Abstract
Background
Patients with hemorrhagic shock from trauma often require balanced blood product transfusion with red blood cells, plasma, and platelets. Resuscitation with whole blood resuscitation is becoming a common practice. We performed a systematic review and meta‐analysis of studies comparing whole blood transfusion with balanced component therapy in patients suffering from traumatic hemorrhagic shock.
Methods
We searched MEDLINE Ovid, EMBASE, and the Cochrane Library for human studies comparing whole blood with component blood therapy published from January 2007 to June 2019. We included studies from both civilian and military settings and that reported 24‐hour, in‐hospital, or 30‐day mortality. We followed the Preferred Reporting Items in Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, assessing study quality, publication bias, and heterogeneity. We used meta‐analytic models to determine the associations (odds ratio [OR] with 95% confidence interval [CI]) between whole blood transfusion and (1) 24‐hour mortality, and (2) in‐hospital or 30‐day mortality.
Results
A total of 1759 identified studies, 12 (reporting on n = 8431 patients) met inclusion criteria. There was heterogeneity in the design, setting, interventions, and outcomes of the studies. On meta‐analysis, whole blood transfusion was not associated with 24‐hour mortality (OR = 0.83; 95% CI = 0.56–1.24) or in‐hospital/30‐day mortality (OR = 0.79; 95% CI = 0.48–1.31).
Conclusion
In this systematic review and meta‐analysis, compared with conventional component transfusion, whole blood was not associated with 24‐hour or in‐hospital mortality. However, there were important limitations with and heterogeneity among the primary studies. Additional study is needed to determine the effectiveness of whole blood.
Keywords: blood products, hemorrhage, meta‐analysis, systematic review, transfusion
1. INTRODUCTION
1.1. Background
Hemorrhage accounts for 30%–40% of total trauma deaths. 1 Blood transfusion with balanced components (red cell concentrate, plasma, platelets, and cryoprecipitate) is the current standard of care for patients suffering from hemorrhagic shock. 2 , 3 , 4 , 5 The United States military is using whole blood, both out‐of‐hospital and in the deployed hospital setting, as a standard of care. 6 , 7 , 8 Recent civilian studies report on the increasing use of whole blood as an alternate approach to trauma resuscitation with component therapy. 6
Fresh whole blood transfusion was first widely used during World War II. 7 In the early 1970s, advancements in the fractionation process led to component therapy becoming usual care for patients in hemorrhagic shock. Fresh whole blood transfusion saw resurgence during the wars in Iraq and Afghanistan, due to easier transfusion logistics and perceived efficacy. 7 Whole blood is now being reintroduced into civilian trauma surgical practice, with reports of improved outcomes. Furthermore, the Joint Trauma System clinical practice guidelines recommend the use of whole blood as the preferred therapy in the out‐of‐hospital treatment of hemorrhagic shock. 8
1.2. Importance
A number of retrospective studies have reported beneficial effects of whole blood in trauma resuscitation, and there is one, small, randomized, single‐center clinical trial. 8 However, there have been few attempts to synthesize the results of the studies. A systematic review and meta‐analysis of these studies could help to highlight the overarching strengths and weaknesses of existing data and estimated associations with outcomes.
1.3. Goals of this investigation
Through a systematic review and meta‐analysis of the existing literature, we sought to determine the association of whole blood with mortality after traumatic hemorrhagic shock.
2. METHODS AND MATERIALS
We conducted a systematic review and meta‐analysis according to Preferred Reporting Items in Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 9 Using a pre‐determined protocol, we performed a systematic search of the OVID Medline, EMBASE, and Cochrane Library databases for studies published from January 2007 to June 2019. The search protocol for this systematic review was registered with the University of York Center for Reviews and Dissemination and the National Institute for Health Research PROSPERO database (registration no. CRD42019136731).
2.1. Study design and data sources
2.2. Selection of studies
The search strategy is summarized in Appendix 1. We structured the search around the population, interest, context (PICO) framework to address the question, “In patients experiencing hemorrhagic shock due to trauma, is whole blood transfusion (compared with component therapy) associated with reduced mortality?” 10 We identified studies evaluating the association of whole blood transfusion with mortality in trauma patients. Eligible studies included randomized control trials, non‐randomized control trials, and retrospective or prospective cohort studies with comparison groups. We defined component therapy as combinations of apheresis platelets (aPLT), packed red blood cells, fresh frozen plasma, and fresh whole blood. We excluded case reports, opinion pieces, review articles, and studies involving non‐human subjects. We did not include abstracts. As resuscitation practices changed dramatically with the advent of damage control resuscitation, we also excluded studies published before 2007. 11 , 12 , 13 There was no language limitation. We included studies from both civilian and military settings.
The lead investigator (EC) identified relevant studies through a review of titles and abstracts against the exclusion criteria. Two different appraisers (EC, HEW) completed a full‐text review of all potentially relevant studies to confirm study inclusion. Using the Newcastle‐Ottawa Scale for non‐randomized cohort studies, the reviewers appraised the quality of each selected study. 14 The Newcastle‐Ottawa Scale awards “stars,” for methodological quality. A third reviewer (SD) resolved discrepancies.
To ensure consistency in the quality review, the reviewers aligned the interpretation of the Newcastle‐Ottawa Scale score among the candidate studies. 14 For representativeness, if a study included civilian patients, we considered the study to be “truly representative of the average trauma in the community.” If a study involved military trauma, the study was considered “somewhat representative of the average trauma in the community.” If the female population was disproportionately under‐represented, we classified the study as “somewhat representative of the average trauma in the community.” For ascertainment of exposure, we accepted trauma registries as “secure records.” For “demonstration that outcome of interest was not present at the start of the study,” we assigned all studies as “yes.” For comparability, we assigned 1 star if a study controlled for 1 factor, 2 stars if the study controlled for > 1 factor, and 0 stars if there was no adjustment. For assessment of outcome, we accepted trauma registries as “independent blind assessment.” Finally, we assumed that all studies had adequate follow‐up long enough for outcomes to occur.
2.3. Data extraction
Three reviewers (EC, HW, and SD) extracted data from the identified papers. We extracted data from each of the identified papers. Data extracted included study design, setting, transfusion quantity, criteria for transfusion, mean/median age of patients, and percent male sex of the population. We also collected data on the types of blood products used in both intervention and control groups, the leukoreduction status of the products, and the titer levels in low titer type O whole blood interventions. Data concerning patient outcomes included 24‐hour mortality, in‐hospital mortality, and 30‐day mortality. We extracted data including the type of statistical analyses and the adjustments used for confounders in each study. Any discrepancies were discussed and resolved by the reviewers.
2.4. Data synthesis
The analysis focused on 2 primary outcomes: (1) 24‐hour mortality and (2) in‐hospital or 30‐day mortality. We included early mortality because it is increasingly recognized to reflect efficacy of hemostatic interventions, and 24‐hour mortality is reported in at least some of the studies published to date. 15 We defined 24‐hour mortality as death occurring within 24 hours of admission to the hospital. In‐hospital mortality included deaths occurring during hospitalization. We defined 30‐day mortality as death occurring within 30 days of hospitalization, or during hospitalization. This combined outcome was used because some papers specifically reported 30‐day mortality, whereas others reported in‐hospital mortality.
2.5. Data analysis
To test for heterogeneity among reported odds ratios (ORs), we calculated I2 and tested its significance. We considered studies with I2 values of < 25%, 25%–75%, and > 75% to represent low, moderate, and high heterogeneity, respectively. Chi‐square tests of heterogeneity with a P‐value < 0.05 were considered to represent heterogeneity higher than expected due to chance, and reason to fit a random effects meta‐analysis of the log ORs. We used the Harbord's test to determine the risk of publication bias for both 24‐hour and in‐hospital/30‐day mortality, separately, despite a small sample size for the former. 16 Where available, we used adjusted outcomes reported by the original studies, converting all results into ORs. We calculated pooled estimates of ORs for both 24‐hour and in‐hospital/30‐day death, by fitting a fixed or random effects meta‐analysis model. 17 We carried out this analysis using Stata v.15.0 (Stata, Inc., College Station, TX).
The Bottom Line
The use of whole blood instead of balanced component therapy during massive transfusion following trauma has been increasing. This meta‐analysis of current studies demonstrates no difference in outcomes when whole blood is used, but it is limited by the small number of existing studies and significant heterogeneity of those studies.
3. RESULTS
3.1. Systematic review
Of the 1759 citations identified in the search, 12 studies (reporting on 8431 patients) met the eligibility criteria of the systematic review (Figure 1; Table 1). All studies were available in English. The types of studies consisted of retrospective cohort (n = 10), prospective cohort (n = 1), and randomized control trial (n = 1). The majority of the studies originated from the civilian setting (n = 7). Of the civilian studies, most were conducted at institutions in the United States (n = 6). The remaining studies used data from military settings in either Afghanistan or Iraq. All of the studies involved mostly male patients (range = 72.7%–100%), and the mean age of patients ranged from 24–50.6 years.
FIGURE 1.
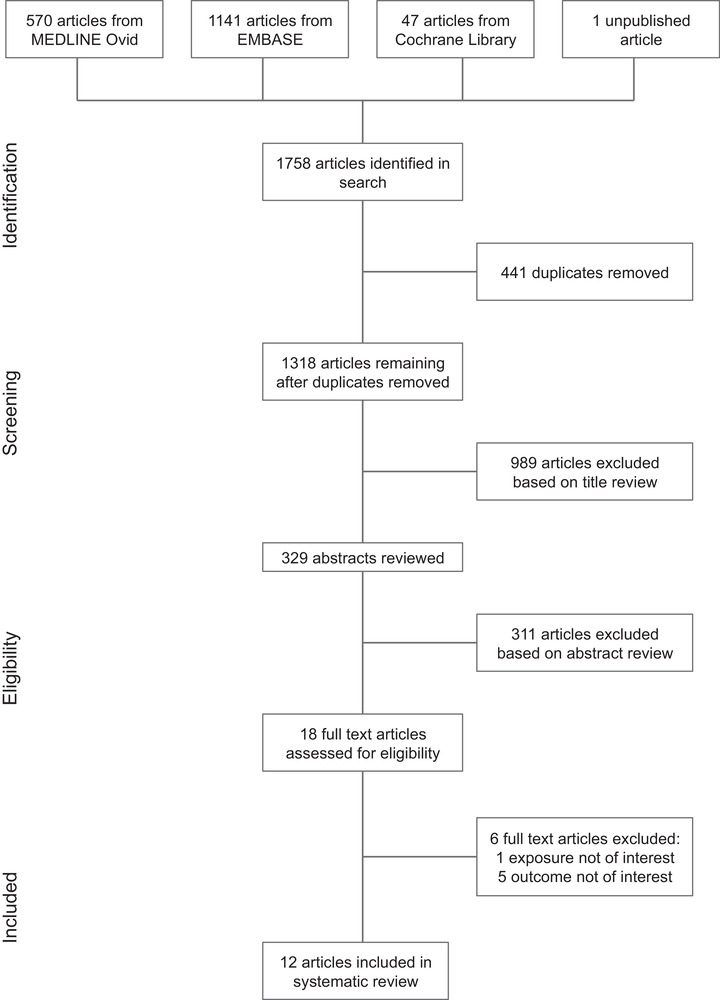
PRISMA flow diagram indicating study selection. *The unpublished article 20 was brought to attention of the reviewers by its authors
TABLE 1.
Characteristics of studies included in the systematic review and meta‐analysis
| Intervention | Comparison/control | Intervention characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Design | Setting | Population | Description | n | Description | n | Age | Male sex (%) | Leukoreduction | Titer (anti‐A & B) |
| Williams et al 21 | Prospec. Observ. | US, civilian | Transfusion ≥1 U | LTO‐WB | 198 | RBC and/or FFP | 152 | 42 (26, 56) | 72 | No | <200 |
| Zhu et al 20 | Retrosp. cohort | US, civilian | MT | LTO‐WB | 25 | CT | 175 | – | – | No | <256 |
| Seheult et al 18 | Retrosp. cohort | US, civilian | Transfusion ≥1 U | LTO‐WB or LTO‐WB + CT | 135 | CT | 135 | 40 (26, 61) | 95.6 | Yes | <50 |
| Yazer et al 19 | Retrosp. cohort | US, civilian | Transfusion ≥1 U | LTO‐WB or LTO‐WB + CT | 47 | CT | 145 | 31 (18, 90) | 100 | Yes | <100 |
| Auten et al 24 | Retrosp. cohort | Afghan., military | MT, ISS ≥15 | FWB + CT | 26 | CT | 35 | 24 ± 3.5 | 100 | No | – |
| Keneally et al 25 | Retrosp. cohort | Afghan., Iraq, military | Transfusion ≥1 U, CRTT | WFWB + CT | 281 | CT | 3656 | 24 (1, 77) | 92 a | No | – |
| Jones et al 23 | Retrosp. cohort | US, civilian | Transfusion ≥1 U, ISS ≥25 | WB | 83 | CT | 1662 | 27 ± 8 | 83 | – | – |
| Cotton et al 8 | RCT | US, civilian | Transfusion ≤4 U 1 h, Level 1 trauma | mWB + PLT (6:1) | 55 | pRBC + FFP + PLT (6:6:1) | 52 | 40 (29, 56) a | 78 | Yes | – |
| Nessen et al 26 | Retrosp. cohort | Afghan., military | Transfusion ≥1 U | FWB + pRBC + FFP | 94 | pRBC + FFP | 394 | 28.1 ± 9.7 | 95.7 | No | – |
| Ho et al 22 | Retrosp. cohort | Australia, civilian | MT | UYWB | 77 | CT | 276 | 50.6 ± 19 | 72.7 | No | – |
| Perkins et al 27 | Retrosp. Cohort | Iraq, military | MT | FWB | 85 | aPLT | 284 | 27.6 ± 7.6 | 96.5 | No | – |
| Spinella et al 28 | Retrosp. cohort | Afghan., Iraq, military | Transfusion ≥1 U | WFWB + pRBC + FFP | 100 | CT | 254 | 24 (21, 29) | – | No | – |
| Outcome (mortality) | Newcastle‐Ottawa scale appraisal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 30 d | In‐hospital | |||||||||
| Analysis, adjustments |
|
|
|
|
|
|
|
|
|
|
|
| Williams et al 21 | MLR: age, AIS, SBP, arrival pH, MI | – | – | 53/198 | 39/152 | – | – | **** | ** | ** | 8 |
| Zhu et al 20 | None | – | – | – | – | Sep‐25 | 100/175 | *** | 0 | ** | 5 |
| Seheult et al 18 | Chi‐square or Fisher exact test | 12/135 | 17/135 | – | – | 25/135 | 33/135 | **** | ** | ** | 8 |
| Yazer et al 19 | Chi‐square or Fisher exact test | – | – | – | – | 17/47 | 40/145 | **** | * | ** | 7 |
| Auten et al 24 | LR, propensity score | Jan‐26 | Feb‐35 | Feb‐26 | Feb‐35 | – | – | *** | ** | *** | 8 |
| Keneally et al 25 | LR, propensity score | – | – | – | – | 60/281 | 468/3656 | *** | ** | ** | 7 |
| Jones et al 23 | MLR: age, sex, ISS, EMS TT, TT | – | – | – | – | 17/83 | 429/1662 | **** | ** | ** | 8 |
| Cotton et al 8 | Chi‐square or Fisher exact test | 11/55 b | 10/52 b | 22/55 b | 14/52 b | – | – | – | – | – | – |
| Nessen et al 26 | LR, propensity score | – | – | – | – | May‐94 | 35/394 | **** | * | *** | 8 |
| Ho et al 22 | Cox regression, propensity score | – | – | – | – | 31/77 | 97/276 | **** | ** | *** | 9 |
| Perkins et al 27 | Cox regression and MLR | 16/85 | 45/284 | 29/68 | 71/177 | – | – | **** | ** | ** | 8 |
| Spinella et al 28 | MLR: sRBC, plasma, aPLT, WFWB, cryo., MT, rFVIIa use, plasma:RBC, PLT:RBC, anti‐coag./add. vol. | 4/100 | 31/254 | 5/100 | 45/254 | – | – | **** | ** | ** | 8 |
aPLT, apheresis platelets; CRTT, combat related thoracic trauma; CT, component therapy; FFP, fresh frozen plasma; FWB, fresh whole blood; LTO‐WB, low‐titer group o‐negative whole blood; MT, massive transfusion; mWB, modified whole blood; PLT, platelets; pRBC, packed red blood cells; UYWB, unrefrigerated young whole blood; WFWB, warm FWB; Cryo., cryoprecipitate; EMS TT, EMS transfer time; LR, logistic regression; MI, mechanism of injury; MLR, multivariable logistic regression; rFVIIa, recombinant factor VIIa; SBP, systolic blood pressure; sRBC, stored RBC; TT, transfusion type.
Contiguous variables reported as mean ± SD or median (IQR)
Indicates data for total patients in the study.
Indicates intent‐to‐treat analysis data.
*, **, ***, **** denote ratings on the Ottawa‐Newcastle Scale.
Some studies included only patients receiving massive transfusion (≥10 U or red cells over 24 hours), while others included those receiving ≥1 U of red blood cells, over any period of time. There was significant variation in the definition of whole blood resuscitation. The types of whole blood in the intervention groups varied from low titer cold stored O‐negative whole blood (n = 2), fresh whole blood (n = 1), unrefrigerated young whole blood (n = 1), and unspecified whole blood (n = 1) to combinations of whole blood with component therapy (n = 7). The latter combinations included fresh whole blood or warm fresh whole blood with component therapy, modified whole blood (mWB) with platelets, fresh whole blood or warm fresh whole blood with packed red blood cells and fresh frozen plasma, and low titer type O whole blood with CT.
Of the 7 studies conducted in the civilian setting, 4 reported on the use of low titer O‐negative whole blood. 18 , 19 , 20 , 21 The study by Williams et al 21 was the only prospective cohort study. The Seheult et al 18 and Yazer et al 19 articles compared low titer O‐negative whole blood, or low titer O‐negative whole blood with component therapy, to patients receiving component therapy alone. Zhu et al 20 compared low titer O‐negative whole blood and component therapy in massively transfused patients without statistical analysis. The Cotton et al 8 study comparing modified whole blood with platelets to packed red blood cells with fresh frozen plasma and platelets was the only randomized controlled trial identified. However, their study was a single center pilot, feasibility trial, and not powered to detect differences in mortality. Ho et al 22 used unrefrigerated young whole blood as the intervention of interest in massively transfused patients. Jones et al 23 conducted a National Trauma Data Bank analysis, using the ICD9 code 99.03 for “Other transfusion of whole blood” to identify civilian patients in the intervention group. The study compared these patients to those receiving component therapies using a multivariable logistic regression.
The studies conducted in the military setting included Auten et al, 24 Keneally et al, 25 Nessen et al, 26 Perkins et al, 27 and Spinella et al. 28 Auten et al, 24 Keneally et al, 25 Nessen et al, 26 and Spinella et al. 28 reported on the use of either fresh whole blood or warm fresh whole blood in combination with component therapy as an intervention. The Auten et al, 24 Keneally et al, 25 and Nessen et al 26 studies performed a propensity score adjusted logistic regression analysis, whereas Spinella et al. 28 compared cohorts via a multivariable logistic regression. Both the Auten et al 24 and Perkins et al 27 studies examined patients undergoing massive transfusion; however, Perkins et al 27 compared the fresh whole blood cohort to patients receiving red blood cells, plasma, and apheresis platelets. Based upon the Newcastle Ottawa Scale, 10 studies were rated as “good” and 2 as “poor.”
3.2. Meta‐analysis
There were 5 studies that reported 24‐hour mortality and 12 studies that reported in‐hospital/30‐day mortality. Harbord's test revealed no significant publication bias for either 24‐hour mortality (P = 0.82) or in‐hospital/30‐day mortality (P = 0.18) (Figure 2). 16 For 24‐hour mortality, there was a small to moderate level of heterogeneity (I2 = 27.2%, P = 0.37) (Figure 2). The fixed effects pooled OR for 24‐hour mortality was 0.83 (95% confidence [CI] = 0.56–1.24). For in‐hospital/30‐day mortality, there was a moderate to high degree of heterogeneity (I2 = 87.3%, P = 0.37) (Figure 3). The DerSimonian and Laird random effects pooled OR for in‐hospital/30‐day mortality was 0.79 (95% CI = 0.49–1.31). 17
FIGURE 2.
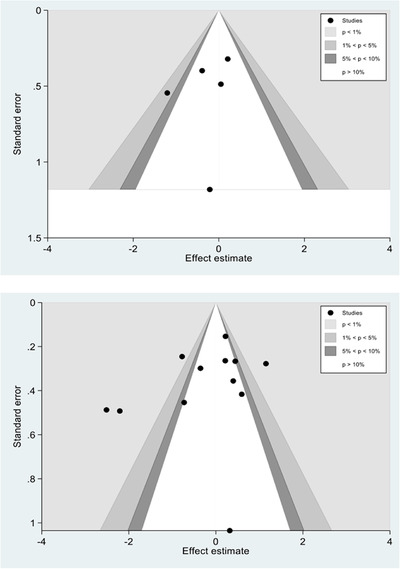
Funnel plots for 24‐hour mortality (upper) and in‐hospital/30‐day mortality (lower pane). P‐values reflect results of Harbord's test
FIGURE 3.
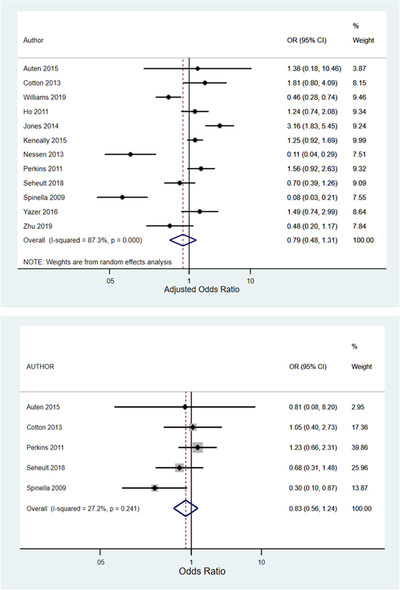
Forest plots illustrating random effects meta‐analyses of the associations between whole blood transfusion and 24‐hour mortality (upper pane) and in‐hospital/30‐day mortality (lower pane)
4. LIMITATIONS
Our study has limitations. Even with a comprehensive search strategy using multiple databases, we may have failed to identify appropriate studies. The included studies were largely retrospective or observational, with only 1 small randomized trial, and the techniques for multivariable adjustment varied. The results of observational studies may be influenced by confounders. However, while observational studies have important limitations, they represent the best data available, and we therefore believe their inclusion to be justifiable. 29 , 30 Many of the studies took place in the military setting, encompassing primarily males with penetrating trauma. The applicability of these studies to civilian trauma may be limited as the latter includes a larger portion of blunt trauma patients, with different demographics and types of whole blood. The definition of whole blood also varied widely across studies. Only 5 studies contributed to the meta‐analysis of 24‐hour mortality. The results of the meta‐analysis were influenced primarily by 2 studies. We did not search clinical trial registries for additional trials.
5. DISCUSSION
Our systematic review and meta‐analysis of 12 studies of whole blood resuscitation in trauma revealed wide heterogeneity in study design, methods, setting, population, interventions, and outcomes. The most striking observation was that the type of whole blood used in each study varied widely, ranging from the fresh warm blood used in military settings to cold stored blood O+ or O‐ used in civilian studies, either leukoreduced or not. The definition of whole blood treatment also varied, with some studies classifying whole blood groups as patients receiving exclusively whole blood and others mixtures of whole blood with other blood product components. Other notable differences included the study setting (military vs civilian) and endpoints (24‐hour, hospital and 30‐day mortality).
Given the extent of heterogeneity across studies, some would consider a meta‐analysis to be inappropriate. However, we feel that the analysis is helpful for illuminating the limitations of the existing literature. For example, the forest plots are useful for visualizing the range of outcomes and the potential pooled effect across the 12 studies. However, we emphasize that, given the limitations of the primary studies, readers should resist from making formal inferences from these data. Rather, they should use the current review as the basis for understanding the limitations of existing data and to guide future research. Given the substantial limitations of observational studies in this population, randomization in a prospective clinical trial is likely the best approach to determining the effectiveness of whole blood therapy. Our review highlights important design considerations for conducting such a trial. If the objective is to evaluate whole blood in civilian trauma, then the trial should take place in trauma systems currently using whole blood, the selection of patients should include both blunt and penetrating trauma patients as well as females of child‐bearing age. Studies must identify whole blood transfusion practices, including the types of whole blood used, the application of leukoreduction and methods of storage. The minimum quantity of whole blood transfused that constitutes the whole blood group should be agreed upon, including the type and number of components allowed before receiving whole blood. Study endpoints might include not just hospital mortality but also early mortality or physiologic measures such thromboelastography. 15 , 31 Because trials of acute therapies may require large sample sizes, innovative trial designs using adaptive and Bayesian techniques might be considered to increase trial efficiency and make the results more interpretable. 32
Although not directly addressed by this systematic review, an important factor influencing the current use of whole blood involves the logistics of trauma blood transfusion; that is, it is simpler to give a single unit of whole blood rather than separate units of red cells, plasma, and platelets. In certain settings, the preparation and storage of whole blood may be less costly than similar amounts of component therapy, and the handling of fewer blood products may be less prone to administrative error. 33 Thus, cost‐effectiveness and logistical burden may represent important outcomes in a prospective study or trial. In conclusion, there is wide heterogeneity in the design, setting, interventions, and outcomes of published studies of whole blood resuscitation. Additional study is needed to determine the effectiveness of whole blood.
AUTHOR CONTRIBUTIONS
HEW, SMD, JOJ, and EC conceived the study. HEW, SMD, and JOJ designed the study. EC, HEW, JOJ, and SMD collected the data and reviewed the articles. AB and SMD conducted the statistical analysis. All authors contributed to the critical review of analytic results. EC drafted the paper. All authors contributed to the critical review and revision of the paper. EC and HEW take overall responsibility for the paper.
CONFLICT OF INTEREST
Dr. Michael Blaivas was the supervising editor for the final review process of this paper. Dr. Wang did not participate in the review process or editorial decision to publish the paper.
Biography
Ellen Crowe is a third year medical student at McGovern Medical School, Houston, Texas.

APPENDIX 1.
1.1.
Publication search strategy
Blood transfusion.ti,ab,kw. or exp Blood Transfusion/
exp Hemorrhage/ or (haemorrhag*.mp. or hemorrhag*).ti,ab,kw.
hemorrhagic shock.ti,ab,kw. or exp Shock, Hemorrhagic/
(“Injur* and Wound*” or “Wound* and Injur*” or Wound* Injur* or Trauma* or Injur* Wound* or Injur* or Wound*).ti,ab,kw.
wounds.ti,ab,kw. or exp “Wounds and Injuries”/
(Personnel Military or Armed Forces Personnel of Personnel Armed Forces or Military or Air Force Personnel or Force Personnel Air or Personnel Air Force or Army Personnel or Personnel Army or Submarin* or Marine* or Navy Personnel or Personnel Navy or Sailor* or Soldier* or Military Deployment* or Deployment* Military or Coast Guard).ti,ab,kw.
military personnel.ti,ab,kw. or exp Military Personnel/
exp Resuscitation/ or resuscitation.ti,ab,kw.
blood preservation.ti,ab,kw. or exp Blood Preservation/
warfare.ti,ab,kw. or exp Warfare/
1 and (2 or 3)
1 and (4 or 5)
1 and (6 or 7)
1 and 8
1 and 9
1 and 10
(6 or 7) and 10
8 and (4 or 5)
8 and 9
9 and (4 or 5)
whole blood.mp.
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 10 or 20
21 and 22
Crowe E, DeSantis SM, Bonnette A, et al Whole blood transfusion versus component therapy in trauma resuscitation: A systematic review and meta‐analysis. JACEP Open. 2020;1:633–641. 10.1002/emp2.12089
REFERENCES
- 1. Centers for Disease Control and Prevention. Injury Prevention & Control. Web‐based Injury Statistics Query and Reporting System (WISQARS). Fatal Injury Data. Fatal Injury Reports, National, Regional and State, 1981‐2018. Available at https://webappa.cdc.gov/sasweb/ncipc/mortrate.html. Accessed May 15, 2020.
- 2. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the proppr randomized clinical trialtransfusion in patients with severe traumatransfusion in patients with severe trauma. JAMA. 2015;313:471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Excellence NIfHa . Major Trauma: Assessment and Initial Management. Managing Haemorrhage in Pre‐hospital and Hospital Settings. Vol NICE Guideline, No. 39. London: National Institute for Healthcare and Excellence; 2016. [PubMed] [Google Scholar]
- 4. Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605‐617. [DOI] [PubMed] [Google Scholar]
- 5. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3‐S11. [DOI] [PubMed] [Google Scholar]
- 6. Black JA, Pierce VS, Kerby JD, Holcomb JB. The evolution of blood transfusion in the trauma patient: whole blood has come full circle. Semin Thromb Hemost. 2020;46(2):215‐220. [DOI] [PubMed] [Google Scholar]
- 7. Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: u.S. military and potential civilian applications. Crit Care Med. 2008;36:S340‐S345. [DOI] [PubMed] [Google Scholar]
- 8. Cotton BA, Podbielski J, Camp E, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258:527‐532. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097‐e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inf Decis Making. 2007;7:16‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685‐686. [DOI] [PubMed] [Google Scholar]
- 12. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307‐310. [DOI] [PubMed] [Google Scholar]
- 13. Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 15. Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC, Group PS . Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47:567‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443‐3457. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 18. Seheult JN, Anto V, Alarcon LH, Sperry JL, Triulzi DJ, Yazer MH. Clinical outcomes among low‐titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018;58:1838‐1845. [DOI] [PubMed] [Google Scholar]
- 19. Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold‐stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81:21‐26. [DOI] [PubMed] [Google Scholar]
- 20. Zhu CS, Pokorny DM, Eastridge BJ, et al. Give the trauma patient what they bleed, when and where they need it: establishing a comprehensive regional system of resuscitation based on patient need utilizing cold‐stored, low‐titer O+ whole blood. Transfusion. 2019;59:1429‐1438. [DOI] [PubMed] [Google Scholar]
- 21. Williams J, Merutka N, Meyer D, et al. Safety Profile and Impact of Low‐Titer Group O Whole Blood for Emergency Use in Trauma. J Trauma Acute Care Surg. 2020;88(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 22. Ho KM, Leonard AD. Lack of effect of unrefrigerated young whole blood transfusion on patient outcomes after massive transfusion in a civilian setting. Transfusion. 2011;51:1669‐1675. [DOI] [PubMed] [Google Scholar]
- 23. Jones AR, Frazier SK. Increased mortality in adult patients with trauma transfused with blood components compared with whole blood. J Trauma Nurs. 2014;21:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Auten JD, Lunceford NL, Horton JL, et al. The safety of early fresh, whole blood transfusion among severely battle injured at US Marine Corps forward surgical care facilities in Afghanistan. J Trauma Acute Care Surg. 2015;79:790‐796. [DOI] [PubMed] [Google Scholar]
- 25. Keneally RJ, Parsons AM, Willett PB. Warm fresh whole blood and thoracic Traumain Iraq and Afghanistan. J Emerg Trauma Shock. 2015;8:21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nessen SC, Eastridge BJ, Cronk D, et al. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion. 2013;53(Suppl 1):107S‐113S. [DOI] [PubMed] [Google Scholar]
- 27. Perkins JG, Cap AP, Spinella PC, et al. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME). Transfusion. 2011;51:242‐252. [DOI] [PubMed] [Google Scholar]
- 28. Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat‐related traumatic injuries. J Trauma. 2009;66:S69‐S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greene TJ, DeSantis SM, Fox EE, Wade CE, Holcomb JB, Swartz MD. Utilizing propensity score analyses in prehospital blood product transfusion studies: lessons learned and moving toward best practice. Mil Med. 2018;183:124‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeSantis SM, Swartz MD, Greene TJ, Fox EE, Holcomb JB, Wade CE, PROHS Study Group . Interim monitoring of non‐randomized prospective studies that invoke propensity scoring for decision‐making. J Trauma Acute Care Surg. 2020;88(2):e46‐e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24:114‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jansen JO, Pallmann P, MacLennan G, Campbell MK, Investigators U‐RT. Bayesian clinical trial designs: another option for trauma trials. J Trauma Acute Care Surg. 2017;83:736‐741. [DOI] [PubMed] [Google Scholar]
- 33. Holcomb JB, Jenkins DH. Get ready: whole blood is back and it's good for patients. Transfusion. 2018;58:1821‐1823. [DOI] [PubMed] [Google Scholar]


