Abstract
Introduction
In this systematic review and meta‐analysis of propensity score‐matched cohort studies, we quantitatively summarize whether venoarterial extracorporeal membrane oxygenation (VA‐ECMO) used as extracorporeal cardiopulmonary resuscitation (ECPR), compared with conventional cardiopulmonary resuscitation (CCPR), is associated with improved rates of 30‐day and long‐term favorable neurological outcomes and survival in patients resuscitated from in‐ and out‐of‐hospital cardiac arrest.
Methods
We searched MEDLINE via PubMed, Embase, Scopus, and Google Scholar for eligible studies on January 14, 2019. All searches were limited to studies published between January 2000 and January 2019. Two investigators independently evaluated the quality (or certainty) of evidence according to GRADE guidelines. Pooled results are presented as relative risks (RRs) with 95% confidence intervals (CIs).
Results
Six cohort studies using propensity score‐matched analysis were included, totaling 1108 matched patients. Pooled analyses showed that ECPR was likely associated with improved 30‐day and long‐term favorable neurological outcome in adults compared to CCPR for in‐ and out‐of‐hospital cardiac arrest (RR = 2.02, 95% CI = 1.29–3.16; I2 = 20%, P = 0.002; very low‐quality evidence) and (RR = 2.86, 95% CI = 1.64–5.01; I2 = 0%, P = 0.0002; moderate‐quality evidence), respectively. When we analyzed in‐ and out‐of‐hospital cardiac arrest separately, ECPR was likely associated with improved 30‐day favorable neurological outcome compared to CCPR for in‐hospital cardiac arrest (RR = 2.18, 95% CI = 1.24–3.81; I2 = 9%, P = 0.006; very low‐quality evidence), but not for out‐of‐hospital cardiac arrest (RR = 2.61, 95% CI = 0.56–12.20; I2 = 59%, P = 0.22; very low‐quality evidence). ECPR was also likely associated with improved long‐term favorable neurological outcome compared to CCPR for in‐hospital cardiac arrest (RR = 2.50, 95% CI = 1.33–4.71; I2 = 0%, P = 0.005; moderate‐quality evidence) and out‐of‐hospital cardiac arrest (RR = 4.64, 95% CI = 1.41–15.25; I2 = 0%, P = 0.01; moderate‐quality evidence).
Conclusions
Our analysis suggests that VA‐ECMO used as ECPR may improve long‐term favorable neurological outcomes and survival when compared to the best standard of care in a selected patient population. Therefore, it is imperative for well‐designed randomized clinical trials to obtain a higher level of scientific evidence to ensure optimal outcomes for cardiac arrest patients.
Keywords: cardiopulmonary resuscitation, extracorporeal membrane oxygenation, extracorporeal life support, in‐hospital cardiac arrest, out‐of‐hospital cardiac arrest
1. INTRODUCTION
Despite significant advances in cardiac arrest resuscitation and post‐arrest care, the majority of in‐ and out‐of‐hospital post‐cardiac arrest patients will succumb to the sequelae of hypoxic‐ischemic brain injury or death before hospital discharge. 1 Cardiac arrest is a major adverse event and a leading cause of morbidity and mortality in the United States. 2 , 3 , 4 Recent progress in advanced perfusion/reperfusion strategies and early implementation of extracorporeal cardiopulmonary resuscitation (ECPR) for the management of refractory cardiac arrest has resulted in increased favorable outcomes. 5 , 6
ECPR was introduced in 1972 7 and was first suggested in 1976 as a therapeutic alternative for refractory cardiac arrest unresponsive to conventional cardiopulmonary resuscitation. 8 , 9 Since 1989, according to the Extracorporeal Life Support Organization registry, 8075 adults have been treated with ECPR, and survival to discharge rate after ECPR for cardiac arrest that was refractory to conventional treatment was 29%. 10 These outcomes in some ways are consistent with recent publications citing a 2‐ to 4‐fold (8%–15% to 30%–45%) increase in survival rates.
The current evidence we have on ECPR for cardiac arrest rests primarily in observational or registry studies with design limitations and potentially confounding selection bias. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 The results of these studies are, by their nature, open to dispute. Furthermore, the lack of randomized controlled studies has resulted in the low‐level recommendation for the use of venoarterial extracorporeal membrane oxygenation (VA‐ECMO) as ECPR for refractory cardiac arrest in current guidelines (Class IIb, LOE C‐LD). 31 , 32 However, the accumulated evidence for the initiation of ECPR has emerged as a salvage intervention in patients with cardiac arrest that is refractory to standard therapies and has introduced significant questions about its role and its potential to improve outcomes in these patients.
An unexplored outcome of this novel approach is long‐term functional and neurologic status following in‐ and out‐of‐hospital cardiac arrest. The primary aim of our study was to conduct a systematic review and meta‐analysis of propensity score‐matched cohort studies to identify whether ECPR, compared with conventional cardiopulmonary resuscitation, is associated with improved rates of 30‐day and long‐term favorable neurological outcomes and survival in patients resuscitated from in‐ and out‐of‐hospital refractory cardiac arrest. Identifying more effective treatments for cardiac arrest and patient‐important outcomes remains a high priority, particularly the role of ECPR for refractory cardiac arrest. 33
2. MATERIALS AND METHODS
Data reporting in this meta‐analysis is consistent with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 34 Our review protocol was drafted by the research team and revised as necessary, before it was registered in the PROSPERO registry of systematic reviews (CRD42020158758). The review question was formulated following the PICO scheme (populations/people/patient/problem, intervention(s), comparison, outcome). Our research question, according to this scheme, is as follows: Among adults resuscitated from in‐ and out‐of‐hospital cardiac arrest (P) and treated with ECPR (I), compared to conventional cardiopulmonary resuscitation (C), what are the rates of 30‐day and long‐term favorable neurological outcome and survival (O)? Because all the analyses were based on previously published studies, no informed consent or ethical approval were required.
2.1. Inclusion and exclusion criteria
All studies that were published in English as full‐text articles in indexed journals, and which used propensity score‐matched analysis as part of the study design and reported neurological outcomes in adults (≥18 years old) who were resuscitated from in‐ and out‐of‐hospital cardiac arrest and received ECPR, were considered for inclusion. Studies that included both in‐ and out‐of‐hospital cardiac arrest were considered if data could be extracted as well as computed separately and either subpopulation was >75% of the total. Publications such as letters, opinions, case reports, case series, review articles, meta‐analyses, and studies that reported insufficient data were excluded. Studies conducted on pediatric populations, pregnancy, presumed pregnancy, or patients with a pulse (eg, cardiogenic shock) were also excluded from the study. Two investigators independently evaluated the criteria for inclusion. Any disagreements regarding inclusion or exclusion were resolved via discussion or by the decision of a third independent investigator.
2.2. Search strategy and study selection
We conducted a comprehensive literature search using MEDLINE via PubMed, Embase, Scopus, and Google Scholar on January 14, 2019, followed by a supplementary search on March 25, 2019, to ascertain that no new literature was published in the interim. We used the PRESS (Peer Review of Electronic Search Strategies) checklist to develop the research strategy. 35 Keywords used in the search were based on the implemented PICO model, which was first defined for use in MEDLINE via PubMed and subsequently adapted for the other databases. All searches were limited to studies published between January 2000 and January 2019. The primary search strategy was in great part limited to English language publications, but key non‐English articles were reviewed at the discretion of the authors. The reference lists of relevant studies were screened to identify other studies of interest. We used EndNote to remove internal (within a database) and external (between databases) duplicates. To identify ongoing clinical trials, we searched the International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) that includes entries in http://ClinicalTrials.gov on March 25, 2019.
Medical subject headings (MeSH) were combined with non‐indexed relevant search terms to create a comprehensive search strategy. Our PubMed search strategy included: (((((((cardiac* OR heart) AND arrest*)) OR heart attack) OR cardiopulmonary arrest) OR (OHCA OR IHCA)) OR out‐of‐hospital cardiac arrest OR in‐hospital cardiac arrest OR out of hospital arrest OR in hospital arrest) OR ((refractory) AND ((ventricular arrhythmia) OR (((tachycardia) OR fibrillation) AND ventricular))))) AND ((((((extracorporeal oxygenation) OR ((“extra corp*”) AND (“membran* oxygenat*” OR “life support*”))) OR (ECPR OR E‐CPR) OR (ECMO OR E‐CMO OR ECLS OR E‐CLS)) OR ((((heart assist devices) OR resuscitation)) AND (((“extra corp*”)) AND ((((cardiopulmonary resuscitation) OR cardiopulm* AND resuscit*) OR cardio‐pulm* AND resuscit*) OR conventional cardiopulmonary resuscitation OR CCPR OR CPR))) AND ((“2000/01/01”[PDat]: “2019/01/14”[PDat]) AND Humans[Mesh] AND adult[MeSH]).
2.3. Data extraction and analysis
Two investigators independently assessed potentially eligible studies. Data extraction was performed by 2 investigators using a standardized Excel form (Microsoft, Redmond, WA). Disagreements between investigators were resolved via discussion or by the decision of a third independent investigator. Data were extracted for the unmatched groups, as well as for the propensity‐matched groups. Extracted information included: first author, year of publication, country, study design, enrollment period, location of arrest, follow‐up period, number of patients, age, sex, witnessed arrest, bystander cardiopulmonary resuscitation (CPR), initial rhythm, time from collapse to initiation of CPR, duration of CPR, recent acute myocardial infarction, reperfusion therapy, and use of ECPR. The primary outcome measures were 30‐day and long‐term favorable neurological outcomes. The secondary outcome measures were 30‐day and long‐term survival.
2.4. Grading
Two investigators independently assessed the methodological qualities of each study using a modified version of the Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies. 36 The Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies quality scores were based on sample selection, comparability between study groups, and assessment of outcome. The quality (or certainty) of evidence for outcomes was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria and each outcome was classified as having high, moderate, low, or very low quality of evidence. 37 , 38 We used the methods and recommendations described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions, employing GRADEpro GDT software. Inconsistencies across studies was graded as serious when heterogeneity was significant (P < 0.10 or I2 > 50%). Imprecision was graded as serious when either the lower or the upper bound of the confidence interval (CI) was <20% or >20% less or more than the point estimate. Two investigators performed the assessment independently, and any conflict or disagreement was resolved via discussion or by the decision of a third independent investigator.
2.5. Strategy for data synthesis
We combined studies using the Review Manager software version 5.3 (RevMan). 39 Dichotomous variables and pooled statistics were calculated as relative risks (RRs) with 95% CIs. Data were pooled using the Mantel‐Haenszel method. We used a random‐effect model for data synthesis and assessed heterogeneity using the Chi2 test and the I2 statistic. We considered a Chi2 test for heterogeneity with a P value of < 0.10 to be indicative of significant heterogeneity. We calculated the I2 statistic to describe the proportion of variability due to heterogeneity and considered statistical heterogeneity relevant with I2 statistic > 50%. 40 We did not include a funnel plot due to the inclusion of only 6 studies. We planned to perform the following subgroup analyses if sufficient data were available: Indications, time periods, and risk factors (age, sex, time on ECMO). A meta‐regression or subgroup analysis to examine the relationship between treatment effects and 1 or more study‐level characteristics was not conducted because few studies were available for inclusion.
3. RESULTS
3.1. Study selection
The initial search was conducted on MEDLINE via PubMed, Embase, and Scopus. The electronic searches of the databases yielded 3021 related articles. Six additional records were identified through forward search. Google Scholar was also used to provide increased access to gray literature, and reference lists of relevant papers were examined to identify additional studies. After duplicate removal, the titles and abstracts of the remaining records were screened for inclusion. Thirty‐four studies were considered for full‐text screening. Among these, 28 studies were excluded because they did not fulfill the inclusion criteria. Ultimately, 6 studies were deemed eligible for the systematic literature review. 11 , 12 , 13 , 14 , 15 , 16 Figure 1 shows the PRISMA flow diagram of the bibliographic search strategy and the results.
FIGURE 1.
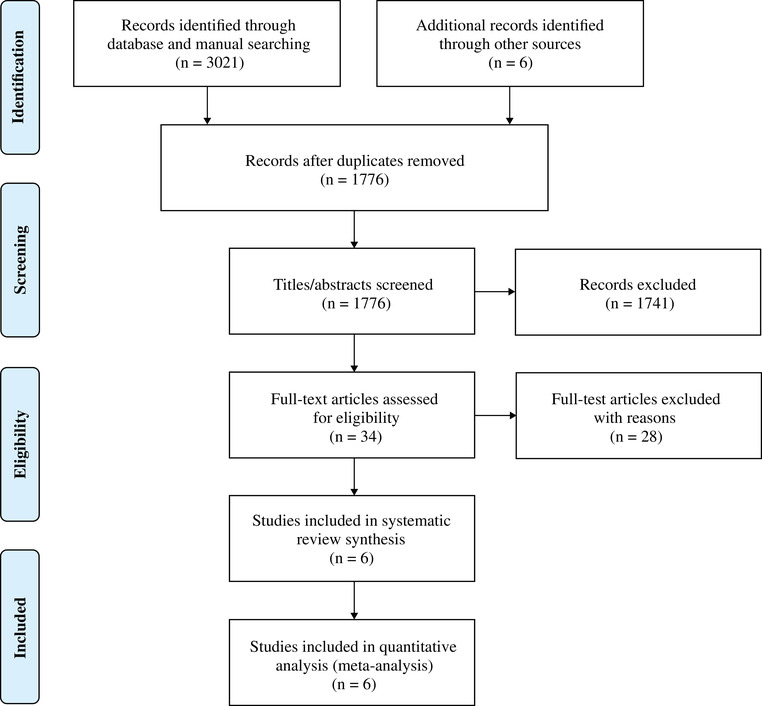
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) study flow diagram.
Notes: Adapted from Moher et al.34
Table 1 provides an overview of the studies selected for inclusion. All studies were observational (non‐randomized) and used propensity score‐matched analysis. For the purposes of this study, we did not identify any set of records describing a controlled clinical study design (randomized controlled trials and non‐randomized controlled trials). All records, identified through http://ClinicalTrials.gov, were indexed by the United States National Library of Medicine and were in progress (ie, had not been completed). We did not identify any specific study that assessed the cost‐effectiveness of ECPR for cardiac arrest, including implementation cost, device/equipment costs, hospital stay costs, costs of treating complications, and long‐term follow‐up after hospital discharge.
TABLE 1.
Baseline clinical characteristics and primary clinical endpoints results of ECPR compared to CCPR after propensity score‐matched analysis
| Author, year, country | Patient population | Primary endpoints | Criteria for ECLS allocation | Propensity score matching variables | Primary endpoint results of the propensity score‐matched analysis of ECPR and CCPR |
|---|---|---|---|---|---|
|
Blumenstein et al. 2016,11 Germany |
This was a retrospective single‐center cohort study that included 353 adult patients (≥18 years old) with witnessed IHCA. Fifty‐two patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | Evaluated survival at 30‐days, long‐term survival, and neurological outcomes. Neurological outcomes were evaluated using the Glasgow‐Pittsburgh CPC scale. Good neurological outcomes were defined as a CPC score of 1–2. | ECPR was considered if witnessed CA did not result in ROSC after undergoing CPR >10 minutes. An arrest was presumed to be of cardiac etiology unless it was known or likely to have been caused by trauma, drug overdose, or any other non‐cardiac cause. | Age, sex, LVEF, and all parameters revealed in the univariate analysis to be predictive of mortality. CPR duration was additionally adjusted during the matching process using the propensity score. | ECMO implantation was the only significant and independent predictor of mortality in multivariate Cox regression analysis (hazard ratio 0.57, 95% CI = 0.35–0.90; P = 0.02). Median follow‐up duration after discharge 1136 days (IQR 823–1416). Short‐term survival (27% vs 17%; P = 0.01), long‐term survival (23.1% vs 11.5%; P = 0.008), CPC of 1 or 2 (83.3% vs 66.7%; P = 0.77). |
|
Chen et al. 2008,12 Taiwan |
This was a retrospective single‐center cohort study that included 172 adult patients (≥18 to 75 years old) with witnessed IHCA of cardiac origin and CPR duration for >10 minutes. Fifty‐nine patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | Survival to hospital discharge, and analysis was by intention to treat. | The decision was made by the attending physician in charge. Exclusion for ECPR included failure to wean from bypass due to post‐cardiotomy shock and patients who experienced shock requiring elective ECPR. | Patient demographics, initial cardiac rhythm, time point of CPR, CPR duration, comorbidities among patients. | Survival to discharge (hazard ratio [HR] 0.51, 95% CI = 0.35–0.74; P < 0.0001), 30‐day survival (HR 0.47, 95% CI = 0.28–0.77; P = 0.003), 1‐year survival (HR 0.53, 95% CI = 0.33–0.83; P = 0.006). |
|
Shin et al. 2013, 13 South Korea |
This was a retrospective single‐center cohort study that included 406 adult patients (≥ 18 to 80 years old) with witnessed IHCA, CPR > 10 minutes. Eighty‐five patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | Survival at two‐years and neurological outcomes. Neurological outcome was defined by the Modified Glasgow Outcome Score. Minimal neurological impairment was defined as a MGOS ≥ 4. Two‐year follow‐up was checked in all survivors. | ECPR was performed according to the discretion of the CPR team leader. ECMO was considered mostly in cases of prolonged arrest and no ROSC within 10–15 minutes after initiation of CPR, when ROSC could not be maintained due to recurrent arrest, or when the recovery without ECMO support was unlikely due to known severe left ventricular dysfunction or coronary artery disease. | Pre‐CPR characteristics and CPR variables. Initial rhythms and study period were exactly matched, and CPR duration was additionally adjusted during the matching process. | In the ECPR group, the independent predictors associated with minimal neurological impairment were age ≤ 65 years (hazard ratio [HR] 0.46; 95% CI = 0.26–0.81; P = 0.008), CPR duration ≤ 35 minutes (HR 0.37; 95% CI = 0.18–0.76; P = 0.007), and subsequent cardiovascular intervention including coronary intervention or cardiac surgery (HR 0.36; 95% CI = 0.18–0.68; P = 0.002). Survival at 2‐year with minimal neurological impairment (20.0% vs 5.0%, HR 0.53, 95% CI = 0.36–0.80; P = 0.002). |
|
Choi et al. 2016,14 South Korea |
This was a retrospective multi‐center cohort study that included 36547 adult patients (≥ 18 years old) with OHCA and presumed cardiac etiology resuscitated by EMS. Data were assessed from the cardiovascular disease surveillance (CAVAS a ) database. Three hundred and twenty patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | Neurologically intact survival to discharge. Neurological outcomes were evaluated using the Glasgow‐Pittsburgh CPC scale. Good neurological outcomes were defined as a CPC score of 1–2. | The decision regarding ECPR implementation depended on the discretion of the attending physicians. | Utstein style guideline defined covariates. Additionally, the following covariables: level of ED (levels 1–3) to adjust for ED performance, community urbanization (metropolitan or not) to adjust for geographical variations in community performance, and resources. | In all of the PSM cohort, there was no statistically significant difference between the ECPR and the CCPR group for neurologically intact survival (OR 1.58, 95% CI = 0.87–2.88) and survival to discharge (OR 1.12, 95% CI = 0.74–1.69). After adjusting for post‐ECPR covariates including reperfusion therapy and TH, there was no statistically significant difference in neurologically intact survival to discharge between the 2 groups (adjusted OR 0.94, 95% CI: 0.41–2.14). |
|
Kim et al. 2014,15 South Korea |
This was a prospective single‐center study based on a prospective cohort. The study included 499 adult patients (≥ 18 years old) with witnessed non‐traumatic OHCA and presumed correctable causes with or without bystander CPR; or no‐flow time was expected to be short, even for unwitnessed arrests. Fifty‐five patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | The primary end point was a good neurological outcome (measured as a CPC score of 1 or 2) at 3 months post‐cardiac arrest. The study aimed to find indications for predicting good neurologic outcome in patients who receive ECPR versus CCPR groups according to the CPR duration and the optimal duration of CPR before considering ECPR. | ECPR was indicated in sudden cardiac arrest with presumed correctable causes, witnessed cardiac arrest with or without bystander CPR, or no‐flow time to CPR by the EMS provider was expected to be short, even for unwitnessed arrests. | Data collected followed Utstein style guidelines. The covariates included age, sex, comorbidity score, bystander CPR, witnessed cardiac arrest, first documented arrest rhythm, presumed etiology of arrest, interval from arrest to CPR started by EMS provider, CPR duration, and therapeutic hypothermia. | In the ECPR group, younger age, witnessed arrest without initial asystole rhythm, early achievement of mean arterial pressure ≥ 60 mmHg, low rate of ECPR‐related complications, and TH were significant factors for expecting a good neurological outcome. The ECPR group with ≥ 21 minutes of CPR duration had a more favorable neurological outcome at 3‐month post‐arrest (15.4% vs 1.9%; P = 0.031), although the rate of survival to discharge was similar in both groups (15.4% vs 7.7%; P = 0.358). |
|
Maekawa et al. 2013,16 Japan |
This was a prospective single‐center study that included 162 adult patients (≥ 16 years old) with witnessed OHCA of presumed cardiac origin and ongoing CPR > 20 minutes. Fifty‐three patients received ECPR (intervention) and were compared to those who received compression‐only CPR. | Neurologically intact survival at 3 months after cardiac arrest (good neurological outcomes were defined as a CPC score of 1–2). Determine potential predictors that can identify candidates for ECPR among patients with OHCA. | Initiation of ECPR was dependent on the attending physicians. ECPR was initiated if ROSC could not be maintained during transportation, if the patient had good activities of daily living before CA, and if the cardiac arrest was clinically presumed as cardiac in origin. | Patient demographics, activities of daily living, witnessed CPR, initial rhythm VF/VT, number of counter shocks, time of arrest to ACLS, CPR duration, therapeutic hypothermia, IABP usage and primary PCI. | According to the predictor analysis, only pupil diameter on hospital arrival was associated with neurological outcome (adjusted hazard ratio, 1.39 per 1‐mm increase; 95% CI = 1.09–1.78; P = 0.008). ROC analysis identified a pupil diameter of < 6 mm as the optimal cut‐off point. Intact survival rate was higher in the matched ECPR group (29.2% [7/24] vs 8.3% [2/24], log‐rank P = 0.018). |
ACLS, advanced cardiovascular life support; CA, cardiac arrest; CCPR, conventional cardiopulmonary resuscitation; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; ED, emergency department; EMS, emergency medical services; IABP, intra‐aortic balloon pump; IHCA, in‐hospital cardiac arrest; LVEF, left ventricular ejection fraction; OHCA, out‐of‐hospital cardiac arrest; PCI, percutaneous coronary intervention; PSM, propensity score‐matched; ROC, Receiver operating characteristic; ROSC, return of spontaneous circulation; TH, therapeutic hypothermia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Notes: All cohort studies included in this review employed propensity score matching; most matched patients 1:1. Some used many‐to‐one matching (1:1, 2:1, and 3:1) on the propensity score to reduce standard error of a treatment effect.
The CAVAS database consists of 3 disease entities including acute myocardial infarction, acute stroke, and a nation‐wide EMS‐assessed OHCA.
A funnel plot did not show skewed distribution, suggesting that publication bias was not likely involved. However, it is important to note that funnel plots are not recommended if there are < 10 studies in a meta‐analysis, hence in this review, the potential impact of publication bias was considered without statistical analysis. 40 Given that the body of evidence was from observational studies, it was initially classified as low quality evidence (ie, permitting low confidence in the estimated effects).
3.2. Study characteristics
The studies included were published from 2008 to 2016, while patient enrollment periods extended from 2000 to 2013. Eligibility criteria for ECPR varied across primary studies. Three studies were performed in South Korea, 1 in Taiwan, 1 in Japan, and 1 in Germany. Regarding confounding variables, 3 papers described prospective cohorts, 12 , 15 , 16 and the remaining described retrospective cohorts. 11 , 13 , 14 Propensity score‐matched analysis was used in all studies to balance observed covariates in the 2 treatment groups. Three studies enrolled patients with in‐hospital cardiac arrest, 11 , 12 , 13 and the remaining studies enrolled patients with out‐of‐hospital cardiac arrest. 14 , 15 , 16 The sample sizes of the unmatched ECPR group ranged from 52–320 and from 109–36,227 in the conventional cardiopulmonary resuscitation group. The mean age of patients in the unmatched ECPR group ranged from 53–72 years and from 60–79 years in the conventional cardiopulmonary resuscitation group; the percentage of male patients in the unmatched ECPR group ranged from 54%–83% and from 61%–73% in the conventional cardiopulmonary resuscitation group. The patient population differed mainly between studies in terms of location of the arrest.
Five studies reported the incidence of witnessed arrest among survivors and non‐survivors. The in‐hospital cardiac arrest studies did not report the time from collapse to initiation of CPR, although it was considered minimal, as per inclusion criteria. The out‐of‐hospital cardiac arrest studies reported no‐flow time. All studies reported the incidence of bystander‐CPR and initial shockable cardiac rhythms but did not include data regarding the timing from collapse to initiation of ECPR. Most of the out‐of‐hospital cardiac arrests (72%) in the ECPR group were witnessed, 14 , 15 even though < 50% of all patients in the ECPR group received bystander‐CPR before emergency medical services (EMS) arrival. 14 , 15 , 16 In the largest study (n = 36,547), cardiac arrest was witnessed in only 71% of patients in the ECPR group and 54% in the control group, only 29% of the patients in the ECPR group had shockable rhythm, and only 30% received bystander‐CPR. Minimizing the time from collapse to restoration of perfusion with ECPR is critical for improving the chances for a good outcome. This study did not report collapse‐to‐ECPR time. Furthermore, ECPR was performed in 29% of unwitnessed cardiac arrest which is not recommended as per current guidelines. These points likely biased the estimate of survival outcomes and suggest that the ECPR group included many patients who would have not benefited from the intervention. 14 Considering that a short or no‐flow time, bystander CPR, and an initial shockable rhythm are among of the most crucial predictors for survival. 13 , 18 , 25 Tables 2 and 3 outline details and baseline clinical characteristics of the unmatched groups.
TABLE 2.
Details and baseline clinical characteristics of the unmatched groups on ECPR for cardiac arrest
| Author, year, country | Enrollment | Location | Study design | Patients, (n) | Patient groups, (n) | Mean age, (y) | Male, (%) | Witnessed arrest, (%) | Bystander CPR, (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totals | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ||||
|
Blumenstein et al. 2016,11 Germany |
2009–2013 | IHCA | Single‐center retrospective cohort | 353a | 52 | 272 | 72 | 75 | 54 | 61 | 100 | 100 | .. | .. |
|
Chen et al. 2008,12 Taiwan |
2004–2006 | IHCA | Single‐center prospective cohort | 172 | 59 | 113 | 57 | 60 | 85 | 65 | 100 | 100 | .. | .. |
|
Shin et al. 2013, 13 South Korea |
2003–2009 | IHCA | Single‐center retrospective cohort | 406 | 85 | 321 | 60 | 62 | 62 | 63 | 100 | 100 | .. | .. |
|
Choi et al. 2016,14 South Korea |
2009–2013 | OHCA | Multi‐center retrospective cohort | 36547 | 320 | 36227 | 67 (54–76) | 67 (54–77) | 81 | 67 | 71 | 54 | 30 | 9 |
|
Kim et al. 2014,15 South Korea |
2006–2013 | OHCA | Single‐center prospective cohort | 499 | 55 | 444 | 53 | 69 | 75 | 64 | 78 | 74 | 42 | 34 |
|
Maekawa et al. 2013,16 Japan |
2000–2004 | OHCA | Single‐center prospective cohort | 162 | 53 | 109 | 54 | 71 | 83 | 73 | … | … | 55 | 39 |
(..), not applicable; (…), data not available; CCPR, conventional cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; IHCA, in‐hospital cardiac arrest; OHCA, out‐of‐hospital cardiac arrest.
Notes: Total percentages refer to studies with available data and continuous variables are reported as mean ± SD or as median interquartile range. None of the patients received mechanical cardiopulmonary resuscitation (mCPR). All studies performed propensity score‐matched analysis.
All patients were admitted to hospital due to cardiovascular reasons.
TABLE 3.
Details and baseline clinical characteristics of the unmatched groups on ECPR for cardiac arrest
| Author, year, country | Asystole (%) | PEA (%) | VF/VT (%) | Time to CPRa (min) | CPR duration (min) | ROSC (ROSB) (%) | AMI (%) | Reperfusion therapy (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | |
|
Blumenstein et al. 2016,11 Germany |
5 | 2 | 2 | 0 | 2 | 3 | … | … | 33 (19–47) | 20 (6–40) | … | … | 29 | 21 | … | … |
|
Chen et al. 2008,12 Taiwan |
22 | 27 | 29 | 41 | 42 | 32 | … | … | 53 ± 37 | 43 ± 31 | 93 | 56 | 63 | 71 | 44b | 6b |
|
Shin et al. 2013, 13 South Korea |
12 | 15 | 59 | 63 | 29 | 3 | … | … | 42 ± 26 | 41 ± 38 | 75 | 52 | 45 | 26 | 21c | 3c |
|
Choi et al. 2016,14 South Korea |
55 | 82 | 16 | 9 | 29 | 9 | 7 (4–10) | 7 (4–9) | 35 (19–56) | 29 (20–38) | … | … | … | … | 31c | 2c |
|
Kim et al. 2014,15 South Korea |
26 | 60 | 18 | 21 | 56 | 19 | 7 (0–13) | 8 (5–12) | 62 (47–89) | 35 (21–50) | 80 | 48 | … | … | … | … |
|
Maekawa et al. 2013,16 Japan |
… | … | … | … | 60 | 22 | 6 (2–9) | 7 (3–10) | 49 (41–59) | 56 (47–66) | … | … | … | … | 40c | 6c |
(…), data not available; AMI, acute myocardial infarction; CCPR, conventional cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation; ECLS, extracorporeal life support; ECPR, extracorporeal cardiopulmonary resuscitation; GABC, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; ROSB, return of spontaneous heartbeat; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Notes: Total percentages refer to studies with available data and continuous variables are reported as mean ± SD or as median interquartile range. Studies reporting in‐hospital cardiac arrest did not report collapsed‐time to CPR though it was considered to be minimal as per inclusion criteria. CPR duration was defined as the interval between initiation of CPR and ROSC or death in the CCPR group and as the interval between initiation of CPR and ECLS implantation in the ECPR group. Return of spontaneous heartbeat was identified by echocardiography in the ECPR group and by palpable central pulse in the CCPR group. Collapsed‐time to ECPR was not reported.
Reported as collapsed‐time to CPR by emergency medical services providers.
Reported as subsequent interventions (PCI or CABG).
Reported as primary PCI.
ECPR patients were more likely to suffer from acute myocardial infarction and to have received primary reperfusion therapy than patients in the control arm in the unmatched groups of the in‐hospital cardiac arrest studies. Specifically, in the unmatched groups, none of the studies of out‐of‐hospital cardiac arrest reported the percentage of patients who suffered acute myocardial infarction, although ECPR patients were more likely to receive primary reperfusion therapy than patients in the control arm. None of the patients received mechanical cardiopulmonary resuscitation. CPR duration was defined as the interval between initiation of CPR and return of spontaneous circulation or death in the conventional cardiopulmonary resuscitation group, and as the interval between initiation of CPR and extracorporeal life support (ECLS) implantation in the ECPR group. Return of spontaneous heartbeat was identified by echocardiography in the ECPR group and by a palpable central pulse in the conventional cardiopulmonary resuscitation group.
Data in terms of invasive vascular access complications, including risks of bleeding or hematoma with a need for transfusion and leg ischemia, were poorly reported. Peripheral vessel complications were only reported by 2 studies. 11 , 16 One study reported renal failure and sepsis/systemic inflammatory response syndrome. 11 The same study was the only one that reported a bridge to long‐term ventricular assist device or heart transplantation. None of the studies included reported specific data about stroke, blood transfusions, or adverse events. One publication mentioned that several related complications were reported during the study period. 13 According to this study, bleeding and hematoma of insertion sites were relatively common; other rare complications mentioned were catheter infection, vascular injury, limb ischemia, gastrointestinal bleeding, hemolysis, and stroke. ECPR patients were more likely to have more complications related to bleeding or hematoma in the leg with the need for transfusion compared to patients in the control arm. 11 It is notable that therapeutic hypothermia was not applied as a standard treatment in 2 studies, 12 , 13 but it was carried out if deemed necessary by the other 4 studies. 11 , 14 , 15 , 16
The sample sizes of the 1:1 matched groups ranged from 24–320. The mean age of patients in the matched ECPR group ranged from 54–72 years and from 54–73 years in the conventional cardiopulmonary resuscitation group, and the percentage of male patients in the matched ECPR group ranged from 54%–81% and from 60%–81% in the conventional cardiopulmonary resuscitation group. Patient populations differed between studies in terms of location of the arrest, witnessed or unwitnessed arrest, presumed cardiac origin, and the length of time CPR was conducted. In the matched group, ECPR patients were almost as likely to suffer from acute myocardial infarction as patients in the control arm, and more likely to receive primary reperfusion therapy than patients in the control arm. Only 1 study included percutaneous coronary intervention as a matching variable in the propensity score matching analysis. 16 All studies reported favorable neurological outcome and survival to discharge; 3 studies reported 30‐day, 3‐month, and long‐term favorable neurological outcome and survival. All of the studies defined favorable neurological outcome as a Cerebral Performance Category score of 1–2, except one, which defined it as a Glasgow Outcome Scale of 4–5. 41 , 42 Tables 4 and 5 outline details and baseline clinical characteristics of the propensity score‐matched groups.
TABLE 4.
Details and baseline clinical characteristics of the propensity score‐matched analysis of ECPR assisted cardiac arrest
| Author, year, country | Matching | Patients (n) | Patient groups (n) | Mean age (y) | Male (%) | Witnessed arrest (%) | Bystander CPR (%) | Weaning from ECPR (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totals | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ||
|
Blumenstein et al. 2016,11 Germany |
1:1 | 104 | 52 | 52 | 72 | 73 | 54 | 60 | 100 | 100 | .. | .. | … | … |
|
Chen et al. 2008,12 Taiwan |
1:1 | 92 | 46 | 46 | 57 | 55 | 85 | 87 | 100 | 100 | .. | .. | … | … |
|
Shin et al. 2013, 13 South Korea |
1:1 | 120 | 60 | 60 | 61 | 61 | 60 | 68 | 100 | 100 | .. | .. | … | … |
|
Choi et al. 2016,14 South Korea |
1:1 | 640 | 320 | 320 | 56 (45–68) | 58 (47–68) | 81 | 81 | 71 | 73 | 30 | 31 | … | … |
|
Kim et al. 2014,15 South Korea |
1:1 | 104 | 52 | 52 | 54 | 54 | 77 | 73 | 81 | 81 | 42 | 31 | … | … |
|
Maekawa et al. 2013,16 Japan |
1:1 | 48 | 24 | 24 | 57 | 57 | 79 | 79 | … | … | 54 | 58 | … | … |
(..), not applicable; (…), data not available; CCPR, conventional cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation.
Notes: Total percentages refer to studies with available data and continuous variables are reported as mean ± SD or as median interquartile range. Propensity score matching analysis was performed to minimize the effect of selection bias and balance observed covariates in the 2 treatment groups.
TABLE 5.
Details and baseline clinical characteristics of the propensity score‐matched analysis on ECPR assisted cardiac arrest
| Author, year, country | Asystole (%) | PEA (%) | VF/VT (%) | Time to CPRa(min) | CPR duration (min) | ROSC (ROSB) (%) | AMI (%) | Reperfusion therapy (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | |
|
Blumenstein et al. 2016,11 Germany |
0 | 4 | 0 | 6 | 2 | 4 | … | … | 33 (19–47) | 37 (30–45) | … | … | 29 | 37 | 32b | 17b |
|
Chen et al. 2008,12 Taiwan |
22 | 20 | 33 | 39 | 46 | 41 | … | … | 53 ± 41 | 47 ± 33 | 91 | 52 | 61 | 72 | 17c | 7c |
|
Shin et al. 2013, 13 South Korea |
10 | 10 | 68 | 68 | 22 | 22 | … | … | 39 ± 21 | 38 ± 21 | 75 | 48 | 43 | 35 | 25d | 3d |
|
Choi et al. 2016,14 South Korea |
55 | 53 | 16 | 19 | 29 | 28 | 7 (4–10) | 7 (4–10) | 35 (19–55) | 28 (15–37) | … | … | … | … | 31d | 9d |
|
Kim et al. 2014,15 South Korea |
25 | 29 | 15 | 15 | 60 | 56 | 7 (0.3–13) | 7 (5–10) | 63 (49–88) | 61 (40–84) | 81 | 39 | 85e | 89e | 56d | 6d |
|
Maekawa et al. 2013,16 Japan |
… | … | … | … | 54 | 58 | 5 (2–10) | 4 (0–8) | 49 (43–66) | 52 (43–65) | … | … | … | … | 21d | 25d |
(…), data not available; AMI, acute myocardial infarction; CCPR, conventional cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation; ECLS, extracorporeal life support; ECPR, extracorporeal cardiopulmonary resuscitation; GABC, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; ROSB, return of spontaneous heartbeat; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Notes: Total percentages refer to studies with available data and continuous variables are reported as mean ± SD or as median interquartile range. Studies reporting IHCA did not report collapsed‐time to CPR although it was considered to be minimal as per inclusion criteria. CPR duration was defined as the interval between initiation of CPR and ROSC or death in the CCPR group, and as the interval between initiation of CPR and ECLS implantation in the ECPR group. Return of spontaneous heartbeat was identified by echocardiography in the ECPR group and by palpable central pulse in the CCPR group. Collapsed‐time to ECPR was not reported.
Reported as collapsed‐time to CPR by emergency medical services providers.
Reported as primary coronary interventions (PCI or CABG + other procedures).
Reported as subsequent interventions (PCI or CABG).
Reported as primary PCI.
Suspected acute coronary syndrome (ACS).
3.3. Primary outcomes
Figure 2 shows a forest plot of the comparison of 30‐day favorable neurological outcome in patients with cardiac arrest. The pooled data from adults with in‐ and out‐of‐hospital cardiac arrest showed that ECPR compared to conventional cardiopulmonary resuscitation was likely associated with increased 30‐day favorable neurological outcome (RR = 2.02, 95% CI = 1.29–3.16; I2 = 20%, P = 0.002; 1060 participants, 5 studies; very low‐quality evidence). When we pooled the data for in‐ and out‐of‐hospital cardiac arrest separately, ECPR compared to conventional cardiopulmonary resuscitation was likely associated with improved 30‐day favorable neurological outcome for in‐hospital cardiac arrest (RR = 2.18, 95% CI = 1.24–3.81; I2 = 9%, P = 0.006; 316 participants, 3 studies; very low‐quality evidence), but not for out‐of‐hospital cardiac arrest (RR = 2.61, 95% CI = 0.56–12.20; I2 = 59%, P = 0.22; 744 participants, 2 studies; very low‐quality evidence). There was low‐to‐moderate heterogeneity in the 30‐day favorable neurological outcome data. We are uncertain of the effects of ECPR on 30‐day favorable neurological outcome, as the quality of the evidence has been assessed as very low (downgraded 1 level for inconsistency).
FIGURE 2.

Forest plot of comparison of 30‐day favorable neurological outcome in adults with cardiac arrest. Squares or diamonds to the right of the solid vertical line favor the intervention group (ECPR) over the control group (conventional cardiopulmonary resuscitation), but this is conventionally significant (P < 0.05) only if the horizontal line or diamond does not overlap the solid line. The result and its 95% confidence interval (CI) are presented by a diamond, with the risk ratio (95% CI) and its statistical significance given alongside. Squares indicate study‐specific risk ratios (RRs). Horizontal lines indicate 95% CIs. A diamond indicates the pooled RR with 95% CI. I2 indicates the percentage of total variations across the studies that are due to heterogeneity rather than change. The weight indicates how much an individual study contributes to the pooled estimate. M‐H stands for the Mantel‐Haenszel method in meta‐analysis. Random indicates that a random‐effects method was adopted for generating the meta‐analysis results. The certainty of evidence for this outcome was graded as very low‐quality based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Figure 3 shows a forest plot of the comparison of long‐term favorable neurological outcome in patients with cardiac arrest. The pooled data from adults with in‐ and out‐of‐hospital cardiac arrest showed that ECPR compared to conventional cardiopulmonary resuscitation was likely associated with increased long‐term favorable neurological outcome (RR = 2.86, 95% CI = 1.64–5.01; I2 = 0%, P = 0.0002; 468 participants, 5 studies; moderate‐quality evidence). When we pooled the data for in‐ and out‐of‐hospital cardiac arrest separately, ECPR compared to conventional cardiopulmonary resuscitation was likely associated with improved long‐term favorable neurological outcome for in‐hospital cardiac arrest (RR = 2.50, 95% CI = 1.33–4.71; I2 = 0%, P = 0.005; 316 participants, 3 studies; moderate‐quality evidence) and for out‐of‐hospital cardiac arrest (RR = 4.64, 95% CI = 1.41–15.25; I2 = 0%, P = 0.01; 152 participants, 2 studies; moderate‐quality evidence). The choice of model for the pooled analysis did not affect the estimate of effect, as the statistical heterogeneity was 0%. We are quite confident that the effect of ECPR on long‐term favorable neurological outcome is close to the true effect. However, it is also possible that it is substantially different as the quality of the evidence has been assessed as moderate (upgraded 1 level due to large magnitude of the effect: RR > 2).
FIGURE 3.

Forest plot of comparison of long‐term favorable neurological outcome in adults with cardiac arrest. Squares or diamonds to the right of the solid vertical line favor the intervention group (ECPR) over the control group (conventional cardiopulmonary resuscitation), but this is conventionally significant (P < 0.05) only if the horizontal line or diamond does not overlap the solid line. The result and its 95% confidence interval (CI) are presented by a diamond, with the risk ratio (95% CI) and its statistical significance given alongside. Squares indicate study‐specific risk ratios (RRs). Horizontal lines indicate 95% CIs. A diamond indicates the pooled RR with 95% CI. I2 indicates the percentage of total variations across the studies that are due to heterogeneity rather than change. The weight indicates how much an individual study contributes to the pooled estimate. M‐H stands for the Mantel‐Haenszel method in meta‐analysis. Random indicates that a random‐effects method was adopted for generating the meta‐analysis results. The certainty of evidence for this outcome was graded as moderate‐quality based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
3.4. Secondary outcomes
Figure 4 shows a forest plot of the comparison of 30‐day survival in patients with cardiac arrest. The pooled data from adults with in‐ and out‐of‐hospital cardiac arrest showed that ECPR compared to conventional cardiopulmonary resuscitation was likely associated with increased 30‐day survival (RR = 1.54, 95% CI = 1.03–2.30; I2 = 48%, P = 0.04; 1108 participants, 6 studies; very low‐quality evidence). When we pooled the data for in‐ and out‐of‐hospital cardiac arrest separately, ECPR compared to conventional cardiopulmonary resuscitation was likely associated with improved 30‐day survival for in‐hospital cardiac arrest (RR = 2.03, 95% CI = 1.03–3.18; I2 = 0%, P = 0.002; 316 participants, 3 studies; very low‐quality evidence), but not for out‐of‐hospital cardiac arrest (RR = 1.18, 95% CI = 0.71–1.97; I2 = 40%, P = 0.53; 792 participants, 3 studies; very low‐quality evidence). There was low to moderate heterogeneity in the 30‐day survival outcome data. We are uncertain of the effects of ECPR on 30‐day survival, because the quality of the evidence has been assessed as very low (downgraded 1 level for inconsistency).
FIGURE 4.

Forest plot of comparison of 30‐day survival in adults with cardiac arrest. Squares or diamonds to the right of the solid vertical line favor the intervention group (ECPR) over the control group (conventional cardiopulmonary resuscitation), but this is conventionally significant (P < 0.05) only if the horizontal line or diamond does not overlap the solid line. The result and its 95% confidence interval (CI) are presented by a diamond, with the risk ratio (95% CI) and its statistical significance given alongside. Squares indicate study‐specific risk ratios (RRs). Horizontal lines indicate 95% CIs. A diamond indicates the pooled RR with 95% CI. I2 indicates the percentage of total variations across the studies that are due to heterogeneity rather than change. The weight indicates how much an individual study contributes to the pooled estimate. M‐H stands for the Mantel‐Haenszel method in meta‐analysis. Random indicates that a random‐effects method was adopted for generating the meta‐analysis results. Notes: The certainty of evidence for this outcome was graded as very low‐quality based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Figure 5 shows a forest plot of the comparison of long‐term survival in patients with cardiac arrest. The pooled data from adults with in‐ and out‐of‐hospital cardiac arrest showed that ECPR compared to conventional cardiopulmonary resuscitation was likely associated with increased long‐term survival (RR = 2.17, 95% CI = 1.37–3.44; I2 = 0%, P = 0.001; 468 participants, 5 studies; low‐quality evidence). When we pooled the data for in‐ and out‐of‐hospital cardiac arrest separately, ECPR compared to conventional cardiopulmonary resuscitation was likely associated with improved 30‐day survival for in‐hospital cardiac arrest (RR = 1.99, 95% CI = 1.16–3.41; I2 = 0% P = 0.01; 316 participants, 3 studies; low‐quality evidence), and for out‐of‐hospital cardiac arrest (RR = 2.47, 95% CI = 1.13–6.67; I2 = 0%, P = 0.03; 152 participants, 2 studies; low‐quality evidence). The choice of model for the pooled analysis did not affect the estimate of effect as the statistical heterogeneity was 0%. The true effect of ECPR on long‐term survival may differ significantly from the estimate as the quality of the evidence has been assessed as low.
FIGURE 5.

Forest plot of comparison of long‐term survival in adults with cardiac arrest. Squares or diamonds to the right of the solid vertical line favor the intervention group (ECPR) over the control group (conventional cardiopulmonary resuscitation), but this is conventionally significant (P < 0.05) only if the horizontal line or diamond does not overlap the solid line. The result and its 95% confidence interval (CI) are presented by a diamond, with the risk ratio (95% CI) and its statistical significance given alongside. Squares indicate study‐specific risk ratios (RRs). Horizontal lines indicate 95% CIs. A diamond indicates the pooled RR with 95% CI. I2 indicates the percentage of total variations across the studies that are due to heterogeneity rather than change. The weight indicates how much an individual study contributes to the pooled estimate. M‐H stands for the Mantel‐Haenszel method in meta‐analysis. Random indicates that a random‐effects method was adopted for generating the meta‐analysis results. Notes: The certainty of evidence for this outcome was graded as low‐quality based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
4. LIMITATIONS
This study should be interpreted in the context of certain limitations. The review was based on non‐randomized, non‐blinded, observational cohort studies that performed propensity score‐matched analysis and were published in English, which may have led to selection bias. Even though propensity score‐matched studies were used to reduce selection bias and confounding factors, an important limitation of our analysis is that bias could remain if there were unmeasured or unknown confounders that were not incorporated into the propensity score analysis. Additionally, of 28 full‐text articles excluded, 2 were associated with cardiac arrest and used propensity score‐matched analysis. 43 , 44 All of the 6 studies used in this review listed at least 1 limitation in the discussion section of the article, with most studies listing 3 or 4 limitations. The most common limitation was a small sample size, followed by missing measures, meaning the authors felt additional measures would have enhanced their research and sample generalizability, and that sample heterogeneity was low.
Furthermore, all studies had different inclusion criteria and methods of intervention. Therefore, subjective selection of ECPR participants likely biased the estimate of survival outcomes. Unfortunately, the vast majority of the studies of in‐ and out‐hospital cardiac arrest, and most certainly those of ECPR, are observational, with the accompanying potential for confounding selection bias. ECMO use cannot be blinded, thus observer bias may confound the interpretation of results. Second, subsequent interventions after ECPR (eg, primary percutaneous coronary intervention, therapeutic hypothermia) or complications could affect the survival and neurological outcome of patients. However, not all patients received primary percutaneous coronary intervention or therapeutic hypothermia, and complications were poorly reported. Consequently, the efficacy of ECPR may be attributed, to some extent, to observer bias and subsequent interventions rather than extracorporeal support. Third, because all patients had cardiac arrest, some important pre‐and post‐resuscitation parameters were incomplete or not reported (eg, no‐flow time, low‐flow time, collapse‐to‐ECPR time). Fourth, inclusion and exclusion criteria of VA‐ECMO used as ECPR for cardiac arrest to identify appropriate candidates have not been established and a protocol has been not standardized; it differs according to the EMS in the out‐of‐hospital setting and medical personnel in the in‐hospital setting, so our results may not be generalizable and should be interpreted with caution in a clinical setting.
In addition to the limitations listed above are those addressed in each individual article; therefore, there is a risk for bias if the authors of any articles included in this review did not include all the true limitations of their studies. Since completing the first literature search, in January 2019, we searched for recent studies on the topic (results are not included in Tables 1, 2, 3, 4, 5) that have been published after the cut‐off day of our prior searches. We identified at least 1 observational study that has been recently published and was therefore not included in our analysis. 45 This study either provides additional evidence for ECPR outcomes in cardiac arrest or evidence supporting our review. It is possible that there are similar unpublished primary studies that we were not able to find, despite our attempt to identify unpublished studies. Finally, no single study, whether meta‐analytic or not, will provide a definitive understanding of responses to treatment. Despite this limitation, meta‐analytic approaches have demonstrable benefits in addressing many of the above‐mentioned limitations.
5. DISCUSSION
In our systematic review and meta‐analysis we both separated and pooled the data from the studies that used propensity score matching analysis for in‐ and out‐of‐hospital cardiac arrest. We limited our inclusion criteria to studies that used propensity score matching analysis in order to use the best available and most relevant evidence on ECPR for in‐ and out‐of‐hospital cardiac arrest. We identified 6 observational studies relating to the research purpose, of which 3 studies were in adults who had suffered in‐hospital cardiac arrest, 11 , 12 , 13 and 3 studies were in adults who had suffered out‐of‐hospital cardiac arrest, 14 , 15 , 16 totaling 38,133 patients, of which 1.6% (n = 624) received VA‐ECMO as ECPR for refractory cardiac arrest. Of these, 1080 patients (554 patients in the ECPR group and 554 patients in the conventional cardiopulmonary resuscitation group) were compared using propensity score‐matched analysis. The results of the ECPR group and the conventional cardiopulmonary resuscitation group were compared further in our quantitative analysis to evaluate our outcomes of interest. Overall, ECLS patients were more likely to have been witnessed by bystanders who performed CPR. In addition, patients were likely to be younger, to be male, to have presented an initial shockable cardiac rhythm, to have suffered from acute myocardial infarction, and to have undergone primary percutaneous coronary intervention. The confounding factors of the characteristics evaluated in the propensity score‐matched analysis were balanced and there were no significant differences between the matched groups. However, in the matched ECPR group, patients were more likely to receive primary reperfusion therapy than patients in the control arm, and only 1 study included primary percutaneous coronary intervention as a matching variable. The GRADE quality of evidence for the majority of the outcomes was graded as low or very low. Table 6 outlines the GRADE summary of findings. There was low to moderate heterogeneity and imprecision in several of the pooled study estimates, which limited confidence in the results, and was reflected in the generally low quality of evidence.
TABLE 6.
GRADE summary of findings
| Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment | No. of patients | Effects | ||||||||
| No. of studies, design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ECPR | Control | Relative (95% CI) | Absolute (95% CI) | Certainty |
| 30‐day favorable neurological outcome | ||||||||||
|
5 observational studies |
No serious limitations |
Serious limitationsa |
No serious limitations |
No serious limitations |
Undetected | 76/530 (14.3%) | 37/530 (7.0%) | RR = 2.02 (1.29–3.16) | 71 more per 1000 (from 20 more to 151 more) |
⊕⊝⊝⊝ Very low |
| Long‐term favorable neurological outcome | ||||||||||
|
5 observational studies |
No serious limitations |
No serious limitations |
No serious limitations |
No serious limitations |
Undetected | 46/234 (19.7%) | 15/234 (6.4%) | RR = 2.86 (1.64–5.01) | 119 more per 1000 (from 41 more to 257 more) |
⊕⊕⊕⊝ Moderateb |
| 30‐day survival | ||||||||||
|
6 observational studies |
No serious limitations |
Serious limitationsa |
No serious limitations |
No serious limitations |
Undetected | 123/554 (22.2%) | 89/554 (16.1%) | RR = 1.54 (1.03–2.30) | 87 more per 1000 (from 5 more to 209 more) |
⊕⊕⊝⊝ Low |
| Long‐term survival | ||||||||||
|
5 observational studies |
No serious limitations |
No serious limitations |
No serious limitations |
No serious limitations |
Undetected | 51/234 (21.8%) | 23/234 (9.8%) | RR = 2.17 (1.37–3.44) | 115 more per 1000 (from 36 more to 240 more) |
⊕⊝⊝⊝ Very low |
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. | ||||||||||
Notes: CI, confidence interval; RR, risk ratio. The risk in the intervention group (and its 95% CI) is based on the comparison group and the relative effect of the intervention (and its 95% CI). The overall certainty of evidence was graded as low to very low for each outcome based on GRADE criteria. In the GRADE approach to quality of evidence the observational studies without special strengths or important limitations provide low quality evidence.
The quality of the evidence was downgraded 1 level for inconsistency.
The quality of the evidence was upgraded 1 level due to the large magnitude of the effect: RR >2.
Our analysis showed that ECPR is likely associated with an improved 30‐day survival (RR = 2.03, 95% CI = 1.03–3.18; I2 = 40%, P = 0.002) and 30‐day favorable neurological outcome (RR = 2.18, 95% CI = 1.24–3.8; I2 = 9%, P = 0.006) for in‐hospital cardiac arrest. However, ECPR had no effect on 30‐day survival (RR = 1.18, 95% CI = 0.71–1.97; I2 = 40%, P = 0.53) or favorable neurological outcome (RR = 2.61, 95% CI = 0.56–12.20; I2 = 59, P = 0.22) for out‐of‐hospital cardiac arrest. ECPR was associated with an improved long‐term survival, (RR = 1.99, 95% CI = 1.16–3.41; I2 = 0%, P = 0.01) and long‐term favorable neurological outcome (RR = 2.50, 95% CI = 1.33–4.71; I2 = 0%, P = 0.005) for in‐hospital cardiac arrest. ECPR was likely associated with improved long‐term survival (RR = 2.47, 95% CI = 1.13–6.67; I2 = 0%, P = 0.03) and favorable neurological outcome (RR = 4.64, 95% CI = 1.41–15.25; I2 = 0%, P = 0.01) for out‐of‐hospital cardiac arrest. These differences in outcome can certainly be attributed, to some extent, to the studies selected for our meta‐analysis. These findings should be interpreted in the context of the quality of evidence, the pooled estimates of reported outcome measures, and the limitations of the individual studies included in our analysis.
The presence of an initial shockable cardiac rhythm, 18 , 46 , 47 low‐flow time, and collapse‐to‐ECPR time are the most crucial predictors of good outcomes in patients treated with ECPR for cardiac arrest. 13 , 18 , 25 , 47 Therefore, the location of cardiac arrest is of great significance for this subgroup of patients. Patients with in‐hospital cardiac arrest tend to have shorter time from collapse to initiation of CPR, duration of resuscitation, collapse‐to‐ECPR time, and percutaneous coronary intervention, and are more likely to have rapid access to a highly specialized response team. 14 , 15 , 16 Patients treated with ECPR for refractory in‐hospital cardiac arrest are more likely to be associated with better survival rates and favorable neurological outcome than patients with refractory out‐of‐hospital cardiac arrest. 48 ECPR is not readily available for out‐of‐hospital use, and patients who experience refractory out‐of‐hospital cardiac arrest are reliant on EMS response time and transportation to ECPR and cardiac catheterization laboratory‐capable hospitals. As such, optimizing EMS response time and the time frame between ECPR attempts may improve outcomes for out‐of‐hospital cardiac arrest. 18 Two different approaches have been implemented to reduce time to initiation of ECPR in patients with refractory out‐of‐hospital cardiac arrest. The first approach uses rapid EMS response time and transportation to the closest highly equipped emergency department or cardiac catheterization laboratory‐capable hospital. The second approach mobilizes ECPR‐equipped emergency response units and the initiation of ECMO in the field. 5 , 6 , 9 , 27 , 49
Studies have shown that patients resuscitated from ventricular fibrillation/pulseless ventricular tachycardia cardiac arrests have clinically significant coronary stenosis due to coronary artery disease. 50 , 51 , 52 , 53 , 54 Patients with refractory ventricular fibrillation/pulseless ventricular tachycardia have been shown to have significantly higher rates of coronary artery disease. 21 , 23 , 25 Therefore, early implementation of ECPR for refractory cardiac arrest will facilitate temporary return of perfusion, minimize the severity of cardiac injuries, including ischemia, and provide protection from progressive myocardial dysfunction to support further resuscitation efforts until definitive therapy, including coronary angiography and percutaneous coronary intervention when indicated. 4 , 5 Interventions aimed at reducing collapse‐to‐ECPR initiation for refractory cardiac arrest may lead to improved outcomes; however, best results have occurred when ECPR has been combined with reperfusion therapies. Furthermore, ECPR should be viewed as a bridge to definitive treatment for cardiac arrest from reversible cardiac etiologies, so rigorous patient selection may be a way to significantly improve the care of this patient population. 4 , 5
Although this paper did not delve into details about patient selection, indication, risk of complications, adverse events, and prognostication related to ECPR for cardiac arrest, 47 , 55 , 56 , 57 , 58 it did make efforts to report the survival rates and functional outcomes of the studies that used propensity score‐matched analysis as part of the study design, to adjust for confounding variables and to reduce treatment selection bias. 11 , 12 , 13 , 14 , 15 , 16 Additionally, this paper did not evaluate the cost‐effectiveness of VA‐ECMO used as ECPR for refractory cardiac arrest. It is important to note that 5 studies conducted cost‐effectiveness analyses for ECPR in non‐cardiac arrest patients; 59 , 60 , 61 , 62 , 63 1 of them partially conducted economic analysis about the cost‐effectiveness of VA‐ECMO used as ECPR for refractory cardiac arrest. 63 Understanding the benefits of this therapy relative to hospital resource utilization and patient outcomes are particularly important given the recent increased use of VA‐ECMO as ECPR for refractory cardiac arrest. Furthermore, we did not provide recommendations on when to establish and maintain an ECPR program for patients with cardiac arrest. This therapy is a complex intervention and its outcomes require a well‐designed protocol and an experienced and dedicated multi‐disciplinary team necessary to sustain a reliable program that is beyond the scope of this paper. 4 , 64
Currently, there is insufficient evidence to recommend routine use of ECPR for patients with cardiac arrest. 31 In settings where it can be rapidly implemented, ECPR may be considered for selected patients for whom the suspected etiology of the cardiac arrest is potentially reversible during a limited period of mechanical cardiorespiratory support (class IIb, LOE C‐LD). 30 , 31 The differences in outcome are likely to disappear after we get a better understanding of the patients that are most likely to benefit from this intervention. 5 The Advanced Reperfusion Strategies for Refractory Cardiac Arrest (the ARREST Trial) under the Center for Resuscitation Medicine at the University of Minnesota Medical School is the largest ongoing randomized clinical trial in the United States evaluating the role of ECPR in patients with out‐of‐hospital cardiac arrest (NCT03880565). This randomized clinical trial is necessary to develop better practices and will address survival to hospital discharge (time frame: 1 week) as a primary outcome measure, as well as survival to discharge with modified Rankin Scale Score (mRS) ≤3 along with functional status (time frame: 1 week, 3 months, 6 months), cost per patient, and cost per life saved (time frame: 6 months). However, the outcomes will be not available until January 2023.
6. CONCLUSIONS
Our meta‐analysis suggests that VA‐ECMO used as ECPR may improve long‐term favorable neurological outcomes and survival compared to the best current standard of care in a select patient population. Nevertheless, there is inconclusive evidence to either support or refute the use of ECPR for in‐ and out‐of‐hospital cardiac arrest. We further conclude that future research has large potential for reducing uncertainty and is very likely to have an important impact on the estimated effect of VA‐ECMO used as ECPR for refractory cardiac arrest. Therefore, it is imperative for well‐designed randomized clinical trials to obtain a higher level of scientific evidence in order to develop best clinical practices and to ensure optimal outcomes for cardiac arrest patients.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
DM, LM, and WA were responsible for the study conception and design. DM and LM were responsible for data abstraction, analysis, interpretation of the data, and drafted the original manuscript. All authors reviewed and approved the final version of the manuscript. DM takes responsibility for the integrity of the data, the accuracy of the data analysis, and for the paper as a whole.
ACKNOWLEDGMENTS
The authors would like to thank the library staff from the Veterans Affairs Caribbean Healthcare System Library Service for assistance with producing the search strategy.
Miraglia D, Miguel LA, Alonso W. Extracorporeal cardiopulmonary resuscitation for in‐ and out‐of‐hospital cardiac arrest: systematic review and meta‐analysis of propensity score‐matched cohort studies. JACEP Open. 2020;1:342–361. 10.1002/emp2.12091
Funding and support: ByJACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Michael R. Gunderson, REMT‐P(Ret.).
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Holmberg MJ, Ross CE, Chan PS, et al. Annual incidence of adult and pediatric in‐hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 3. Neumar RW. Doubling Cardiac Arrest Survival by 2020. Circulation. 2016;134:2037‐2039. [DOI] [PubMed] [Google Scholar]
- 4. Merchant RM, Yang L, Becker LB, et al. American Heart Association Get With The Guidelines‐Resuscitation Investigators. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yannopoulos D, Bartos JA, Martin C, et al. Minnesota Resuscitation Consortium's advanced perfusion and reperfusion cardiac life support strategy for out‐of‐hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5:e003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out‐of‐hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109‐1117. [DOI] [PubMed] [Google Scholar]
- 7. Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post‐traumatic respiratory failure (shock‐lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629‐634. [DOI] [PubMed] [Google Scholar]
- 8. Fagnoul D, Taccone FS, Belhaj A, et al. Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation. 2013;84:1519‐1524. [DOI] [PubMed] [Google Scholar]
- 9. Lamhaut L, Jouffroy R, Kalpodjian A, et al. Successful treatment of refractory cardiac arrest by emergency physicians using pre‐hospital ECLS. Resuscitation. 2012;83:e177‐e178. [DOI] [PubMed] [Google Scholar]
- 10. Extracorporeal Life Support Organization . ECLS Registry Report: International Summary. https://www.elso.org/. Accessed March 2019.
- 11. Blumenstein J, Leick J, Liebetrau C, et al. Extracorporeal life support in cardiovascular patients with observed refractory in‐hospital cardiac arrest is associated with favourable short and long‐term outcomes: a propensity‐matched analysis. Eur Heart J Acute Cardiovasc Care. 2016;5:13‐22. [DOI] [PubMed] [Google Scholar]
- 12. Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554‐561. [DOI] [PubMed] [Google Scholar]
- 13. Shin TG, Jo IJ, Sim MS, et al. Two‐year survival and neurological outcome of in‐hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol. 2013;168:3424‐3430. [DOI] [PubMed] [Google Scholar]
- 14. Choi DS, Kim T, Ro YS, et al. Extracorporeal life support and survival after out‐of‐hospital cardiac arrest in a nationwide registry: a propensity score‐matched analysis. Resuscitation. 2016;99:26‐32. [DOI] [PubMed] [Google Scholar]
- 15. Kim SJ, Jung JS, Park JH, Park JS, Hong YS, Lee SW. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out‐of‐hospital cardiac arrest: a propensity‐matched study. Crit care. 2014;18:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out‐of‐hospital cardiac arrest of cardiac origin: a propensity‐matched study and predictor analysis. Crit Care Med. 2013;41:1186‐1196. [DOI] [PubMed] [Google Scholar]
- 17. Chen YS, Yu HY, Huang SC, et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529‐2535. [DOI] [PubMed] [Google Scholar]
- 18. Park SB, Yang JH, Park TK, et al. Developing a risk prediction model for survival to discharge in cardiac arrest patients who undergo extracorporeal membrane oxygenation. Int J Cardiol. 2014;177:1031‐1035. [DOI] [PubMed] [Google Scholar]
- 19. Kagawa E, Dote K, Kato M, et al. Should we emergently revascularize occluded coronaries for cardiac arrest? Rapid‐response extracorporeal membrane oxygenation and intra‐arrest percutaneous coronary intervention. Circulation. 2012;126:1605‐1613. [DOI] [PubMed] [Google Scholar]
- 20. Avalli L, Maggioni E, Formica F, et al. Favorable survival of in‐hospital compared to out‐of‐hospital refractory cardiac arrest patients treated with extracorporeal membrane oxygenation: an Italian tertiary care centre experience. Resuscitation. 2012;83:579‐583. [DOI] [PubMed] [Google Scholar]
- 21. Haneya A, Philipp A, Diez C, et al. A 5‐year experience with cardiopulmonary resuscitation using extracorporeal life support in non‐postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331‐1337. [DOI] [PubMed] [Google Scholar]
- 22. Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out‐of‐ hospital cardiac arrest: a comparison with that for extracorporeal rescue for in‐hospital cardiac arrest. Resuscitation. 2014;85:1219‐1224. [DOI] [PubMed] [Google Scholar]
- 23. Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out‐of‐hospital and emergency department cardiac arrest. Resuscitation. 2014;85:1527‐1532. [DOI] [PubMed] [Google Scholar]
- 24. Sakamoto T, Morimura N, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out‐of‐hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762‐768. [DOI] [PubMed] [Google Scholar]
- 25. Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88‐94. [DOI] [PubMed] [Google Scholar]
- 26. Lee JJ, Han SJ, Kim HS, et al. Out‐of‐hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal membrane oxygenation: focus on survival rate and neurologic outcome. Scand J Trauma Resusc Emerg Med. 2016;24:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamhaut L, Hutin A, Puymirat E, et al. A pre‐hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109‐117. [DOI] [PubMed] [Google Scholar]
- 28. Schober A, Sterz F, Herkner H, et al. Emergency extracorporeal life support and ongoing resuscitation: a retrospective comparison for refractory out‐of‐hospital cardiac arrest. Emerg Med J. 2017;34:277‐281. [DOI] [PubMed] [Google Scholar]
- 29. Pozzi M, Koffel C, Armoiry X, et al. Extracorporeal life support for refractory out‐of‐ hospital cardiac arrest: should we still fight for? A single‐centre, 5‐year experience. Int J Cardiol. 2016;204:70‐76. [DOI] [PubMed] [Google Scholar]
- 30. Leick J, Liebetrau C, Szardien S, et al. Door‐to‐implantation time of extracorporeal life support systems predicts mortality in patients with out‐of‐hospital cardiac arrest. Clin Res Cardiol. 2013;102:661‐669. [DOI] [PubMed] [Google Scholar]
- 31. Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S444‐S464. [DOI] [PubMed] [Google Scholar]
- 32. Soar J, Nolan JP, Bottiger BW, et al. European resuscitation council guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation. 2015;95:100‐147. [DOI] [PubMed] [Google Scholar]
- 33. Kagawa E. Extracorporeal cardiopulmonary resuscitation for adult cardiac arrest patients. World J Crit Care Med. 2012;1:46‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 36. Wells GA, Shea BJ, O'Connell D, et al. The Newcastle—Ottawa scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. Appl Eng Agri. 2000;18:727‐734. [Google Scholar]
- 37. Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration; 2011. www.cochrane-handbook.org. [Google Scholar]
- 38. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407‐415. [DOI] [PubMed] [Google Scholar]
- 39. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 40. Sterne JAC, Egger M, Moher D, Boutron I. Chapter 10: Addressing reporting biases In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017). Cochrane, 2017. www.training.cochrane.org/handbook. [Google Scholar]
- 41. Becker LB, Aufderheide TP, Geocadin RG, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between cerebral performance category, modified Rankin scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin JW, Wang MJ, Yu HY, et al. Comparing the survival between extracorporeal rescue and conventional resuscitation in adult in‐hospital cardiac arrests: Propensity analysis of three‐year data. Resuscitation. 2010;81:796‐803. [DOI] [PubMed] [Google Scholar]
- 44. Shin TG, Choi JH, Jo IJ, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Critical Care Medicine. 2011;39:1‐7. [DOI] [PubMed] [Google Scholar]
- 45. Patricio D, Peluso L, Brasseur A, et al. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a retrospective propensity score matched study. Crit Care. 2019;23:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fjolner J, Greisen J, Jorgensen MR, et al. Extracorporeal cardiopulmonary resuscitation after out‐of‐hospital cardiac arrest in a Danish health region. Acta Anaesthesiol Scand. 2017;61:176‐185. [DOI] [PubMed] [Google Scholar]
- 47. D'Arrigo S, Cacciola S, Dennis, M , et al. Predictors of favourable outcome after in‐hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta‐analysis. Resuscitation. 2017;121:62‐70. [DOI] [PubMed] [Google Scholar]
- 48. Kagawa E, Inoue I, Kawagoe T, et al. Assessment of outcomes and differences between in‐and out‐of‐hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010;81:968‐973. [DOI] [PubMed] [Google Scholar]
- 49. Lamhaut L, Jouffroy R, Soldan M, et al. Safety and feasibility of prehospital extra corporeal life support implementation by non‐surgeons for out‐of‐hospital refractory cardiac arrest. Resuscitation. 2013;84:1525‐1529. [DOI] [PubMed] [Google Scholar]
- 50. Dumas F, Cariou A, Manzo‐Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out‐of‐ hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3:200‐207. [DOI] [PubMed] [Google Scholar]
- 51. Camuglia AC, Randhawa VK, Lavi S, Walters DL. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: review and meta‐analysis. Resuscitation. 2014;85:1533‐1540. [DOI] [PubMed] [Google Scholar]
- 52. Callaway CW, Schmicker RH, Brown SP, et al. ROC Investigators. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out‐of‐hospital cardiac arrest. Resuscitation. 2014;85:657‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dumas F, Bougouin W, Geri G, Lamhaut L, et al. Emergency percutaneous coronary intervention in post‐cardiac arrest patients without ST‐segment elevation pattern: insights from the PROCAT II Registry. JACC Cardiovasc Interv. 2016;9:1011‐1018. [DOI] [PubMed] [Google Scholar]
- 54. Garcia S, Drexel T, Bekwelem W, et al. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: the Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J Am Heart Assoc. 2016;5:e002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim SJ, Kim SL, Lee HY, Ahn HS, Lee SW. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: a meta‐analysis. Resuscitation. 2016;103:106‐116. [DOI] [PubMed] [Google Scholar]
- 56. Wang GN, Chen XF, Qiao L, et al. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a meta‐analysis of 2 260 patients with cardiac arrest. World J Emerg Med. 2017;8:5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Debaty G, Babaz V, Durand M, et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out‐of‐hospital refractory cardiac arrest. A systematic review and meta‐analysis. Resuscitation. 2017;112:1‐10. [DOI] [PubMed] [Google Scholar]
- 58. Ouwenell DM, Schotborgh JV, Limpens L, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta‐analysis. Intensive Care Med. 2016;42:1922‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nance JR, Sistino JJ. Heparin‐coated adult ECMO vs. ventricular assist devices: a decision analysis modeling approach. J Extra Corpor Technol. 2006;38:33‐37. [PMC free article] [PubMed] [Google Scholar]
- 60. Roos JB, Doshi SN, Konorza T, et al. The cost‐effectiveness of a new percutaneous ventricular assist device for high‐risk PCI patients: mid‐stage evaluation from the European perspective. J Med Econ. 2013;16:381‐390. [DOI] [PubMed] [Google Scholar]
- 61. Maini B, Gregory D, Scotti DJ, Buyantseva L. Percutaneous cardiac assist devices compared with surgical hemodynamic support alternatives: cost‐effectiveness in the emergent setting. Catheter Cardiovasc Interv. 2014;83:E183‐E192. [DOI] [PubMed] [Google Scholar]
- 62. Chang HH, Chen PL, Chen IM, et al. Cost‐utility analysis of direct ventricular assist device vs double bridges to heart transplantation in patients with refractory heart failure. Clin Transplant. 2017;31(12). [DOI] [PubMed] [Google Scholar]
- 63. St‐Onge M, Fan E, Megarbane B, Hancock‐Howard R, Coyte PC. Venoarterial extracorporeal membrane oxygenation for patients in shock or cardiac arrest secondary to cardiotoxicant poisoning: a cost‐effectiveness analysis. J Crit Care. 2015;30:437.e7‐e14. [DOI] [PubMed] [Google Scholar]
- 64. Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;4:717‐729. [DOI] [PubMed] [Google Scholar]


