Abstract
Renewal of the intestinal epithelium occurs approximately every week and requires a careful balance between cell proliferation and differentiation to maintain proper lineage ratios and support absorptive, secretory, and barrier functions. We review models used to study the mechanisms by which intestinal stem cells (ISCs) fuel the rapid turnover of the epithelium during homeostasis and might support epithelial regeneration after injury. In anatomically defined zones of the crypt stem cell niche, phenotypically distinct active and reserve ISC populations are believed to support homeostatic epithelial renewal and injury-induced regeneration, respectively. However, other cell types previously thought to be committed to differentiated states might also have ISC activity and participate in regeneration. Efforts are underway to reconcile the proposed relatively strict hierarchical relationships between reserve and active ISC pools and their differentiated progeny; findings from models provide evidence for phenotypic plasticity that is common among many if not all crypt-resident intestinal epithelial cells. We discuss the challenges to consensus on ISC nomenclature, technical considerations and limitations inherent to methodologies used to define reserve ISCs, and the need for standardized metrics to quantify and compare the relative contributions of different epithelial cell types to homeostatic turnover and post-injury regeneration. Increasing our understanding of the high-resolution genetic and epigenetic mechanisms that regulate reserve ISC function and cell plasticity will help refine these models and could affect approaches to promote tissue regeneration following intestinal injury.
Renewal of the intestinal epithelium occurs approximately weekly and requires a tightly controlled balance of proliferation and differentiation to maintain proper lineage ratios and support absorptive, secretory, and barrier functions. The intestinal epithelium has evolved in a luminal environment that exposes its cells to microbiota, naturally occurring toxins, and physical stresses that continually challenge epithelial integrity. In addition, man-made stressors such as contemporary chemotherapies and radiation treatments for cancer can have devastating consequences on epithelial integrity and renewal. In response to physiologic challenge or injury, intestinal stem cells (ISCs) repair damaged tissue and replace lost cells. ISC researchers investigate the specific intestinal cell types and mechanisms involved in the homeostatic renewal and injury-induced regeneration of the intestine.
Decades of research have generated competing models for how ISCs fuel homeostatic renewal and regeneration in the intestine. Based on 1 model, all ISCs are essentially similar in identity and provide a common functionally homogeneous pool of actively proliferating ISCs (aISCs), which continuously self-renew and give rise to all differentiated intestinal lineages. The rate of intestinal turnover is determined largely by parameters of ISC self renewal, cell differentiation, and death. In a separate model, aISCs mediate the normal homeostatic turnover of the intestinal epithelium, whereas a population of normally non- or slowly-proliferating reserve ISCs (rISCs) fulfill regenerative tasks specifically after tissue injury or extreme physiologic stress. In the most recent model, differentiated epithelial cell types have a large degree of phenotypic plasticity and can therefore re-acquire stem cell characteristics and function in response to injury. There is evidence to support of each of these models, indicating evolutionary selection for different pathways of regenerative responses in the intestine.
We review the evidence and concepts related to rISCs in intestinal homeostasis and injury and discuss the technical advances made and challenges to studying these. We introduce main concepts in ISC heterogeneity, regarding actively cycling vs quiescent ISCs, and explain the development of a hierarchical model, in which aISCs and rISCs are functionally separate ISC pools. We review rISC identity, function, and regulation because many intestinal cell types, including those previously thought to be committed to post-mitotic differentiated secretory cell (Paneth, enteroendocrine, tuft, and goblet) and absorptive enterocyte lineages, might have the capacity to participate in regeneration after tissue injury. We also review approaches for discriminating among cells that mediate the ISC regeneration response, and for identifying the signaling mechanisms that activate and direct regeneration. The complex nature of ISC dynamics is becoming clear—we discuss the difficulties in building consensus on ISC nomenclature, heterogeneity, and function. In this article, term reserve ISC (rISC) refers to cells that make no or a minimal contribution to homeostatic epithelial renewal, but mediate epithelial regeneration following damaging injury or stress, whereas active ISC (aISC) refers to cells that divide regularly during homeostatic epithelial renewal and are sensitive to and selectively compromised by damage or stress.
Identity, Function, and Regulation aISCs
Homeostatic renewal of the intestinal epithelial monolayer is mediated by a pool of ISCs located at the base of micro-anatomical units called crypts. These ISCs, originally classified as crypt base columnar cells (CBCs), are a highly proliferative population of cells intercalated between Paneth cells in the crypt base1,2 (Figure 1). Barker et al found that CBCs express high levels of the leucine rich repeat containing G protein-coupled receptor 5 gene (LGR5). Genetic lineage tracing of Lgr5-expressing CBCs demonstrated the long-term self renewal and multipotency of these cells in mice3, thereby defining CBCs as a bona fide ISC pool. ISC function in single, cultured Lgr5-expressing cells was confirmed based on their ability to give rise to intestinal organoids, which are stem cell-containing epithelial units with the capacity for multi-lineage differentiation and long-term passage4,5. Although these studies established the Lgr5-expressing CBCs as a predominant aISC population responsible for the homeostatic renewal of the entire intestinal epithelium, surprisingly, ablation of Lgr5-expressing cells, through diphtheria toxin-induced cell death, had little effect on homeostatic renewal of the epithelium6. These findings provided evidence for a pool of reserve ISCs that could contribute to epithelial renewal when the Lgr5-expressing ISC population was lost.
Figure 1. Role of crypt-localized cells in epithelial homeostasis and injury-induced regeneration.
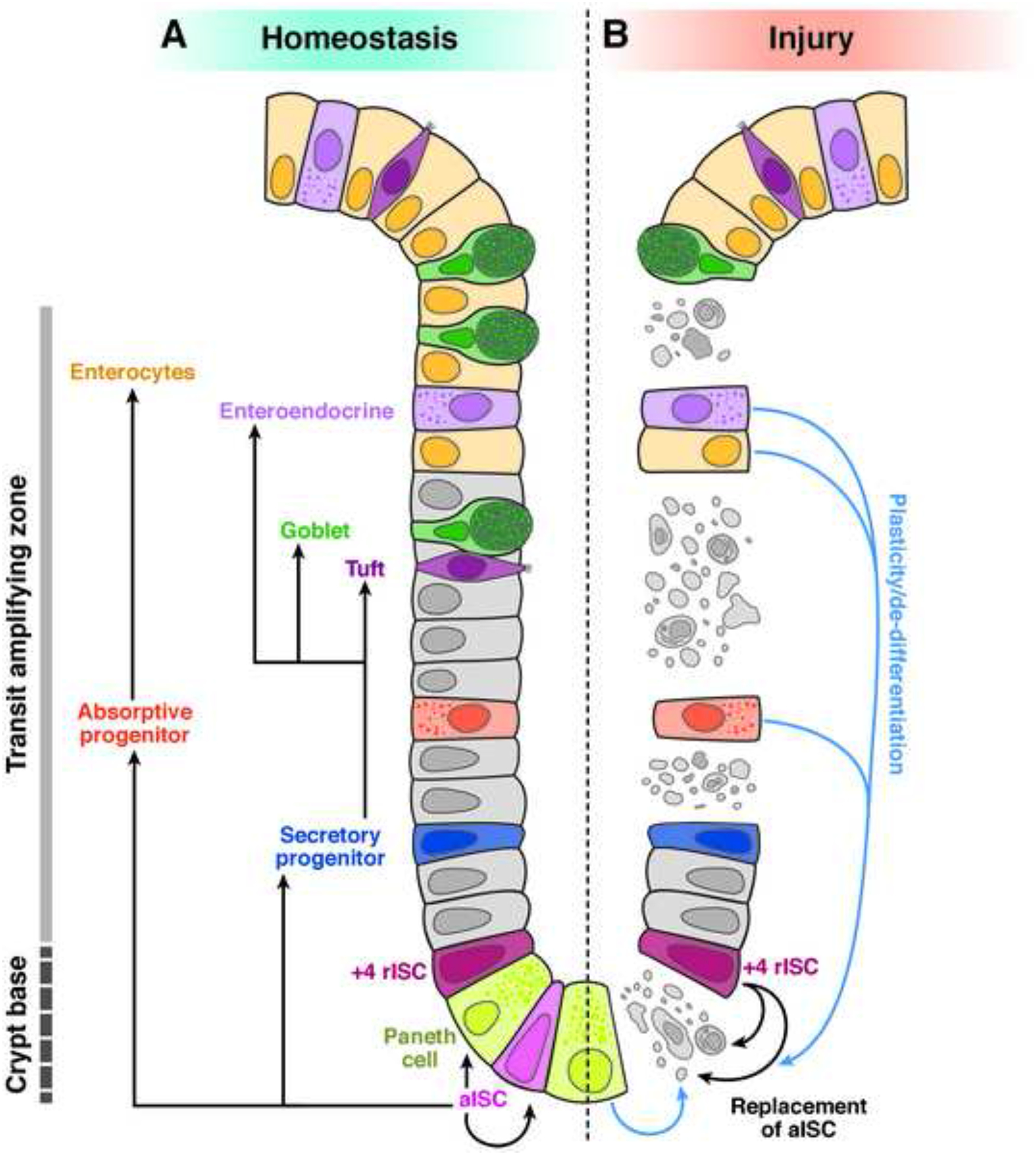
A) Homeostatic renewal of the intestinal epithelium (comprising ISCs, transit-amplifying progenitors, absorptive enterocytes, Paneth, goblet, enteroendocrine, and tuft cells) is mediated by active ISCs (aISC) in the crypt base. A rare pool of ISCs located near the +4 position is maintained in a quiescent state (+4 ISC), making only minimal contributions to the homeostatic maintenance of aISC and renewal of differentiated lineages. B) During injury-induced regeneration, when aISCs are compromised or lost, rISCs become a primary cell source that mediates regeneration. Plastic non-ISC cell types can also contribute to regenerating epithelial tissue (blue arrows), presumably through the generation of an aISC intermediate. The +4 position is an approximation, referring to a region of the crypt from +4 to +7 nuclear positions, counted upward from the bottom-most nucleus in the crypt base.
ISCs fuel a complete turnover of the epithelium approximately every 4–7 days7. How do ISCs divide to achieve and maintain such high rates of turnover without becoming depleted? Studies are underway to determine the mechanisms by which self renewal and cell-lineage allocation are balanced under normal physiologic conditions. Lgr5-expressing ISCs can self renew by symmetric cell division to yield 2 equipotent stem cell progeny7,8,9. This mode of self renewal generates monoclonal crypts, over time, according to neutral drift kinetics, indicating that symmetric divisions are prevalent in the Lgr5-expressing population8,9,10. Asymmetric ISC divisions occur frequently in the Drosophila gut11, but the extent to which asymmetric division is a primary mechanism of intestinal epithelial turnover in higher organisms is not well-established12.
Lineage tracing of individual Lgr5-expressing ISCs have indicated that the decision to remain a stem cell or differentiate might be affected by competition for signals that are spatially patterned within the crypt niche-microenvironment12,13. In this case, Lgr5-expressing ISCs positioned near the niche boundary (border cells) are more likely to be passively displaced out of the niche by newly born cells in the crypt base. These displaced ISCs ostensibly become transit-amplifying cells without a requirement for prior cell division. Conversely, ISCs at the bottom of the crypt receive signals that make them more likely to undergo self renewal via symmetric division13. It will be important to learn whether and how specific signals within the niche promote one process vs another—this information could help us build more accurate models for studying regulation of intestinal turnover and the pathogenic effects of defects in ISC regulation.
Although many signaling pathways are involved in regulating ISCs, we focus on Wnt signaling and how alterations in this pathway affect stem cell function and intestinal homeostasis. For more detailed review of other signaling pathways involved, see14,15,16,17,18,19,20. The functions of aISCs are regulated by the canonical Wnt to beta-catenin signaling pathway. Although there has been much attention on Paneth cells as a predominant source of Wnt in the crypt, there are other cell sources for Wnt in the intestinal epithelium and parenchyma, indicating its importance in the ISC niche21,22,23,24,25,26. Wnt ligands associate with frizzled class receptors and LDL receptor related protein 5 (LRP5) and LRP6 to induce axin-dependent stabilization and nuclear translocation of beta-catenin, which complexes with lymphoid enhancer binding factor (LEF/TCF) transcription factors to induce expression of genes regulated by Wnt signaling27. Tissue-specific deletion of the transcription factor 7 like 2 gene (TCF7L2 or TCF4), which encodes a protein that activates Wnt-dependent gene expression, resulted in loss of aISCs and blocked intestinal crypt development in mice28. Likewise, transgenic expression of dickkopf WNT signaling pathway inhibitor 1 (DKK1) resulted in progressive and pervasive loss of Lgr5-expressing ISCs and subsequent failure in homeostatic epithelial maintenance in mice29,30,31. These loss of function studies established Wnt signaling as a cornerstone of ISC function.
Gain of function studies have provided more evidence for the importance of Wnt in ISC functions. R-spondins 1–4 (RSPO1–4) are secreted agonists of Wnt signaling that associate with and inhibit transmembrane E3 ligase receptors ZNRF3 and RNF43—these otherwise promote internalization and degradation of frizzled class receptors and LRP5 and LRP632,33,34,35,36,37. Transgenic overexpression of RSPO1 stimulates crypt cell proliferation and increased Lgr5-expressing cell numbers, eventually resulting in intestinal hypertrophy in mice30,33. Conversely, combined disruption of the RSPO receptor genes (Lgr4 and Lgr5) is lethal; more crypts and ISCs are lost in double mutants (LGR4 and LGR5 double knockout) than in single mutants35,38,39. Wnt signaling therefore balances aISC proliferation to promote normal homeostatic turnover of the intestinal epithelium, and is likely required to re-establish aISC function following cell loss due to disease, damage, or injury.
History on the conceptualization of rISCs
The concept that there is a pool of replicating cells that are distinct from aISCs/CBCs arose from experiments designed to locate and track dividing intestinal cell populations. In these, radiolabeled nucleotide analogues incorporated into DNA during S-phase are used to characterize differentiation, division, and migration kinetics of replicating cells40. These analyses led to a model in which most replicating intestinal cells cycle every 24 hours40, and cells migrating upward from the crypt originate from approximately 4 nucleus positions from the crypt base (the +4 position)128. Some cells at or near +4, moreover, had a slowly cycling or quiescent phenotype, indicated by their ability to retain DNA label for extended time periods41. Together these early studies provided evidence for the existence of a long-lived, slowly cycling, label-retaining cell (LRC) population that resides at or near the +4 position.
Additional characterization of +4 LRCs suggested that at least some LRCs asymmetrically segregate their DNA strands during division—the old DNA strand is retained in the mother cell and new DNA strand moves to the daughter progeny, presumably to protect the DNA of the replicating mother stem cell from the accumulation of mutations acquired during S phase42,43,44,45. In this immortal DNA strand model, +4 cells are a dormant and potentially damage-resistant cell population. There is little evidence, however, beyond the original studies to support asymmetric DNA segregation as a prevalent behavior of these cells. In fact, multi-isotope mass spectrometry evaluation of DNA label retention in ISCs indicated that ISCs dilute label in a manner consistent with random DNA strand segregation. The studies found no evidence for label retention in any non-Paneth crypt cells regardless of anatomic position46. It wasn 201367, almost 5 decades after the +4 model was proposed, that there was functional evidence for ISC activity in +4 LRCs.
A genetic marker for +4 ISCs
In validating the +4 ISC model, much effort has been directed toward identifying +4 cell-specific biomarkers, to allow studies of cell-cycling, label-retention, and lineage-tracing behaviors. The first direct evidence for the existence of +4-resident stem cells was reported in 200847. BMI1 protooncogene, polycomb ring finger (BMI1) is a member of the polycomb repressor complex 1, which regulates chromatin silencing and has been implicated in regulating stem cell function in numerous systems, including the intestine47,48,49,50,51,52. In tracing the Bmi1-IRES-CreER lineage at specific time points soon after tamoxifen administration, researchers found Bmi1-expressing cells predominantly at the +4 to +5 locations of many crypts in the proximal small intestine30. Progeny of Bmi1-expressing cells traced for extended periods gave rise to differentiated lineages in the villus, demonstrating self renewal and multipotency of the Bmi1-expressing population. Notably, the kinetics of lineage tracing from Bmi1-expressing cells was significantly slower than Lgr5-expressing ISCs, suggesting that Bmi1 marks ISCs that proliferate and differentiate less frequently than the Lgr5-expressing ISC pool3. Direct comparison between Lgr5-marked and Bmi1-marked ISCs incorporating 5-ethynyl-2 -deoxyuridine (EdU) confirmed that Bmi1-expressing populations were essentially quiescent, and far less likely than Lgr5-expressing cells to enter S-phase or allocate differentiated cells under homeostatic conditions30.
Single isolated Bmi1+ cells could generate organoids that were self renewing and possessed all post-mitotic cell lineages, further implicating ISC function in Bmi1-expressing cells30. Bmi1-expressing ISCs, however, were relatively insensitive to canonical Wnt stimulation, compared with Lgr5-expressing ISCs, revealing an important phenotypic difference between proposed +4 ISC and aISC populations30. Together, these studies provided evidence that ISCs with nonequivalent proliferation profiles coexisted in the intestinal crypt.
Conversion of ISCs between active and quiescent states
The initial report on BMI1 was followed by the discovery of additional biomarkers for slowly replicating stem cells located at or near +4. These markers include, but are not limited to, the HOP homeobox (HOPX), telomerase reverse transcriptase (TERT), leucine rich repeats and immunoglobulin like domains 1 (LRIG1), phosphatase and tensin homolog (PTEN), doublecortin like kinase 1 (DCLK1), protein phosphatase, and Mg2+/Mn2+ dependent 1D (PPMID or WIP1), as well as high-level expression of the transcription factors SRY-box 9 (SOX9) and mex-3 RNA binding family member A (MEX3A) (reviewed in53,54). We focus on expression of Hopx to illustrate the concept that active and +4 quiescent ISCs can switch between phenotypes. Hopx is an atypical homeobox gene—its expression marks a population of +4 ISCs along essentially the entire intestine55. Studies of Hopx lacZ knock-in mice revealed enrichment of Hopx-expressing cell numbers around +4 to +5, and an overlap in Hopx-reporter expression in LRCs, consistent with a quiescent +4 ISC phenotype. Notably, these studies used a combination of lineage tracing, gene-expression profiling, and laser capture microdissection to demonstrate that Hopx-expressing cells were capable of generating Lgr5-expressing CBCs, in addition to all the differentiated lineages of the intestine55.
As with the Bmi1-expressing cells, however, a direct comparison of lineage-tracing kinetics revealed that the progeny of Hopx-expressing ISCs take longer to populate crypts and villi than Lgr5-expressing CBCs3. Isolated Hopx-expressing cells were also shown to be multipotent in culture. Importantly, actively dividing Lgr5-expressing cells could also give rise to new quiescent Hopx-expressing progeny in culture55. These collective observations supported a model in which 2 populations of stem cells coexist in the crypt, each capable of converting between actively cycling and quiescent phenotypes.
Aside from their capacity for self-renewal and multipotency, ISCs have been extensively classified based on their proliferative behavior (actively cycling vs quiescent). The identification and use of biomarkers, lineage-tracing tools, and ex vivo ISC culture models have refined our understanding of ISC heterogeneity in terms of anatomical and numerical relationships, marker-identity, and stem cell function. In the latest model, quiescent +4 ISCs and aISC/CBCs can be thought of as 2 anatomically and functionally distinct ISC pools. How is homeostatic turnover of the intestinal epithelium, or its regeneration after damage, fueled by these respective populations? The ability of putative quiescent +4 ISC to convert to and from aISCs suggests that each population of ISCs can sense and adapt to fluctuations in physiologic demand for stem cell activity56 (Figure 1B, Figure 2). Such fluctuations might occur during homeostatic conditions such as during periods of nutrient deprivation or during circadian cycles63,130,131, or alternatively under conditions of extreme injury and stress. The concept that +4 ISCs are dedicated to the reserve function of replacing lost or compromised aISCs to support epithelial regeneration is of great interest.
Figure 2. Models for Conversion Between aISCs and rISCs.
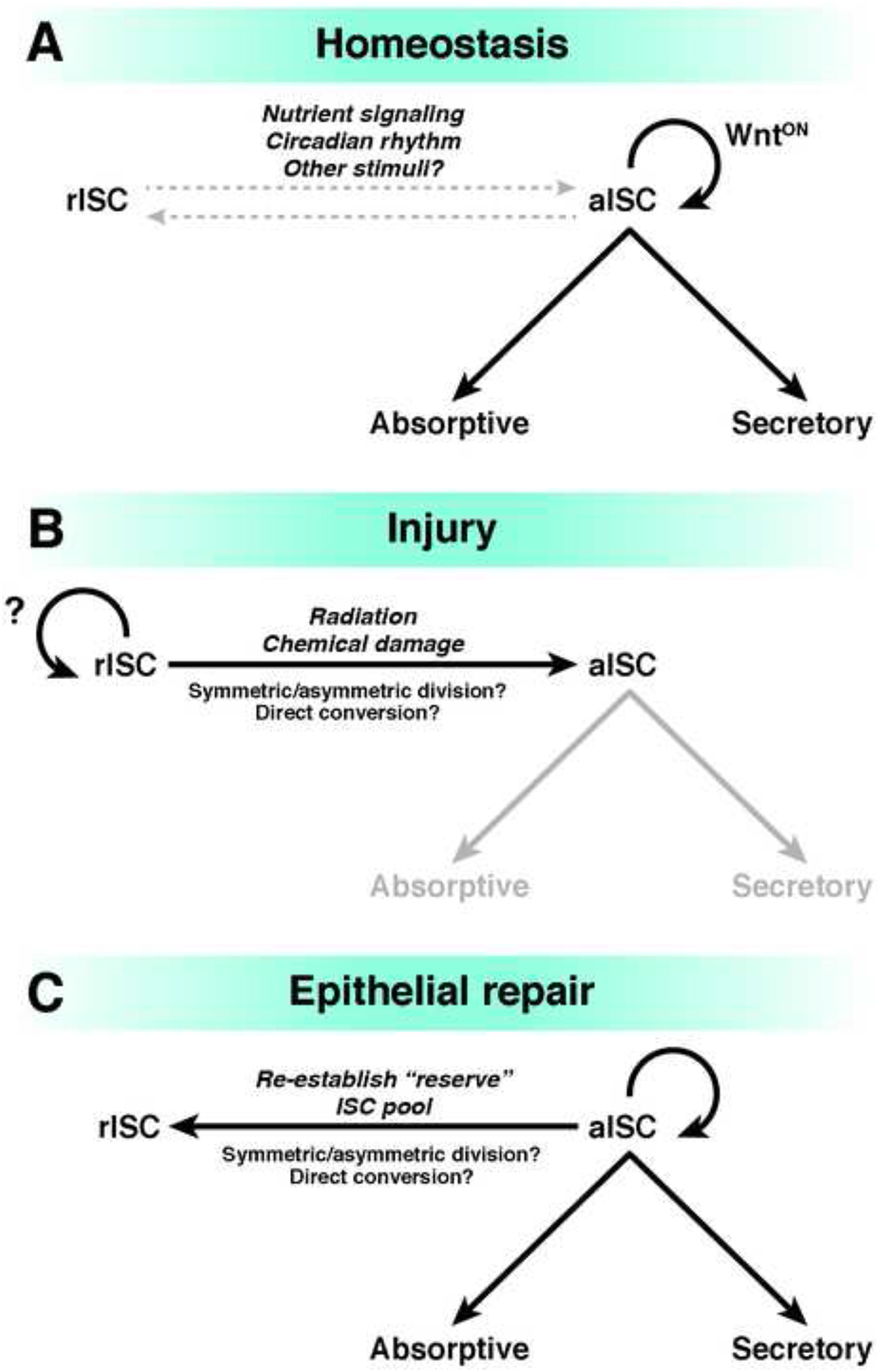
A) Canonical Wnt signaling mediates self renewal of the aISC pool, which is the predominant source of all differentiated lineages. Conversion between aISCs and rISCs can occur, but is rarely observed under normal physiologic conditions. B) When aISCs are selectively lost after injury, rISCs become activated and convert to aISCs via unclear mechanisms. C) Epithelial repair is achieved as the aISC pool is replenished by rISCs, and regeneration of the full complement of epithelial cell types is completed. The rISC pool at this stage is presumably re-established, though this has not been formally tested. Note: transit-amplifying populations are not shown in this diagram.
Identity, Function, and Regulation of rISCs
The rISC phenotype is defined as a dedicated feature of cells that maintain stem cell capacity in a quiescent state under homeostatic conditions, and can re-acquire a proliferative aISC state when the resident aISC population is compromised. Although rISCs have been primarily associated with +4 ISCs, there is increasing evidence that many intestinal cell types, including progenitors of differentiated lineages and differentiated cell types themselves, possess rISC-like activity. It is therefore unclear whether rISC refers to a single and homogenous stem cell type. Does a larger collection of cell types exist, with varying degrees of ISC activity? If so, these cells might be used in regenerative therapeutic strategies for gastrointestinal disorders. A range of cell types have rISC-like activity, supporting a model in which crypt-based cells have phenotypic plasticity.
+4 ISCs
Experiments in which Lgr5-expressing aISCs (with a diphtheria toxin receptor gene knocked into the Lgr5 locus) could be selectively ablated by administration of diphtheria toxin provided evidence for the existence of a reserve ISC pool that does not express Lgr56. These experiments showed that Lgr5-expressing ISCs, which existed before diphtheria toxin administration, were dispensable for maintenance of the intestinal epithelium, and that other epithelial cells could compensate for their loss—specifically Bmi1-expressing cells6. Further evidence for this concept came from lineage tracing of +4 quiescent ISCs after radiation-induced damage. In this situation, Lgr5-expressing ISCs were particularly susceptible to radiation exposure, and could be almost completely ablated by high doses of radiation30,58,59. In contrast, Bmi1-expressing ISCs were not only radio-resistant, but lineage tracing output increased following exposure to radiation30, indicating that the +4 quiescent ISCs were at least partly responsible for the regenerative response. In addition to cells that express Bmi130, cell populations that express Hopx55,60,61, mouse Tert62,63, and other genes59,64,65,66 were found to have regenerative, ISC functions after epithelial damage, supporting a model in which quiescent +4 ISCs can survive and participate in regeneration after loss of aISCs. Notably, regeneration of Lgr5-expressing ISCs was essential for subsequent post-radiation epithelial repair132, consistent with a model in which rISCs repopulate Lgr5-expressing aISCs6. Altogether, these studies provide evidence for rare damage-resistant and quiescent +4 rISCs that can re-activate to replenish the aISC pool and facilitate regeneration.
Label-retaining secretory progenitors
In 2013, researchers confirmed that Lgr5-expressing populations could be subdivided into either actively cycling or quiescent label-retaining ISC fractions, based on an ability to retain an inducible H2B-YFP label67. Lgr5-expressing H2B-YFP+ LRCs had patterns of gene expression consistent with secretory progenitors of the Paneth and enteroendocrine cell lineages, indicating that these cells are transient intermediates on the path to terminal differentiation. Single H2B-YFP LRCs, isolated by flow cytometry, could form organoids in culture and had stem cell-like functions in vitro. Using a split-Cre lineage tracing approach, these authors showed that LRCs were capable of extensive proliferation and multilineage differentiation after chemical injury in mice67. The LRCs produced multi-lineage clones distributed along the crypt–villus axis, similar to conventional Lgr5-expressing aISCs. This study provided intriguing evidence that secretory precursor populations could be captured in an Lgr5-expressing and label-retaining state, and that these cells can recall a functional stem-like regenerative program in response to damage. It was not shown whether multiple subtypes of secretory progenitors were capable of this behavior, or whether the secretory progenitors investigated are a common pool for all secretory cell types.
A subsequent study compared transcription profiles and stem-cell functions between so-called short-term (10 days post label-induction) and long-term (up to 3 months) H2B-LRCs, with other ISC populations traced using Lgr5, Bmi1, and Hopx promoters to induce CreER expression. Short-term H2B-LRCs had a secretory progenitor-like gene signature and stem cell capacity61. These findings were consistent with those from a previous study showing that secretory precursors are plastic prior to terminal differentiation. Long-term LRCs were largely Paneth cells, which were not shown to have stem cell activity in culture or injury-induced regenerative capacity in this report61.
LRCs have been associated with quiescent ISCs, but tools were not always available to directly test their stem cell function. The studies we described were the first to use tracing techniques to determine whether LRCs had injury-induced stem cell potential. It is important to remember that a heterogeneous mixture of cell lineages is likely to be captured in the LRC assay, depending on the specific labeling parameters used as well as the replication, label-dilution, and cell-differentiation characteristics of various cell types under study. Studies are underway to identify genetic and epigenetic mechanisms that regulate the quiescent label-retaining rISC state—these studies could uncover the mechanisms of plasticity in LRCs.
Dll1+ secretory progenitors
The allocation of stem cell progeny toward the secretory lineages requires, at least in part, that Notch signaling activity is reduced or blocked68,69,70,71. The delta-like canonical Notch ligand 1 gene (DLL1) encodes a transmembrane protein involved in Notch pathway activation and signaling. DLL1 is thought to be normally expressed by a subset of transit-amplifying secretory precursors that are the immediate progeny of aISCs72,73,74. In studies of Dll1GFP-ires-CreER knock-in mice and in situ hybridization experiments, Dll1highCD24mid populations were enriched for markers of progenitor cells and multiple secretory lineages75. Lineage tracing showed that these cells generate only short-lived proliferative clones, which quickly differentiate into secretory cell types. These results support the concept that transit-amplifying secretory precursors are committed to differentiation, and are not fully multipotent for all intestinal lineages or normally capable of prolonged self-renewal.
Sorted Dll1highCD24mid cells, however, could form organoids that contain Lgr5-expressing ISCs following addition of exogenous WNT75. Thus, ex vivo, Wnt-pathway stimulation induces stem cell activity in an otherwise committed Dll1high population of secretory precursor cells. In mice, when lineage-tracing of Dll1high cells was initiated (using a tamoxifen-inducible R26RLacZ transgene) just prior to administration of sub-lethal doses of radiation, rare tracing events consistent with stem cell multipotency were observed, in addition to LacZ+ Lgr5-expressing cells. These results indicate that at least some secretory progenitors are plastic and can regain stem cell identity and function in response to damage. It was not determined whether Dll1high cells generate slowly replicating or otherwise label-retaining cells, in addition to the Lgr5-expressing aISCs75.
SOX9+ secretory progenitor cells
The Sry-box-containing proteins are a family of conserved transcription factors that function broadly during embryonic development and in adult stem cells. SOX9, a member of this family, has been shown via lineage tracing to mark adult and/or facultative stem cells in the intestine, colon, pancreas and liver76,77,78. Cell populations that express high levels of a Sox9EGFP reporter transgene (Sox9EGFP-HI), contain putative label-retaining ISCs as well as secretory progenitors, differentiated enteroendocrine cells, and tuft cells79,80. Single cell transcriptomic profiling of Sox9EGFP-HI cells revealed that a small population of cells from crypt-enriched epithelial fractions have a secretory progenitor signature similar to those reported to participate in regeneration after injury65. There is evidence that SOX9 might have direct roles in regulating ISC behaviors in response to stimuli such as nutrient-dependent signaling and damage63,81,82,83,84.
Isolated Sox9EGFP-HI cells rarely proliferate or form organoids in vitro, but have increased organoid formation after stimulation with insulin like growth factor (IGF) and radiation exposure84. IGF also stimulates DNA synthesis in Sox9EGFP-HI cells and facilitates intestinal regeneration in mice exposed to radiation. Therefore, it appears that Sox9EGFP-HI cells can respond to stimuli by activating the cell cycle and participating in regenerative processes84. No lineage tracing experiments have been performed to confirm this interpretation, due to the lack of a specific marker for SOX9HI cells.
Perhaps more importantly, SOX9 protein is required for intestinal regeneration after radiation injury in mice65. Because the SOX9-deficient epithelium is defective in generation of LRCs, SOX9 might preserve radio-resistance in ISCs, at least in part, by promoting a slower cycling state, and thereby increasing protection of the genome. It is also possible that specific functions for SOX9 during Paneth cell differentiation could be required to generate mature Paneth cells that are support regenerative processes in the intestine85,86. Studies are needed to determine the roles for SOX9 in regulating the ISC cell cycle, in conferring ISC radio-resistance, and in regulating Paneth cell differentiation from ISCs, to define the mechanisms through which SOX9 regulates intestinal homeostasis and regeneration.
Paneth cells
Differentiated Paneth cells might also provide a source for new intestinal tissue after damage87. Researchers used villin-rtTA/TRE-H2B-GFP mice, with doxycycline dosing and withdrawal strategies, to label long-lived LRCs in the crypt base. These LRCs, which persisted in the crypt for up to 100 days, were shown to be differentiated Paneth cells. Although these label-retaining Paneth cells expressed low levels of the stem cell markers Lgr5, Bmi1, Tert, and Lrig1, they did express high levels of the stem cell marker musashi RNA binding protein 1 (MSI1)87,88. Label-retaining Paneth cells in mice could re-enter the cell cycle following radiation damage, and the numbers of H2B-GFP label-positive cells increased over 3–5 days. The intensity of GFP also decreased in these cells over time, presumably due to replication-associated dilution of H2B-GFP signal. These findings indicate active regeneration from the Paneth cell population. Eventually, the label-retaining Paneth cells lost Paneth cell markers and began to express Bmi1 during regeneration87. Therefore, Paneth cells and/or their immediate precursors might reacquire stem cell function after radiation exposure to participate directly in epithelial regeneration. Further studies are needed.
Enteroendocrine cells
Post-mitotic enteroendocrine cells have also been reported to have injury-induced stem cell activity84,89—specifically enteroendocrine subsets marked by neurogenin3-Cre90 or Nkx2.2-Cre91. Using a Bmi1-GFP knock-in allele to allow Bmi1-expressing cell isolation without relying on Bmi1-CreER-mediated lineage tracing30,47, researchers showed that single GFP+ intestinal cells could undergo clonogenic organoid formation indicative of stem cell function93. GFP+ cells also had a gene expression signature associated with enteroendocrine cells, indicating that quiescent and regeneration-competent Bmi1-expressing subpopulations might be relatively differentiated enteroendocrine cells92,93. Although the formal equivalence of the Bmi1-GFP+ knock-in and Bmi1-CreER+ alleles, in terms of cell specificity, has not been demonstrated, analyses of transcriptomes of Bmi1-CreER+ cells revealed expression of enteroendocrine cell-associated genes, although this was accompanied by Paneth cell gene expression patterns not usually associated with Bmi1-GFP+ cells. This might be due to imperfect overlap between true Bmi1+ ISCs, Bmi1-GFP+ cells, and the cells that become labeled by Bmi1-CreER+ after exposure to tamoxifen.
Bmi1-GFP+ cells also expressed the homeobox transcription factor prospero homeobox 1 (PROX1)93, which is expressed by differentiated enteroendocrine cells94. In Drosophila, the Prox1 ortholog, prospero, is required for generation of midgut intestinal enteroendocrine cells95,96,97. In intestines of mice, Prox1-expressing cells exhibit clonogenic growth into organoids and radiation-inducible lineage tracing similar to Bmi1-GFP+ cells93. Bulk and single-cell RNA sequencing revealed that Bmi1-GFP+ cells were predominantly lineage-committed Neurod1+Neurog3− enteroendocrine cells, whereas Prox1-GFP+ cells comprise cells with a Bmi1-like mature enteroendocrine phenotype, but also contain a subset that expresses tuft-cell associated genes with low levels of enteroendocrine gene expression. Separately, it was shown that the differentiated chromatin and enhancer occupancy profile of Bmi1-GFP+ cells and of CD69+CD274+ goblet cells reverts to a distinct Lgr5-expressing ISC-like pattern upon radiation exposure92. The post-mitotic and differentiated state of at least some enteroendocrine cells is therefore not hard wired, and dynamic chromatin changes accompany the plastic conversion of these cells toward a more ISC-like condition during regeneration.
Absorptive Enterocytes
Damage to Lgr5-expressing ISCs induces crypt regeneration—not only from ISCs and secretory progenitor/precursor populations, as described above, but also from the progenitors of absorptive enterocytes98. Alkaline phosphatase, intestinal (ALPI) has been widely used as a marker for enterocytes and their progenitors98,99. Upon diphtheria toxin receptor-mediated ablation of Lgr5-expressing ISCs, Alpi-expressing cells de-differentiate into proliferative Lgr5-expressing ISC and Paneth-like cells in mice98. During their conversion, Alpi-expressing cells upregulate stem cell-specific genes and downregulate enterocyte-specific genes. Because absorptive enterocytes and their progenitors constitute a majority of the intestinal epithelium, the concept that they can serve as a reservoir for new ISCs after damage is potentially of high significance.
Relevance of Reserve Stemness and Cell Plasticity to Regeneration
With a body of evidence showing that various intestinal cell types can participate in epithelial regeneration, questions are being pursued as to which cell-type or types represent the primary sources for generating new stem cells and differentiated tissue. On the one hand, it is possible that requisite populations of dedicated rISCs are maintained in the niche so that they can become activated in response to damage and fulfill regenerative tasks. On the other hand, it is also possible that regeneration could be accomplished by a concerted effort among many if not all epithelial cell types, ostensibly through the injury-induced activation of a plasticity program that is shared across multiple stem and differentiated cell types. Data indicate that elements of both processes occur to at least some extent (Table 1).
Table 1.
Contribution of regenerative populations to epithelial homeostasis and repair
| Contribution to epithelial lineages | ||||||
|---|---|---|---|---|---|---|
| Biomarker | Model | Homeostasis | Regeneration | Injury Type | Citation | |
| LGR5 |
Lgr5EGFP-IRES-CreER; Rosa26lsl.tdTom or Rosa26lsl.YFP |
Tracing Period | 7 Days | 4–7 Days | 12 Gy Whole body IR | 30 |
| Trace frequency | ~95% C/V Units | Eradicated | ||||
| Trace Type | Ribbons | ------------- | ||||
| BMI1 |
Bmi1CreER; Rosa26lsl.YFP |
Tracing Period | 7 Days | 7 Days | 12 Gy Whole body IR | 30 |
| Trace frequency | ~18% C/V Units | ‘more extensive’ | ||||
| Trace Type | Ribbons | Ribbons | ||||
| BMI1 | Bmi1CreER;Rosa26R | Tracing Period | 6 Days | 6 Days | Diptheria Toxin ablation of Lgr5* ISCs | 6 |
| Trace frequency | ~3% fully labeled crypts | ~15-fold increase | ||||
| Trace Type | Labeled crypts | Labeled Crypts | ||||
| HOPX |
HopxCreER; Rosa26lsl.tdTom or Rosa26R |
Tracing Period | 2 Months | 4 Days | 12 Gy Whole body IR | 55,60,61 |
| Trace frequency | Rare | Broad contribution | ||||
| Trace Type | Ribbons | Labeled cells in all C/V units | ||||
| PROX1 |
Prox1CreER; Rosa26lsl.tdTom |
Tracing Period | 2–395 Days | 7 Days | 12 Gy Whole body IR | 93 |
| Trace frequency | ~5% C/V Units | “Similar to Bm/130” | ||||
| Trace Type | Ribbons by 395 days | Ribbons and large cell-clusters | ||||
| ALPI | AlpiCreER;Rosa26R | Tracing Period | 14 Days | 14 Days | Diptheria Toxin ablation of Lgr5* ISCs | 99 |
| Trace frequency | None | 500–900 per mouse | ||||
| Trace Type | None in crypts | Ribbons | ||||
| DLL1 |
Dll1GFP-IRES-CreER; Rosa26R |
Tracing Period | 28 Days | 14 Days | 6 Gy Whole body IR | 75 |
| Trace frequency | Frequently Paneth Cells | ~100 crypts per duodenum | ||||
| Trace Type | Paneth cells only | Ribbons | ||||
| MEX3A |
Mex3atdTOM-P2A-CreER; Rosa26mTmG |
Tracing Period | 7–14 Days | 7–14 Days | 5-fluorouracil | 120 |
| Trace frequency | Not reported | Not reported | ||||
| Trace Type | Varies, eventual ribbons | More rapid clone and ribbon formation | ||||
Note: Tabulation of reported lineage tracing behaviors from intestinal epithelial cell populations proposed to contribute to homeostasis and/or regeneration after injury or cell ablation interventions. Values are approximations in some cases. C/V units refers to “crypt-villus” units, which are ribbon-like stripes of lineage labeled cells extending from individual crypt-bases upward toward the tips of villi.
Lineage-tracing of Bmi1-CreER6,30 and Hopx-CreER55,60,61 cells after injury with high-dose radiation (12 Gy) showed that regeneration from these purported rISC populations occurs frequently and broadly within the intestine. Moreover, knockout of MSI1 from Hopx-expressing cells perturbs intestinal regeneration after injury in mice88. These findings show that Bmi1 and Hopx are markers of cells that mediate epithelial tissue formation during regeneration. In contrast, secretory progenitor cells marked by Dll1-CreER75, Prox1-CreER93, or H2B-label67 have many fewer tracing events after injury. However, a caveat to comparison of these distinct putative rISCs is the heterogeneous nature of different ROSA reporter alleles used in the various studies (Table 1). Moreover, studies in which Lgr5-expressing cells were ablated and regenerative Alpi1-expressing enterocytes were traced indicated that Alpi1-expressing cells only rarely acquire functional stem cell characteristics after injury98. A recent report suggested that a slowly cycling, multipotent subset of Lgr5-expressing ISCs, located nearer the crypt base (at and below +4) and expressing high levels of Mex3a, were resistant to radiation and the chemotherapeutic agent 5-fluorouracil—these cells converted to fast-cycling ISCs and participated in regeneration after chemotherapy-induced injury in mice (Ref119) Therefore, subsets of Lgr5-expressing cells (presumably a subpopulation of aISCs) can participate in epithelial regeneration following injury. Development of rigorous metrics for regeneration, used in combination with focused comparative analyses based on markers of intestinal cell sub-populations and different types or severity of damage, will provide more information on the frequency with which individual cell types acquire stem cell potential in response to injury.
Technical Approaches and Limitations
Until recently, the identification of aISCs and rISCs, has been based primarily on the presence or absence of single or small numbers of biomarkers (such as Lgr5, Bmi1, etc.). A more complex picture of intestinal epithelial cell heterogeneity, however, is now developing based on global transcriptomic analyses at single cell resolution. Cells expressing biomarkers once proposed to be restricted only to aISCs were found to also be expressed by quiescent and putative rISCs and differentiated cell populations (Figure 3). Biomarkers such as Bmi1 and Hopx, originally proposed to mark +4 quiescent ISCs, were found to be broadly expressed within the crypt. Bmi1 and Hopx mRNAs are expressed in most, if not all CBCs, as well as in transit-amplifying populations71,100,101,102,103,129.
Figure 3. Expression patterns of biomarkers commonly associated with rISCs.
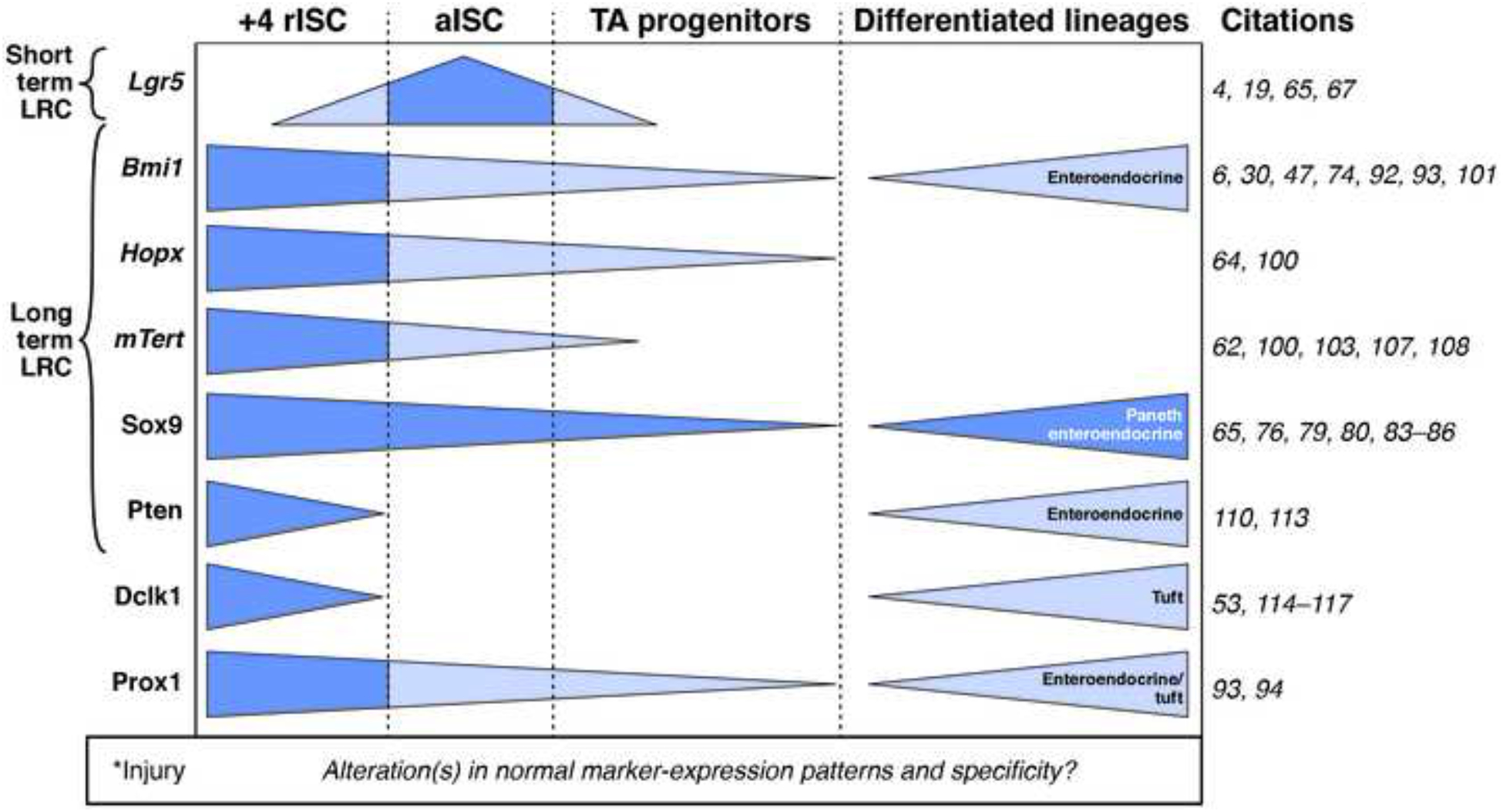
Originally reported biomarker specificity is indicated in dark blue. Marker expression patterns reported afterward are indicated in light blue. It is not clear whether and how gene expression patterns are altered, especially within short time periods, during injury (*).
Moreover, there are questions about the reproducibility of lineage-tracing initiation from the +4 position using Bmi1-CreER lines46,71. Other proposed markers for ISC subpopulations have met similar criticisms, indicating the limitations of reliance on biomarkers and CreER-based lineage tracing to define specific cells or populations104,105,106. For example, the mouse Tert gene was proposed, based on analyses of mTert-EGFP and mTert-CreER mouse lines, to mark a rare subpopulation of multipotent +4 ISCs distinct from Hopx-expressing cells (but with overlapping expression of Bmi1)62,107. Tert mRNA, however, was found throughout the crypt—even at relatively high levels in Lgr5-expressing CBCs100,108. This finding is perhaps not surprising, given that telomerase protects the genomes of replicating cells109.
Other proposed +4 markers such as PTEN, DCLK1, and WIP1 were identified based on their expression by rare populations of +4-localized cells with label-retaining characteristics110,111,112. Subsequent validation of these markers by antibody labeling or gene-expression analyses, however, indicated less specificity in their +4 location than previously reported. In the case of PTEN, antibody labeling studies found that PTEN marks, in addition to +4 localized LRCs, cells expressing enteroendocrine markers in the crypt base113. DCLK1 was originally found to be highly expressed in micro-dissected samples of fixed tissue sections, with a protein localization pattern consistent with rare +4 LRC, but was later found to mark rare populations of tuft cells53,114,115,116. This result was confirmed by lineage tracing using a CreER knock-in line regulated by Dclk1 promoter sequences117. Although knock-in studies have identified cell populations that are induced in response to injury, these and other cases indicate a need for caution in relying on biomarkers to define subpopulations of regenerative cells within the intestine biomarkers alone should not be used to define subpopulations of cells within the intestine.
ISC researchers have used flow cytometry and fluorescence-activated cell sorting methods to study stem cell identity and function. Cell sorting-based enrichment of cell populations, using fluorescent reporter lines such as Lgr5EGFP, Bmi1EGFP, HopxEGFP, and Sox9EGFP cells, is common practice. These techniques have been critical for validating cell identity, function, and heterogeneity53,118,120. Several protocols use fluorescent reporter lines in conjunction with cell-surface antibody labeling to identify and isolate specific populations of cells. Although few if any current primary antibodies are specific for single subpopulations of ISCs, markers such as CD24122, CD44123, GRP78121 and CD166124 have been used to identify crypt-localized stem cell populations in mouse, and in some cases human, tissues118,122,123.
Other, non-fluorescence-based sorting methods, such as side population (SP) sorting, have also been used to isolate and characterize various stem cell populations. Briefly, side populations are sorted based on the presence of efflux transporters on the plasma membrane of stem cells, to isolate stem cells from bone marrow and many solid tissues124. SP sorting has been used to isolate crypt base ISCs125,126, and more recently to segment populations of ISCs into cycling a non-cycling or slowly-cycling states127. As the repertoire of genetic and antibody markers for specific subsets of stem and differentiated cell types grows, our understanding of the potential multitude of mechanisms underlying intestinal renewal and regeneration will improve.
Future Directions
The seeming diversity of cell populations in the intestine that have the demonstrated capacity to participate in regeneration has raised numerous questions regarding the dedicated functions of specific cell types in the contexts of homeostasis and injury. It is important to establish whether dedicated active and reserve ISC populations exist and function in homeostatic renewal and regeneration after injury132, or whether it is more accurate to consider reserve stemness more broadly—as cellular plasticity that is a shared among many cell types in the intestinal epithelium. Multiple mechanisms appear to have evolved to maintain and quickly repair the epithelial lining after damage. It is therefore not surprising that multiple cell types in the damaged epithelium contribute to repair. However, just because 1 cell type can participate in regeneration, it does not necessarily mean that this cell type is dominant in or required for reestablishment of the epithelial lining.
To the extent possible, it will be necessary to rigorously establish the relative and essential contributions of distinct cell populations to the regenerative response, and especially to determine whether similar or different molecular mechanisms are involved in each. Moreover, focused investigations into stem cell heterogeneity and the existence of rISCs in colonic crypts are needed to further studies of cancer stem cells and tumor initiation, progression, and response to treatment. It will also be important to identify the signals and niche cell types that regulate conversion between rISCs and aISCs—these might vary during homeostasis and repair. Such investigations will continue to refine prevailing models, challenge overly simplistic definitions of reserve and active stem cell states, and inform strategies for regenerative medicine.
Footnotes
The authors declare no conflicts of interest
REFERENCES
- 1.Cells IP, Cheng H. Origin, Differentiation and Renewal of the Four Main Epithelial Cell Types in the Mouse Small Intestine . Dev. Dynamics 1974; 141:537–561. [DOI] [PubMed] [Google Scholar]
- 2.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat 1981;160:51–63. [DOI] [PubMed] [Google Scholar]
- 3.Barker N, Es van JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–265. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, Clevers H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science (80−) 2013;340:1190–1194. [DOI] [PubMed] [Google Scholar]
- 6.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2012;478:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013;154:274–284. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Garcia C, Klein AM, Simons BD, et al. Intestinal Stem Cell Replacement Follows a Pattern of Neutral Drift. Science 2010;330:822–825. [DOI] [PubMed] [Google Scholar]
- 9.Snippert HJ, Flier van der LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010;143:134–144. [DOI] [PubMed] [Google Scholar]
- 10.Kozar S, Morrissey E, Nicholson AM, et al. Continuous Clonal Labeling Reveals Small Numbers of Functional Stem Cells in Intestinal Crypts and Adenomas. Cell Stem Cell 2013;13:626–633. [DOI] [PubMed] [Google Scholar]
- 11.Hou SX. Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J Cell Physiol 2010;224:581–584. [DOI] [PubMed] [Google Scholar]
- 12.Fre S, Hannezo E, Sale S, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One 2011;6:e25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritsma L, Ellenbroek SIJ, Zomer A, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 2014;507:362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mah AT, Yan KS, Kuo CJ. Wnt pathway regulation of intestinal stem cells. J Physiol 2016;594:4837–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung TM, Chia LA, Kosinski CM, et al. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci 2011;68:2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sailaja BS, He XC, Li L. The regulatory niche of intestinal stem cells. J Physiol 2016;594:4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosnier C, Stamataki D, Lewis J. Organizing Self renewal in the intestine: stem cells, signals, and combinatorial control. Nat. Rev. Genetics 2006;7:349–359. [DOI] [PubMed] [Google Scholar]
- 18.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol 2016;594:4791–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Date S, Sato T. Mini-Gut Organoids: Reconstitution of the Stem Cell Niche. Annu Rev Cell Dev Biol 2015;31:269–89. [DOI] [PubMed] [Google Scholar]
- 20.Flier Van Der LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol 2009;71:241–260. [DOI] [PubMed] [Google Scholar]
- 21.Farin HF, Jordens I, Mosa MH, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016;530:340–343. [DOI] [PubMed] [Google Scholar]
- 22.Aoki R, Shoshkes-Carmel M, Gao N, et al. Foxl1-Expressing Mesenchymal Cells Constitute the Intestinal Stem Cell Niche. Cell Mol Gastroenterol Hepatol 2016;2:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabiri Z, Greicius G, Madan B, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 2014;141:2206–2215 [DOI] [PubMed] [Google Scholar]
- 24.Lei NY, Jabaji Z, Wang J, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.San Roman AK, Jayewickreme CD, Murtaugh LC, et al. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports 2014;2:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler KM, Schenhals EL, Furstenberg von RJ et al. , Tissue underlying the intestinal epithelium elicits proliferation of intestinal stem cells following cytotoxic damage. Cell Tissue Res 2015;361:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H, Loh KM, Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012–1248012. [DOI] [PubMed] [Google Scholar]
- 28.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998;19:379–383. [DOI] [PubMed] [Google Scholar]
- 29.Kuhnert F, Davis CR, Wang H-T, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci 2004;101:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan K, Chia L, Li X. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. PNAS 2012;109:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes and Development 2003:1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazanskaya O, Glinka A, del Barco Barrantes I, et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell 2004;7:525–534. [DOI] [PubMed] [Google Scholar]
- 33.Yan KS, Janda CY, Chang J, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5 + intestinal stem-cell self-renewal. Nature 2017;545:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmon KS, Gong X, Lin Q, et al. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. PNAS 2011;108:11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau De W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011;476:293–297. [DOI] [PubMed] [Google Scholar]
- 36.Peng WC, deLau W, Forneris F, et al. Structure of Stem Cell Growth Factor R-spondin 1 in Complex with the Ectodomain of Its Receptor LGR5. Cell Rep 2013;3:1885–1892. [DOI] [PubMed] [Google Scholar]
- 37.Hilkens J, Timmer NC, Boer M, et al. RSPO3 expands intestinal stem cell and niche compartments and drives tumorigenesis. Gut 2016:gutjnl-2016–311606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia MI, Ghiani M, Lefort A, et al. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol 2009;331:58–67. [DOI] [PubMed] [Google Scholar]
- 39.Mustata RC, Loy Van T, Lefort A, et al. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep 2011;12:558–56452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairnie A, Lamerton L, Steel G. Cell proliferation studies in the intestinal epithelium of the rat I. Determination of the kinetic parameters. Exp Cell Res 1965;39:528–538. [DOI] [PubMed] [Google Scholar]
- 41.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and γ irradiation. Nature 1977;269:518–521. [DOI] [PubMed] [Google Scholar]
- 42.Potten CS, Gandara R, Mahida YR, et al. The stem cells of small intestinal crypts: Where are they? Cell Prolif 2009;42:731–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potten CS, Hume WJ, Reid P, et al. The segregation of DNA in epithelial stem cells. Cell 1978;15:899–906. [DOI] [PubMed] [Google Scholar]
- 44.Tomasetti C, Bozic I. The (not so) immortal strand hypothesis. Stem Cell Res 2015;14:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yennek S, Tajbakhsh S. DNA asymmetry and cell fate regulation in stem cells. Semin Cell Dev Biol 2013;24:627–642. [DOI] [PubMed] [Google Scholar]
- 46.Steinhauser ML, Bailey AP, Senyo SE, et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 2012;481:516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008;40:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest 2004;113:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Arribillaga E, Rodilla V, Pellegrinet L, et al. Bmi1 regulates murine intestinal stem cell proliferation and self-renewal downstream of Notch. Development 2015;142:41–50. [DOI] [PubMed] [Google Scholar]
- 50.Yu T, Chen X, Zhang W, et al. Regulation of the potential marker for intestinal cells, Bmi1, by β-catenin and the zinc finger protein KLF4: Implications for colon cancer. J Biol Chem 2012;287:3760–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharya R, Banerjee Mustafi S, Street M, et al. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis 2015;2:225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Cao R, Wang M, et al. Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J Biol Chem 2006;281:20643–20649. [DOI] [PubMed] [Google Scholar]
- 53.Barker N, Oudenaarden Van A, Clevers H. Identifying the stem cell of the intestinal crypt: Strategies and pitfalls. Cell Stem Cell 2012;11:452–460. [DOI] [PubMed] [Google Scholar]
- 54.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014;15:19–33. [DOI] [PubMed] [Google Scholar]
- 55.Takeda N, Jain R, LeBoeuf MR, et al. Interconversion between intestinal stem cell populations in distinct niches. Science 2011;334:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010;327:542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao S, Tang D, Morita Y, et al. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J 2015;34:624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asfaha S, Hayakawa Y, Muley A, et al. Krt19+/Lgr5- Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell 2015;16:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li N, Yousefi M, Nakauka-Ddamba A, et al. Single-cell analysis of proxy reporter allele-marked epithelial cells establishes intestinal stem cell hierarchy. Stem Cell Reports 2014;3:876–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li N, Nakauka-Ddamba A, Tobias J, et al. Mouse Label-Retaining Cells Are Molecularly and Functionally Distinct From Reserve Intestinal Stem Cells. Gastroenterology 2016;151:298–310.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montgomery RK, Carlone DL, Richmond Ca, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A 2011;108:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richmond CA, Shah MS, Deary LT, et al. Dormant Intestinal Stem Cells Are Regulated by PTEN and Nutritional Status. Cell Rep 2015;13:2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powell AE, Wang Y, Li Y, et al. The pan-ErbB negative regulator lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012;149:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roche KC, Gracz AD, Liu XF, et al. SOX9 Maintains Reserve Stem Cells and Preserves Radioresistance in Mouse Small Intestine. Gastroenterology 2015;149:1553–1563.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yousefi M, Li L, Lengner CJ. Hierarchy and Plasticity in the Intestinal Stem Cell Compartment. Trends Cell Biol 2017;27:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buczacki SJA, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013; 495:65–69. [DOI] [PubMed] [Google Scholar]
- 68.Carulli AJ, Keeley TM, Demitrack ES, et al. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol 2015;402:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 2012;139:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian H, Biehs B, Chiu C, et al. Opposing activities of notch and wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep 2015;11:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srinivasan T, Than EB, Bu P, et al. Notch signalling regulates asymmetric division and inter-conversion between lgr5 and bmi1 expressing intestinal stem cells. Sci Rep 2016;6:26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riccio O, Gijn van ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 2008;9:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milano J, McKay J, Dagenais C, et al. Modulation of Notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 2004;82:341–358. [DOI] [PubMed] [Google Scholar]
- 74.Es Van JH, Gijn Van ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature;435:959–963. [DOI] [PubMed] [Google Scholar]
- 75.Es Van JH, Sato T, van de Wetering M, Luybimova A et al. Dll1 marks early secretory progenitors in gut crypts that can revert to stem cells upon tissue damage. Nat Cell Biol 2012;14:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2010;3:34–41. [DOI] [PubMed] [Google Scholar]
- 77.Gracz AD, Magness ST. Sry-box (Sox) transcription factors in gastrointestinal physiology and disease. Am J Physiol Gastrointest Liver Physiol 2011;300:G503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gracz AD, Magness ST. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol 2014;307:G260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 2009;296:G1108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gerbe F, Es Van JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011;192:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mah AT, Landeghem Van L, Gavin HE, et al. Impact of diet-induced obesity on intestinal stem cells: Hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 2014;155:3302–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhongcheng Shi MA, Chiang CI, Mistretta TA, et al. SOX9 directly regulates IGFBP-4 in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol Shi Z Am J Physiol Gastrointest Liver Physiol 2013;305:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landeghem Van L, Santoro MA, Mah AT, et al. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J 2015;29:2828–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landeghem Van L, Santoro M a., Krebs a. E, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. AJP Gastrointest Liver Physiol 2012;302:G1111–G1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 2007;178:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mori-Akiyama Y, Born van den M, Es van JH, et al. SOX9 Is Required for the Differentiation of Paneth Cells in the Intestinal Epithelium. Gastroenterology 2007;133:539–546. [DOI] [PubMed] [Google Scholar]
- 87.Roth S, Franken P, Sacchetti A, et al. Paneth cells in intestinal homeostasis and tissue injury. PLoS One 2012;7:e38965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yousefi M, Li N, Nakauka-Ddamba A, et al. Msi RNA-binding proteins control reserve intestinal stem cell quiescence. J Cell Biol 2016;215:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sei Y, Lu X, Liou A, et al. A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am J Physiol Gastrointest Liver Physiol 2011;300:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 2004;270:443–454. [DOI] [PubMed] [Google Scholar]
- 91.Gross S, Balderes D, Liu J, et al. Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential. Am J Physiol - Gastrointest Liver Physiol 2015;309:G975–G987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jadhav U, Saxena M, O’Neil NK, et al. Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells. Cell Stem Cell 2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan KS, Gevaert O, Zheng G et al. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity. Cell Stem Cell 2017;21:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrova TV, Nykänen A, Norrmén C, et al. Transcription Factor PROX1 Induces Colon Cancer Progression by Promoting the Transition from Benign to Highly Dysplastic Phenotype. Cancer Cell 2008;13:407–419. [DOI] [PubMed] [Google Scholar]
- 95.Guo Z, Lucchetta E, Rafel N, et al. Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Curr Opin Genet Dev 2016;40:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo Z, Ohlstein B. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 2015;350:aab0988–aab0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeng X, Hou SX. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 2015;142:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tetteh PW, Basak O, Farin HF, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 2016;18:203–213. [DOI] [PubMed] [Google Scholar]
- 99.Narisawa S, Huang L, Iwasaki A, et al. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol 2003;23:7525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muñoz J, Stange DE, Schepers AG, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent “+4” cell markers. EMBO J 2012;31:3079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grün D, Lyubimova A, Kester L, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 2015;525:251–255. [DOI] [PubMed] [Google Scholar]
- 102.Flier van der LG, Gijn van ME, Hatzis P, et al. Transcription Factor Achaete Scute-Like 2 Controls Intestinal Stem Cell Fate. Cell 2009;136:903–912. [DOI] [PubMed] [Google Scholar]
- 103.Itzkovitz S, Lyubimova A, Blat I, et al. Single molecule transcript counting of stem cell markers in the mouse intestine. Nat Cell Biol 2012;14:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J, Willet SG, Bankaitis ED, et al. Non-parallel recombination limits cre-loxP-based reporters as precise indicators of conditional genetic manipulation. Genesis 2013;51:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Comai G, Sambasivan R, Gopalakrishnan S, et al. Variations in the Efficiency of Lineage Marking and Ablation Confound Distinctions between Myogenic Cell Populations. Dev Cell 2014;31:654–667. [DOI] [PubMed] [Google Scholar]
- 106.Smith L. Good planning and serendipity: Exploiting the Cre/Lox system in the testis. Reproduction 2011;141:151–161. [DOI] [PubMed] [Google Scholar]
- 107.Breault DT, Min IM, Carlone DL, et al. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci U S A 2008;105:10420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schepers AG, Vries R, Born van den M, et al. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 2011;30:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maciejowski J, Lange de T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 2017;18:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He XC, Zhang J, Tong W-G, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nat Genet 2004;36:1117–1121. [DOI] [PubMed] [Google Scholar]
- 111.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 2007;39:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demidov ON, Timofeev O, Lwin HNY, et al. Wip1 Phosphatase Regulates p53-Dependent Apoptosis of Stem Cells and Tumorigenesis in the Mouse Intestine. Cell Stem Cell 2007;1:180–190. [DOI] [PubMed] [Google Scholar]
- 113.Bjerknes M, Cheng H. Re-examination of P-PTEN staining patterns in the intestinal crypt. Nat Genet 2005;37:1016–1017. [DOI] [PubMed] [Google Scholar]
- 114.Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 2006;281:11292–11300. [DOI] [PubMed] [Google Scholar]
- 115.May R, Riehl TE, Hunt C, et al. Identification of a Novel Putative Gastrointestinal Stem Cell and Adenoma Stem Cell Marker, Doublecortin and CaM Kinase-Like-1, Following Radiation Injury and in Adenomatous Polyposis Coli/Multiple Intestinal Neoplasia in Mice. Stem Cells 2008;26:630–637. [DOI] [PubMed] [Google Scholar]
- 116.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 2009;27:2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakanishi Y, Seno H, Fukuoka A, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2012;45:98–103. [DOI] [PubMed] [Google Scholar]
- 118.Henning SJ, Furstenberg von RJ. GI stem cells - new insights into roles in physiology and pathophysiology. J Physiol 2016;0:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barriga MF, Montagni E, Mana M, et al. Mex3a Marks a Slowly Dividing Subpopulation of Lgr5+ Intestinal Stem Cells. Cell Stem Cell, 2017;20:801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang F, Scoville D, He XC, et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 2013;145:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furstenberg von RJ, Gulati AS, Baxi A, et al. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. AJP Gastrointest Liver Physiol 2011;300:G409–G417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gracz AD, Fuller MK, Wang F, et al. Brief Report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 2013;31:2024–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin TG, Powell AE, Davies PS, et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 2010;139:2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Challen G a, Little MH. A side order of stem cells: the SP phenotype. Stem Cells 2006;24:3–12. [DOI] [PubMed] [Google Scholar]
- 125.Dekaney CM, Rodriguez JM, Graul MC, et al. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 2005;129:1567–1580. [DOI] [PubMed] [Google Scholar]
- 126.Gulati AS, Ochsner SA, Henning SJ. Molecular properties of side population-sorted cells from mouse small intestine. Am J Physiol Gastrointest Liver Physiol 2008;294:G286–94. [DOI] [PubMed] [Google Scholar]
- 127.Furstenberg Von RJ, Gulati AS, Baxi A, et al. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 2011;300:G409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qiu JM, Roberts SA, Potten CS. Cell migration in the small and large bowel shows a strong circadian rhythm. Epithelial Cell Biol. 1994;3(4):137–48. [PubMed] [Google Scholar]
- 129.Haber AL, Biton M, Rogel N et al. A single-cell survey of the small intestinal epithelium. Nature. 2017, 551;333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pagel R, Bar F, Schroder T et al. Circadian rhythm disruption impairs homeostasis and exacerbates chronic inflammation in the intestine. FASEB 2017;31:4707–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bishehsari F, Levi F, Turek FW, Keshavarzian Ali. Circadian Rhythms in GI Health and Diseases. Gastroenterology 2016;151:e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ Stem Cells are Indispensable for Radiation-Induced Intestinal Regeneration. Cell Stem Cell 2014;14:149–159. [DOI] [PubMed] [Google Scholar]


