Abstract
Background
In 2006, the Centers for Disease Control and Prevention (CDC) recommended non‐targeted, opt‐out HIV screening in all healthcare settings, including emergency departments (EDs). Multiple HIV testing programs have been implemented in EDs across the United States with varying designs and testing platforms. We report findings from a free, non‐targeted, rapid HIV testing program in 2 EDs in the Southeastern United States.
Methods
From 2008 to 2012, adults ≥18 years of age were offered free rapid HIV testing using an oral swab test (OraQuick ADVANCE Rapid HIV‐1/2 antibody test) in the EDs of a large academic medical center and an affiliated community hospital in Durham, North Carolina.
Results
In total, 5443 ED patients were offered HIV testing. The overall acceptance rate was 66.9% (3639/5443). Younger persons were significantly more likely to accept testing (78.2% for 18–29 years old vs 67.1% for ≥30 years old; P < 0.001) as were Black participants (72.6% Black vs 66.5% White; P < 0.001). Acceptance rates improved significantly after opt‐out oral consent replaced written consent (71.3% vs 63.1%; P < 0.001). Seven new HIV diagnoses were confirmed during the testing program, resulting in a seropositivity rate of 0.19% (7/3639). There were 8 false–positive rapid oral HIV tests (positive predictive value = 46.7%).
Conclusions
Although the number of new HIV diagnoses was low, implementation of this rapid, non‐targeted ED screening program was feasible with high acceptance rates, particularly after introducing the opt‐out oral consent approach.
Keywords: acceptance rates, emergency department, HIV, HIV testing, non‐targeted, rapid, Southeast
1. BACKGROUND
Testing for HIV infection in emergency departments appears to be a relatively high yield strategy, in part because the population seen in EDs includes those at higher risk for HIV (racial and ethnic minorities, socio‐economically disadvantaged persons, and young people). 1 , 2 Recognizing that persons with undiagnosed HIV contribute significantly to HIV transmission, the Centers for Disease Control and Prevention (CDC) recommended non‐targeted, routine opt‐out HIV screening for adults ages 13–64 in all healthcare settings, including EDs in 2006. 3
The proportion of people living with HIV who are undiagnosed has diminished in the past decade, falling from 21% in 2006 to 14% in 2016. 4 , 5 This is largely attributed to enhanced HIV screening programs that have been implemented in various healthcare settings, including EDs. These programs have varied greatly in design and include testing based on risk‐assessment versus non‐targeted screening, rapid versus conventional assay testing, and HIV antibody testing versus nucleic acid amplification (NAAT) testing. The latter approach offers the advantage of facilitating early diagnosis, as was demonstrated in a review of NAAT HIV testing across 9 EDs in which 15% of all HIV diagnoses were in persons with acute HIV infection. 6
An additional benefit of HIV testing in EDs is the identification of people living with HIV who have been lost to follow‐up, providing an opportunity to link them back into care. In a large cohort study, HIV testing in the ED was associated with improved linkage to care, retention, and virologic suppression among persons previously diagnosed with HIV who were “re‐diagnosed” as HIV‐infected during an ED visit. 7
Published reports of ED HIV testing programs have shown variable test acceptance rates (53%–91%), 8 , 9 , 10 , 11 and rates of new HIV diagnoses have been relatively modest (0.14%–1.7%). 9 , 10 , 12 , 13 , 14 , 15 Successful ED HIV testing program are tailored to the characteristics of their specific sites, optimizing use of scarce resources and maximizing test acceptance rates and numbers of new diagnoses. 16
Here, we report findings from a free, non‐targeted, rapid HIV testing program conducted in 2 academic EDs in the Southeastern United States, the region with the highest rates of new HIV infections and people living with HIV. 17 The objective was to help identify a successful testing program that could be implemented in our ED setting, thereby increasing the rate of new HIV diagnoses in the ED. Data collected included basic demographics, clinical characteristics, test acceptance rates, and reasons for declining testing. Due to a hospital policy change approximately half‐way through the study period, the testing strategy was changed to eliminate the need for written informed consent, replacing it with an opt‐out oral consent approach. Test acceptance rates were compared before and after this change occurred.
2. METHODS
2.1. Study design and setting
This was a prospective observational study of a rapid HIV screening program at Duke University Hospital and Duke Regional Hospital EDs, both located in Durham, North Carolina. Duke University Hospital is a 900‐bed urban tertiary care hospital with a level‐1 trauma center, caring for 75,000 ED patients per year. Duke Regional Hospital is a 369‐bed acute care hospital, treating more than 64,000 ED patients annually. The HIV testing program was initiated at Duke University Hospital ED in 2008 and at Duke Regional Hospital in 2010; as such, the number of tests done during the study was higher at Duke University Hospital than at Duke Regional Hospital.
Eligible participants for HIV screening in this study were 18 years or older, mentally competent, not known to be HIV‐positive, and had not been approached by our testing program in the previous 6 months. After being signed in for care in the ED and placed in private exam rooms, patients were approached by test counselors (both Duke University Health System employees and medical and physician assistant student volunteers) and offered HIV testing. The majority of HIV testing took place during weekday business hours (8 am–5 pm) with occasional night and weekend coverage. Patients with serious illnesses or injuries were excluded (eg, patients in the trauma beds or undergoing acute medical interventions). Patients could also be excluded from testing at the judgement of the treating medical team, nursing staff, and/or at the discretion of the test counselor. Additionally, patients whose visits lasted <20 minutes and who spoke languages other than English and Spanish were excluded. Testing was performed using the OraQuick ADVANCE Rapid HIV‐1/2 antibody test (oral swab), provided by the North Carolina Department of Health and Human Services through a CDC testing grant.
THE BOTTOM LINE
In 2006, the Centers for Disease Control and Prevention (CDC) recommended non‐targeted, opt‐out HIV screening in all health care settings, including emergency departments. This paper shows that implementation of this rapid, non‐targeted ED screening program at two North Carolina EDs was feasible with high acceptance rates, particularly after introducing the opt‐out oral consent approach.
All preliminary positive oral HIV tests were confirmed by ELISA/western blot using the standard algorithm in place at the time of this study. All participants with preliminary reactive tests were given a follow‐up appointment at the Infectious Diseases Clinic at Duke University where the results from the confirmation test were provided.
Written informed consent for HIV testing was required by the Duke University Health System until August 2010 when the institutional HIV testing policy was changed to incorporate HIV testing into the general medical consent document. As a result, enrolled participants in the first 2 years of the study signed a written informed consent, which served as both consent for HIV testing and participation in a research study mandated by the Duke Institutional Review Board (IRB); in the second half of this study participants were asked for verbal consent to collect their data as required by the IRB, and no written consent was required.
2.2. Data collection
Demographics (age, race/ethnicity, and sex) and prior testing history were recorded on all participants providing consent. Reason for ED admission (“chief complaint”), vital signs, and HIV‐related symptoms (using a checklist which included recurrent fevers, night sweats, fatigue, rash, sore throat, diarrhea, >10 lb weight loss in last 6 months, and swollen lymph nodes) were obtained and reviewed from both the participant and medical record on all patients at time of testing. If the person declined testing, they were asked why they declined, and the response was categorized into 1 of the following: (1) not interested or scared, (2) not perceived at risk, (3) recently tested, (4) too sick, (5) other, or (6) no reason given. For persons with newly diagnosed HIV infection, ED discharge diagnoses, initial CD4 lymphocyte counts, and baseline HIV RNA levels were collected.
2.3. Statistical analysis
Data were analyzed by using SAS (version 9.1.3; SAS Institute, Inc., Cary, NC). Descriptive statistics were calculated including: number of participants approached, number tested, number of preliminary reactive rapid HIV tests, number of positive results confirmed by ELISA/western blot, and reasons for declining the test. The χ2 test was used to assess association between selected demographics (age, race/ethnicity, sex, history of prior testing) and whether a participant accepted testing and reasons for refusing testing. The Mantel–Haenszel test was used to determine whether the change in acceptance rates under the new consent procedure varied by demographic group. Acceptance rates among participants who were re‐approached for testing (if >6 months since last test) were also analyzed.
The number of participants approached who chose not to enter the study was recorded in order to calculate the overall acceptance rate. Participants who declined testing could elect or decline to provide their demographic information (age, race/ethnicity, and sex), whether they had ever been previously tested, and reason for declining testing.
3. RESULTS
Between 2008 and 2012, a total of 5443 ED patients were offered HIV testing (4237 at Duke University Hospital; 1206 at Duke Regional Hospital). The median age was 35.9 years old. The majority were Black (67.2%), female (60.6%), and reported having previously been tested for HIV infection (68.8%). The number of persons with race other than Black or White was extremely low and removed from the analyses, as was the number who reported being Hispanic/Latino.
Overall, HIV testing acceptance rates were high at both EDs (Duke University Hospital: 66%; Duke Regional Hospital: 69.8%) and did not differ significantly among the various demographic groups (Table 1), except for younger persons (aged 18–29 years old) and Black participants, both of whom had significantly higher acceptance rates. Women also had a slightly higher acceptance rate than men (71.4% vs 69.3%; P = 0.1052). Acceptance rates improved after written informed consent was replaced with verbal consent (71.3% vs 63.1%; P < 0.001).
TABLE 1.
Demographics with acceptance rates
| Variables | n (%) | Accepted | P |
|---|---|---|---|
| Age | |||
| 18–29 years old | 1620 (31) | 1266 (78) | <0.001 |
| ≥30 years old | 3535 (69) | 2373 (67) | |
| Missing | 288 | ||
| Race | |||
| White | 1694 (33) | 1127 (67) | <0.001 |
| Black | 3461 (67) | 2512 (73) | |
| Missing | 288 | ||
| Gender | |||
| Male | 2030 (39) | 1407 (69) | 0.105 |
| Female | 3124 (61) | 2231 (71) | |
| Missing | 289 | ||
| Testing history | |||
| Hx of prior testing | 3201 (69) | 2344 (73) | 0.255 |
| No hx prior testing | 1453 (31) | 1087 (75) | |
| Missing a | 789 | ||
| Total | 5443 | 3639 (67) |
Other races/ethnicity not included due to low numbers.
Missing includes unknown.
3.1. Reactive oral HIV tests
Fifteen participants had reactive oral swab tests; 7 were confirmed to be HIV‐infected and 8 were confirmed to be HIV‐uninfected by subsequent ELISA/western blot testing. With the 7 new HIV infections, the overall seropositivity rate for the testing program was 0.19% (7/3639); 5 new diagnoses were made at Duke University Hospital (0.18%; 5/2797) and 2 at Duke Regional Hospital (0.24%; 2/842). All 7 newly diagnosed HIV infections occurred in Blacks, 4/7 were males (57%). (Table 2). The median age was 26 years old (range = 25–55). The median initial CD4 lymphocyte count was 49 cells/mm3 (range = 9–985 cells/mm3). Six of the 7 newly diagnosed persons presented with HIV‐related signs and symptoms including: esophageal candidiasis, genital/anal herpes, severe pharyngitis, and bacterial pneumonia. Three persons were diagnosed with presumptive Pneumocystis jiroveci pneumonia (PJP) in the ED.
TABLE 2.
Characteristics of newly diagnosed HIV+ patients
| Sex | Race | Age | Previous HIV test | Initial CD4 count (cells/mm3) | Symptoms at diagnosis |
|---|---|---|---|---|---|
| Female | Black | 55 | No | 49 | Oropharyngeal thrush, PJP |
| Male | Black | 26 | Yes | 25 | PJP |
| Male | Black | 25 | Yes | 9 | PJP |
| Female | Black | 38 | Unknown | 34 | Genital/anal herpes, oropharyngeal thrush |
| Female | Black | 46 | No | 985 | Abdominal pain |
| Male | Black | 25 | Yes | 291 | Bacterial pneumonia |
| Male | Black | 25 | Yes | 360 | Severe pharyngitis |
PJP, Pneumocystis jiroveci pneumonia.
Regarding the 8 false–positives, 4 occurred in each ED test site. Three false–positives occurred in 2009 during a period when HIV testing centers reported higher false–positive rates when using OraQuick test kits close to their expiration dates. 18 One false–positive occurred in a person with dengue fever, and another in a patient with active systemic lupus erythematosus. The remaining 3 false–positives had no apparent explanation. HIV‐1 RNA viral load testing was not performed in any of the 8 patients with false–positive tests to rule out acute HIV infection at the time of testing. Notably, none of the patients had symptoms suggestive of acute HIV infection (such as fever, night sweats, or lymphadenopathy).
3.2. Reasons for declining free HIV testing
Reasons for declining HIV testing included having had “recent testing” (43%), being “not perceived at risk” (20%), being “not interested/scared” (19%), “too sick” (14%), or offering “other or no reason” (4%). Younger persons (aged 18–44 years) were more likely to decline due to recent testing than were older persons (≥45 years) (46% vs 23%; P < 0.001). Compared to the younger group, older persons were more likely to decline testing due to being “not interested/scared” (30% vs 19%; P < 0.001) or not perceiving themselves to be at risk (29% vs 18%; P < 0.001).
Reasons for declining testing varied by race (Figure 1). Black participants were significantly more likely to decline testing due to recent testing than were White participants (44% vs 30%; P < 0.001). White participants more commonly listed “not perceived at risk” as a reason for declining testing (White [31%] vs Black [14%]; P < 0.001). These differences based on race were more apparent among younger persons. Disinterest or fear of testing accounted for a similar and substantial proportion of both Black (23%) and White persons (24%). As mentioned, the numbers of study participants from other racial/ethnic groups were insufficient to allow comparisons to be made.
FIGURE 1.
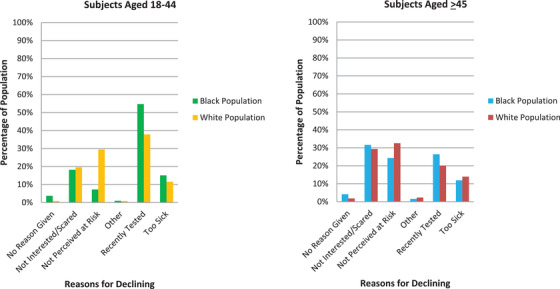
Reasons for declining testing compared for age and race
Some ED patients were approached for HIV testing more than once during the study if >6 months had elapsed since the initial testing request. Of 41 patients who initially declined testing, 22 (54%) accepted testing the second time they were approached (Figure 2).
FIGURE 2.
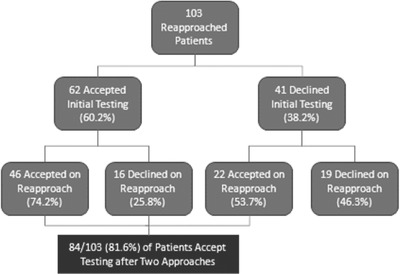
Acceptance rates of re‐approached participants
4. DISCUSSION
Persons presenting for care in EDs are, as a group, at higher risk for HIV infection than the general population. In a 2013 study conducted at the same health system, 71% of persons newly diagnosed with HIV who were seen in the Duke Infectious Diseases Clinic completed at least 1 healthcare encounter in the previous 12 months during which they were not tested for HIV. The mean number of healthcare visits per patient in the year before their diagnosis was 2.75 and 51% had ≥1 visit to the ED. 19 Earlier diagnosis of HIV infection during an ED encounter could improve individual patient outcomes and help reduce HIV transmission rates.
Although testing was only offered to a fraction of the total number of ED patients seen during this 4‐year period, high acceptance rates for HIV testing were noted among all demographic groups in this free, ED‐based rapid HIV testing program in North Carolina, a state with high rates of HIV incidence and prevalence overall. 20 Importantly, younger persons and Blacks had the highest acceptance rates, 2 groups at higher risk for HIV in the United States. Test acceptance rates did not differ between those not previously tested and those who had been tested for HIV before. These data are now more than 5 years old but we feel it is still relevant in the Southeast where the rates of new diagnoses have not changed from 2012 to 2016. 17
Few new HIV diagnoses were made in this non‐targeted screening program (seropositivity rate 0.19%) despite the fact that Durham County ranks fourth among 100 counties in North Carolina in newly diagnosed HIV rates. 21 In 2010, halfway through the study, the rate of HIV‐positive tests at the Durham County Health Department was 0.5%, 22 although this number undoubtedly included persons already known to be HIV‐infected (vs new diagnoses). The relatively low rate of new HIV diagnoses in this ED‐based testing program may have been influenced by a number of factors including exclusion of patients with serious illness or injury, a group that may represent a higher risk population. Another potential factor may be the timing during which the majority of testing was offered (daytime hours during weekdays). Although rates of new HIV diagnoses have been similarly modest in other ED testing programs, 9 , 10 , 13 , 14 , 15 , 23 all these rates are consistently above the CDC threshold of 0.1% for instituting routine HIV screening. 3 Notably, seroprevalence rates among persons who decline testing have been found to be 1.4–18 times higher than those who accept testing, even when reporting similar risk. 12 , 24 , 25 , 26 , 27
The most common reason for declining testing in this study was due to ED patients reporting having been previously tested, which may be a reasonable reason for not being tested based on the CDC recommendations that persons should be tested at least once as adults. Although we were unable to verify prior testing unless the testing had been done during this study, patient reliability has been demonstrated in both self‐reported testing history and behavioral risk factors. 27 , 28 However, many people declined testing due to disinterest/fear and/or not perceiving themselves to be at risk. Current public awareness of HIV testing recommendations remains quite low. 29 Scaling up public health education efforts about the realities of today's HIV epidemic may improve public awareness of the importance of getting tested and the need for routine HIV testing programs.
Reasons for declining testing in this program differed by age and race. Older persons were significantly more likely to perceive themselves “not at risk” for having HIV infection. However, in 2016, persons aged 50 and older accounted for 17% of all new diagnoses and 47% of all persons living with HIV. 30 These rising numbers may reflect changing attitudes regarding sexuality in older populations, misinformation or lack of knowledge of risky sexual behaviors among seniors and the inattention of healthcare providers regarding sexual health in their older patients. 31 , 32 , 33 , 34 Although some of the increase of identified HIV infections among older persons may be due to expanded HIV testing programs, the median CD4 count at initial presentation among persons aged >50 years old is consistently lower than that seen in younger adults, with a greater proportion diagnosed with AIDS at the time of or within 3 months of initial presentation, indicative of late diagnosis. 35
White participants were also more likely to decline testing due to not perceiving themselves to be at risk for HIV infection. The incidence and prevalence of HIV infection is lower among Whites, 36 but it is also true that Whites in this study and others have lower rates of previous HIV testing. Denial of risk is associated with late diagnosis and more advanced HIV infection at time of diagnosis. 37 Moreover, HIV testing programs that do not apply equally to all socioeconomic groups may increase stigma and diminish overall rates of HIV testing in the population. The finding in this study that White persons and older persons were more likely to have never been tested and more likely to decline testing, even in this universal testing paradigm, is concerning and should serve as a reminder for healthcare providers to encourage testing of all persons.
According to the data we collected, one effective way to encourage more people to test may be to increase the number of times that persons are asked to test. We found that participants who initially declined and were approached a second time had a higher acceptance rate, suggesting that frequent approach can be effective. By re‐approaching patients and reinforcing the universal nature of our testing program, the testing seems normative rather than selective. Stigma associated with being asked to be tested is decreased considerably with repeated approaches, and refusing the test, rather than accepting, adopts the stigma of appearing to possess heightened risk‐factors for HIV infection. 38 Our results and others have shown that even being approached only 2 times may be sufficient enough to reduce stigma or encourage testing acceptance for a significant number of people. 39
More false–positive tests were observed in this study than true–positive tests, yielding a positive predictive value (PPV) of ∼47%. This was much lower than the performance characteristics reported by the manufacturer and others including in low‐risk populations. 10 , 40 , 41 However, multiple “real‐world” settings have experienced similar issues with false–positives while using the oral version of the OraQuick test. 42 , 43 It is notable that since this study was concluded, the NC DHHS no longer recommends use of oral swab testing for HIV screening programs in North Carolina (personal correspondence). Since this study was conducted, both EDs are now using a fourth‐generation HIV test, which is a combined antigen/antibody test. The antigen/antibody test can identify HIV infection earlier (typically 2 weeks from time of infection) and has improved performance characteristics. The primary disadvantage is that the results take over 2 hours, and thus it is not considered a rapid test. For this free HIV testing program with external test counselors in which tests were provided for free by the state, the OraQuick was felt to the best option at the time.
We would like to emphasize that all the new HIV diagnoses in this study were made in persons with HIV‐associated symptoms. As such, in our setting it appears to be more appropriate to consider a more targeted testing program focused on persons presenting to the ED with such symptoms. The obvious disadvantage of a symptom‐targeted approach to HIV testing is that asymptomatic patients presenting earlier in the course of HIV infection would likely be missed. Notably, the median CD4 lymphocyte count of those diagnosed in this study was only 49 cells/mm 3 , indicative of late diagnosis. Additionally, when considering implementation of a HIV testing program, it is important to recognize that trying to embed HIV testing into routine ED clinical practice requires significant time and effort. 44 Our program with high rates of test acceptance were due to the work of dedicated HIV test counselors. Without such counselors in place, it has proven difficult to maintain consistent HIV testing in our EDs.
5. CONCLUSIONS
Incorporating HIV testing into the workflow of EDs offers significant health benefits to persons seeking care who are unaware of their HIV diagnosis, but ED HIV testing programs may need to be suspended when circumstances demand a different focus. For example, the current COVID‐19 pandemic has tasked EDs with enormous patient care responsibilities, requiring strict attention to infection control measures including minimizing non‐essential contact between health care personnel and potentially infected persons. Obviously, this necessitates deferral of population‐based HIV screening, as ED resources must be concentrated on managing the pandemic. Flexibility with regard to testing strategies will allow EDs to maximize the benefits of HIV screening over time while ensuring that EDs focus on critical needs during mass casualties, natural disasters, and epidemics.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MM takes responsibility of the final manuscript.
Biography
Mehri Sadri McKellar, MD, is an Associate Professor of Medicine at Duke University School of Medicine, Durham, NC.

Safeek R, Hill T, Hendricks A, et al. Testing for HIV infection in the emergency departments of 2 hospitals in the Southeastern United States. JACEP Open. 2020;1:487–493. 10.1002/emp2.12102
Funding and support: MM received grant funding from Janssen Services, LLC; Duke University Center for AIDS Research (CFAR) 5P30 AI064518. Senior author MM has served on the advisory boards for Gilead Sciences, Inc., ViiV Healthcare, and Thera Technologies. CH currently works at Viiv Healthcare.
Supervising Editor: Junichi Sasaki, MD.
REFERENCES
- 1. Torres M. Rapid HIV screening in the emergency department. Emerg Med Clin North Am. 2010;28(2):369‐380. [DOI] [PubMed] [Google Scholar]
- 2. Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: should emergency departments conduct routine HIV screening? a systematic review. Acad Emerg Med. 2003;10(3):278‐285. [DOI] [PubMed] [Google Scholar]
- 3. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health‐care settings. MMWR Recomm Rep. 2006;55(RR‐14):1‐17; quiz CE11‐14. [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC) . New analysis highlights the power of testing and treatment to end the HIV epidemic in the U.S. https://www.cdc.gov/media/releases/2019/p0315-gaps-hinder-hiv-testing.html. Published 2019. Accessed January 7, 2020.
- 5. CDC . HIV Prevalence Estimates – United States, 2006. MMWR. 2008;57(39):1073‐1076. [PubMed] [Google Scholar]
- 6. White DAE, Giordano TP, Pasalar S, et al. Acute HIV discovered during routine hiv screening with HIV antigen‐antibody combination tests in 9 US emergency departments. Ann Emerg Med. 2018;72(1):29‐40 e22. [DOI] [PubMed] [Google Scholar]
- 7. Flash CA, Pasalar S, Hemmige V, et al. Benefits of a routine opt‐out HIV testing and linkage to care program for previously diagnosed patients in publicly funded emergency departments in Houston, TX. J Acquir Immune Defic Syndr. 2015;69(Suppl 1):S8‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) . Rapid HIV testing in emergency departments—three U.S. sites, January 2005‐March 2006. MMWR. 2007;56(24):597‐601. [PubMed] [Google Scholar]
- 9. Walensky RP, Reichmann WM, Arbelaez C, et al. Counselor‐ versus provider‐based HIV screening in the emergency department: results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med. 2011;58(1 Suppl 1):S126‐132 e121‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sattin RW, Wilde JA, Freeman AE, Miller KM, Dias JK. Rapid HIV testing in a southeastern emergency department serving a semiurban‐semirural adolescent and adult population. Ann Emerg Med. 2011;58(1 Suppl 1):S60‐S64. [DOI] [PubMed] [Google Scholar]
- 11. Brown J, Kuo I, Bellows J, et al. Patient perceptions and acceptance of routine emergency department HIV testing. Public Health Rep. 2008;123(suppl 3):21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Czarnogorski M, Brown J, Lee V, et al. The prevalence of undiagnosed HIV infection in those who decline HIV screening in an urban emergency department. AIDS Res Treat. 2011;2011:879065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wheatley MA, Copeland B, Shah B, Heilpern K, Del Rio C, Houry D. Efficacy of an emergency department‐based HIV screening program in the Deep South. J Urban Health. 2011;88(6):1015‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. d'Almeida KW, Kierzek G, de Truchis P, et al. Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch Intern Med. 2012;172(1):12‐20. [DOI] [PubMed] [Google Scholar]
- 15. Brown J, Magnus M, Czarnogorski M, Lee V. Another look at emergency department HIV screening in practice: no need to revise expectations. AIDS Res Ther. 2010;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christopoulos KA, Koester K, Weiser S, Lane T, Myers JJ, Morin SF. A comparative evaluation of the process of developing and implementing an emergency department HIV testing program. Implement Sci. 2011;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) . HIV Surveillance Report, 2018 (Preliminary) . https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updatedvol-31.pdf. November 2019. Accessed January 7, 2020.
- 18. Facente SN, Dowling T, Vittinghoff E, Sykes DL, Colfax GN. False positive rate of rapid oral fluid HIV tests increases as kits near expiration date. PLoS One. 2009;4(12):e8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin T, Hicks C, Samsa G, McKellar M. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDs. 2013;27(7):392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. HIV/STD/Hepatitis Surveillance Unit Division, North Carolina Department of Health and Human Services . 2017 North Carolina HIV/STD/Hepatitis Surveillance Report. September 2018. https://epi.dph.ncdhhs.gov/cd/stds/figures/std17rpt_rev12142018.pdf. Accessed November 19, 2019. [Google Scholar]
- 21. HIV/STD/Hepatitis Surveillance Unit Division, North Carolina Department of Health and Human Services. 2018 North Carolina HIV/STD/Hepatitis Surveillance Report . August 2019. https://epi.dph.ncdhhs.gov/cd/stds/figures/hiv18rpt_02042020.pdf. Accessed November 19, 2019.
- 22. North Carolina Department of Health and Human Services. Epidemiologic Profile for HIV/STD Prevention and Care Planning . https://epi.dph.ncdhhs.gov/cd/stds/figures/Epi_Profile_2011.pdf. December 2011. Accessed November 19, 2019.
- 23. Haukoos JS. The impact of nontargeted HIV screening in emergency departments and the ongoing need for targeted strategies. Arch Intern Med. 2012;172(1):20‐22. [DOI] [PubMed] [Google Scholar]
- 24. Groseclose SL, Erickson B, Quinn TC, Glasser D, Campbell CH, Hook EW 3rd. Characterization of patients accepting and refusing routine, voluntary HIV antibody testing in public sexually transmitted disease clinics. Sex Transm Dis. 1994;21(1):31‐35. [DOI] [PubMed] [Google Scholar]
- 25. Hull HF, Bettinger CJ, Gallaher MM, Keller NM, Wilson J, Mertz GJ. Comparison of HIV‐antibody prevalence in patients consenting to and declining HIV‐antibody testing in an STD clinic. JAMA. 1988;260(7):935‐938. [PubMed] [Google Scholar]
- 26. Weinstock H, Dale M, Linley L, Gwinn M. Unrecognized HIV infection among patients attending sexually transmitted disease clinics. Am J Public Health. 2002;92(2):280‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ubhayakar ND, Lindsell CJ, Raab DL, et al. Risk, reasons for refusal, and impact of counseling on consent among ED patients declining HIV screening. Am J Emerg Med. 2011;29(4):367‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanable PA, Carey MP, Brown JL, et al. Test‐retest reliability of self‐reported HIV/STD‐related measures among African‐American adolescents in four U.S. cities. J Adolesc Health. 2009;44(3):214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calderon Y, Leider J, Hailpern S, et al. High‐volume rapid HIV testing in an urban emergency department. AIDS Patient Care STDs. 2009;23(9):749‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) . HIV Among People Aged 50 and Older. https://www.cdc.gov/hiv/group/age/olderamericans/index.html. November 2019. Accessed January 7, 2020.
- 31. Patel D, Crane LR. Growing old with HIV. Curr Infect Dis Rep. 2011;13(1):75‐82. [DOI] [PubMed] [Google Scholar]
- 32. Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindau ST, Leitsch SA, Lundberg KL, Jerome J. Older women's attitudes, behavior, and communication about sex and HIV: a community‐based study. J Womens Health (Larchmt). 2006;15(6):747‐753. [DOI] [PubMed] [Google Scholar]
- 34. Henderson SJ, Bernstein LB, George DM, Doyle JP, Paranjape AS, Corbie‐Smith G. Older women and HIV: how much do they know and where are they getting their information? J Am Geriatr Soc. 2004;52(9):1549‐1553. [DOI] [PubMed] [Google Scholar]
- 35. Althoff KN, Gebo KA, Gange SJ, et al. CD4 count at presentation for HIV care in the United States and Canada: Are those over 50 years more likely to have a delayed presentation? AIDS Res Ther. 2010;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention (CDC) . HIV Testing in the United States (CDC Fact Sheet) . 2016. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-testing-us-508.pdf. Accessed January 7, 2020.
- 37. Branson B. Current HIV epidemiology and revised recommendations for HIV testing in health‐care settings. J Med Virol. 2007;79(Suppl 1):S6‐S10. [DOI] [PubMed] [Google Scholar]
- 38. Young SD, Monin B, Owens D. Opt‐out testing for stigmatized diseases: a social psychological approach to understanding the potential effect of recommendations for routine HIV testing. Health Psychol. 2009;28(6):675‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calderon Y, Cowen E, Simberlund J, et al. Assessing the impact of inpatient HIV testing in an urban hospital with an ED‐based rapid HIV testing and counseling program 2011. CROI 2011 Paper #1050. Accessed January 7, 2020. [Google Scholar]
- 40. Nkenfou CN, Kembou JE, Temgoua ES, et al. Evaluation of OraQuick(R) HIV‐1/2 as Oral Rapid Test. Afr J Infect Dis. 2013;7(2):27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pant Pai N, Balram B, Shivkumar S, et al. Head‐to‐head comparison of accuracy of a rapid point‐of‐care HIV test with oral versus whole‐blood specimens: a systematic review and meta‐analysis. Lancet Infect Dis. 2012;12(5):373‐380. [DOI] [PubMed] [Google Scholar]
- 42. Delaney KP, Branson BM, Uniyal A, et al. Performance of an oral fluid rapid HIV‐1/2 test: experience from four CDC studies. AIDS. 2006;20(12):1655‐1660. [DOI] [PubMed] [Google Scholar]
- 43. Walensky RP, Arbelaez C, Reichmann WM, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med. 2008;149(3):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rayment M, Rae C, Ghooloo F, et al. Routine HIV testing in the emergency department: tough lessons in sustainability. HIV Med. 2013;14(Suppl 3):6‐9. [DOI] [PubMed] [Google Scholar]


