Abstract
Background
Evaluate an indication‐based clinical decision support tool to improve antibiotic prescribing in the emergency department.
Methods
Encounters where an antibiotic was prescribed between January 2015 and October 2017 were analyzed before and after the introduction of a clinical decision support tool to improve clinicians’ selection of a guideline‐approved antibiotic based on clinical indication. Evaluation was conducted on a pre‐defined subset of conditions that included skin and soft tissue infections, respiratory infections, and urinary infections. The primary outcome was ordering of a guideline‐approved antibiotic prescription at the drug and duration of therapy level. A mixed model following a binomial distribution with a logit link was used to model the difference in proportions of guideline‐approved prescriptions before and after the intervention.
Results
For conditions evaluated, selection rate of a guideline‐approved antibiotic for a given indication improved from 67.1% to 72.2% (P < 0.001). When duration of therapy is included as a criterion, selection of a guideline‐approved antibiotic was lower and improved from 24.7% to 31.4% (P < 0.001), highlighting that duration of therapy is often missing at the time of prescribing. The most substantial improvements were seen for pneumonia and pyelonephritis with an increase from 87.9% to 97.5% and 62.8% to 82.6%, respectively. Other significant improvements were seen for abscess, cellulitis, and urinary tract infections.
Conclusion
Antibiotic prescribing can be improved both at the drug and duration of therapy level using a non‐interruptive and indication based‐clinical decision support approach. Future research and quality improvement efforts are needed to incorporate duration of therapy guidelines into the antibiotic prescribing process.
1. INTRODUCTION
1.1. Background
Emergency physicians are among the top 5 prescribers of antibiotics with an estimated 14.7 million antibiotic prescriptions written each year. 1 Antibiotic therapy is often the first‐line treatment for many types of common infections with emergency departments (ED) being where patients first seek care. 2 , 3 Unfortunately, current estimates indicate that upward of 47 million antibiotics prescribed each year are either unnecessary or inappropriate. 4 , 5 , 6 , 7 , 8 , 9 , 10 In addition, antibiotics are one of the most costly drug classes, exceeding chemotherapeutic agents and blood clotting modifiers, 11 with the United States spending $10 billion annually, including $6.5 billion in the ambulatory setting. 12 , 13 The Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) have recognized antibiotic resistance as one of three global threats to human health. 14 , 15
Antibiotic stewardship programs have used a multi‐faceted approach, including both restrictive and enablement strategies, with improvement in infection treatment, reduced adverse events, 16 , 17 improved patient safety, reduced treatment failure, reduced rates of antibiotic resistance, 18 , 19 and cost savings for hospitals. 16 , 20 , 21 , 22 , 23 A 2017 Cochrane review evaluated 221 studies using antibiotic stewardship program interventions to improve prescribing practices for hospital in patients and concluded these interventions are effective in decreasing antibiotic treatment duration and increasing compliance with antibiotic guidelines/policy. 16 Additionally, interventions directed to physicians decreased patient length of stay. The use of both enablement and restriction interventions were independently associated with improvements in interrupted time series studies with enablement consistently increasing the effect of the interventions. 16
1.2. Importance
Innovative use of health information technology (HIT) has been recognized as one of the cornerstones of antibiotic stewardship programs. 24 , 25 , 26 , 27 , 28 , 29 However, to date, many electronic health record systems fall short in their ability to provide real‐time antimicrobial recommendations targeted to a clinical indication or to incorporate guidelines into clinical workflow. Rather, interventions have taken the form of an alert, often met with implementation and adoption issues, and alert fatigue. 27 , 30 Prior research has shown drug alerts, even for severe allergic reactions, are overridden upward of 90% of the time. 31
In this study, we use an indication‐based prescribing approach that allows the clinician to access antibiotic recommendations based on current guidelines. Because the platform is non‐interruptive and optional, there are no alerts or overrides triggered by its use. Indication‐based prescribing is a new paradigm of thinking about how we prescribe medications, and current research suggests that indication‐based prescribing can, in fact, be safer and more efficient. 32 , 33 Efforts to include indication when prescribing a medication have received support by the National Coordinating Council on Medication Errors Reporting and Prevention 34 and the National Association of Boards of Pharmacy. 35 However, to the best of our knowledge, an indication‐based approach to prescribing antibiotics in the ED has not been studied.
The Bottom Line
Antibiotic prescribing is ubiquitous in emergency departments, but keeping up with local antibiotic recommendations can be difficult. This study demonstrated improved selection of guideline‐approved antibiotics after implementing an indication‐based clinical decision support tool.
1.3. Goals of this investigation
The goal of this study is to evaluate an indication‐based antibiotic prescribing platform in the ED and measure the improvement in antibiotic selections made by clinicians before and after its implementation, considering both the drug selection and duration of therapy.
2. MATERIALS AND METHODS
2.1. Study design and setting
This study took place at the University of Colorado Hospital in Aurora, Colorado, a level 1 trauma center and tertiary academic teaching hospital with ≈149,275 ED visits annually. The facility is staffed by board‐certified emergency physicians, residents in their post graduate training years (1–4), and advanced practice providers (certified physician assistants or nurse practitioners). The Colorado Multiple Institutional Review Board approved this study.
2.2. Selection of participants
Adult patients (>18 years) were included in this study. Eligible visits included patient encounters between February 1, 2015 and January 31, 2017 where a clinician prescribed an antibiotic. A subset of common infections was selected for our evaluation that included skin and soft tissue infections, respiratory infections, and urinary infections. Each type of infection was mapped to its corresponding ICD‐10 diagnosis codes using the clinical classification groupers (CCS) accessible from the Agency for Health Research and Quality. 36 Encounters in which multiple infectious diagnoses existed during the encounter were excluded from subsequent analyses.
2.3. Intervention
The clinical decision support intervention implemented was a software application, SwiftRx (Denver, CO), embedded in our institution's electronic health record (Epic Systems, Verona, WI). The platform uses an indication‐based prescribing approach to assist clinicians with selection of an antibiotic based on the diagnosis they enter. For example, if a clinician enters the diagnosis of pneumonia, the platform will use the ICD‐10 code recorded in the electronic health record for pneumonia and generate a prioritized list of guideline‐based recommendations based on our local antibiogram for the clinician to select from. Recommendations were provided by the hospital's department of infection prevention and antimicrobial stewardship team that reviews the current literature and hospital recommendations, and consensus is reached during a committee review.
Clinicians can view the recommendations in one of two ways. In the first, clinicians click on an activity button located in the left rail of their screen that on selection, opens the platform and displays recommendations with the ability to place orders from the list of recommendations directly into the electronic health record. In the second, users access the activity in their discharge workflow where they have a button (a hyperlink) that resides directly above the orders activity (Figures 1A and 1B). Clicking on this button takes the user into the clinical decision support platform where they can order an antibiotic. The application is loaded directly within the user's screen, limiting the number of clicks and screens the clinician must navigate through. Once the order is accepted, the clinician is brought back to the discharge screen where they can sign the order and complete the patient's discharge instructions.
FIGURE 1.
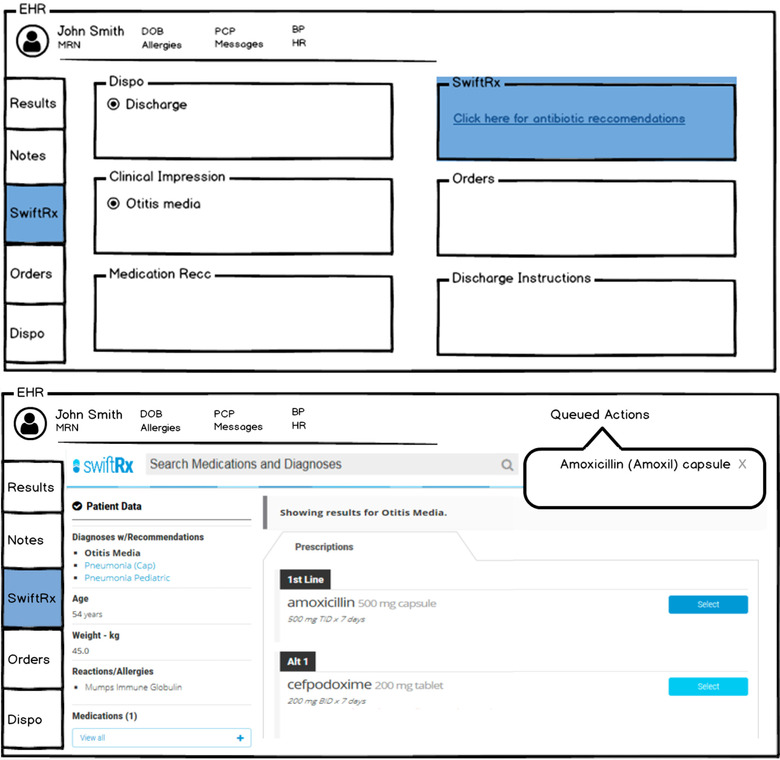
(A) Accessibility of the clinical decision support intervention (SwiftRx) from within activity tab in the patient's chart and in the disposition screen. (B) View of clinical decision support intervention (SwiftRx) during ordering process with options for user to select a recommended antibiotic or an acceptable alternative
In contrast to the typical electronic health record prescription workflow (ie, where an antibiotic is selected followed by entering dose, duration, dispense amount, refills, and indication), with the clinical decision support intervention, after an indication is entered (eg, pneumonia) and recommendation selected (eg, doxycycline), the indication, dose, duration, dispense amount, and any refills are all pre‐populated, saving the clinician time and additional clicks. Training for attendings, advanced practice providers, and residents was performed during a faculty meeting and a weekly morbidity and mortality conference by the medical director and faculty champions. The training presentation lasted ≈15 minutes with time allotted for any follow‐up questions on September 15, 2016. Use of the tool was made optional and available to clinicians for use within the ED only.
2.4. Outcome measures
The primary outcome measures for this study was clinicians’ adherence to our institution's treatment guidelines for a given infection in alignment with our local antibiogram. Stratifying by diagnosis, we analyzed the number of antibiotic prescriptions ordered and classified selections into a guideline‐approved or unapproved selection for each encounter (ie, according to the department of infection prevention and antimicrobial stewardship recommendations). Approved was defined as the optimal/first‐line agent or an acceptable‐alternative. Unapproved was defined as a suboptimal selection that should not be used for the considered infection (eg, first‐line should have been selected). For example, when the clinician enters their diagnosis of pneumonia, the platform recommends doxycycline as the optimal/first‐line agent and azithromycin as an acceptive alternative. Other acceptable alternatives include Augmentin XR and Levofloxacin (in cases of severe beta lactam allergy). Two analysis were then conducted with the first evaluating the selection of the antibiotic and the second considering if the selection and the duration of therapy were correct. We used the CDC days of therapy metric 37 to assess how often the duration of therapy was correct (eg, 7 days for pneumonia, 5 days for cellulitis). Days of therapy was not used as a confounder in our analyses.
2.5. Primary data analysis
Antibiotic selection choices were analyzed according to our classification schema above. Data fields extracted from the electronic health record during this time included encounter ID, patient ID, generic medication name, medication ID, medication dose prescribed, quantity, date of prescription, primary diagnosis (ICD‐10 code), and allergies. Selections were evaluated both on choice of the antibiotic and days of therapy. To meet the criteria for a given selection, the prescription order must meet all medication and days of therapy requirements. If any of the requirements were not met, the prescription would be considered unapproved. If a medication did meet all requirements for a recommendation line, it was considered approved.
A pre‐/post‐variable was the primary predictor of interest and was created by determining whether the encounter occurred before or after October 4, 2016. The intervention period included a pre‐period of 9 months that was selected to establish a baseline before the intervention date. The intervention date was based on contractual agreements between the health system and vendor, and the post‐period was selected based on data available to the study group at the time of analysis. A mixed‐model following a binomial distribution with a logit link was used to model the difference in proportions of approved prescriptions pre‐ and post‐ the clinical decision support intervention. Physician correlation was adjusted for using an exchangeable covariance structure within the mixed model. To account for within‐provider correlation, a logistic regression mixed‐model with a random intercept was used to model the difference in proportions of approved prescriptions pre‐ and post‐ the clinical decision support intervention. All analyses were conducted using SAS version 9.4 and R version 3.5.2.
3. RESULTS
Over the study period, there were 53,266 eligible encounters where an antibiotic was prescribed. Among these, 12,016 included a diagnosis that was an exact match to one of the pre‐specified infection types (eg, skin and soft tissue infections, respiratory infections, or urinary infection). A total of 472 encounters were excluded as they contained multiple diagnoses (eg, urinary tract infection, suicidal ideations, gastritis) leaving 11,544 eligible encounters available for evaluation (Figure 2). Conditions by infection type are shown in Table 1 with urinary tract infections and cellulitis being the most frequent. We found that prior to the clinical decision support intervention, a guideline‐approved antibiotic was selected 67.1% of the time (Table 2). After the introduction of our clinical decision support intervention, approved selections rose to 72.2% (P < 0.001). When days of therapy were included as a criterion, guideline‐approved antibiotic selection was 24.7%. After introduction of the clinical decision support intervention, approved selections increased to 31.4% (P < 0.001).
FIGURE 2.
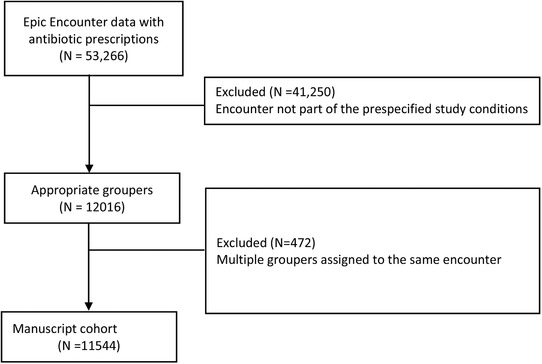
Enrollment flow diagram showing eligible encounters and manuscript cohort
TABLE 1.
Frequency of conditions evaluated in study cohort
| Infection type | Condition | Pre‐intervention (n, %) | Post‐intervention (n, %) |
|---|---|---|---|
| Skin and soft tissue infections | Abscess | 774 (11.1) | 587 (12.0) |
| Cellulitis | 1468 (21.1) | 1096 (22.4) | |
| Impetigo | 33 (0.5) | 19 (0.4) | |
| Periorbital cellulitis | 42 (0.6) | 50 (1.0) | |
| Respiratory infections |
Bronchitis Influenza |
61 (0.9) 8 (0.1) |
24 (0.5) 8 (0.2) |
| Mastoiditis | 2 (0.0) | 3 (0.1) | |
| Otitis media | 309 (4.4) | 206 (4.5) | |
| Peritonsillar abscess | 60 (0.9) | 55 (1.2) | |
| Pharyngitis | 433 (6.2) | 304 (6.6) | |
| Pneumonia | 420 (6.0) | 285 (6.2) | |
| Sinusitis | 232 (3.3) | 111 (2.4) | |
| Tonsillitis | 42 (0.6) | 30 (0.6) | |
| Upper respiratory infection | 221 (3.2) | 141 (3.1) | |
| Urinary infections | Pyelonephritis | 911 (13.1) | 501 (10.9) |
| Urethritis | 62 (0.9) | 37 (0.8) | |
| Urinary tract infection | 1874 (27.0) | 1135(24.7) | |
| Total | 6952 | 4592 |
TABLE 2.
Estimated population means and confidence intervals based on duration of therapy as a classification criterion
| Duration as criteriona | Duration not a criterion | |||||
|---|---|---|---|---|---|---|
| Mean (%) (CI) | Difference (%) | P value* | Mean (%) (CI) | Difference (%) | P value* | |
| Pre‐ | 24.7 (0.22, 0.27) | 6.7 | <0.001 | 67.1 (0.65, 0.69) | 5.1 | <0.001 |
| Post‐ | 31.4 (0.28, 0.34) | 72.2 (0.70, 0.74) | ||||
Duration as a criterion implies the correct antibiotic and days of therapy were selected by the clinician for a given indication. Duration not as a criterion requires only that the correct antibiotic was selected and not the days of therapy.
P values using a Wald test.
Conditions with the largest percent improvements after the clinical decision support intervention at the drug and days of therapy level included pyelonephritis (23.1% to 46.8%), abscess (8.3% to 27.6%), and cellulitis (8.1% to 15.6%). When only the appropriate drug selection is considered, the largest improvements were seen for the indication of pneumonia (87.9% to 97.5%), pyelonephritis (62.8% to 82.6%), urinary tract infection (75.4% to 82.8%), and abscess (82.0% to 88.9%) (P < 0.001). These findings are summarized in Table 3. Our results indicate that although clinicians often select a guideline‐appropriate antibiotic, they often miss entering the correct duration of therapy.
TABLE 3.
Condition‐specific mixed‐model estimates based on classification criterion
| Conditionb | Duration as criteriona | Duration not a criterion | |||||
|---|---|---|---|---|---|---|---|
| Mean (%) (CI) | Difference (%) | P value* | Mean (%) (CI) | Difference (%) | P value* | ||
| Abscess | Pre‐ | 8.4 (5.3, 13.0) | 19.31 | <0.001 | 82.0 (77.4, 85.4) | 6.91 | 0.001 |
| Post‐ | 27.7 (20.1, 36.9) | 89.0 (85.9, 91.8) | |||||
| Cellulitis | Pre‐ | 8.05 (5.7, 11.2) | 7.58 | <0.001 | 84.1 (81.4, 86.4) | 2.84 | 0.099 |
| Post‐ | 15.6 (12.1, 20.0) | 86.9 (84.1, 89.2) | |||||
| Pneumonia | Pre‐ | 67.3 (61.7, 72.5) | 12.29 | 0.005 | 87.9 (84.2, 91.0) | 9.68 | 0.001 |
| Post‐ | 79.6 (73.3, 84.8) | 97.5 (95.1, 98.8) | |||||
| Otitis media | Pre‐ | 49.9 (42.5, 58.0) | 2.90 | 0.66 | 62.4 (54.3, 69.8) | 4.63 | 0.39 |
| Post‐ | 52.8 (41.8, 62.9) | 67.0 (57.9, 75.0) | |||||
| Tonsillitis | Pre‐ | 16.7 (8.1, 30.8) | 0.64 | 0.94 | 43.8 (28.4, 61.0) | 2.90 | 0.81 |
| Post‐ | 17.3 (8.2, 33.3) | 46.7 (29.0, 65.3) | |||||
| Urinary tract infection | Pre‐ | 41.2 (36.9, 45.3) | −0.18 | 0.95 | 75.4 (72.2, 78.3) | 7.48 | <0.001 |
| Post‐ | 41.0 (37.2, 45.3) | 82.9 (80.1, 85.3) | |||||
| Pyelonephritis | Pre‐ | 23.1 (18.9, 27.9) | 23.68 | <0.001 | 62.8 (57.6, 68.7) | 19.76 | <0.001 |
| Post‐ | 46.8 (38.8, 54.9) | 82.6 (77.1, 87.0) | |||||
Duration as a criterion implies the correct antibiotic and days of therapy were selected by the clinician for a given indication. Duration not as a criterion requires only that the correct antibiotic was selected and not the days of therapy.
Conditions evaluated before and after the clinical decision support intervention, with means, percent difference, and statistical significance reported.
P values using a Wald test.
4. LIMITATIONS
Our study was limited by several factors. Specifically, the post‐intervention group (3 months) was a smaller sample than the pre‐intervention group (9 months). This smaller sample may not only increase variability but could also be subject to seasonal variations that are not accounted for because of the restricted date range. The lack of a suitable control group restricted the ability to do more traditional inferential statistics on this specific analysis. This, in part, was because of the lack of independence between the two groups (the same clinicians are in both pre‐ and post‐intervention groups as well as the lack of complete pairing of subjects pre‐ and post‐intervention). In other words, it was not guaranteed that a clinician who made a prescription decision in the pre‐intervention group was also included in the post‐intervention group (and vice versa). Another limitation is that our study only evaluated a subset of infectious conditions and did not account the presence of multiple overlapping infections. Future studies will be needed to evaluate if an indication‐based prescribing approach will be generalizable to a larger number of infectious conditions or when multiple infections are present during the encounter (eg, urinary tract infection, pneumonia) that may influence antibiotic selection. Last, our analysis did not consider existing contraindications or comorbidities that could limit prescribing choices through our intervention.
5. DISCUSSION
We evaluated 11,544 eligible encounters where an antibiotic was prescribed for a urinary, respiratory or skin and soft tissue infection and found that an indication‐based clinical decision support approach can improve the selection of an appropriate antibiotic (P < 0.001). The most substantial improvements were seen for pneumonia and pyelonephritis with an increase from 87.9% to 97.5% and 62.8% to 82.6%, respectively. Other significant improvements were seen for abscess, cellulitis, and urinary tract infections. When days of therapy was included as an additional criterion, selection of an approved antibiotic for a given indication was markedly lower but improved after the introduction of the clinical decision support intervention (P < 0.001). Our findings highlight a gap in antibiotic prescribing where clinicians often fail to enter the correct days of therapy for a given infection, an important factor to reducing antibiotic resistance and maintaining stewardship.
Prior studies on acceptance of clinical decision support recommendations to improve antibiotic prescribing have seen mixed and variable results. In a study by Butler et al, an alert implemented in the electronic health record to trigger appropriate antibiotic treatment from an order‐set for Clostridium difficile increased use of the order‐set but did not change guideline compliance. 38 Davis et al evaluated the use of clinical decision support to improve guideline adherence for the treatment of upper respiratory infections, urticaria, and constipation and found marginal increases in the control group (39% to 40%) and intervention group (38% to 42%), respectively. 39 Buising et al found an increase in antibiotic guideline compliance following introduction of their clinical decision support intervention (65% to 85% in the intervention group, P = 0.05). 40 In a meta‐analysis by Curtis et al, antibiotic prescribing with clinical decision support improved from 15% to 85% in the non‐interventional group to 32% to 92% in the interventional group. 40 Chow et al found a marked increase in adoption of their intervention after removing the ability of the user to bypass or close the application (ie, a hard‐stop intervention) with an increase from 23% to 87% while maintaining an acceptance rate of recommendations was 67%. 41 In contrast, our study showed an improvement from 67% to 72% with optional use of the intervention and without the introduction of an alert or hard‐stop, highlighting the value of clinical decision support when designed around clinical workflows.
An important finding from our study was the lack of days of therapy being correctly entered for a given infection, a recommendation that may change annually depending on antibiotic resistance patterns and guidelines. One explanation is that antibiotic orders are often created in the electronic health record with a generic duration or frequency (7 or 14 days) and not based on indication. This frequency dates back to the Roman Emperor Constantine who decided a week contains 7 days. Although the application of Constantine units for antibiotic prescribing remains unknown, we do know it is not evidenced‐based. Current research has shown that shorter durations are equally efficacious to longer durations. A recent study by Walk‐Dickler et al found ten conditions in which shorter duration of therapy was equivalent to longer durations in prospective randomized controlled trials. 42 Another explanation is that clinicians may know the antibiotic that would be appropriate for a given infection but not know the frequency or appropriate duration. Having to seek out this information requires clinicians to go outside of their workflow, increasing their time on task, and reducing their efficiency. A study by Garabedian et al compared an indication‐based approach to an electronic health record vendor and the need to access outside references dropped from 58.8% to 28.8% (P < 0.001). Similarly, the number of clicks required using an indication‐based approach was significantly less (18.39 vs 46.50; P < 0.001). 43 Last, clinicians may be extending the duration of an existing antibiotic that the patient is currently taking. However, encounters that represent this scenario we believe are likely to be few and not representative of the overall prescribing patterns of our cohort.
Standardizing treatment decisions using clinical indication has the potential to improve not only quality of care but patient safety. Schiff et al found that clinical decision support systems that use an indication‐based prescribing approach required fewer pharmacy interventions to correct the clinician's prescription (5%) compared to those using a standard electronic health record order entry process (15%–40%). 44 To standardize antibiotic ordering, many health systems use order sets; however, these lack the breadth to encompass the large number of infectious conditions typically seen in the ED and are usually focused on key quality and process measures (eg, stroke, sepsis, acute coronary syndrome). In our study, we found improvements in adherence to guideline recommendations and while some improvements were not statistically significant, one could contend that they were clinically significant as patients were getting the appropriate antibiotic more often after the intervention, potentially limiting treatment failures, adverse events, or readmissions. Despite modest overall improvements (5.1% without days of therapy, 6.7% with days of therapy), we believe there is value to improving clinician's adherence to guidelines for a specific condition and in some instances, the improvement was considerable (>20%).
Last, there is a cost associated with medication errors that now exceeds $40 billion each year. 45 Although many recognize the value of clinical decision support to improve appropriate prescribing, 46 few clinical decision support implementations to date have fully delivered on the promise to improve healthcare processes and outcome. 47 Those that have, have struggled to be widely scaled. 48 To date, there are no payment polices that support clinical decision support implementation, but as more organizations take on risk in the new value‐based care environment, these tools may generate more interest. To improve health outcomes, decrease avoidable costs, and improve antibiotic prescribing behavior, future clinical decision support solutions will need to be highly focused on user interface design, mature beyond the order set, consider both drug and indication, and be aligned with the prescriber's clinical workflow.
6. CONCLUSION
Antibiotic prescribing can be improved both at the drug and duration of therapy level using a non‐interruptive and indication‐based clinical decision support approach. Future research and quality improvement efforts are needed to incorporate duration of therapy guidelines into the antibiotic prescribing process.
CONFLICT OF INTEREST
FRG has been funded by the Agency for Healthcare Research and Quality during this study period. During the study period, he received no funding or equity from the company RxREVU (SwiftRx), which he now serves as a consultant and receives equity compensation. KB also received no funding or equity from the company RxREVU (SwiftRx) during the study period but now receives equity compensation. FRG and KB's financial interests have been reviewed by University of Colorado Hospital and University of Colorado School of Medicine in accordance with their institutional policies. No other disclosures were reported.
AUTHOR CONTRIBUTIONS
FRG, KB, and JW conceived of the study, collected the data and wrote the paper. MK and KW conducted the statistical analysis. All authors contributed substantially to its revision. FRG and JW take responsibility for the paper as a whole.
ACKNOWLEDGMENTS
We would like to thank the UCHealth Care Innovation Center and the Center for Innovative Design and Analysis for their support in this study. This work was funded in part by a local grant by the Colorado Office of Economic Development and International Trade ‐ Advanced Industries.
Biography
Foster R. Goss, DO, MMSc is an Associate Professor of Emergency Medicine at University of Colorado School of Medicine.

Goss FR, Bookman K, Baron M, et al. Improved antibiotic prescribing using indication‐based clinical decision support in the emergency department. JACEP Open 2020;1:214–221. 10.1002/emp2.12029
[Correction added on 17 March 2020, after first online publication: “department” has been added in the title of the article.]
Supervising Editor: Faheem W. Guirgis, MD.
REFERENCES
- 1. Centers for Disease Control and Prevention . Outpatient Antibiotic Prescriptions ‐ United States. https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2016.html. Published 2016. Accessed August 22, 2019.
- 2. Hersh AL, Fleming‐Dutra KE, Shapiro DJ, Hyun DY, Hicks LA. Frequency of first‐line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176(12):1870‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic‐associated adverse events. Clin Infect Dis. 2008;47(6):735‐743. [DOI] [PubMed] [Google Scholar]
- 4. Camins BC, King MD, Wells JB, et al. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(10):931‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ingram P, Seet J, Budgeon C, Murray R. Point‐prevalence study of inappropriate antibiotic use at a tertiary Australian hospital. Intern Med J. 2012;42(6):719‐721. [DOI] [PubMed] [Google Scholar]
- 6. Levin PD, Idrees S, Sprung CL, et al. Antimicrobial use in the ICU: indications and accuracy—an observational trial. J Hosp Med. 2012;7(9):672‐678. [DOI] [PubMed] [Google Scholar]
- 7. Dellit TH, Owens RC, McGowan JE, Jr. , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159‐177. [DOI] [PubMed] [Google Scholar]
- 8. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194‐200. [PMC free article] [PubMed] [Google Scholar]
- 9. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972‐978. [DOI] [PubMed] [Google Scholar]
- 10. Fleming‐Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864‐1873. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman JM, Doloresco F, Vermeulen LC, et al. Projecting future drug expenditures—2010. Am J Health‐Syst Pharm. 2010;67:919‐928. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Fast Facts: Antibiotic Use and Resistance. https://www.cdc.gov/getsmart/community/about/fast-facts.html. Accessed Nov 4, 2019.
- 13. Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger LH. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68(3):715‐718. [DOI] [PubMed] [Google Scholar]
- 14. So A, Furlong M, Heddini A. Globalisation and antibiotic resistance. BMJ. 2010;341:c5116. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Antimicrobial resistance. http://www.who.int/mediacentre/factsheets/fs194/en/. Published 2016. Accessed November 4, 2019.
- 16. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital's antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145‐148. [DOI] [PubMed] [Google Scholar]
- 18. DiazGranados CA. Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes. Am J Infect Control. 2012;40(6):526‐529. [DOI] [PubMed] [Google Scholar]
- 19. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad‐spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(04):354‐361. [DOI] [PubMed] [Google Scholar]
- 20. Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10(1):63‐73. [DOI] [PubMed] [Google Scholar]
- 21. Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial‐resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49(8):1175‐1184. [DOI] [PubMed] [Google Scholar]
- 22. Sick AC, Lehmann CU, Tamma PD, Lee CK, Agwu AL. Sustained savings from a longitudinal cost analysis of an internet‐based preapproval antimicrobial stewardship program. Infect Control Hosp Epidemiol. 2013;34(6):573‐580. [DOI] [PubMed] [Google Scholar]
- 23. Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7‐year program. Infect Control Hosp Epidemiol. 2012;33(4):338‐345. [DOI] [PubMed] [Google Scholar]
- 24. Agwu AL, Lee CK, Jain SK, et al. A World Wide Web‐based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis. 2008;47(6):747‐753. [DOI] [PubMed] [Google Scholar]
- 25. Amadeo B, Dumartin C, Parneix P, Fourrier‐Réglat A, Rogues A‐M. Relationship between antibiotic consumption and antibiotic policy: an adjusted analysis in the French healthcare system. J Antimicrob Chemother. 2011;66(2):434‐442. [DOI] [PubMed] [Google Scholar]
- 26. Pestotnik SL. Expert clinical decision support systems to enhance antimicrobial stewardship programs: insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2005;25(8):1116‐1125. [DOI] [PubMed] [Google Scholar]
- 27. Kullar R, Goff DA, Schulz LT, Fox BC, Rose WE. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis. 2013;57(7):1005‐1013. [DOI] [PubMed] [Google Scholar]
- 28. Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis. 2014;58(3):401‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans RS, Pestotnik SL, Classen DC, et al. A computer‐assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232‐238. [DOI] [PubMed] [Google Scholar]
- 30. Carspecken CW, Sharek PJ, Longhurst C, Pageler NM. A clinical case of electronic health record drug alert fatigue: consequences for patient outcome. Pediatrics. 2013;131(6):e1970‐1973. [DOI] [PubMed] [Google Scholar]
- 31. Topaz M, Seger DL, Slight SP, et al. Rising drug allergy alert overrides in electronic health records: an observational retrospective study of a decade of experience. J Am Med Inform Assoc. 2016;23(3):601‐608. [DOI] [PubMed] [Google Scholar]
- 32. Kron K, Myers S, Volk L, et al. Incorporating medication indications into the prescribing process. Am J Health Syst Pharm. 2018;75(11):774‐783. [DOI] [PubMed] [Google Scholar]
- 33. McEvoy GK. Bringing medication prescribing out of the dark: time for full disclosure. Am J Health Syst Pharm. 2018;75(11):739‐740. [DOI] [PubMed] [Google Scholar]
- 34. National Coordinating Council on Medication Errors Reporting and Prevention. https://www.nccmerp.org/. Accessed November 4, 2019.
- 35. National Association of Boards of Pharmacy. https://nabp.pharmacy/. Accessed November 4, 2019.
- 36. Agency for Healthcare Research and Quality . Clinical Classifications Software (CCS) https://www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp. Accessed June 24, 2018.
- 37. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Revolinski S. Implementation of a clinical decision support alert for the management of Clostridium difficile infection. Antibiotics (Basel). 2015;4(4):667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davis RL, Wright J, Chalmers F, et al. A cluster randomized clinical trial to improve prescribing patterns in ambulatory pediatrics. PLoS Clin Trials. 2007;2(5):e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buising K, Thursky K, Robertson M, et al. Electronic antibiotic stewardship—reduced consumption of broad‐spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J Antimicrob Chemother. 2008;62(3):608‐616. [DOI] [PubMed] [Google Scholar]
- 41. Chow AL, Ang A, Chow CZ, et al. Implementation hurdles of an interactive, integrated, point‐of‐care computerised decision support system for hospital antibiotic prescription. Int J Antimicrob Agents. 2016;47(2):132‐139. [DOI] [PubMed] [Google Scholar]
- 42. Wald‐Dickler N, Spellberg B. Short course antibiotic therapy—replacing constantine units with “shorter is better”. Clin Infect Dis. 2019;69(9):1476‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garabedian PM, Wright A, Newbury I, et al. Comparison of a prototype for indications‐based prescribing with 2 commercial prescribing systems. JAMA Netw Open. 2019;2(3):e191514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schiff G. Why Am I Taking This Drug? Incorporating Indications in CPOE. Paper presented at: HIMSS2018; Las Vegas, NV.
- 45. Tariq RA, Scherbak Y. Medication Errors. In: StatPearls [Internet]. StatPearls Publishing; 2019. [PubMed]
- 46. American Health Information Community Quality Workgroup Vision Summary. http://www.hhs.gov/healthit/ahic/materials/qual_vision_summary.pdf. Published 2007. Accessed January 5, 2019.
- 47. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742‐752. [DOI] [PubMed] [Google Scholar]
- 48. Sittig DF, Wright A, Osheroff JA, et al. Grand challenges in clinical decision support. J Biomed Inform. 2008;41(2):387‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]


