The Third Affiliated Hospital of Qiqihar Medical University, and all operations were in accordance with the regulations of the Ethics Committee. All patients volunteered to participate and signed informed consent.
RNA extraction and real-time fluorescence quantitative polymerase chain reaction (PCR)
The bone tissue was ground into powder under liquid nitrogen, while the blood sample was not ground. The nucleated cells were obtained after the red blood cells were lysed using the low-osmosis method, and the total RNA was extracted using TRIzol from the tissue and blood samples. 500 ng RNA and 4 μL reverse transcription reagent were added into a 200 μL EP tube according to the instructions of the kit, and the autoclaved distilled water was added till the total volume reached 10 μL. Then the EP tube was placed in a reverse transcription machine for reverse transcription at 50°C for 15 min and 85°C for 5 s. After RNA was reversely transcribed into cDNA, qRT-PCR was performed. 10 μL SYBR Green reagent, 1 μL cDNA, 7 μL distilled water, 1 μL forward primer and 1 μL reverse primer were added into the PCR plate. The qRT-PCR conditions are as follows: 95°C for 30 s, 95°C for 5 s, 60°C for 30 s, 72°C for 30 s, a total of 40 cycles, 72°C for 30 s.
GAPDH was used as an internal reference gene, and the expression levels of miR-214, Sox4, bone sialoprotein (BSP) and bone morphogenetic protein 2 (BMP2) were detected using the 2-ΔΔCt method. All primer sequences used in this experiment are shown in Table 2.
Table 2.
Primer sequences in qRT-PCR.
| Primer | Forward | Reverse |
|---|---|---|
| miR-214 | ACAGCAGCACAGACAGGCAGT | GTCTACGGCCATACCACCCTGA |
| GAPDH | TCCACTGGCGTCTTCACC | GGCAGAGATGATGACCCTTTT |
| Sox4 | CTTGACATGATTAGCTGGCATGATT | CCTGTGCAATATGCCGTGTAGA |
| BSP | CTATGAAGGCTACGAGGGTCAG | GCAAAGACAGAATGGGGAGTC |
| BMP2 | CAGCGAGTTTGAGTTGAGG | CGGTACAGGTCGAGCATAT |
Cell culture
The human osteoblast cell line MC3T3-E1 was cultured in the α-MEM containing 10% FBS and 100 U/mL penicillin-streptomycin, inoculated into a 25 cm2 cell culture flask, and incubated in an incubator (Thermo, USA) at 37°C. After 24 h, the cells were observed under an inverted optical microscope (Nikon, Japan), followed by passage when 80-90% cells were fused at a ratio of 1:2 or 1:3.
Cell transfection
After the cells were inoculated into the culture plate and 50-60% cells were fused, the transfection was performed using the transfection reagent X-treme, serum-free Opti medium and miRNAs. The cells were divided into miR-214 mimics (final concentration: 50 nM) group, mimics NC (final concentration: 50 nM) group, miR-214 AMO (final concentration: 100 nM) group and AMO NC (final concentration: 100 nM) group. At 6 h after transfection, the original medium was replaced with the fresh serum medium, followed by subsequent experiments after 24 h. miR-214 mimic, forward: 5′-ACAGCAGGCACAGACAGGCAG-3’, reverse: 5′-GCCUGUCUGUGCCUGCUGUUU-3’.
miR-214 inhibitor, 5′-CUGCCUGUCUGUGCCUGCUGU-3’;
mimics-NC, forward: 5′-UUCUCCGAACGUGUCACGUTT-3’,
reverse: 5′-ACGUGACACGUUCGGAGAATT-3’;
NC inhibitor, 5′-CAGUACUUUUGUGUAGUACAA-3’.
MTT assay
After the cells were inoculated into a 96-well plate, they were transfected with miR-214. After 24 h, 10 μL MTT staining solution was added into each well, followed by incubation at 37°C for another 4 h. After 4 h, the liquid in the well was discarded, 150 μL DMSO was added and the mixture was shaken to fully dissolve the crystals. Then the optical density (OD) value was measured at a wavelength of 490 nm using a microplate reader (TECAN, Germany).
TUNEL assay
After the cells were inoculated into the small dish, they were transfected with miR-214 mimics, AMO and corresponding NC for 24 h, and the apoptosis level was detected using the TUNEL kit. The culture medium in the dish was discarded, and cells were washed with PBS and fixed with 4% paraformaldehyde. After the cells were sealed and penetrated, Vial1 and Vial2 were mixed at a ratio of 1:9 according to instructions of the kit to incubate cells in a dark place for 1 h. After the cells were washed with PBS, they were incubated with Hoechst staining solution, followed by observation under the inverted fluorescence microscope (Nikon, Japan) and photography.
Prediction of target gene
The target gene of human miR-214 was predicted through the TargetScan Human7.0 online website (http://www.targetscan.org/vert_70/), and finally Sox4 was screened as the target gene according to references. Among the target genes of miR-214, it has been reported that Sox4 can inhibit bone formation and destroy osteogenic differentiation[14-16]. Therefore, Sox4 was screened as the target gene in this experiment for subsequent research.
Statistical analysis
All data were expressed as Mean±SD. The comparison between sample means was performed by t-test. Comparisons among multiple groups were analyzed by variance (ANOVA) followed by Student-Newman Keuls (SPSS 19.0, U.S.A) post hoc test. When *p<0.05, **p<0.01 and ***p<0.001, the difference was statistically significant.
Results
Expression of miR-214 in bone tissue and blood of patients with fragility fracture
The results of qRT-PCR shown that the expression of miR-214 in the bone tissue and blood were significantly decreased in the fragility fracture group compared with those in the control group, and the differences were statistically significant (p<0.001) (Figure 1A & 1B).
Figure 1.
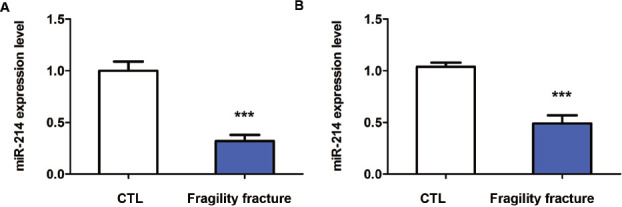
Expression of miR-214 in patients with fragility fracture. (A) Expression of miR-214 in bone tissue of patients in control group and fragility fracture group. (B) Expression of miR-214 in blood of patients in control group and fragility fracture group. ***p<0.001: The difference is statistically significant.
Expression of miR-214 in blood of patients with fragility fracture at different stages
The expression of miR-214 in the blood of patients with traumatic fracture was detected at admission and at 7 d, 14 d and 21 d after standardized fixation treatment. The results showed that there were no changes in the miR-214 expression at 7 d, 14 d and 21 d compared with that at admission, and the differences were not statistically significant (p>0.05) (Figure 2). Compared with that in patients with traumatic fracture, the miR-214 expression was gradually increased in a time-dependent manner in patients with fragility fracture at 7 d, 14 d and 21 d after standardized fixation treatment, and reached the peak at 21 d (Figure 2).
Figure 2.
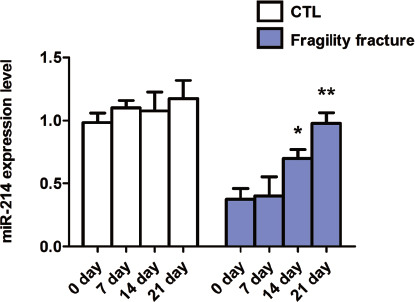
Expression of miR-214 in blood of patients with fragility fracture and traumatic fracture. Expression of miR-214 in blood of patients with fragility fracture and traumatic fracture within 24 h after admission and at 7 d, 14 d and 21 d after treatment. **p<0.01 and ***p<0.001: The difference is statistically significant.
Effect of miR-214 on proliferation and apoptosis of osteoblasts
To further explore the effects of miR-214 on proliferation and apoptosis of osteoblasts, we transfected miR-214 mimics, miR-214 AMO, mimics-NC and AMO-NC into osteoblasts. Compared with mimics-NC, the expression of miR-214 in osteoblasts transfected with miR-214 mimics increased significantly, while the expression of miR-214 in osteoblasts transfected with miR-214 AMO decreased significantly compared with AMO-NC (Figure 3A). The results of MTT assay manifested that compared with mimics NC, miR-214 mimics could significantly increase the OD value (Figure 3B), while miR-214 AMO could reduce the OD value (Figure 3A). The results of TUNEL staining showed that miR-214 mimics could reduce the number of TUNEL-positive cells, while miR-214 AMO could significantly increase the number of TUNEL-positive cells (Figure 3C).
Figure 3.
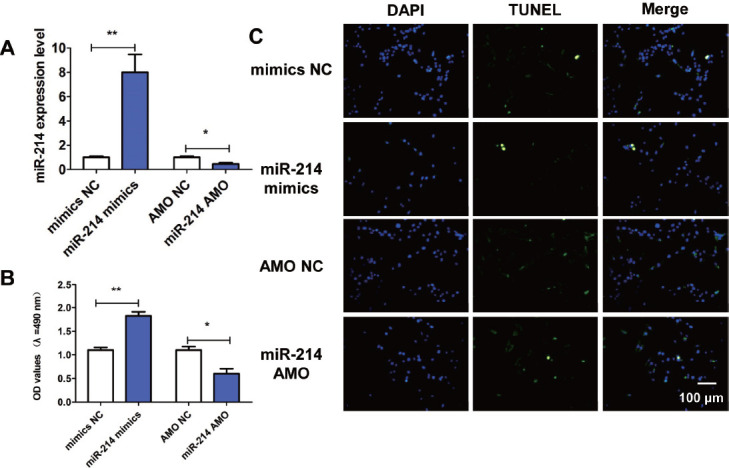
Influences of miR-214 on proliferation and apoptosis of osteoblasts. (A) Transfection efficiency of miR-214. (B) Influence of miR-214 on proliferation of osteoblasts detected via MTT assay. (C) Influence of miR-214 on apoptosis of osteoblasts detected via TUNEL staining. *p<0.01 and **p<0.001: The difference is statistically significant.
Effect of miR-214 on bone formation-related genes
The results of qRT-PCR showed that the expressions of BSP and BMP2 in miR-214 mimic group were increased, while miR-214 AMO could reduce their expressions (Figure 4), indicating that miR-214 is involved in bone formation and promotes the bone formation.
Figure 4.
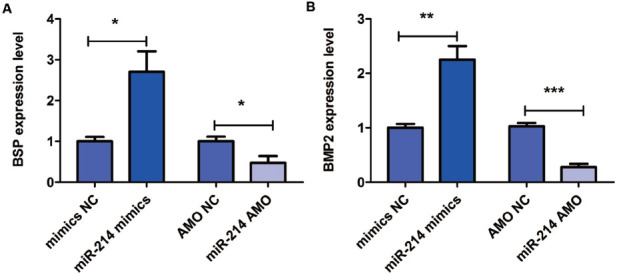
Influence of miR-214 on bone formation. (A) Influences of miR-214 mimics and AMO on expression level of bone formation-related gene BSP detected via qRT-PCR. (B) Influences of miR-214 mimics and AMO on expression level of bone formation-related gene BMP2 detected via qRT-PCR. *p<0.05, **p<0.01 and ***p<0.001: The difference is statistically significant.
Mechanism of miR-214 in regulating fracture healing
The target prediction showed that Sox4 was the target gene of miR-214, and the binding sites of miR-214 and Sox4 are shown in Figure 5A. MiR-214 could remarkably reduce the Sox4 mRNA level, while miR-214 AMO could increase the Sox4 mRNA level (Figure 5B). The aforementioned results suggest that miR-214 is involved in the biological function of osteoblasts through inhibiting its target gene Sox4.
Figure 5.
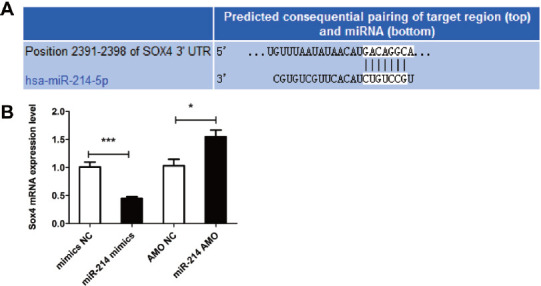
Mechanism of miR-214 in osteoblasts. (A) Binding sites of miR-214 and its target gene Sox4. (B) Influence of miR-214 on Sox4 mRNA detected via qRT-PCR. *p<0.05 and ***p<0.001: The difference is statistically significant.
Discussion
MiRNAs can induce mRNA degradation or inhibit its further translation through the complete or incomplete complementary pairing with the 3’ untranslated region of target mRNA, thereby participating in a variety of important biological processes, such as cell proliferation, differentiation, apoptosis, migration, invasion and adsorption[17,18]. A large number of studies have reported that miRNAs are closely related to bone formation, resorption and metabolism, and play an important regulatory role in the occurrence and development of osteoporosis, osteoarthritis and osteosarcoma[19,20]. It has been reported that miR-142-5p in bone mesenchymal stem cells reduces the cell migration ability through targeted regulation on the vascular cell adhesion molecule-1, thereby promoting the development of osteoporosis[21]. MiR-137 leads to the activity and expression dysregulation of alkaline phosphatase through inhibiting the leucine-rich complex G-protein-coupled receptor 4, thus participating in the occurrence of osteoporotic fracture[22]. MiR-299-5p acts on the CDK family to regulate the proliferation and cycle of osteosarcoma cells, which has great clinical significance[17]. Also, miR-6 inhibits the migration and invasion of osteosarcoma cells by reducing the expression of RAB23, thus providing new targets and ideas for the clinical treatment of osteosarcoma[23].
It has been reported that the PI3K/Akt signal transduction pathway plays an essential regulatory role in cell metabolism, proliferation, apoptosis and adhesion[24]. Akt is one of the key proteins in the PI3K/Akt signaling pathway and is a key kinase downstream of PI3K, which is involved in cell proliferation, growth and survival[25]. p-Akt is a mediator of Akt activation, which activates the mammalian target of rapamycin (mTOR) downstream of the PI3K/Akt signaling pathway, thereby interfering with life activities such as apoptosis[26]. Studies have shown that the PI3K/Akt signal transduction pathway plays an important role in bone formation and bone regeneration[27,28]. It was found in this study that the miR-214 expression in the bone tissue and blood of patients with fragility fracture was significantly decreased, so it is believed that miR-214 may be related to the occurrence of fragility fracture. Further studies showed that the expression of miR-214 was gradually increased with the extension of regular treatment time for patients with fragility fracture. The expression of miR-214 was increased by about twice at 21 d after treatment compared with that before treatment. Therefore, it is believed that miR-214 plays an important role in the occurrence and development of fragility fracture and it may serve as an important target for the clinical diagnosis and treatment of fragility fracture. Moreover, the results of MTT and TUNEL assays revealed that miR-214 could regulate the biological functions of osteoblasts, promote its proliferation and inhibit its apoptosis. The results of qRT-PCR proved that the transfection with miR-214 could up-regulate the expressions of bone formation-related genes BSP and BMP2 in osteoblasts. According to the mechanism research, miR-214 could down-regulate the expression of its target gene Sox4. The above results indicate that miR-214 regulates the osteoblast proliferation, apoptosis and bone formation through inhibiting the expression of Sox4, thus participating in the occurrence and development of fragility fracture.
In conclusion, miR-214 is significantly reduced in the bone tissue and blood of patients with fragility fracture, which can regulate the osteoblast proliferation, apoptosis and bone formation, thereby playing an important role in fragility fracture. This study introduce a new index and target for clinical diagnosis and treatment of fragility fracture, and provides an experimental basis and theoretical knowledge for the research on fragility fracture in the future.
Authors’ contributions
ZX and WS were responsible for PCR. DC and JW helped with cell culture and transfection. LM worked on prediction of target gene; FS, CT and LH contributed to MTT assay and TUNEL assay. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Shu X, Mei M, Ma L, Wang Z, Yang S, Hu J, Song Y, He W, Luo T, Cheng Q, Wang Y, Zhen Q, Li Q Chongqing Primary Aldosteronism Study(CONPASS) Group. Postmenopausal osteoporosis is associated with elevated aldosterone/renin ratio. J Hum Hypertens. 2018;32:524–530. doi: 10.1038/s41371-018-0069-7. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou T, Macdonald EM, Yao Z, Gomes T, Tadrous M, Ho JM, Mamdani MM, Juurlink DN. A population-based study of the risk of osteoporosis and fracture with dutasteride and finasteride. BMC Musculoskelet Disord. 2018;19:160. doi: 10.1186/s12891-018-2076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, Maasalu K, Bolognese MA, Woodson G, Bone H, Ding B, Wagman RB, San Martin J, Ominsky MS, Dempster DW Denosumab?Phase 3?Bone?Histology Study Group. Effects of denosumab on bone histomorphometry:the FREEDOM and STAND studies. J Bone Miner Res. 2010;25:2256–2265. doi: 10.1002/jbmr.149. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Gori F. Targeting WNT signaling in the treatment of osteoporosis. Curr Opin Pharmacol. 2018;40:134–141. doi: 10.1016/j.coph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Gross T. [Patient- vs Physician-Reported Implementation of and Compliance to Anti-Osteoporotic Medication One Year after Sustained Fragility Fracture] Praxis (Bern 1994) 2018;107:573–584. doi: 10.1024/1661-8157/a002992. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama T. Observational studies investigating hip fracture risk:a fundamental methodological issue? J Intern Med. 2018;284:325–326. doi: 10.1111/joim.12773. [DOI] [PubMed] [Google Scholar]
- 7.Sultan AA, Whittle R, Muller S, Roddy E, Mallen CD, Bucknall M, Helliwell T, Hider S, Paskins Z. Risk of fragility fracture among patients with gout and the effect of urate-lowering therapy. Cmaj. 2018;190:E581–E587. doi: 10.1503/cmaj.170806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanazawa I, Sugimoto T. Diabetes Mellitus-induced Bone Fragility. Intern Med. 2018;57:2773–2785. doi: 10.2169/internalmedicine.0905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Beom J, Kim BR, Lim SK, Lim JY. Comparative effectiveness of fragility fracture integrated rehabilitation management for elderly individuals after hip fracture surgery:A study protocol for a multicenter randomized controlled trial. Medicine (Baltimore) 2018;97:e10763. doi: 10.1097/MD.0000000000010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge DW, Wang WW, Chen HT, Yang L, Cao XJ. Functions of microRNAs in osteoporosis. Eur Rev Med Pharmacol Sci. 2017;21:4784–4789. [PubMed] [Google Scholar]
- 11.Cai M, Yang L, Zhang S, Liu J, Sun Y, Wang X. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int J Nanomedicine. 2017;12:7469–7482. doi: 10.2147/IJN.S139775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolamperti S, Villa I, Spinello A, Manfredini G, Mrak E, Mezzadri U, Ometti M, Fraschini G, Guidobono F, Rubinacci A. Evidence for Altered Canonical Wnt Signaling in the Trabecular Bone of Elderly Postmenopausal Women with Fragility Femoral Fracture. Biomed Res Int. 2016;2016:8169614. doi: 10.1155/2016/8169614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocijan R, Muschitz C, Geiger E, Skalicky S, Baierl A, Dormann R, Plachel F, Feichtinger X, Heimel P, Fahrleitner-Pammer A, Grillari J, Redl H, Resch H, Hackl M. Circulating microRNA Signatures in Patients With Idiopathic and Postmenopausal Osteoporosis and Fragility Fractures. J Clin Endocrinol Metab. 2016;101:4125–4134. doi: 10.1210/jc.2016-2365. [DOI] [PubMed] [Google Scholar]
- 14.Nissen-Meyer LS, Jemtland R, Gautvik VT, Pedersen ME, Paro R, Fortunati D, Pierroz DD, Stadelmann VA, Reppe S, Reinholt FP, Del Fattore A, Rucci N, Teti A, Ferrari S, Gautvik KM. Osteopenia, decreased bone formation and impaired osteoblast development in Sox4 heterozygous mice. J Cell Sci. 2007;120:2785–2795. doi: 10.1242/jcs.003855. [DOI] [PubMed] [Google Scholar]
- 15.Kodric K, Camernik K, Cerne D, Komadina R, Marc J. P4 medicine and osteoporosis:a systematic review. Wien Klin Wochenschr. 2016;128:480–491. doi: 10.1007/s00508-016-1125-3. [DOI] [PubMed] [Google Scholar]
- 16.Jemtland R, Holden M, Reppe S, Olstad OK, Reinholt FP, Gautvik VT, Refvem H, Frigessi A, Houston B, Gautvik KM. Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. J Bone Miner Res. 2011;26:1793–1801. doi: 10.1002/jbmr.396. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CL, Li LB, She C, Xie Y, Ge DW, Dong QR. MiR-299-5p targets cell cycle to promote cell proliferation and progression of osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22:2606–2613. doi: 10.26355/eurrev_201805_14954. [DOI] [PubMed] [Google Scholar]
- 18.Wei GJ, Zheng KW, An G, Shi ZW, Wang KF, Guan Y, Wang YS, Li PF, Dong DM. Comprehensive Effects of Suppression of MicroRNA-383 in Human Bone-Marrow-Derived Mesenchymal Stem Cells on Treating Spinal Cord Injury. Cell Physiol Biochem. 2018;47:129–139. doi: 10.1159/000489756. [DOI] [PubMed] [Google Scholar]
- 19.Qian M, Gong H, Yang X, Zhao J, Yan W, Lou Y, Peng D, Li Z, Xiao J. MicroRNA-493 inhibits the proliferation and invasion of osteosarcoma cells through directly targeting specificity protein 1. Oncol Lett. 2018;15:8149–8156. doi: 10.3892/ol.2018.8268. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Zhou S, Xiong M, Dai G, Yu L, Zhang Z, Chen J, Guo W. MicroRNA-192-5p suppresses the initiation and progression of osteosarcoma by targeting USP1. Oncol Lett. 2018;15:6947–6956. doi: 10.3892/ol.2018.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng Z, Xie X, Zhu Y, Liu J, Hu X, Na Q, Zhang X, Wei G, Xu S, Liu Y, Zhang X, Xian CJ. miR-142-5p in Bone Marrow-Derived Mesenchymal Stem Cells Promotes Osteoporosis Involving Targeting Adhesion Molecule VCAM-1 and Inhibiting Cell Migration. Biomed Res Int. 2018;2018:3274641. doi: 10.1155/2018/3274641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Xu X. MicroRNA-137 dysregulation predisposes to osteoporotic fracture by impeding ALP activity and expression via suppression of leucine-rich repeat-containing G-protein-coupled receptor 4 expression. Int J Mol Med. 2018;42:1026–1033. doi: 10.3892/ijmm.2018.3690. [DOI] [PubMed] [Google Scholar]
- 23.Jiao ZH, Wang JD, Wang XJ. MicroRNA-16 suppressed the invasion and migration of osteosarcoma by directly inhibiting RAB23. Eur Rev Med Pharmacol Sci. 2018;22:2598–2605. doi: 10.26355/eurrev_201805_14953. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Qin F, Liu A, Sun Q, Wang Q, Xie S, Lu S, Zhang D, Lu Z. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sci. 2019;217:169–175. doi: 10.1016/j.lfs.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Qiu S. Activation of GPR120 promotes the metastasis of breast cancer through the PI3K/Akt/NF-kappaB signaling pathway. Anticancer Drugs. 2019;30:260–270. doi: 10.1097/CAD.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal S, John S, Sapra L, Sharma SC, Das SN. Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemother Pharmacol. 2019;83:451–461. doi: 10.1007/s00280-018-3746-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Yu W, Niibe K, Zhang W, Egusa H, Tang T, Jiang X. The Effects of Platelet-Derived Growth Factor-BB on Bone Marrow Stromal Cell-Mediated Vascularized Bone Regeneration. Stem Cells Int. 2018;2018:3272098. doi: 10.1155/2018/3272098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen Y, Xu X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018;27:1634–1645. doi: 10.1089/scd.2018.0119. [DOI] [PubMed] [Google Scholar]


