Abstract
The anterior insular cortex (AIC) mediates various social, emotional, and interoceptive components of addiction. We recently demonstrated a disruption of prosocial behavior following heroin self-administration in rats, as assessed by examining the animals’ propensity to rescue its cagemate from a plastic restrainer while having simultaneous access to heroin. To examine the possibility that heroin-induced deficits in prosocial function are mediated by the AIC, the present study examined the effects of chemogenetic activation or inhibition of excitatory AIC pyramidal neurons on heroin-induced prosocial deficits. After establishment of baseline rescuing behavior, rats received bilateral infusions of viral vectors encoding either a control virus (AAV-CaMKIIα-GFP), stimulatory DREADD (AAV-CaMKIIα-hM3Dq-mCherry) (Experiment 1), or inhibitory DREADD (AAV-CaMKIIα-hM4Di-mCherry) (Experiment 2), into the AIC. Rats were then allowed to self-administer heroin (0.06 mg/kg/infusion) 6 hr/day for 2 weeks. Prior to re-assessment of prosocial behavior, animals were administered clozapine-N-oxide (1.5 mg/kg, i.p.) to assess effects of chemogenetic activation or inhibition of the AIC. Relative to control animals, chemogenetic activation of the AIC reversed deficits in rescuing behavior induced by heroin, whereas chemogenetic inhibition of the AIC had no effect. We hypothesize that stimulatory neuromodulation of the AIC may be a novel approach for restoring prosociality in opiate abuse.
Keywords: heroin, rescue behavior, rodent, self-administration, chemogenetics, insula
Introduction
Heroin abuse and overdose-related deaths have reached epidemic levels in the United States and elsewhere (Jalal, Buchanich, Roberts, Balmert, Zhang and Burke, 2018). The feelings of euphoria elicited by heroin use believed to result from increased dopamine release throughout the mesolimbic reward circuitry in the brain (Compton and Volkow, 2006; Corre, van Zessen, Loureiro, Patriarchi, Tian, Pascoli et al., 2018; Johnson and North, 1992). Chronic drug use alters brain structure and chemical neurotransmission via depletions in both gray and white matter volume, as well as disrupts connectivity in regions important for decision-making, impulse-control and executive function (Upadhyay, Maleki, Potter, Elman, Rudrauf, Knudsen et al., 2010; Wollman, Alhassoon, Hall, Stern, Connors, Kimmel et al., 2017). One brain region gaining traction for its potential role in driving addiction is the insular cortex (Droutman, Read and Bechara, 2015; Kroll, Nikolic, Bieri, Soyka, Baumgartner and Quednow, 2018; Naqvi, Gaznick, Tranel and Bechara, 2014) which has been shown to be important for integrating the interoceptive constructs of motivation and emotion, as well as those produced by drugs of abuse, into conscious feelings of craving (Koob and Volkow, 2016).
In humans, activity in the insular cortex, particular its anterior subdivision, is correlated with both addiction-related behaviors and abilities needed for prosocial behavior (Heilig, Epstein, Nader and Shaham, 2016). Here, we define prosocial behavior as those that occur with the intent to interact with others. One important aspect of prosocial behavior is empathy, the ability to perceive and understand the emotions or situations of others. fMRI studies have shown that the insula is consistently activated during empathy-related information processing (Fan, Duncan, de Greck and Northoff, 2011). Interestingly, individuals with a history of opioid use demonstrate lower levels of empathetic ability. For example, when levels of empathy were assessed in heroin-dependent individuals, marijuana users, psychiatric patients, prison inmates, and police officers, it was shown that heroin-dependent participants exhibited among the lowest levels of empathy and sociability (Kurtines, Hogan and Weiss, 1975). The loss of empathy and other prosocial behaviors following heroin intake is likely a result of opioid-induced changes in brain mechanisms mediating these behaviors. Recently, Kroll and colleagues (Kroll et al., 2018) further investigated the neural substrates of prosociality, including empathy and the impact of opioids, by administering a battery of neuropsychological test to participants with a history of non-medical prescription opioid use compared to opioid-naïve controls. Results of this study demonstrated opioid-related deficits in the ability to recognize emotions in facial expressions, prosody of voices, and other emotion recognition tasks, and that these deficits were dose-dependent. These data lend further support to the notion that opioid abuse and dependence can induce impairments in prosocial functioning.
In an attempt to lend insight into the neural mechanisms of impaired prosocial function in opioid addiction, we previously utilized an established paradigm in rodents developed by Ben-Ami Bartal and colleagues (Ben-Ami Bartal, Decety and Mason, 2011) in which a rat will release or rescue a conspecific from a plastic restrainer instead of receiving food or other palatable rewards. Our results showed that rats with a history of sucrose self-administration continued to release their cagemate from the restrainer when given the opportunity to perform both tasks simultaneously, whereas rats with a history of heroin self-administration did not continue to release their cagemate, but rather continued to self-administer heroin instead (Tomek et al. 2019). These results are consistent with a study in which rescuer rats administered the abused benzodiazepine midazolam, but not the beta-adrenergic antagonist nadolol, released their conspecific from a restrainer less often, while still opening the restrainer for a palatable food (Ben-Ami Bartal, Shan, Molasky, Murray, Williams, Decety et al., 2016). The authors interpreted these results as a drug-induced interruption or dysregulation social affective processing that appears necessary to motivate the rat to open the restraint door for its cagemate.
In order to further examine the role of the insula in both heroin addiction-related and prosocial behaviors, the current study used the above-mentioned rodent prosocial paradigm as previously published (Tomek, Stegmann and Olive, 2019), in conjunction with chemogenetic approaches to selectively activate or inhibit the excitatory pyramidal neurons within the insula. In this study, we specifically targeted the anterior insular cortex (AIC), due its prominent role in both opioid addiction- and prosocial-related behaviors (Droutman, Read and Bechara, 2015; Naqvi, Gaznick, Tranel and Bechara, 2014; Wollman et al., 2017). Chemogenetic approaches harness the utility of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), which are genetically engineered muscarinic acetylcholine receptors (hM3Dq and hM4Di). These engineered receptors are not activated by endogenous neurotransmitters, but are only activated by an otherwise exogenous inert ligand, such as clozapine-N-oxide (CNO). Here, we refer to hM3Dq as a “stimulatory DREADD”, and hM4Di as an “inhibitory DREADD”, as they are considered stimulatory and inhibitory respectively as a result of activation of Gq and Gi signaling pathways, respectively. Plasmids encoding DREADDS were packaged into an adeno-associated viral vector (AAV), along with cell-type specific promoter (calcium/calmodulin-dependent protein kinase II alpha, CaMKIIα) which allows for selective expression in cortical excitatory neurons (Liu and Jones, 1996), and a fluorescent reporter protein (mCherry). A viral vector lacking the DREADD transgene and encoding green fluorescent protein (GFP) was used as a control. In vitro slice electrophysiology was used to verify DREADD functionality. Based on the existing literature reviewed above, we hypothesized that chemogenetic stimulation of the AIC would improve deficits in prosocial behavior induced by heroin self-administration, whereas chemogenetic inhibition of this region would exacerbate heroin-induced prosocial deficits.
Methods
Animals
A total of n=99 male Sprague Dawley rats (Charles River Laboratories) were used for these studies. Ninety-six rats were used for Experiments 1 and 2, where one half of this number were the rats located inside the restrainer, and thus never underwent surgical procedures or virus infusions. An additional n=3 were used infused with the viral constructs for electrophysiological validation of DREADD function. Rats weighed approximately 250 g upon arrival and were pair-housed upon arrival on a 12-hour reversed light–dark cycle (lights on at 0700 hr) and given ad libitum access to food and water during all experimental procedures except during behavioral testing. All experimental sessions took place during the dark phase of the light-dark cycle. Upon arrival, one rat of each pair was randomly selected and its tail was marked with a permanent marker. These rats were designated the “rescuer rats” while the other rat in each pair was designated the “trapped” rat. Rats were individually handled and weighed for 5 min daily for two weeks post-arrival to allow them to acclimate to experimental procedures. A timeline of all behavioral experiments is provided in Figure 1.
Figure 1.
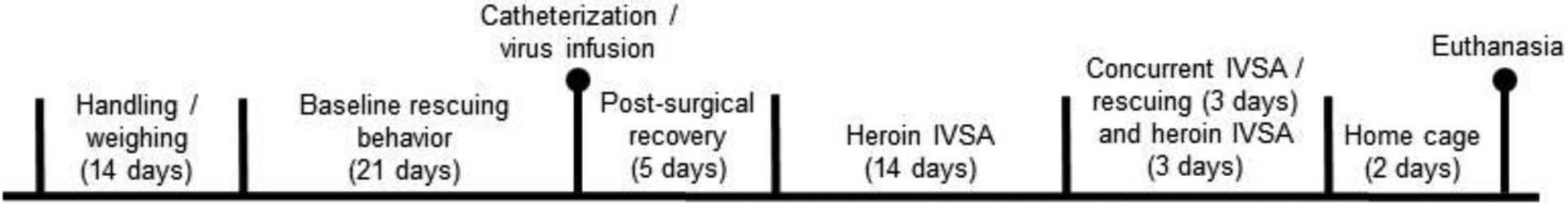
Timeline of Experiments 1 and 2 in the present study.
Assessment of baseline prosocial behavior
Following habituation to handling, animals underwent baseline assessment of prosocial behavior. Testing occurred at the same time every day for 3 weeks. In this procedure, each designated trapped rat was placed in a plastic restrainer (Harvard Apparatus, Holliston, MA; modified to 13.3 cm × 8 cm × 8.9 cm) equipped with a removable door, and the restrainer was placed in an operant conditioning chamber (Med Associates, St. Albans, VT; model ENV-008, modified to 43.2 cm × 20.3 cm × 22.9 cm). All rescuing sessions were video recorded. Following placement of the trapped rat in the restrainer into the operant chamber, the rescuer rat was then placed in the chamber, followed by illumination of a house light. The occurrence of and latency to rescue the trapped rat by opening of the restrainer door was recorded for each session. The maximum amount of time allowed in the operant chamber for each session was initially 1 hr, but then reduced to 45 min and ultimately 30 min over the course of the 3 weeks of testing. The session time was only reduced if the rat began rescuing immediately at the beginning of each session. In order to reduce the possibility that removal from the testing apparatus was a motivating factor for rescuing behavior, upon freeing of the trapped rat, rats remained in the chambers for the duration of the session. After the first week, if the randomly assigned rescuer rat had not shown rescuing behavior at least one time, the roles were reversed where the rescuer rat was assigned to be the trapped rat and its cagemate was assigned to be the rescuer rat. Previous research indicates that rats which experiences the same stressful situation will learn to rescue its cagemate from that situation that more quickly than a naïve rat (Sato, Tan, Tate and Okada, 2015). Rescuer rats were allowed to undergo 2 weeks of baseline rescuing behavior. If after switching the rescuer and trapped rats, the new rescuer rat failed to release the trapped rat across the last two weeks of baseline rescue testing, the pair of rats was removed from the study.
Surgical procedures
After 2 weeks of baseline rescue testing, all rescuer animals were surgically implanted with intravenous catheters into the jugular vein according to our previously published procedures (Tomek, Stegmann and Olive, 2019). Briefly, rats were anesthetized with isoflurane (2% v/v) vaporized in oxygen at a flow rate of 2 l/min. Rats received pre-incision, subcutaneous, injections of buprenorphine (0.05 mg/kg, s.c., Henry Schein Animal Health, Dublin, OH) and meloxicam (1 mg/kg, s.c., Henry Schein Animal Health,). Surgical sites were shaved and disinfected with 1% iodine. An approximately 2 cm incision was made in order to locate and isolate the right or left jugular vein. A sterile silastic catheter filled with 100 U/ml heparin was inserted 2.5 cm into the vein. The catheter was secured to the surrounding tissue with silk sutures, and the opposite end of the catheter was tunneled subcutaneously to the dorsum where it exited the skin between the scapulae. The catheter was secured to the vascular access port subcutaneously and sutured into place. The wound was then closed with Ethicon nylon sutures and topically treated with topical lidocaine and triple antibiotic ointment. A 2.5-cm incision was made between the scapulae for implantation of a threaded vascular access port (Instech Laboratories, Plymouth Meeting, MA). The port was covered with an aluminum cap to prevent damage from cagemate chewing.
Virus infusion procedures were conducted during the same surgical procedure as catheter implantation. Prior to surgery, half of the rescuer rats were randomly assigned to receive either the control virus (AAV8-CaMKIIα-EGFP, ≥1×1012 vg/ml, Addgene #50469), stimulatory DREADD virus (AAV8-CaMKIIα-hM3Dq, ≥3×1012 vg/ml, Addgene #50476), or inhibitory DREADD virus (AAV8-CaMKIIα-hM4Di, ≥2×1012 vg/ml, Addgene #50477) by use of a random number generator. The goal of this randomized approach was to provide an unbiased method for assigning individual rats to treatment groups and experimental conditions (i.e., trapped vs. rescuer rat, type of virus infused). Thus, any individual variability in prosocial behavior and heroin intake, as well as subject attrition due to loss of catheter patency or erroneous virus placement, would be unrelated to experimental group assignment.
The skin overlying the skull was shaved and scrubbed with betadine, and an incision was made to expose the skull surface. After appropriately placed holes were drilled into the skull, a 10 μl Hamilton syringe was lowered into the anterior insular cortex (AP: +2.8, ML: +/− 3.5, DV: −6 mm) from skull surface and bregma according to a stereotaxic atlas (Paxinos and Watson, 2014). A total of 0.5 μl of the appropriate virus was infused into each hemisphere at a rate of 0.25 μl/min. Cranial holes were covered with bone wax dental cement and the wound was sutured closed. The wound was then treated with 2% bacitracin/polymyxin B/neomycin and 5% xylocaine, and sutured closed with 3–0 Vicryl sutures. Animals received meloxicam (2.5 mg/ml, s.c.) once daily for 5 days to minimize post-surgical pain and discomfort. All rats were allowed to recover from surgery for 5 days prior to the initiation of drug self-administration. During this time, the animals received daily intravenous infusions of 70 U/ml heparinized Timentin (66 mg/ml, dissolved in sterile saline containing 70 U/ml heparin, 0.1 ml volume) to maintain catheter patency and protect against infection. Rats were single housed during recovery. After 6 days of post-operative care to recover from surgery, all rats were returned to their respective pair-housing conditions.
Self-administration procedures
Following recovery from surgery, rats underwent 6 hr daily heroin self-administration sessions. All self-administration chambers were located inside sound-attenuating cubicles equipped with a house light and exhaust fan designed to mask external noise and odors and were interfaced to a personal computer. Rats performed the rescuing paradigm in the same chamber as self-administration to avoid any potential environmental or context confounds. Chambers were equipped with two nosepoke holes on one wall with a 4.2 × 5 cm food pellet receptacle equipped with head entry detector and placed between the nosepoke holes. Each response hole was located approximately 7 cm above a stainless steel grid floor, and positioned above each lever was a 2.5-cm diameter white stimulus light. Located near the top of the self-administration chambers was a Sonalert speaker that provided an auditory stimulus during reinforcer delivery. Located outside each chamber was a syringe pump interfaced to the computer. When attached to the tether for heroin, the syringe pump delivered the drug solution via a single-channel liquid swivel mounted atop the chamber via polyethylene tubing. In each session, nosepokes into the designated active hole resulted in delivery of heroin (Cayman Chemical, Ann Arbor, MI) at a dose of 0.06 mg/kg per infusion. Heroin was dissolved in sterile saline and delivered in a volume of 0.06 ml over a 2 sec period. Self-administration was conducted on a fixed ratio 1 (FR1) schedule of reinforcement. Each heroin infusion was followed by a 20-sec timeout period, during which additional active nosepokes were recorded but produced no drug infusions. Each reinforcer delivery was accompanied by concurrent illumination of a stimulus light located directly above the active hole, and simultaneous presentation of an auditory tone (2900 Hz, ~65 dB) for 2 sec. Nosepokes into a separate inactive hole had no programmed consequences at any time during the experiment. Self-administration sessions were conducted 7 days per week for 14 consecutive days, and data (i.e., number of reinforcers obtained) were recorded in 1-min time bins across each session. To verify catheter patency in the heroin group, rats were periodically administered sodium methohexital (10 mg/ml i.v., 0.2 ml volume) and observed for brief periods of immobility.
Re-assessment of prosocial behaviors
Following acquisition of heroin self-administration across 14 consecutive days, the rescue paradigm was repeated as described above, with the exception that rats were attached to the infusion tethers as during the self-administration phase. Animals were tested for prosocial behaviors in one-hour sessions for 3 consecutive days, while being allowed concurrent access to heroin. Twenty minutes prior to being placed into the operant chambers, rats were administered clozapine-N-oxide (CNO) (1.5 mg/kg i.p., dissolved in sterile saline) for DREADD activation. One half of the group was randomly assigned to receive CNO and placed into the operant chamber and tested for rescuing behavior for 3 consecutive days (1 hr /day rescuing, 6 hr/day heroin self-administration); the other half of the group received CNO and only access to heroin without assessing rescuing behavior for 3 consecutive days (6 hr/day). These procedures were conducted in a counterbalanced designed in order to identify and control for any potential effects of CNO on heroin intake, resulting in 6 total days of heroin self-administration, three of which assessed rescuing behavior. During sessions in which rescuing behavior was assessed, after 1 hr of access to rescue, the rat in the restrainer was removed from the chamber (unless it was released by the experimental animal prior to the 1 hr elapsing), and the rescuer rat was allowed to remain in the chamber to continue heroin self-administration for the remainder of the 6 hr session to avoid the potential influence of drug withdrawal throughout the reassessment of the prosocial behavior. The latency of rescue was timed and recorded, as well as the number of infusions each rat received the first hour in the chamber.
Electrophysiological Recordings
For verification of DREADD functionality, whole-cell patch clamp electrophysiology was performed in brain tissue slices prepared from experimentally naïve rats infused with one of the three DREADD constructs as described above (stimulatory, inhibitory or control). Following viral infusion, animals were given at least 3 weeks of postsurgical recovery to allow optimal virus expression. Animals were then anesthetized with CO2 and rapidly decapitated. Brains were rapidly removed and submerged in ice-cold carbogen (95% O2 / 5% CO2) saturated cutting solution (cutting artificial cerebrospinal fluid, aCSF) containing (in mmol/L): NaCl, 120; NaHCO3, 25; Dextrose, 10; KCl, 3.3; NaH2PO4, 1.23; CaCl2, 1.8; MgCl2, 2.4. Solution osmolarity was adjusted to 295±5 mOsm and pH adjusted to 7.40±0.03. Brains were then transferred to a cutting chamber of a vibrating tissue slicer (Leica, VT1000S) and 300 μm thick coronal slices containing the insular cortex were prepared in ice-cold cutting aCSF. Slices were then placed in a holding chamber filled with recording aCSF solution containing (in mmol/L): NaCl, 120; NaHCO3, 25; KCl, 3.3; NaH2PO4, 1.23; CaCl2, 0.9; MgCl2, 2.0; dextrose, 10, osmolarity adjusted to 295±5 mOsm and pH adjusted to 7.40±0.03. The holding chamber aCSF was continuously bubbled with carbogen and incubated at 34°C for 45 minutes and then allowed to cool to room temperature before recording. Prior to recording, slices were transferred to a recording chamber where they were perfused continuously at a flow rate of 1–2 mls/min with filtered, carbogen-saturated recording aCSF solution.
DREADD-expressing pyramidal cells within the insula were visually identified using infrared DIC microscopy with an Olympus BX51WI microscope. Fluorescence (mCherry or GFP) was visualized using light emitted from a collimated LED (ThorLabs). Whole-cell recordings were made from the soma of identified virus-expressing pyramidal neurons after establishing a seal (resistance range: 1–10 GΩ). Recording pipettes (<20 mΩ), made from thin-walled capillary tubes were filled with an intracellular solution containing (in mmol/L): K-gluconate, 135; NaCl, 12; K-EGTA, 1; HEPES, 10; Mg-ATP, 2 and tris-GTP, 0.38. Osmolarity was adjusted to 285±5 mOsm and pH adjusted to 7.30±0.01. All recordings were conducted using Axograph software. Responses were digitized at 10kHz and saved on a disk using a digidata interface (Axon Instruments) and analyzed offline using Axograph.
Upon membrane rupture, cells were allowed to equilibrate for a minimum of 5 min prior to recording. During equilibration time, resting membrane potential, capacitance and membrane resistance were continually monitored. All recordings were conducted in current clamp, where cell membrane potentials for labeled cells were maintained at either hyperpolarizing potentials (−80 mV) for hM3Dq, spiking threshold potentials (−45 mV) for hM4Di, or resting potentials (−65 mV) for GFP controls. After membrane potential stabilization was achieved, the DREADD agonist clozapine-N-oxide (CNO) was bath applied at 10 μM for a minimum of 5 min. Changes in membrane potential and spontaneous activity were observed.
Verification of Virus Placement
Two days following the last behavioral test session in Experiments 1 and 2, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused via the transcardial route with phosphate-buffered saline (PBS) followed by 4% w/v paraformaldehyde in PBS (pH=7.4). Brains where then removed, placed in the same fixative for at least 48 hr, then transferred to PBS containing 30% w/v sucrose) for at least 48 hr. Coronal brain sections (40 μm thickness) containing the AIC were then obtained on a cryostat, mounted onto microscope slides, coverslipped with Vectashield (Vector Laboratories) antifade mounting medium, and viewed under epifluorescence using a Leica MZ FLIII stereozoom microscope equipped with a digital camera. The spread of virus as indicated by mCherry expression (stimulatory and inhibitory DREADDs) or EGFP expression (control virus) was then assessed in individual hemispheres by overlaying a transparent version of the vector diagram of the corresponding coronal section from the atlas of Paxinos and Watson (2014). The shapes of the corpus callosum and lateral fissure between the insular cortex and olfactory bulb served as anatomical landmarks for section alignment. Virus placements were considered to be erroneous either if no fluorescent signal in the AIC could be discerned, or if significant virus expression was observed in neighboring brain regions such as the olfactory bulb or sensorimotor cortex.
Data analyses
For heroin self-administration data, the dependent variables were the number of reinforcers obtained per session during the 14 days of acquisition, as well as the number of reinforcers obtained during the first hour of sessions where animals were provided simultaneous access to heroin and the trapped cagemate. The rationale for analysis of only the first hour of the 6 hr session following acquisition of heroin self-administration was that rescuing was only measured for the first hour of this session, and the primary objective of this experimental phase was to measure heroin intake in the presence of a cagemate. Self-administration data were analyzed by multilevel ANOVA, with virus infused as a between-subjects factor and session as a within-subjects factor. Baseline differences in rescue rates (proportion of rats exhibiting rescue behavior) were also assessed as part of the analysis. Due to differences in baseline rescuing behavior exhibited prior to the initiation of heroin self-administration in rats in Experiment 1 (see Results), it was necessary to separate animals into two different cohorts, and therefore cohort was analyzed as a factor for this experiment.
For rescuing behavior, dependent measures were observation of freeing the trapped rat and latency to rescue. Given the structure of the data in which each rat was measured multiple times in each treatment condition and stage of the study, a multilevel model was used nesting observations within rats. In order to account for observation of rescuing behavior, as well as the latency to rescue, a survival analysis using the accelerated failure time (AFT) model was used. This allowed for modeling of the rate of rescue as predicted by the treatment condition (stimulatory or inhibitory DREADDs, or control virus), stage in the study (before and after acquisition of heroin self-administration), and interactions between these two variables. We have previously used similar analyses as described elsewhere (Tomek, Stegmann and Olive, 2019); also see (Allison, 2010) for details on survival analysis and the AFT model). For graphical representation of survival data, the average proportion of completed rescues per rat at each time point throughout the 60-minute period are shown. Since self-administration data (i.e., number of reinforcers obtained) were recorded in 1-min time bins across each session, while the occurrence of and latency to rescue were video recorded and were assessed on a continues timescale, analyses of post-rescue rates of self-administration could not be accurately calculated.
The primarily outcome of interest was the interaction between the treatment condition and the stage of the study, as this would indicate whether the change of rate in rescue from the pre-acquisition to the post-acquisition stages differed across DREADD virus versus control conditions. In Experiment 1, as a result of cohort differences in baseline rescuing behavior, cohort membership was included as a variable. All analyses were conducted in SAS Version 9.4.
Results
Electrophysiological validation of DREADD function:
All DREADD expression (hM3Dq, hM4Di and control) were readily identifiable within the AI of recorded slices. A representative image DREADD expression within the AIC as well as individual cells is shown in Fig. 2A. As shown in Fig. 2B, AIC pyramidal neuron activity can be modulated via chemogenetic approaches, where we observed expected neuronal activation, inhibition, and no effects in hM3Dq, hM4Di, or control virus expressing cells, respectively. For cells expressing hM3Dq, CNO bath application readily induced cell depolarization and elicited action potentials, whereas CNO bath application inhibited action potential firing in cells expressing hM4Di. Furthermore, CNO bath application had no effect on membrane potential and did not elicit action potentials in neurons expressing the control DREADD vector. Representative electrophysiological traces are shown in Fig. 2B.
Figure 2.
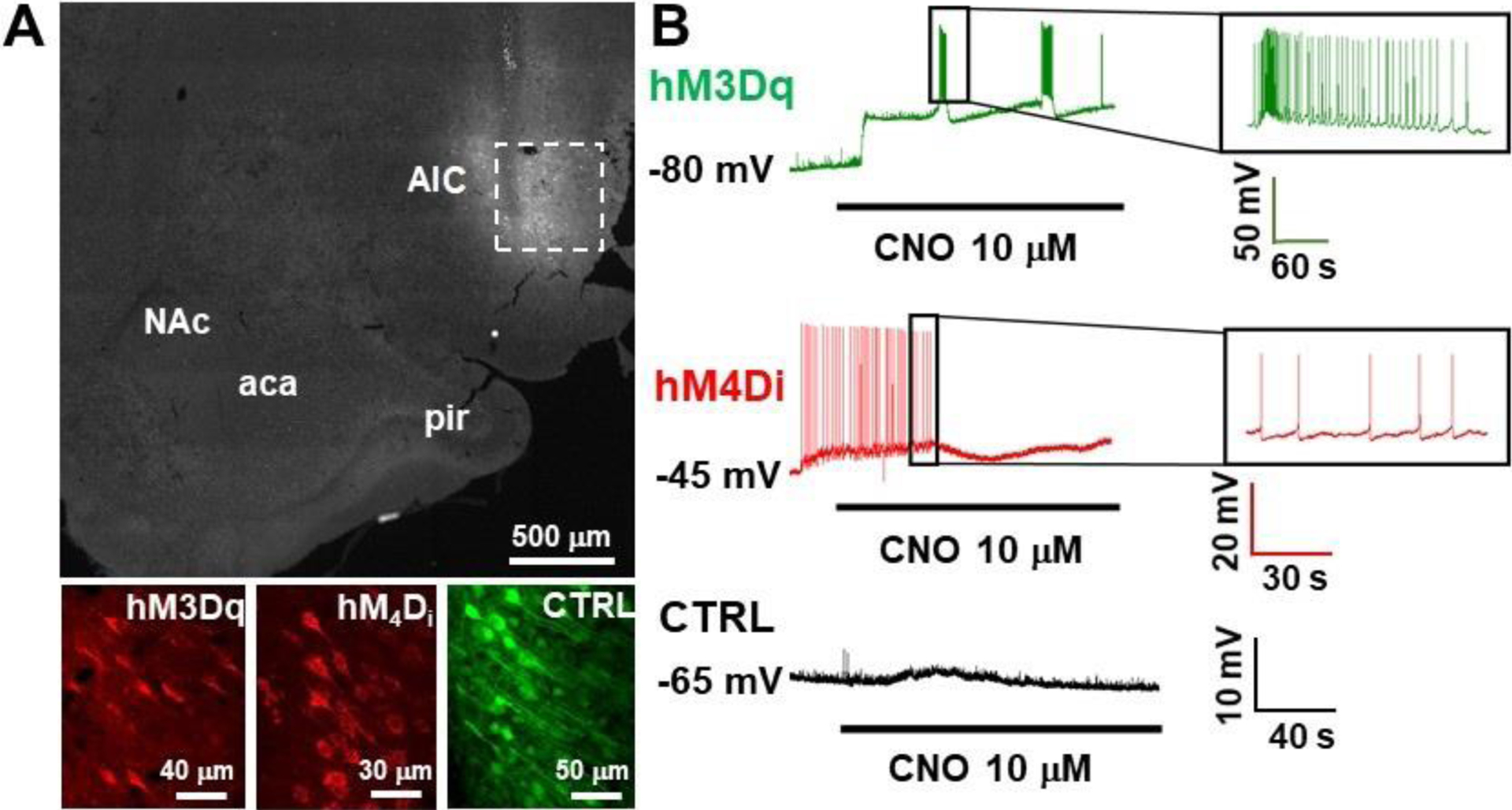
Validation of DREADD functionality using whole cell patch clamp electrophysiology. A) Representative low magnification images of DREADD expression within the AIC (dashed box), and high magnification images in the AIC of each of the viral vectors utilized (bottom). B) Representative electrophysiological traces for hM3Dq (green; top), hM4Di (red; middle) and control (black; bottom) are shown. Black line below traces represents CNO bath application.
Experiment 1 -. Effects of chemogenetic activation of the anterior insula on prosocial behavior
A total of n=48 animals were used to examine the effects of the stimulatory DREADD, with two cohorts of n=12 animals each initially being assigned to be the rescuer rats, and n=12 animals each initially being assigned to be the trapped rats. A total of n=2 rats designated to receive the stimulatory DREADD virus, and n=2 rats designated to receive the control virus, underwent switching from being the trapped vs. rescuer rat due to lack of initial establishment of baseline rescuing behavior. Of these, n=14 rats were removed from the study due to failure to establish baseline rescuing behavior. Two rats (and their cagemates) were excluded from the analysis due to loss of catheter patency. Therefore, a total of n=13 animals were used for the analysis, where n=8 received the stimulatory DREADD virus and n=5 received the control virus. All rats in Experiment 1 were found to have correct virus placement confined to the AIC. Localization of virus placements is shown in Fig. 3.
Figure 3.
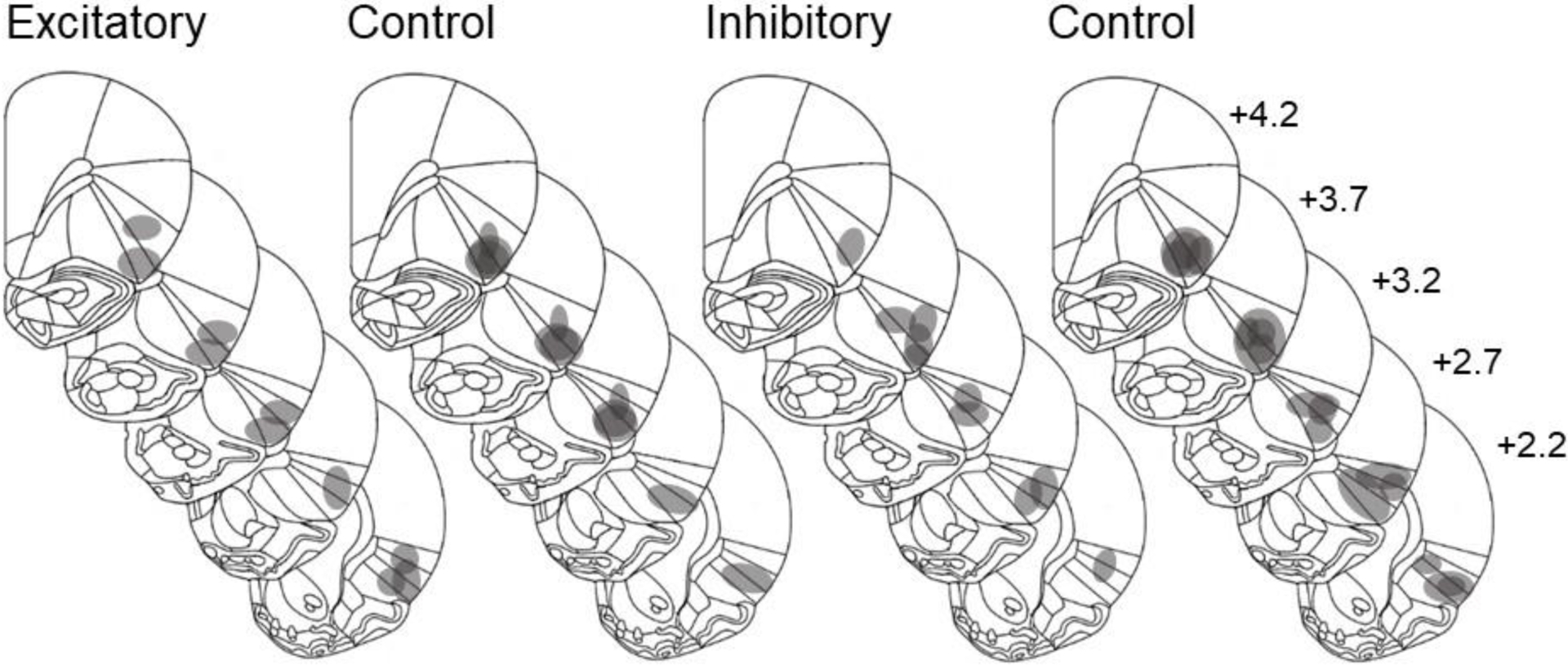
Anatomical localization of virus placement in rats receiving the stimulatory DREADD or control virus in Experiment 1 (left two panels) and Experiment 2 (right two panels). Numbers next to each coronal brain section represent distance of that section in mm from bregma. Diagrams modified from Paxinos and Watson (2014).
Fig. 4A shows heroin intake across the initial 14 sessions of self-administration for both cohorts combined. No effects of either virus infused (control or stimulatory DREADD; p>0.05) or cohort (p>0.05) on acquisition of heroin self-administration were observed. However, for all groups, heroin intake increased gradually across all sessions (F(1,168)=34.86, p<0.0001), indicating acquisition of heroin self-administration. Fig. 4B shows the amount of heroin self-administered in both cohorts following acquisition across the 3 sessions where rats were provided simultaneous access heroin self-administration and the cagemate trapped in the restrainer. No significant differences were observed when heroin intake data were analyzed as a function of virus infused or session (all p-values >0.05). The mean proportion of rats demonstrating rescuing behavior across these 3 sessions is shown in Fig. 4C.
Figure 4.
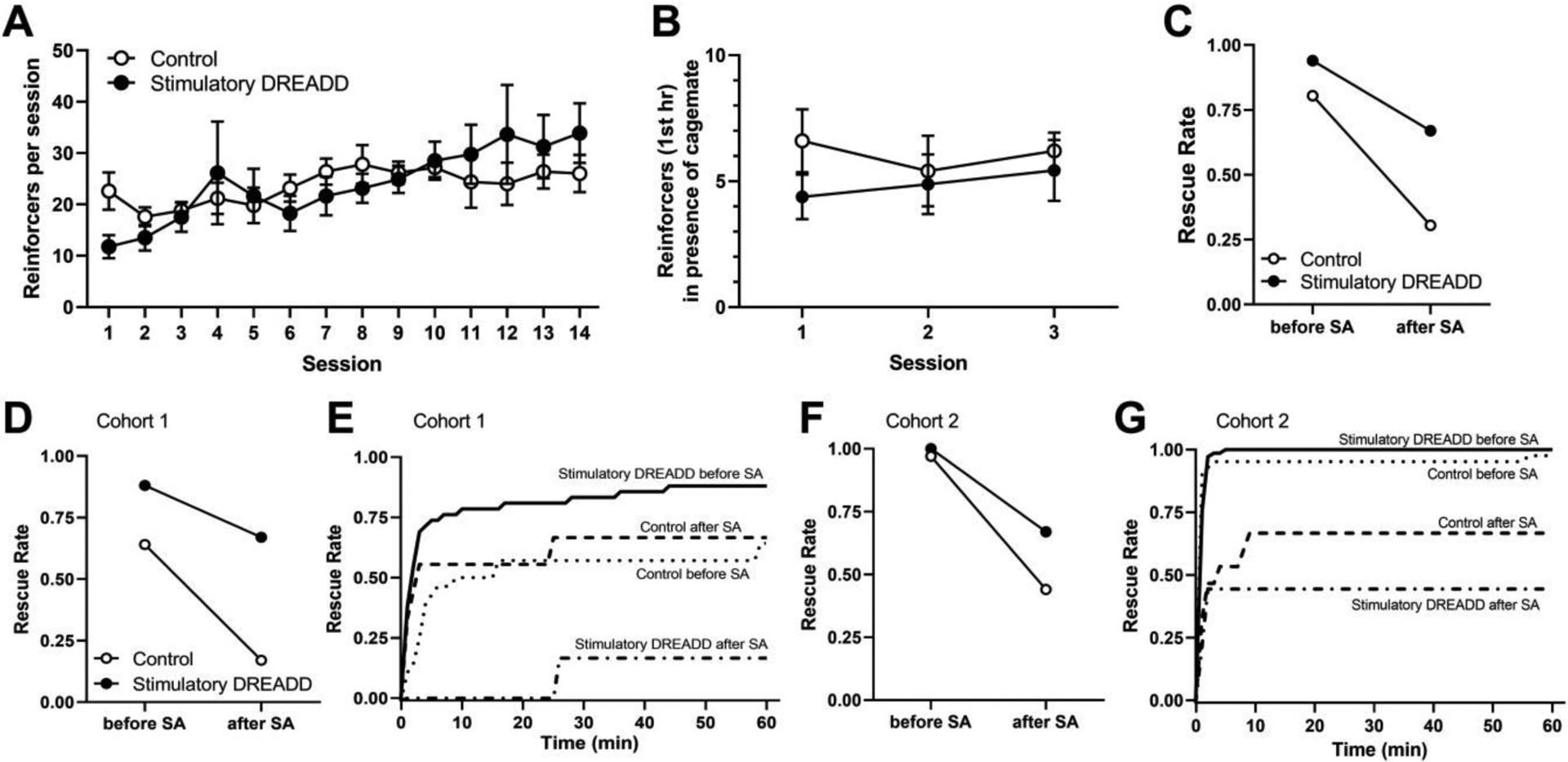
A) Average heroin intake per session during the initial acquisition of heroin self-administration in Experiment 1 (n=8 for stimulatory DREADD, n=5 for control virus). B) Average heroin intake in the first hour during the 3 test sessions for both cohorts where rats were given simultaneous access to receiving heroin and rescuing its cage-mate. C) Rescue rate (average proportion of rescues) before and after the self-administration phase of the experiment for each treatment condition in both cohorts. D) Rescue rates before and after the self-administration phase of the experiment for each treatment condition in cohort 1 (n=3 for stimulatory DREADD, n=2 for control virus). E) Rescue rate across the 60 min session in cohort 1, in each virus conditions before and after the self-administration phase of the experiment. F) Rescue rates before and after the self-administration phase of the experiment for each treatment condition in cohort 2 (n=5 for stimulatory DREADD, n=3 for control virus). G) Rescue behavior across the 60 min session in cohort 2, respectively, in each virus conditions before and after the self-administration phase of the experiment. Survival lines represent the average proportion of completed rescues per rat within each group at each time point throughout the 60-minute period, averaged across the 3 sessions.
However, we observed differences in baseline rescue rates across the two cohorts of animals that were used as subjects in Experiment 1, which required us to control for cohort in the analyses. Cohort 1 consisted of n=3 rats infused with the stimulatory DREADD virus and n=2 rats infused with the control virus. Cohort 2 consisted of n=5 rats infused with the stimulatory DREADD virus and n=3 rats infused with the control virus.
Fig. 4D and 4F show rescue rates for cohorts 1 and 2, respectively, during the first hour of the three post-acquisition self-administration session during which cagemates located inside the restrainer were also present. Fig. 4E and 4G show rescue rates across the two treatment conditions and the two stages of study for the first and second cohorts. In the first cohort of animals, prior to heroin self-administration, the average rescue rates were 88% and 64% for rats receiving the stimulatory DREADD or control virus, respectively. Following acquisition of heroin self-administration, these proportions decreased to 67% and 17%, respectively. In the second cohort of animals, prior to acquisition of heroin self-administration, the average rescue rates were 100% and 97% for rats receiving the stimulatory DREADD or control virus respectively. Following acquisition of heroin self-administration, these rates decreased to 67% and 44%, respectively. Table 1 shows the descriptive statistics for each treatment condition and stage of the study, including the average proportion of rescues per rat, and the mean, median, and standard deviation for the latency in rescue (in seconds) for the rats that rescued in both cohorts of rats.
Table 1.
Rescue rate (average proportion of rescues per rat) across treatment conditions and self-administration phase, and median and mean latency to rescue.
| Cohort | treatment condition | stage (before or after self-administration) | proportion of rescues (%) | median latency to rescue (sec) | mean (SD) latency to rescue (sec) |
|---|---|---|---|---|---|
| 1 | stimulatory DREADD | before | 88 | 106 | 320 (596) |
| after | 67 | 76 | 316 (584) | ||
| control | before | 64 | 226 | 608 (924) | |
| after | 17 | 1546 | 1546 (n/a) | ||
| 2 | stimulatory DREADD | before | 100 | 35.5 | 48 (43) |
| after | 67 | 115 | 307 (525) | ||
| control | before | 97 | 28.5 | 32 (18) | |
| after | 44 | 49 | 55 (35) |
Note that an SD value of n/a indicates only one animal in that experimental group and condition demonstrated rescuing behavior during the post-acquisition sessions.
As described above, a multilevel survival analysis was conducted controlling for the two cohorts. The effect of interest was the interaction effect between the treatment condition and self-administration stage, as this would indicate whether rats in the two treatment conditions responded differently to the self-administration procedures with regard to rescuing behaviors. This interaction effect was found to be significant (t12 = −2.32, p < 0.05), indicating that across the two cohorts, animals in the two treatment conditions reacted differently to the self-administration stage, although all rats decreased their rescuing behavior following acquisition of heroin self-administration (t12 = 6.9, p < 0.05).
Experiment 2 -. Effects of chemogenetic inhibition of the anterior insula on prosocial behaviors
A total of n=48 animals were used in this study, with two cohorts of n=12 animals each initially being assigned to be the rescuer rats, and n=12 animals each initially being assigned to be the trapped rats. A total of n=7 animals received the active inhibitory DREADD virus and n=11 received the control virus. A total of n=4 rats designated to receive the inhibitory DREADD virus, and n=3 rats designated to receive the control virus, underwent switching from being the trapped vs. rescuer rat due to lack of initial establishment of baseline rescuing behavior. Of these, n=4 rats were removed from the study due to failure to establish baseline rescuing behavior. All rats in Experiment 2 were found to have correct virus placement in the AIC. Localization of virus placements are shown in Fig. 3.
As shown in Fig. 5A, heroin intake increased across the initial 14 sessions of self-administration. No effects of virus infused (control or inhibitory DREADD; F(1,89)=0.06, p=0.81) was observed. However, for both groups, heroin intake increased gradually across all sessions (F(1,89)=18.71, p<0.0001), indicating acquisition of heroin self-administration. Fig. 5B shows heroin intake during the first hour of the three post-acquisition self-administration session during which cagemates located inside the restrainer were also present. No significant differences were observed when heroin intake data were analyzed as a function of virus infused or session (all p-values >0.05), and no baseline differences in rescue rate were observed (p>0.05).
Figure 5.
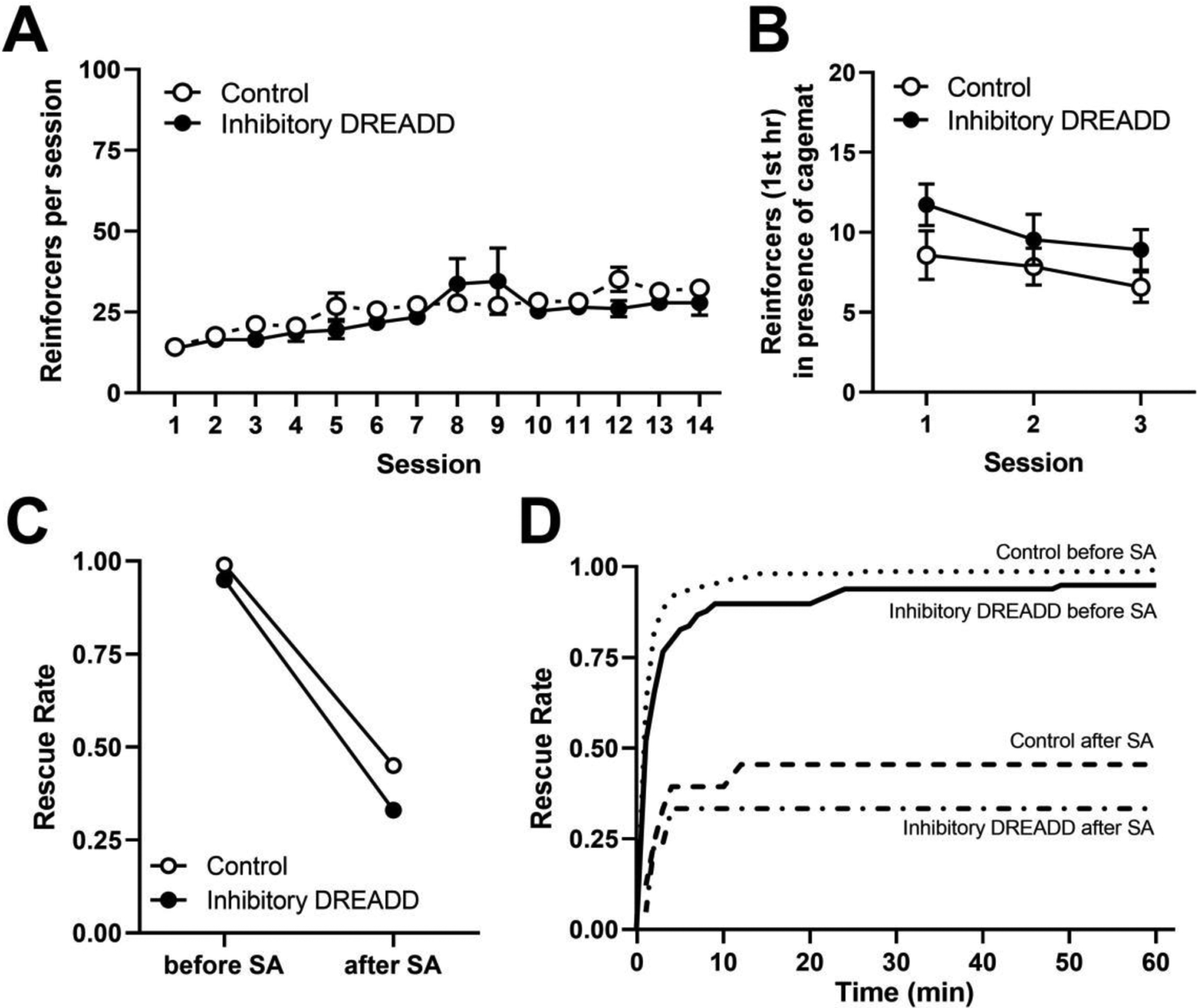
A) Average heroin intake per session during the initial acquisition of heroin self-administration (n=7 for inhibitory DREADD, n=11 for control virus). B) Average heroin intake in the first hour during the 3 test sessions where rats were given simultaneous access to receiving heroin and rescuing its cage-mate. C) Average rescue rates before and after the self-administration phase of the experiment for each treatment condition. D) Rescue behavior across the 60 min session in each virus conditions before and after the self-administration phase of the experiment. Survival lines represent the average proportion of completed rescues per rat within each group at each time point throughout the 60-minute period, averaged across the 3 sessions.
For rescuing behavior, a multilevel survival analysis was conducted using the AFT model to account for observation of rescuing behavior as well as the latency to rescue, modeling the rate of rescue as predicted by the treatment condition (control virus or inhibitory DREADD), the stage in the study (before and after/during heroin acquisition via self-administration), and the interaction between these two variables. The primary measure of interest was the interaction between the treatment condition and the stage of the study, as this would indicate whether the change of rate in rescue from the pre self-administration to the post self-administration stages differed across virus condition. Table 2 shows the descriptive statistics for each treatment condition and stage of the study, including the average rescue rate, and the mean, median, and standard deviation for the latency in rescue (in seconds) for the rats that rescued.
Table 2.
Rescue rate (average proportion of rescues per rat) across treatment conditions and self-administration phase, and median and mean latency to rescue.
| treatment condition | stage (before or after self-administration) | proportion of rescues (%) | median latency to rescue (sec) | mean (SD) of latency to rescue (sec) |
|---|---|---|---|---|
| inhibitory DREADD | before | 99 | 53 | 186 (402) |
| after | 45 | 102 | 118 (63) | |
| control | before | 95 | 39 | 118 (336) |
| after | 33 | 80 | 204 (272) | |
As shown in Fig. 5C, prior to heroin self-administration, the average rescue rate was 99% and 95% for rats receiving the inhibitory DREADD or control virus respectively. Following acquisition of heroin self-administration, these rates decreased to 45% and 33%, respectively. No baseline differences in rescuing behavior across cohorts of animals were observed. An interaction between self-administration stage and treatment group was not observed (t17 = 0.2, p>0.05), indicating that the rats from the two treatment conditions (inhibitory DREADD or control virus) did not react differently to the self-administration stage. All rats decreased their rescuing behavior after acquisition of heroin self-administration (t17 = 11.03, p < 0.05) as compared to before self-administration, regardless of whether they received the inhibitory DREADD or control virus. Fig. 5D shows rescue rates during the 60-min period for each treatment condition before and after acquisition of heroin self-administration.
Discussion
The current study confirms and extends previous findings that a history of heroin self-administration decreases prosocial behaviors in rats (Tomek, Stegmann and Olive, 2019). Further, these data demonstrate that selectively chemogenetic activation of the AIC can restore heroin-induced deficits in prosocial behaviors. These findings shed new light on mechanisms and brain regions underlying heroin-induced impairments in prosocial functioning and lend support of the role of the insula in these prosocial behaviors. Endogenous opioids, specifically through activation of the mu opioid receptor (MOR), are known to play a role in prosocial functioning (Heilig, Epstein, Nader and Shaham, 2016). For example, MOR knockout mice are unable to form normal attachments with their own mothers (Moles, Kieffer and D’Amato, 2004). Conversely, systemic treatment with MOR agonists reduce signs of stress from socially isolated rat pups (Carden, Barr and Hofer, 1991). In humans, endogenous opioids peptides acting through MOR in the insula appear to mediate social attachment and bonding (Hsu, Sanford, Meyers, Love, Hazlett, Wang et al., 2013; Nummenmaa, Manninen, Tuominen, Hirvonen, Kalliokoski, Nuutila et al., 2015). The AIC has reciprocal connections to limbic regions such as the amygdala, the anterior cingulate cortex, ventral striatum, and the dorsolateral prefrontal cortex (Gogolla, 2017). These regions play a role in motivational, emotional, and cognitive functions, and thus chemogenetic activation of neurons in the insula may, to some degree, restore heroin-induced impairments in function or connectivity between these regions, motivating the rats to resume opening the restrainer door for their trapped cagemate.
In the present study, chemogenetic inhibition of the AIC did not produce any significant differences in heroin intake or prosocial behaviors. These observations were somewhat surprising, given the wealth of evidence that the AIC is involved in prosocial related behaviors in humans (de Waal and Preston, 2017; Rogers-Carter and Christianson, 2019). Previous studies in rodents that have shown reduced baseline activity of AIC is predictive of the ability of social reward to dissuade methamphetamine self-administration (Venniro, Zhang, Caprioli, Hoots, Golden, Heins et al., 2018), and pharmacological inactivation of the AIC can attenuated cue-induced reinstatement of opiate-seeking (Zhang, Jia, Wang, Zhu, Liu, Li et al., 2019). Likewise, while chemogenetic activation of the AIC facilitates social exploration in juvenile rats (Rogers-Carter, Djerdjaj, Gribbons, Varela and Christianson, 2019), optogenetic silencing of this region prevents social affective preference (Rogers-Carter, Varela, Gribbons, Pierce, McGoey, Ritchey et al., 2018). We speculate that the lack of effects of chemogenetic inactivation of the AIC in the present study may be due to fact that inhibitory DREADDs have been found to suppress only about ~60% of firing rates in affected cells after CNO i.p. injections, resulting in a reduction of neuronal activity rather than a complete cessation (Chang, Todd, Bucci and Smith, 2015; Smith, Bucci, Luikart and Mahler, 2016). Alternatively, heroin-induced deficits in prosocial behavior were already at low levels in these animals, and inhibiting the insula did not produce changes due to floor effects.
One limitation of the current study is the difference in baseline rescue rates (before self-administration) observed between the two cohorts of rats in the stimulatory DREADD study (Experiment 1). While these baseline differences were controlled for statistically in the data analyses, the reason for their occurrence is currently unknown, and we observed no cohort differences in acquisition of heroin self-administration. Regardless of the causative factor, these observations underscore the importance of assessment of baseline prosociality, as well as individual variability in these measures, when assessing effects of experimental manipulations. Indeed, other investigators using the current or similar paradigms have also noted the importance of assessing baseline prosocial behavior (Ben-Ami Bartal, Rodgers, Bernardez Sarria, Decety, and Mason, 2014; Hiura, Tan, and Hackenberg, 2018).
Another limitation to this study was the potential biological effects of the DREADD agonist CNO. While initially believed to be physiologically inert, various studies have emerged demonstrating that at some doses and in some species, CNO is reverse metabolized to clozapine (Chen, Choo, Huang, Yang, Stone, Roth et al., 2015; MacLaren, Browne, Shaw, Krishnan Radhakrishnan, Khare, Espana et al., 2016; Thompson, Khajehali, Bradley, Navarrete, Huang, Slocum et al., 2018), which can exert physiological effects that could potentially confound behavioral experiments. Taking this into consideration along with the recommended efficacy doses of CNO being between 0.1 to 3 mg/kg (Smith, Bucci, Luikart and Mahler, 2016), our study was conservatively designed by including a dose of CNO within this recommended range (1.5 mg/kg), and the use of control virus lacking the coding sequence for either DREADD.
In the current study, we show that chemogenetic activation of the AIC restored prosocial behaviors following heroin intake. However, there were no significant difference in heroin intake between animals receiving the active or control virus, and chemogenetic inhibition of the insula had no effect on prosocial behaviors or heroin intake. As a result, future studies should investigate alternative ways to modulate the insula, such as selective modulation of local GABAergic interneuron or glial cell activity in this region, in attempts to further refine and elucidate its role in addiction and related behaviors. In addition, investigations into the role of other forebrain and limbic regions, particularly those with anatomical and functional connectivity with the AIC, in regulation of prosociality and deficits induced by heroin or other drugs of abuse are needed. Overall, these experiments help narrow the quest for more anatomically targeted strategies in the attenuation and treatment of opioid use disorders.
Acknowledgements
This work was supported by Public Health Service grant DA042172 to MFO, a research grant award to SET from The American Psychological Association, Society of Addiction Psychology, Division 50, and funds from the College of Liberal Arts and Sciences at Arizona State University. The authors would like to acknowledge Matt Zucker, Kyle Backman, Kylie Snow, Vince Carfagno, and Leanna Monahan, for their laboratory assistance, and Mark Namba for his surgical assistance.
Footnotes
Disclosure of interest
The authors report no conflict of interest of any kind, including the funding agencies that supported this work (NIH Public Health Service grant DA042172, American Psychological Association, and the College of Liberal Arts and Sciences at Arizona State University)
References
- Allison PD Survival Analysis Using SAS: A Practice Guide, 2nd Ed. Cary, NC: SAS Institute; 2010. [Google Scholar]
- Ben-Ami Bartal I, Decety J, Mason P (2011). Empathy and pro-social behavior in rats. Science, 334, 1427–30. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Shan H, Molasky NM, Murray TM, Williams JZ, Decety J, Mason P (2016). Anxiolytic treatment impairs helping behavior in rats. Front Psychol, 7, 850. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P (2014) Pro-social behavior in rats is modulated by social experience. eLife, 3, e01385. doi: 10.7554/eLife.01385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA (1991). Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res, 62, 17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, Smith KS (2015). Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. Eur J Neurosci, 42, 3105–16. doi: 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J (2015). The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci, 6, 476–84. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND (2006). Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend, 81, 103–7. doi: 10.1016/j.drugalcdep.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, Luscher C (2018). Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife, 7, e39945. doi: 10.7554/eLife.39945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM, Preston SD (2017). Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci, 18, 498–509. doi: 10.1038/nrn.2017.72 [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A (2015). Revisiting the role of the insula in addiction. Trends Cogn Sci, 19, 414–20. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev, 35, 903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Gogolla N (2017). The insular cortex. Curr Biol, 27, R580–R586. doi: 10.1016/j.cub.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, Shaham Y (2016). Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci, 17, 592–9. doi: 10.1038/nrn.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura LC, Tan L, Hackenberg TD (2018) To free, or not to free: Social reinforcement effects in the social release paradigm with rats. Behav Processes, 152, 37–46. doi: 10.1016/j.beproc.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, . . . Zubieta JK (2013). Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry, 18, 1211–7. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science, 361. doi: 10.1126/science.aau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci, 12, 483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 3, 760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll SL, Nikolic E, Bieri F, Soyka M, Baumgartner MR, Quednow BB (2018). Cognitive and socio-cognitive functioning of chronic non-medical prescription opioid users. Psychopharmacology (Berl), 235, 3451–3464. doi: 10.1007/s00213-018-5060-z [DOI] [PubMed] [Google Scholar]
- Kurtines W, Hogan R, Weiss D (1975). Personality dynamics of heroin use. J Abnorm Psychol, 84, 87–9. doi: 10.1037/h0076258. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG (1996). Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not γ-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci U S A, 93, 7332–6. doi: 10.1073/pnas.93.14.7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD (2016). Clozapine N-oxide administration produces behavioral effects in Long-Evans rats: implications for designing DREADD experiments. Eneuro, 3. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR (2004). Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science, 304, 1983–6. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A (2014). The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci, 1316, 53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Manninen S, Tuominen L, Hirvonen J, Kalliokoski KK, Nuutila P, . . . Sams M (2015). Adult attachment style is associated with cerebral mu-opioid receptor availability in humans. Hum Brain Mapp, 36, 3621–8. doi: 10.1002/hbm.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C The Rat Brain in Stereotaxic Coordinates. 7th ed. San Diego: Elsevier Academic Press; 2014. [Google Scholar]
- Rogers-Carter MM, Christianson JP (2019). An insular view of the social decision-making network. Neurosci Biobehav Rev, 103, 119–132. doi: 10.1016/j.neubiorev.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, Christianson JP (2019). Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. J Neurosci, 39, 8717–8729. doi: 10.1523/JNEUROSCI.0316-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, Christianson JP (2018). Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci, 21, 404–414. doi: 10.1038/s41593-018-0071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, Okada M (2015). Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn, 18, 1039–47. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016). DREADDS: use and application in behavioral neuroscience. Behav Neurosci, 130, 137–55. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S, . . . Tobin AB (2018). DREADD agonist 21 Is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol Transl Sci, 1, 61–72. doi: 10.1021/acsptsci.8b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomek SE, Stegmann GM, Olive MF (2019). Effects of heroin on rat prosocial behavior. Addict Biol, 24, 676–684. doi: 10.1111/adb.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, . . . Borsook D (2010). Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain, 133, 2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, . . . Shaham Y (2018). Volitional social interaction prevents drug addiction in rat models. Nat Neurosci, 21, 1520–1529. doi: 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, . . . Radua J (2017). Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. Am J Drug Alcohol Abuse, 43, 505–517. doi: 10.1080/00952990.2016.1245312. [DOI] [PubMed] [Google Scholar]
- Zhang R, Jia W, Wang Y, Zhu Y, Liu F, Li B, . . . Tan Q (2019). A glutamatergic insular-striatal projection regulates the reinstatement of cue-associated morphine-seeking behavior in mice. Brain Res Bull, 152, 257–264. doi: 10.1016/j.brainresbull.2019.07.023 [DOI] [PubMed] [Google Scholar]


