Abstract
Objective
Research evaluating the relationship between vasopressor initiation timing and clinical outcomes is limited and conflicting. We investigated the association between time to vasopressors, worsening organ failure, and mortality in patients with septic shock.
Methods
This was a retrospective study of patients with septic shock (2013–2016) within 24 hours of emergency department (ED) presentation. The primary outcome was worsening organ failure, defined as an increase in Sequential Organ Failure Assessment (SOFA) score ≥2 at 48 hours compared to baseline, or death within 48 hours. The secondary outcome was 28‐day mortality. Time to vasopressor initiation was categorized into 6, 4‐hour intervals from time of ED triage. Multiple logistic regression was used to identify predictors of worsening organ failure.
Results
We analyzed data from 428 patients with septic shock. There were 152 patients with the composite primary outcome (SOFA increase ≥2 or death at 48 hours). Of these, 77 patients died in the first 48 hours and 75 patients had a SOFA increase ≥2. Compared to the patients who received vasopressors in the first 4 hours, those with the longest time to vasopressors (20–24 hours) had increased odds of developing worsening organ failure (odds ratios [OR] = 4.34, 95% confidence intervals [CI] = 1.47–12.79, P = 0.008). For all others, the association between vasopressor timing and worsening organ failure was non‐significant. There was no association between time to vasopressor initiation and 28‐day mortality.
Conclusions
Increased time to vasopressor initiation is an independent predictor of worsening organ failure for patients with vasopressor initiation delays >20 hours.
Keywords: hypotension, organ failure, sepsis, septic shock, vasopressors
1. INTRODUCTION
1.1. Background
There are an estimated 1.7 million annual sepsis cases in the United States with an overall mortality rate around 20%. 1 , 2 , 3 When septic shock is present, mortality exceeds 40%. 2 , 4 The fundamental components of sepsis resuscitation include intravenous fluids, antibiotics, and vasopressors for fluid‐resistant septic shock. Early management of sepsis with bundled care, including early identification, intravenous fluids, broad spectrum antibiotics, and source control has been demonstrated to improve outcomes. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
1.2. Importance
Despite evidence in favor of early initiation of other septic shock therapies, the impact of earlier vasopressor initiation on patient outcomes remains unclear. The limited studies on vasopressor timing yield conflicting results. 13 , 14 , 15 , 16 , 17 , 18 , 19 Despite conflicting evidence, the Surviving Sepsis Campaign Bundle currently recommends vasopressors to maintain a mean arterial pressure ≥65 mm Hg within the first hour of care, a highly controversial recommendation. 20 , 21 , 22
Current practice for the early management of sepsis‐associated hypotension varies widely. 23 Based on the physiologic understanding of the Starling curve, and the desire to provide sufficient intravascular volume to maximize stroke volume, clinicians often administer intravenous fluids without vasopressors in the initial phase of resuscitation. Consensus guidelines recommend that resuscitation of septic shock patients begin with a 30 mL/kg bolus of crystalloids within the first 3 hours, with the caveat that many patients will require more fluid than the initial amount. Guidelines further recommend that the adequacy of fluid resuscitation be assessed with dynamic rather than static variables. After adequate fluid resuscitation, persistent sepsis‐induced hypotension should be treated with vasoactive agents. The most recent Surviving Sepsis Campaign update consolidates the previous 3‐ and 6‐hour bundles into a single “hour 1 bundle,” recommending that bundled resuscitation begin immediately. 20 , 21 This update specifically includes the initiation of vasoactive agents for hypotension in the “hour 1 bundle.”
Despite the adoption of these recommendations, early vasopressor initiation has not been consistently associated with improved outcomes. Although some studies suggest increased mortality associated with hourly delays in vasopressors, 15 others demonstrate that delays in vasopressor administration are only harmful for those with the longest delays, 14 and still other studies have shown that early initiation is associated with harm. 16 , 17 Furthermore, these existing studies do not use time from triage to determine time to vasopressor initiation.
1.3. Goals of this investigation
We sought to evaluate the association between the timing of vasopressor initiation in septic shock and subsequent worsening organ failure and death using metrics consistent with current consensus definitions. We hypothesized that increased time to vasopressor initiation in septic shock would be associated with worsening organ failure and increased 28‐day mortality.
2. MATERIALS AND METHODS
2.1. Study design and setting
We conducted a retrospective review of all patients treated for septic shock within 24 hours of emergency department presentation at University of Florida Health Jacksonville, an urban, not‐for‐profit academic medical center and regional referral center, from October 1, 2013 to May 12, 2016. Our approach and reporting follows STROBE guidelines. 24 The study was approved by the University of Florida institutional review board (IRB 201701712) with a full waiver of informed consent.
2.2. Patient selection
This was a secondary analysis of a retrospective dataset obtained to evaluate sepsis outcomes before and after implementation of a hospital quality‐improvement sepsis alert program. 25 The methods and data extraction plans have been described previously. 25 Briefly, patients with any of 28 explicit International Statistical Classification of Disease codes for sepsis (Supplement 1) and 2 or more systemic inflammatory response syndrome (SIRS) criteria were included in the initial dataset. Patients younger than 18 years of age or incarcerated patients were excluded.
2.3. Exposure
Of the patients in the parent dataset, those who received vasoactive medications (norepinephrine, vasopressin, dopamine, phenylephrine, dobutamine) in the first 24 hours of admission were assigned cardiovascular Sequential Organ Failure Assessment (SOFA) scores based on vasopressor and dose. 26 , 27 Patients with a cardiovascular SOFA score ≥2 were considered to have septic shock and included in this study. Time to vasopressor initiation was categorized into 6, 4‐hour intervals from the time of ED triage. We defined time to vasopressor administration based on time from triage, in accordance with national guidelines. 20 , 21
2.4. Measurements
We collected demographic data, clinical information, vital signs, laboratory values, Charlson comorbidity index scores, and SOFA scores. SOFA scores were calculated at baseline and at 48 hours according to standard criteria. 26 , 27 Pulse oximetry (SpO2) was used when arterial partial pressure of oxygen (PaO2) data were not available. An SpO2/FiO2 (fraction of inspired oxygen) ratio was then calculated, a previously validated approach for calculating respiratory SOFA scores. 28 As vasopressor initiation is closely related to intravenous fluid resuscitation in early septic shock, we collected the volume of intravenous fluids administered in the first 6 and 24 hours from triage. In addition to time to vasopressors, we retrieved other relevant treatment data including time to antibiotics, mechanical ventilation use, and sepsis alert bundle utilization. Treatment data and corresponding times were obtained using data from the electronic medical record system.
2.5. Outcomes
The primary outcome was worsening organ failure, defined as an increase in 48‐hour SOFA score ≥2 points from enrollment. This outcome was chosen based on its association with mortality, 29 and because the most recent Sepsis‐3 consensus definitions use a SOFA score increase of 2 or more points from baseline. 2 , 30 We included patients who died within the first 48 hours, and therefore did not have 48‐hour SOFA scores available for analysis, in the worsening organ failure group. The secondary outcome was 28‐day mortality.
The Bottom Line
Although the 2018 “Hour‐1 Bundle” recommends urgent vasopressor use within the first hour of care for hypotensive septic patients, the relationship between vasopressor initiation timing and clinical outcomes is limited and conflicting. This retrospective study of 428 patients with septic shock found that >20 hours delay in starting vasopressors was associated with worsening organ failure.
2.6. Analysis
Categorical variables were summarized using counts and percentages, and analyzed using Pearson's χ2 or Fisher's exact tests. Continuous data were summarized using means, SDs, or medians and interquartile ranges, depending on the normality of the data. Continuous data were analyzed using Wilcoxon rank‐sum test or Student t test depending on data normality. We used multivariable logistic regression to investigate associations between time from triage to vasopressor initiation and outcomes. To assess the best predictive model, we used backward variable elimination methods with a P‐value threshold of 0.05. Candidate predictors included age, sex, race, initial vital signs, relevant comorbidities, initial lactate, mechanical ventilation dependence, volume of resuscitative intravenous fluids, time from triage to antibiotic administration, and time from triage to vasopressor initiation. We included the volume of intravenous fluids administered and time to antibiotic administration in the regression model to account for related aspects of resuscitation and because earlier time to antibiotics has been associated with improved outcomes. To facilitate clinical interpretation, we included time to vasopressors categorized into sextiles by time from triage in hours (0–4, 4–6, 6–12, 12–16, 16–20, 20–24 hours) in the regression model. We described the magnitude of the associations using odds ratios (OR), along with 95% confidence intervals (CI). To evaluate the independent effect of delays in vasopressor initiation on worsening organ failure by categories of time to vasopressor initiation, we used the Stata margins command to generate probabilities of worsening organ failure by vasopressor initiation time. 31 Stata version 15 (College Station, TX) and SAS version 9.4 (Cary, NC) were used for analysis.
3. RESULTS
3.1. Characteristics of study subjects
There were 467 patients diagnosed with septic shock within 24 hours of ED presentation. Of those, 39 patients were excluded from the analysis due to missing components of the SOFA score (Supplement 2). The median age of the remaining 428 patients was 65 years; 51% (217) were female, 52% were black (222), 42% were white (179), and 6% (27) were other races. The most prevalent comorbidities were diabetes mellitus (41%), chronic obstructive pulmonary disease (38%), and congestive heart failure (28%) (Table 1).
TABLE 1.
Baseline patient characteristics by worsening organ failure at 48 hours
| Variable | Category | Overall (n = 428) | Worsening organ failure (n = 152) | No worsening organ failure (n = 276) | P‐value |
|---|---|---|---|---|---|
| Age^ | 65 (14) | 67 (14) | 63 (14) | 0.007 a | |
| Sex | Female | 217 (51) | 79 (52) | 138 (50) | 0.696 b |
| Race | Black | 222 (52) | 81 (53) | 141 (51) | 0.162 b |
| White | 179 (42) | 66 (43) | 113 (41) | ||
| Other | 27 (6) | 5 (2) | 22 (8) | ||
| Comorbidities | |||||
| AIDS | Yes | 8 (2) | 3 (2) | 5 (2) | 1.000 c |
| Cancer | Yes | 46 (11) | 19 (13) | 27 (10) | 0.392 b |
| CHF | Yes | 121 (28) | 37 (24) | 84 (31) | 0.173 b |
| COPD | Yes | 164 (38) | 58 (38) | 106 (39) | 0.937 a |
| CVD | Yes | 44 (10) | 9 (6) | 35 (13) | 0.027 b |
| Diabetes mellitus | Yes | 174 (41) | 54 (36) | 120 (43) | 0.109 a |
| Dementia | Yes | 32 (7) | 6 (4) | 26 (9) | 0.039 b |
| ESRD | Yes | 51 (12) | 23 (15) | 28 (10) | 0.128 b |
| Liver disease | Yes | 73 (17) | 34 (22) | 39 (14) | 0.030 a |
| Myocardial infarction | Yes | 50 (12) | 22 (14) | 28 (10) | 0.187 b |
| Metastatic cancer | Yes | 13 (3) | 7 (5) | 6 (2) | 0.999 c |
| Charlson comorbidity indexΨ | 3 (1;4) | 3 (1;4) | 3 (1;4) | 0.842 b |
Data are counts (percentages), unless otherwise specified ^mean (SD), Ψfor median (first quartile; third quartile). AIDS, acquired immune deficiency syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; ESRD, end stage renal disease.
Student t test.
Pearson's χ2 test.
Fisher's exact test.
The overall 28‐day mortality rate was 39% (166/428). The median baseline SOFA score was 9 (interquartile range (IQR) 7–11] and the median change in SOFA score was a decrease by 2 (IQR 1, −4) at 48 hours, representing improvement in organ dysfunction. There were 152 patients with the composite primary outcome (SOFA increase ≥2 or death at 48 hours). Of these, 77 patients died in the first 48 hours and 75 patients had a SOFA increase ≥2.
Baseline characteristics were similar among patients who did and did not meet the primary outcome of worsening organ failure with a few exceptions (Table 1). Patients who experienced the primary outcome of worsening organ failure were slightly older and were more likely to have liver disease, while a greater percentage of patients with a history of cerebrovascular disease and dementia did not develop worsening organ failure. Clinical variables between groups were similar at baseline (Table 2) with 2 notable exceptions. Initial lactate levels were significantly higher in patients with worsening organ failure compared to those without the primary outcome (4.8 mmol/L [IQR 2.6–8.3] vs 2.4 mmol/L [IQR 1.5–4.1]; P < 0.001). Initial heart rate was also higher in patients with worsening organ failure (110 bpm [IQR 96–135] vs 104 bpm [IQR 82–124]; P = 0.003).
TABLE 2.
Clinical variables by worsening organ failure at 48 hours
| Variable | Overall (n = 428) | Worsening organ failure (n = 152) | No worsening organ failure (n = 276) | P‐value |
|---|---|---|---|---|
| Initial vital signs* | ||||
| SBP (mm Hg) | 100 (81;121.5) | 100 (80;122) | 100 (83;121) | 0.997a |
| HR (beats/min) | 106 (87;127) | 110 (96;135) | 104 (82;124) | 0.003a |
| RR (breaths/min) | 20 (18;26) | 21 (18;26) | 20 (18;26) | 0.491a |
| Temperature (°F) | 98.3 (97.3;100.2) | 98.1 (97;99.7) | 98.4 (97.3;100.4) | 0.085a |
| SpO2 (%) | 96 (92;100) | 96 (91;100) | 97 (92;100) | 0.189a |
| Lab findings | ||||
| Initial WBC* (thousand/mm3) | 13.3 (8.3;18.1) | 12.5 (6.5;19.2) | 13.3 (8.9;17.9) | 0.107a |
| Lactate*, (mmol/L) | 2.9 (1.7;6.0) | 4.8 (2.6;8.3) | 2.4 (1.5;4.1) | <0.001a |
| Lactate, (mmol/L) | ||||
| ≥4 versus other | 133 (38) | 74 (59) | 59 (26) | <0.001b |
| 2–3.9 versus other | 109 (31) | 29 (23) | 80 (36) | 0.015b |
| ˂2 versus other | 109 (31) | 23 (18) | 86 (38) | <0.001b |
| Any positive culture | 557 | 196 | 361 | |
| Blood | 219 (39) | 77 (39) | 142 (39) | 0.991a |
| Respiratory | 179 (32) | 71 (36) | 108 (30) | 0.128a |
| Urine | 153 (27) | 47 (24) | 106 (29) | 0.174a |
| Wound | 6 (1) | 1 (1) | 5 (1) | 0.671c |
| Sepsis management | ||||
| Mechanical ventilation | 84 (20) | 36 (24) | 48 (17) | 0.117b |
| Time to antibiotics (min)*, d | 156 (95;244) | 155 (100;232) | 156 (95;253) | 0.642a |
| Time to vasopressors (h)*, d | 6.1 (3.1;11.1) | 6.2 (3.5;10.4) | 5.7 (2.1;11.7) | 0.343a |
| Fluids in first 6 h (mL)*, d | 3000 (2000;4000) | 3000 (1250;4000) | 3000 (2000;4000) | 0.523a |
| Fluids in first 24 h (mL)*, d | 5000 (3000;7000) | 5000 (3000;6500) | 5500 (4000;7000) | 0.016a |
Data are counts (percentages), unless otherwise specified *for median (first quartile; third quartile). HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; WBC, white blood cell count.
Wilcoxon rank‐sum test.
Pearson's χ2 test.
Fisher's exact test.
Time from triage.
Overall, the median time to vasopressor initiation was 6 hours (IQR 3.13–11.06) and norepinephrine was the most commonly used vasopressor (91%). Features of sepsis resuscitation were similar between groups (Table 2), although patients without worsening organ failure at 48 hours received 500 mL more intravenous fluids in the first 24 hours (5500 [IQR 4000–7000] vs 5000 [IQR 3000–6500]; P = 0.02). There was no difference in time to antibiotics between groups. Baseline SOFA scores were generally similar between patients with and without worsening organ failure; all SOFA score components were significantly different between groups by 48 hours (Table 3).
TABLE 3.
Baseline and 48 hour SOFA score components by organ failure
| Variable | Overall (n = 428) | Worsening organ failure (n = 152) | No worsening organ failure (n = 276) | P‐value* |
|---|---|---|---|---|
| Baseline | ||||
| Neurologic | 1 (0;3) | 1 (0;3) | 1 (0;3) | 0.7150 |
| Cardiovascular | 3 (3;4) | 3 (3;4) | 3 (3;4) | 0.8414 |
| Coagulation | 0 (0;1) | 0 (0;1) | 0 (0;0) | <0.0001 |
| Liver | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0.0437 |
| Renal | 1 (0;3) | 1.5 (0.5;3) | 1 (0;3) | 0.9513 |
| Respiration | 2 (0;3) | 2 (0;3) | 2 (0;3) | 0.1916 |
| 48 ha | n = 351a | n = 75a | n = 276 | |
| Neurologic | 2 (1;3) | 3 (2;3) | 2 (0;3) | <0.0001 |
| Cardiovascular | 1 (1;4) | 4 (4;4) | 1 (1;4) | <0.0001 |
| Coagulation | 1 (0;2) | 1 (0;3) | 0 (0;1) | 0.0001 |
| Liver | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0.0003 |
| Renal | 1 (0;2) | 2 (0;2) | 0 (0;2) | <0.0001 |
| Respiration | 0 (0;2) | 2 (1;3) | 0 (0;1) | <0.0001 |
Data are median (IQR). *Wilcoxon rank‐sum for all tests of significance.
a = 77 patients who died early no longer included.
3.2. Predictors of worsening organ failure
Significant independent predictors of worsening organ failure included age (OR = 1.02, 95% CI = 1.00–1.04, P = 0.032), initial heart rate (OR = 1.01, 95% CI = 1.01–1.02, P = 0.005), and lactate (OR = 1.16, 95% CI = 1.09–1.23, P < 0.001). Time to vasopressor initiation was a significant independent predictor of worsening organ failure only for those with the longest time to vasopressor initiation (20–24 hours). For all other categories of time to vasopressor initiation the association with worsening organ failure was non‐significant (Table 4). Compared to those who received vasopressors within the first 4 hours, patients with the longest time to vasopressor initiation had over 4 times the odds of developing worsening organ failure (OR = 4.34, 95% CI = 1.47–12.79, P = 0.008). Controlling for all other variables in the model, the predicted marginal probabilities of worsening organ failure increased with longer delays to vasopressor initiation (Figure 1). The final model also included gender (OR = 1.17, 95% CI = 0.726–1.916, P = 0.506) and volume of intravenous fluids in the first 24 hours (OR = 1.00, 95% CI = 1.00–1.00, P = 0.056) as fluid resuscitation and vasopressor initiation are interrelated. The model showed no evidence of overfitting, Hosmer‐Lemeshow test P = 0.650 (using the standard 10 groups).
TABLE 4.
Odds of worsening organ failure at 48 hours by categories of time to vasopressor initiationa
| Time to vasopressor initiation | OR | 95% CI | P‐value |
|---|---|---|---|
| 4–8 h | 0.62 | 0.33–1.16 | 0.137 |
| 8–12 h | 0.59 | 0.28–1.25 | 0.168 |
| 12–16 h | 0.77 | 0.35–1.71 | 0.518 |
| 16–20 h | 0.83 | 0.28–2.46 | 0.736 |
| 20–24 h | 4.34 | 1.47–12.79 | 0.008 |
Results from the multivariable logistic regression model adjusting for other predictors in the model. CI, confidence interval; OR, odds ratio.
aCompared to the reference time to vasopressor initiation group of 0–4 h.
FIGURE 1.
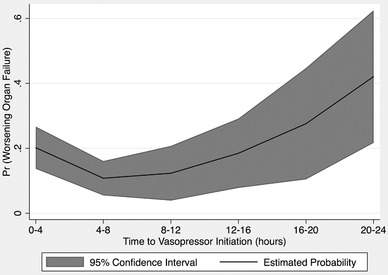
Probability of worsening organ failure at 48 hours by categories of time to vasopressor initiation
3.3. Predictors of mortality
There was no significant association between time to vasopressors and 28‐day mortality. We conducted a post‐hoc power analysis based on observed mortality rates and time to vasopressor variance and determined that we had >90% power to detect a 2‐hour difference in time to vasopressor initiation between patients that died and those that survived. Significant independent predictors of 28‐day mortality included age (OR = 1.03, 95% CI = 1.01–1.05, P = 0.001), history of cancer (OR = 2.80, 95% CI = 1.32–5.90, P = 0.007), myocardial infarction (OR = 2.92, 95% CI = 1.47–5.81, P = 0.002), liver disease (OR = 1.92, 95% CI = 1.03–3.58, P = 0.041), and lactate (OR = 1.18, 95% CI = 1.10–1.26, P < 0.001).
4. LIMITATIONS
We limited our analysis to patients with an admission diagnosis of sepsis. This may have resulted in missed cases of patients admitted for sepsis but without an admitting diagnosis. We did not use a lactate cutoff as 1 of our inclusion criteria although the Sepsis‐3 definition of septic shock uses a lactate threshold of ≥2 mmol/L after fluid resuscitation. Similarly, the parent study included SIRS as part of the enrollment criteria. At the time of initial enrollment, this was consistent with consensus guidelines. 32 Because this analysis was limited to patients requiring vasopressors, corresponding to a SOFA score of at least 2, we do not believe this significantly impacted our findings. Calculating fluid volume and vasopressor timing in a retrospective study has inherent difficulties as these are predicated on timely nursing documentation. Given the retrospective nature of the study, we did not have information on whether static or dynamic indicators of fluid responsiveness were used to determine the adequacy of fluid resuscitation prior to vasopressor initiation. We defined time to vasopressor administration based on time from triage in keeping with national guidelines despite a lack of convincing evidence in favor of this criteria. 20 , 21
5. DISCUSSION
Our findings suggest that profound delays in vasopressor administration impact organ failure progression. Time from triage to vasopressor initiation was associated with an increased risk of worsening organ failure at 48 hours only for patients with delays in vasopressor initiation of >20 hours. Shorter delays in vasopressor initiation did not predict worsening organ failure in this cohort of ED patients with septic shock. Although consensus guidelines and national metrics support earlier vasopressor initiation, we did not find improved outcomes in the group with the earliest time to vasopressor initiation.
Our findings are similar to those of Beck et al 14 who showed a significant association between increased time to vasopressor administration and hospital mortality and organ failure. In their retrospective study, the increased odds of organ failure and mortality were driven by the subset of patients with the longest time to vasopressors (>14 hours). 14 Our findings support their conclusions with a more recent cohort of septic shock patients. Their study was based on septic shock cases from 1996–2008, a period of time that encompassed substantial changes in sepsis management.
Although both our findings and those of Beck et al 14 question whether there is an association between more modest delays in vasopressor initiation and adverse outcomes, the existing evidence on the impact of vasopressor timing is inconsistent. A study by Bai et al 15 demonstrated that each hour delay in vasopressor initiation was associated with a 5.3% increase in mortality in septic shock patients. However, their study was a retrospective review of patients enrolled exclusively from 2 surgical intensive care units that primarily admitted surgical and traumatic complications. Our study population included cases of community‐acquired sepsis and was not restricted to either a medical or surgical intensive care unit. Another study found no association between increased time to vasopressor administration and mortality. 13 However, the detectable effect was limited by sample size (160 patients), where our study includes more than twice as many patients.
Waechter et al 16 compared vasopressor timing between 3 groups (0–1 hours, 1–6 hours, and 6–24 hours). They found higher mortality rates if vasopressors were initiated within the first hour or after 6 hours and lower mortality rates when vasopressors were started between 1 and 6 hours after persistent hypotension. 16 Their findings are supported by some evidence that suggests outcomes may be worse with early vasopressor therapy, particularly if initiated before achieving adequate global perfusion. 17 , 33 However, if the most severely ill patients were started on vasopressors within the first hour, this may have influenced the results. Another recent, smaller, retrospective study also found increased mortality among patients who received vasopressors >6 hours after hypotension compared to those with a time to vasopressor initiation of >6 hours. 34 The median time to vasopressor initiation in these 2 groups is not reported, and the maximum time included in the >6 hours group is not explicitly stated. 34 These results and those of Waechter et al 16 may be cofounded by the unbalanced structure of the groups with regard to time. It is possible that a later time to vasopressors within the 6 or more hours group is driving these findings.
Although we found the effect of vasopressor timing on organ failure was limited to those with the longest delays, it suggests that vasopressor timing may influence overall septic shock morbidity. However, we caution against interpreting these findings as evidence that earlier vasopressor initiation is beneficial. Whether earlier vasopressor use can prevent adverse outcomes cannot be inferred from existing literature, despite the adoption of the recommendation for earlier vasopressor therapy into current sepsis management guidelines. Previous studies demonstrate that increased hypotension exposure is associated with increased organ failure and mortality. 35 , 36 , 37 , 38 , 39 However, whether or not earlier vasopressor initiation can mitigate these adverse outcomes is unclear and an area in need of future research.
Our study adds to the limited existing body of evidence on the impact of vasopressor timing in septic shock using metrics consistent with consensus guidelines and including more than twice as many patients as most of the other studies on this topic. Although 2 of the existing retrospective studies included >2000 patients, they both used the same database of patients from the same research group for their analyses. 14 , 16 Despite using the same database, these studies came to slightly different conclusions. Our study also provides more granular information on later times to vasopressor initiation than most other studies. Our findings suggest that analyzing patients who receive vasopressors after 6 hours as a unit may be an artificial construct that limits our ability to make more sophisticated recommendations for vasopressor initiation timing.
Generating broad recommendations may be challenging due to the heterogeneity of the septic shock population. It is possible that some patients would benefit from earlier vasopressors while others may not. Elucidating metabolic differences in host responses to sepsis may identify a subgroup of early responders to vasopressor therapy. Furthermore, on a human factors level, barriers need to be identified and strategies developed to improve appropriate initiation of vasopressors.
In summary, time to vasopressor initiation was only a significant independent predictor of worsening organ failure for those with the longest delays of >20 hours. Time to vasopressor initiation was not associated with increased mortality in this retrospective population of septic shock patients. Future prospective studies are needed to validate our findings.
CONFLICTS OF INTEREST
The authors have no conflicts of interest or financial interests to disclose, except for RF, who reports personal payment from Physio‐Control, Inc. for speaker fees.
AUTHOR CONTRIBUTIONS
Contributions of all authors are compliant with the Journal's Authorship policy as per the Editorial Policies and Ethical Considerations. LPB, FWG, MP, and RF conceptualized and designed the study. FWG, MP, and RF provided expert guidance in study design and conduct. LPB and CS analyzed the study data. FG, TM, CS, and LPB conceptualized the data acquisition plan. FG, TM, CS, and LPB contributed to data cleaning and conditioning. LPB drafted the manuscript and all others contributed substantially to its revision. LPB takes final responsibility for the article.
Supporting information
Supporting Information
Supporting Information
Biography
Lauren P. Black, MD, MPH, is an assistant professor in the department of emergency medicine at the University of Florida College of Medicine‐Jacksonville.

Black LP, Puskarich MA, Smotherman C, Miller T, Fernandez R, Guirgis FW. Time to vasopressor initiation and organ failure progression in early septic shock. JACEP Open. 2020;1:222–230. 10.1002/emp2.12060
Optional Twitter‐compliant text: Does time to vasopressor initiation impact downstream organ failure in septic shock? Study shows only for those with the longest delays.
Study Site: All patients were enrolled at UF Health Jacksonville, 655 West 8th Street, Jacksonville, FL 32209. The study was approved by the UF Jacksonville IRB.
Funding and support: There was no external funding for this study but the authors report the following grant support for effort—LPB: NCATS KL2; MP: NIGMS K23 and the Minneapolis Medical Research Foundation; RF: Agency for Healthcare Research and Quality and the Department of Defense; FG: NIGMS K23.
Supervising Editor: Junichi Sasaki, MD.
REFERENCES
- 1. Gaieski D, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the united states. Crit Care Med. 2013;41(5):1167‐1174. [DOI] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar G, Kumar N, Taneja A, Al E. Nationwide Trends of Severe Sepsis in the 21st Century (2000–2007). Chest. 2011;140(5):1223‐1231. [DOI] [PubMed] [Google Scholar]
- 4. Shankar‐Hari M, Phillips GS, Levy ML, et al. Developing a newdefinition and assessing newclinical criteria for Septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315(8):775‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller RR, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu VX, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367‐374. [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589‐1596. [DOI] [PubMed] [Google Scholar]
- 9. Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2012;39(9):2066‐2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall JC, Maier RV, Jimenez M, Dellinger EP. Source control in the management of severe sepsis and septic shock: an evidence‐based review. Crit Care Med. 2004;32(11):S513‐526. [DOI] [PubMed] [Google Scholar]
- 11. Leisman DE, Goldman C, Doerfler ME, et al. Patterns and outcomes associated with timeliness of initial crystalloid resuscitation in a prospective sepsis and septic shock cohort. Crit Care Med. 2017;45(10):1596‐1606. [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146(4):908‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel JJ, Kurman JS, Biesboer A, et al. Impact of duration of hypotension prior to norepinephrine initiation in medical intensive care unit patients with septic shock: a prospective observational study. J Crit Care. 2017;40:178‐183. [DOI] [PubMed] [Google Scholar]
- 14. Beck V, Chateau D, Bryson GL, et al. Timing of vasopressor initiation and mortality in septic shock: a cohort study and The Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Crit Care. 2014;18(3):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai X, Yu W, Ji W, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18(5):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waechter J, Kumar A, Lapinsky SE, et al. Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Crit Care Med. 2014;42(10):2158‐2168. [DOI] [PubMed] [Google Scholar]
- 17. Subramanian S, Yilmaz M, Rehman A, Hubmayr RD, Afessa B, Gajic O. Liberal vs. conservative vasopressor use to maintain mean arterial blood pressure during resuscitation of septic shock: an observational study. Intensive Care Med. 2008;34:157‐162. [DOI] [PubMed] [Google Scholar]
- 18. Morimatsu H, Singh K, Uchino S, Bellomo R, Hart G. Early and exclusive use of norepinephrine in septic shock. Resuscitation. 2004;62(2):249‐254. [DOI] [PubMed] [Google Scholar]
- 19. Abid O, Akca S, Haji‐Michael P, Vincent JL. Strong vasopressor support may be futile in the intensive care unit patient with multiple organ failure. Crit Care Med. 2000;28(4): 947‐949. [DOI] [PubMed] [Google Scholar]
- 20. Levy MM, Evans LE, Rhodes A. The surviving sepsis Campaign Bundle: 2018 Update. Intensive Care Med. 2018;44(6):925‐928. [DOI] [PubMed] [Google Scholar]
- 21. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit Care Med. 2018;46(6):997‐1000. [DOI] [PubMed] [Google Scholar]
- 22. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock: 2016. Int Care Med. 2017;43(3):304‐377. [DOI] [PubMed] [Google Scholar]
- 23. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486‐552. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344‐349. [DOI] [PubMed] [Google Scholar]
- 25. Guirgis FW, Jones L, Esma R, et al. Managing sepsis: electronic recognition, rapid response teams, and standardized care save lives. J Crit Care. 2017;40:296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707‐710. http://www.ncbi.nlm.nih.gov/pubmed/8844239. [DOI] [PubMed] [Google Scholar]
- 27. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate Clearance vs Central Venous Oxygen Saturation as Goals of Early Sepsis Therapy: a Randomized Clinical Trial. JAMA. 2010;303(8):739‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pandharipande PP, Shintani AK, Hagerman HE, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009;37(4):1317‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315(8):762‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308‐333. [Google Scholar]
- 32. Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2001;31(4):1250‐1256. [DOI] [PubMed] [Google Scholar]
- 33. Hinder F, Stubbe HD, Van Aken H, et al. Early multiple organ failure after recurrent endotoxemia in the presence of vasoconstrictor‐masked hypovolemia. Crit Care Med. 2003;31(3):903‐909. [DOI] [PubMed] [Google Scholar]
- 34. Colon Hidalgo D, Patel J, Masic D, Park D, Rech MA. Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J Crit Care. 2020;55:145‐148. [DOI] [PubMed] [Google Scholar]
- 35. Varpula M, Tallgren M, Saukkonen K, Voipio‐Pulkki LM, Pettil V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066‐1071. [DOI] [PubMed] [Google Scholar]
- 36. Jones AE, Yiannibas V, Johnson C, Kline JA. Emergency department hypotension predicts sudden unexpected in‐hospital mortality: a prospective cohort study. Chest. 2006;130(4):941‐946. [DOI] [PubMed] [Google Scholar]
- 37. Jones AE, Aborn LS, Kline JA. Severity of emergency department hypotension predicts adverse hospital outcome. Shock. 2004;22(5):410‐414. [DOI] [PubMed] [Google Scholar]
- 38. Marchick MR, Kline JA, Jones AE. The significance of non‐sustained hypotension in emergency department patients with sepsis. Intensive Care Med. 2009;35(7):1261‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maheshwari K, Nathanson BH, Munson SH, et al. The relationship between ICU hypotension and in‐hospital mortality and morbidity in septic patients. Intensive Care Med. 2018;44(6):857‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


