Abstract
Type A aortic dissection is an uncommon cause of chest pain that carries a high morbidity and mortality rate. A previous history of hypertension and coronary artery bypass grating (CABG) are recognized risk factors for Type A aortic dissection. We present a case of an elderly man who presents with acute onset chest pain and was found to have an acute ruptured Type A aortic dissection that has a “saddle pulmonary embolism”‐like appearance on computed tomography (CT) imaging. We also describe the clinical, laboratory, and radiological workup done leading up to the diagnosis of Type A aortic dissection in the emergency setting.
Keywords: Type A Aortic Dissection, Mediastinal Hematoma, Pulmonary Artery Compression, Point of Care Ultrasound (POCUS), Aortic Dissection Detection Risk Score (ADD‐RS)
1. INTRODUCTION
Type A aortic dissection is a rare but fatal disease associated with a high mortality rate. Fewer than half of all patients with ruptured Type A aortic dissection arrive at the hospital alive, where the majority of death occurs within 6‐hour from time of rupture. 1 Previous studies have shown that transesophageal echocardiogram and magnetic resonance aortography (MRA) are the most reliable imaging modalities for diagnosing aortic dissection. However, computed tomography (CT) aortography is the imaging method of choice during emergency settings because of its wide availability, rapid turnover time, and high negative predictive value. 2 , 3 , 4 We present the case of an elderly man who presented with chest pain and was found to have ruptured Type A aortic dissection with “saddle pulmonary embolism”‐like appearance on CT imaging.
2. CASE DESCRIPTION
A 74‐year‐old man presented to the emergency department with a 1‐day history of acute onset chest pain, nausea, and vomiting. He denied any radiation of chest pain to his back, arms, neck, or abdomen. Review of systems was negative for any reports of dyspnea, palpitations, headache, dizziness, blurry vision, paralysis, or paresthesia of extremities. His medical history was significant for hypertension, obesity, and coronary artery disease requiring coronary artery bypass grafting (CABG) a year prior. He had no history of any murmurs or valvular heart disease but had a 50‐pack‐a‐year smoking history.
Blood pressure on admission was 140/80 measured on both arms, respiratory rate was 20, and heart rate was 90 without radio‐femoral or radial‐radial delay. His oxygen saturation was 94% in room air. Physical examination revealed early diastolic heart murmur heard loudest at the lower left sternal border. Lungs were clear to auscultation, abdomen was soft and non‐tender.
Laboratory testing was positive for an elevated D‐dimer at 2000 ng/mL. Complete blood count and complete metabolic panel were within normal limits. He had an ischemic workup done involving electrocardiogram and serial troponins that were negative. Admission chest radiograph demonstrated no acute cardiopulmonary pathology. Point‐of‐care transthoracic echocardiogram (POCUS) of the heart was done before any additional radiologic testing (eg, CT chest), to rule out any cardiopulmonary pathology that was missed on chest radiograph and determine the etiology for the new early diastolic murmur appreciated on physical exam.
POCUS (Figure 1A) showed aortic regurgitation and dilated ascending aorta with possible intimal flap. CT angiography of the thoracic aorta was pursued because of the findings seen on the point‐of‐care transthoracic echocardiogram, positive D‐dimer, and patient's calculated aortic dissection detection risk score (ADD‐RS) of 2. The ADD‐RS was scored based on the new onset of acute severe chest pain and aortic insufficiency murmur as shown in Table 1. CT angiography (Figure 2) showed an acute ruptured Type A aortic dissection with mediastinal hematoma that compressed the pulmonary artery bifurcation adjacent to it.
FIGURE 1.

(A) Transthoracic echocardiogram parasternal long view demonstrating dilated ascending aorta (AA). (B) Transesophageal echocardiogram mid esophageal view. (C) Transesophageal echocardiogram transverse cut of ascending aorta. white arrow shows location of intimal flap. AA, aortic aneurysm; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; TL, true lumen
TABLE 1.
Aortic dissection detection risk score (ADD‐RS)
| Three criteria in aortic dissection detection risk score | Points |
|---|---|
| High‐risk predisposing conditions | 1 |
| Marfan syndrome | |
| Family history of aortic disease | |
| Known aortic valve disease | |
| Recent aortic manipulation | |
| Known thoracic aortic aneurysm | |
| High‐risk pain features | 1 |
| Abrupt, severe intensity, or ripping/tearing onset of chest, back, or abdominal paina | |
| High‐risk physical exam features | 1 |
| Pulse deficit | |
| Systolic BP differential | |
| Focal neuro deficits plus pain | |
| New aortic insufficiency murmur with paina | |
| Hypotension/shock |
Patient scored two‐thirds based on high risk pain features and high risk physical exam features
FIGURE 2.
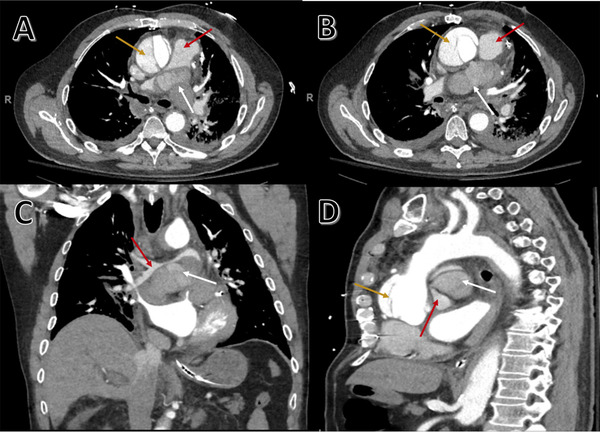
CT angiography of thoracic aorta showing acute Type A aortic dissection. Axial view (A and B), coronal view (C), and sagittal view (D). White arrows show mediastinal hematoma that compresses and obliterates 90% of the bifurcation of pulmonary artery (A–D), red arrows show location of pulmonary artery in relation to mediastinal hematoma, and yellow arrows show location of false lumen (A, B, and D). Mediastinal hematoma extrinsically compressing the pulmonary bifurcation (A–C) giving it a “saddle pulmonary embolism”‐like appearance on CT. Dissection involving the aortic root (D) with defect in posterior wall of proximal ascending aorta forming mediastinal hematoma
Urgent cardiothoracic consultation was obtained and patient was rushed for emergent cardiac surgery repair. He had a transesophageal echocardiogram done during surgery (Figures 1B and 1C). His surgery was complex and lasted for >24 hours. During his lengthy surgery, the patient developed multiple complications such as excessive amount of blood loss and coagulopathy requiring multiple blood transfusions. He died during surgery.
3. DISCUSSION
The differential diagnoses for any elderly male who presents with chest pain includes pneumonia, coronary arterial disease, pulmonary embolism, pneumothorax, trauma to chest wall, and aortic dissection. Pneumonia usually presents with fever, productive cough, and leukocytosis with sepsis syndrome. A chest radiograph will typically show focal or diffuse infiltrate with air bronchogram. None of these were present in our patient. Cardiac workup including serial EKGs, and troponins were negative for coronary artery disease. Chest radiograph showed no evidence of pneumothorax or rib fractures to suspect traumatic chest injury. Moreover, lack of history of recent fall or trauma and unremarkable physical examination for trauma supports an alternative diagnosis. The diagnosis of Type A aortic dissection was made based on (1) clinical presentation of acute onset of chest pain, (2) physical examination revealing early diastolic murmur from dilated aortic root leading to aortic regurgitation, (3) elevated D‐dimer on laboratory workup, and (4) point‐of‐care transthoracic echocardiogram with CT angiography showing dilated ascending aorta with intimal flap consistent with Type A aortic dissection.
Aortic dissection is a rare disease with an incidence of 3.5/100,000 person/years. Type A dissection (62%) is more prevalent than Type B. 2 , 5 Type A aortic dissection, as per Stanford Classification, is defined as primary intimal tear that begins in the ascending aorta. In Type B, it originates elsewhere. 2 , 3 Aortic dissection begins in the aortic intima from degeneration of underlying aortic media. Pulsatile arterial blood tracks along the tear and separates the aortic media creating a false lumen. Sinotubular junction of the ascending aorta is the most common site of intimal tear because of high shear forces exerted by left ventricle during systole. The false lumen can spread to the aortic arch and its branching vessels or proximally to the aortic valve and pericardial space. This is responsible for end‐organ ischemia (coronary, cerebral, spinal, or visceral), aortic regurgitation, and cardiac tamponade. 2 , 4 Saddle pulmonary embolism is defined as a large thromboembolus that straddles the bifurcation of the main pulmonary artery trunk. 6 Figure 3 shows how similar a mediastinal hematoma compressing the pulmonary artery from ruptured Type A aortic dissection is compared to saddle pulmonary embolism.
FIGURE 3.
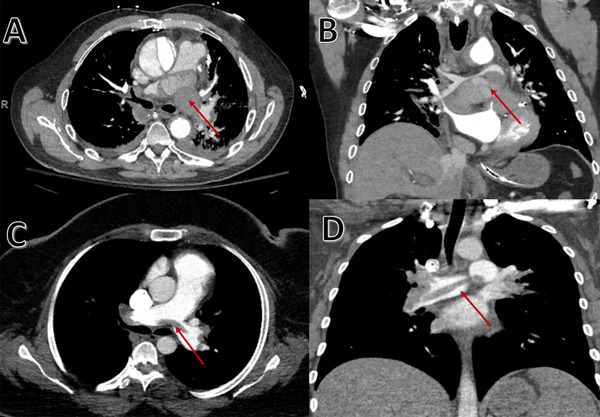
CT angiography of chest. Axial view (A) and coronal view (B) shows mediastinal hematoma with compression of pulmonary artery (red arrow) from ruptured Type A aortic dissection that mimics a "saddle pulmonary embolism" like appearance. The similarities are highlighted when comparing it to an actual saddle pulmonary embolism case seen in CT chest axial view (C) and coronal view (D)
Based on the review of International Registry of Acute Aortic Dissection (IRAD), in Type A aortic dissection, common presentation includes acute onset chest pain (85%), radiation to back (43%) or abdomen (22%), syncope (19%), and focal neurologic deficits (3%). Pulse deficit define as a difference in systolic blood pressure by >20 mmHg on both arms is an infrequent finding in up to 30% of cases. 5 , 7 Risk factors for aortic dissection include hypertension, hyperlipidemia, tobacco dependence, advanced age, and CABG. 8 Based on a single‐center study, 20% of patients with Type A aortic dissection have a CABG history within 2–5 years prior. Despite surgical intervention, mortality remained high (20%) over 5‐year follow‐up. Location of intimal tears was common at partial occlusion clamp site (58%) and cross‐clamping site (20%). 8 A clinical scoring system, termed aortic dissection detection risk score (ADD‐RS), was described as a diagnostic tool for acute aortic dissection based on high‐risk predisposing conditions, pain, and physical examination features. Based on IRAD study, a ADD‐RS risk score of 1 and more had 96% sensitivity and 64% specificity in diagnosing acute aortic dissection. 9
The workup for Type A aortic dissection often varies depending on the clinical suspicion. Chest radiograph (sensitivity 65%) often shows a widened mediastinum. Based on previous studies, CT aortography (sensitivity, 94%; specificity, 87%) is the preferred diagnostic test (65%) in the emergency setting because of its wide availability, non‐operator dependence, and rapid turnover time. 2 , 4 This is followed by echocardiogram (33%) and magnetic resonance aortography (MRA) (2%). Transthoracic echocardiography (sensitivity, 60%; specificity, 83%) and transesophageal echocardiogram (sensitivity, 98%; specificity, 90%) are rarely used because of lack of accessibility, highly operator‐dependent, and the need for preprocedural sedation with transesophageal echocardiogram. Despite the similar reliability of MRA (sensitivity, 98%; specificity, 98%) to transesophageal echocardiogram, it is rarely used because of lack of availability, cost, and slow turnover. 2 , 4 , 10 D‐dimer is a product of degraded fibrin formed during activation of the coagulation cascade by tissue factor release during intimal tear. It can be elevated in both aortic dissection and pulmonary embolism. A meta‐analysis study showed that D‐dimer (sensitivity, 97%; specificity, 56%) is useful in screening patients to rule out aortic dissection. 11 The ADvISED Trial combined ADD‐RS followed by the serum biomarker of D‐dimer where an ADD‐RS of 1>and negative D‐dimer had the sensitivity of 98.8%, the negative predictive value of 99.7%, and the likelihood ratio of −0.02 in ruling out aortic dissection. 12 Therefore, any patient with a positive ADD‐RS of 1 and more with elevated D‐dimer requires a point‐of‐care transthoracic echocardiogram to rule out aortic dissection, especially in the setting of normal chest radiograph and negative coronary artery disease workup. However, if the capability of using point‐of‐care transthoracic echocardiogram is not available during the emergency setting, then recommendations would be to directly pursue CT aortography to rule out aortic dissection, if aortic dissection detection risk score and D‐dimer return positive.
The use POCUS has been growing in the field of emergency and critical medicine to provide a fast and non‐invasive tool for the diagnosis of aortic dissection. Traditionally, physicians have relied on CT aortography to diagnose aortic dissection, but these modalities require considerable length of time, use of contrast material, and transportation of an unstable patient. Studies using POCUS have shown that the presence of aortic root dilation (sensitivity, 95%; specificity, 51%), intimal flap (sensitivity, 67%; specificity, 100%), and both aortic regurgitation and pericardial effusions (sensitivity, 37%; specificity, 94%) were helpful for bedside diagnosis of aortic dissection. 13 To date, however, no set criteria have been used for cutoff measurements. POCUS among emergency physicians had an 88% sensitivity in diagnosing Type A aortic dissection. When combined with aortic dissection detection risk, sensitivity improved to 94% with 98% specificity. 14 POCUS has also been demonstrated to significantly reduce the time of diagnosis of aortic dissections by >140 minutes. This study showed significant improvement in mortality without any incidence of misdiagnosis in the point‐of‐care transthoracic echocardiogram group compared to the non‐point‐of‐care transthoracic echocardiogram group. 15
A few case reports exist regarding complexity in managing patients with a concurrent diagnosis of acute aortic dissection and pulmonary embolism that are rare, but fatal, diseases. Tudoran et al.16 reported a patient diagnosed with both Type A aortic dissection and pulmonary embolism where intravenous heparin was administered with aggressive blood pressure management under close inpatient observation for at least 2 weeks. Patient was discharged with oral anticoagulation and repeat CT aortography was done a month later that showed stability of dissection. Oral anticoagulation was continued for 6 months before a surgical repair of Type A aortic dissection was scheduled. 16 A case report by Suárez‐Barrientos and Vilacosta17 described a patient with concurrent diagnoses of both ruptured Type B aortic dissection and pulmonary embolism where 1 month of intravenous heparin was given under close observation as an inpatient before being discharged with oral anticoagulation. A repeat CT aortography was done 3 months later that showed worsening intramural hematoma requiring indefinite cessation of anticoagulation therapy. 17 Another case report described acute pulmonary embolism diagnosed 2 days post‐operatively after open heart surgery for acute Type A aortic dissection repair. Embolectomy and thrombolytic therapy was not applied to the patient because of stable hemodynamics where intravenous heparin was started. CT pulmonary angiography was repeated every 2–3 days until improvement in the size of the pulmonary thromboembolism was appreciated radiographically. 18 There is currently no consensus in how to treat a patient diagnosed with both acute aortic dissection and pulmonary embolism other than starting anticoagulation under close supervision, aggressive blood pressure management, and stopping anticoagulation after a minimum of 3–6 months of therapy (or any signs of active bleeding are noted). The risk and benefits of fibrinolytic therapy have been acknowledged in previous case reports but was not administered due to concerns of causing worsening hematoma or bleeding. The utility of placing an inferior vena cava filter (IVC) was not described in previous case reports.
Acute Type A aortic dissection requires both medical and surgical intervention. Anti‐hypertensive such as beta‐blocker is essential in reducing the shear stress exerted on the aortic wall to decrease the tendency for dissection to propagate and minimizing cardiac contraction to reduce myocardial ischemia. Mortality is 50% within 48 hours without surgery, and even with surgery, mortality remains high (9%–25%). The goal of surgery is to prevent aortic rupture and minimize aortic regurgitation. 3 If aortic rupture has occurred, mortality reaches 100%, regardless of surgical intervention.
4. CONCLUSION
Acute Type A aortic dissection is a rare detrimental disease that can present with little to no symptoms. It is associated with a high mortality rate regardless of medical and surgical intervention. CT aortography is the imaging of choice because of its reliability in the emergency settings. This article highlights the importance of clinical, laboratory, and radiologic workups including point‐of‐care transthoracic echocardiogram for diagnosing Type A aortic dissection.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Chong WH, Saha BK, Wang C, Beegle S. Type A aortic dissection mimicking saddle pulmonary embolism on CT imaging. JACEP Open 2020;1:132–136. 10.1002/emp2.12026
Supervising editor: Michael Blaivas, MD, MBA.
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1. Johansson G, Markström U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg. 1995;21(6):985‐988. [DOI] [PubMed] [Google Scholar]
- 2. Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112(24):3802‐3813. [DOI] [PubMed] [Google Scholar]
- 3. Fukui T. Management of acute aortic dissection and thoracic aortic rupture. J Intensive Care. 2018;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628‐635. [DOI] [PubMed] [Google Scholar]
- 5. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897‐903. [DOI] [PubMed] [Google Scholar]
- 6. Ryu JH, Pellikka PA, Froehling DA, Peters SG, Aughenbaugh GL. Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features and outcome. Respir Med. 2007;101(7):1537‐1542. [DOI] [PubMed] [Google Scholar]
- 7. Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: a 20‐year experience of collaborative clinical research. Circulation. 2018;137(17):1846‐1860. [DOI] [PubMed] [Google Scholar]
- 8. Hagl C, Ergin MA, Galla JD, et al. Delayed chronic type A dissection following CABG: implications for evolving techniques of revascularization. J Card Surg. 2000;15(5):362‐367. [DOI] [PubMed] [Google Scholar]
- 9. Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline‐based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123(20):2213‐2218. [DOI] [PubMed] [Google Scholar]
- 10. Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 11. Shimony A, Filion KB, Mottillo S, Dourian T, Eisenberg MJ. Meta‐analysis of usefulness of d‐dimer to diagnose acute aortic dissection. Am J Cardiol. 2011;107(8):1227‐1234. [DOI] [PubMed] [Google Scholar]
- 12. Nazerian P, Mueller C, Soeiro AM, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D‐dimer for acute aortic syndromes: the ADvISED Prospective Multicenter Study. Circulation. 2018;137(3):250‐258. [DOI] [PubMed] [Google Scholar]
- 13. Roudaut RP, Billes MA, Gosse P, et al. Accuracy of m‐mode and two‐dimensional echocardiography in the diagnosis of aortic dissection: an experience with 128 cases. Clin Cardiol. 1988;11(8):553‐562. [DOI] [PubMed] [Google Scholar]
- 14. Nazerian P, Vanni S, Castelli M, et al. Diagnostic performance of emergency transthoracic focus cardiac ultrasound in suspected acute type A aortic dissection. Intern Emerg Med. 2014;9(6):665‐670. [DOI] [PubMed] [Google Scholar]
- 15. Pare JR, Liu R, Moore CL, et al. Emergency physician focused cardiac ultrasound improves diagnosis of ascending aortic dissection. Am J Emerg Med. 2016;34(3):486‐492. [DOI] [PubMed] [Google Scholar]
- 16. Tudoran M, Tudoran C. High‐risk pulmonary embolism in a patient with acute dissecting aortic aneurysm. Niger J Clin Pract. 2016;19(6):831. [DOI] [PubMed] [Google Scholar]
- 17. Suárez‐Barrientos A, Vilacosta I. [Aortic intramural hematoma and pulmonary embolism. Diagnostic challenge and therapeutic dilemma]. Rev Esp Cardiol. 2011;64(12):1227‐1229. [DOI] [PubMed] [Google Scholar]
- 18. Yang WJ, Duan QJ, Cheng HF, Dong AQ. A case study of pulmonary embolism from the right atrial shunt after acute type a aortic dissection surgery. J Cardiothorac Surg. 2014;9(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]


