Abstract
With an increasing number of left ventricular assist devices (LVADs) being placed every year, emergency clinicians are increasingly likely to encounter them in their practice. Patients may present to the emergency department (ED) with significant hemodynamic perturbations with an LVAD and it is imperative that emergency clinicians are able to assess and treat conditions contributing to low cardiac output states. This review describes the important aspects of the third generation of LVADs and their complications as well as common management approaches for the emergency physician.
Keywords: left ventricular assist device, LVAD, mechanical circulatory support
1. INTRODUCTION
With approximately six million Americans diagnosed with heart failure, emergency physicians are acutely aware of its impact. 1 , 2 Three million patients are admitted annually for heart failure and this number is projected to increase. 3 Given the morbidity and mortality associated with chronic heart failure, many patients are being considered for advanced therapies including left ventricular assist devices (LVADs). Though initially used as a bridge to transplant, LVAD indications have expanded beyond bridge to transplant to bridge to recovery, bridge to (transplant) candidacy, and destination therapy (DT). 4
The mortality of patients with end‐stage heart failure has been improved by LVADs. 5 In fact, 36.6% of patients who received heart transplants from 2009 to June 2014 were transplanted from an LVAD ‐ nearly three times as many compared to 1992–2003. 6 According to Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) data, from 2006 to 2016, an estimated 17,634 LVADs have been implanted. 7 Given the increasing number of LVAD implantations nationwide, emergency physicians are increasingly likely to encounter them in practice. This manuscript reviews the third‐generation continuous flow LVADs (cf‐LVADs) presently implanted and their imminent significance to emergency physicians.
2. LVAD GENERATIONS
LVADs have undergone significant structural evolution since their first implant in 1963. 8 The difference in each generation of LVADs pertain to their pump mechanics and their implant sites. The first‐generation LVADs are characterized by pulsatile flow into the ascending aorta and established their utility in patients who were deemed eligible for DT after the REMATCH trial in 2001. 9 , 10 Given the numerous complications of the first‐generation devices, namely burdensome device design, proclivity for infection, and device failure after 18–30 months after implantation, first‐generation devices are no longer used. 11
The flagship device of the second generation of LVADs is the Thoratec HeartMate II. 12 The second‐generation LVADs utilize a continuous (rather than a pulsatile) flow into the ascending aorta by way of an axial flow pump and are generally smaller than their predecessors. In addition, second‐generation LVADs are implanted in the preperitoneal upper abdomen. The majority of LVADs implanted in recent years have been cf‐LVADs owing to lower incidences of adverse events. 7 In 2014, an acute increase in HeartMate II thromboses three months after their implantations was observed by several heart failure centers, the etiology of which remains a subject of debate. 13 The higher rates of device thrombosis in concert with increased rates of gastrointestinal bleeding led to a decrease in use of the HeartMate II. 12
The third (and current) generation of devices are cf‐LVADs, which have a centrifugal flow of blood through the pump, as opposed to an axial flow of blood through the pump as seen in previous LVAD models. The existing third‐generation LVADs are the HeartMate 3 and the HeartWare (HVAD). Antecedent devices are either no longer being implanted, being exchanged for newer models, or are being explanted for patients who receive heart transplants.
3. LVAD ANATOMY
Regardless of the manufacturer, the third‐generation LVADs are structurally similar. Third‐generation LVAD pump design and pump implant location are displayed in Figures 1 and 2 respectively. Blood from the left ventricular cavity is delivered to the pump by way of an inflow cannula, implanted into the left ventricular apex and directed at the mitral valve. In previous generations, the inflow cannula was separate from the pump; however, third‐generation LVAD inflow cannulae are integrated into the pump housing. Given the inflow cannula's proximity to the pump housing, this allows for another unique feature of the third‐generation devices: intrapericardial pump location. A representative chest X‐ray of a third‐generation LVAD is in Figure 3.
FIGURE 1.
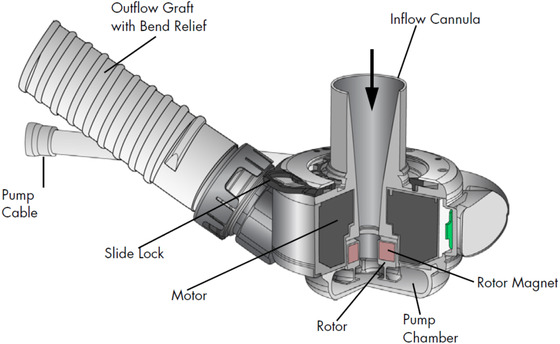
HeartMate 3 pump design. Reproduced with permission of Abbott
FIGURE 2.
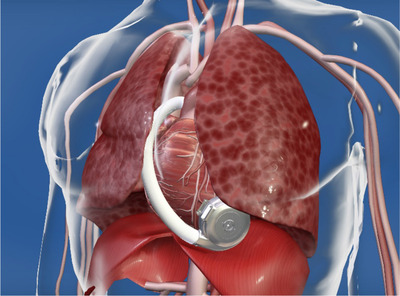
HVAD pump location. Reproduced with permission of Medtronic, Inc.
FIGURE 3.
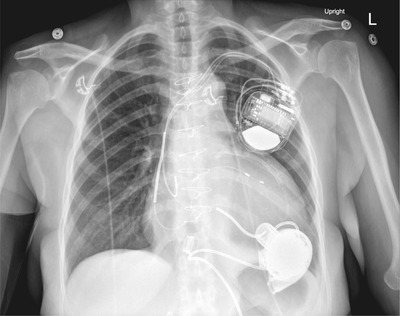
Third‐generation LVAD chest X‐Ray. Case courtesy of Dr Jayanth Keshavamurthy, rID 43083, Radiopaedia.org
An additional improvement in the third‐generation LVAD design was the elimination of contact of the rotor with the pump housing. The newer mechanism is entirely magnetic and lowers the risk for pump thrombus. 12 In fact, the MOMENTUM 3 trial demonstrated that the HeartMate 3 (centrifugal flow) had a significantly better 24‐month event‐free survival compared to the HeartMate II (axial flow). 14
An LVAD driveline, as seen in Figure 4, contains the wires that connect the pump to an external controller. The external controller features a display with indicators of LVAD function as seen in Figure 5. Each controller requires either AC wall outlet input or dual battery input to provide energy to the pump. Both the HVAD and HeartMate 3 require two batteries that simultaneously discharge in order to power the controller. 15 , 16 After the blood is impelled through the pump it goes through an outflow graft into the ascending aorta where it mixes with blood that is ejected out of the aortic valve. Aortic regurgitation leads to recirculation from the aorta through the pump and is competitive with function. There are surgical and interventional techniques to minimize this occurrence.
FIGURE 4.

HVAD with driveline. Reproduced with permission of Medtronic, Inc.
FIGURE 5.
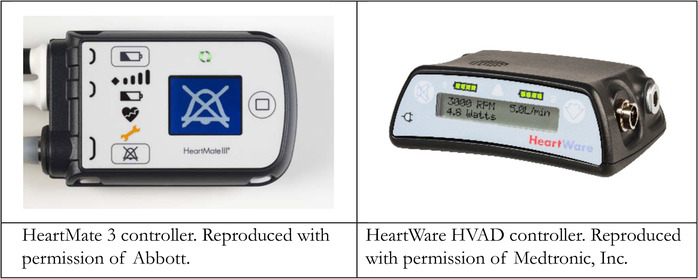
Third‐generation LVAD controllers
FIGURE 6.
Third‐generation LVAD consoles
4. LVAD PARAMETERS
Common to all LVADs are four canonical settings available in Table 1. The only setting that clinicians can adjust on an LVAD is the speed of the motor in revolutions per minute (RPM). Both third‐generation LVADs have preprogrammed features that periodically vary clinician‐set RPMs in order to decrease stagnant areas of blood in the left ventricle. 15 , 17 Based on the patient's cardiovascular dynamics, several dependent variables are recorded by the LVAD system.
TABLE 1.
Left ventricular assist device parameters
| Characteristics | |
|---|---|
| Speed (RPM) | Set by clinician. |
| Flow (LPM) | Calculated. |
| Power (Watts) | Energy applied to pump to maintain flow. |
| Pulsatility index | Proportional to the left ventricle's contribution to cardiac output. |
The LVAD power (reported in Watts) is the amount of energy that is consumed by the pump to keep the RPM constant. Power consumption varies during the cardiac cycle and peaks with systole where the controller increases available current to keep RPM constant when additional blood flows through the pump. The pulsatility index (PI) is a quotient of cardiac cycle powers that is proportional to the inherent LV contractility and preload status of the left ventricle. 18 The PI value may increase with a higher LV contribution or a lower pump contribution to flow. Flow is an estimated value (in liters per minute) reported by the LVAD that is proportional to power.
5. PATIENT ASSESSMENT AND WORKUP
When a patient with an LVAD presents to the ED, the patient should be placed into a resuscitation bay and connected to an LVAD bedside console if available. The precordium should be auscultated for an expected LVAD hum. Standard telemetry including continuous ECG, pulse oximetry, and blood pressure monitoring should be used for every patient. Intravenous access should be obtained early and laboratory studies should be sent for routine chemistries, lactic acid, liver function tests, complete blood count, coagulation profile (prothrombin time/international normalized ratio [PT/INR], partial thromboplastin time, fibrinogen, plasma‐free hemoglobin, haptoglobin, lactate dehydrogenase), troponin, and a type and screen. Regardless of the reason for presentation, assessment of device function is required. A general patient assessment algorithm can be seen in Figure 7. A list of emergent diagnoses to both consider and rule‐out can be seen in Table 2.
FIGURE 7.
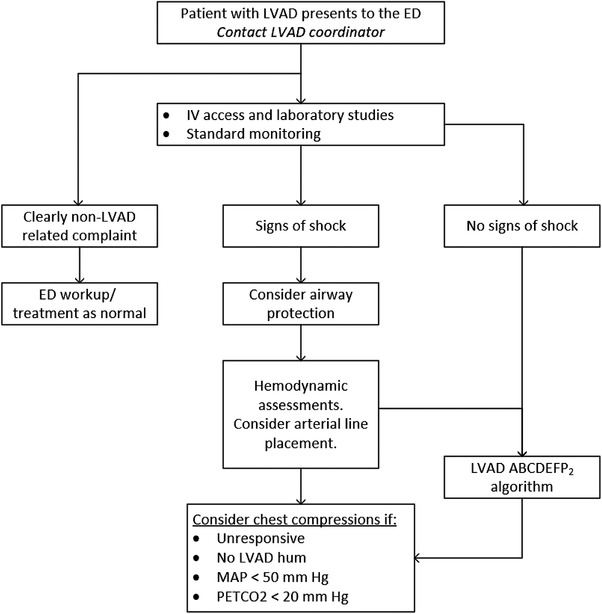
Emergency department approach to a patient with an LVAD
TABLE 2.
Emergent left ventricular assist device diagnoses
| Emergent diagnoses in patients with left ventricular assist devices |
|---|
|
Gastrointestinal bleeding/hemorrhage • Septic shock • RV failure • Arrhythmia • Pump thrombosis • Stroke • Driveline injury/malfunction |
After LVAD implantation, patients and their families are discharged from the hospital by their advanced heart failure team with a contact person (a “VAD coordinator”) for when questions arise. A patient's VAD coordinator should be involved as early as possible in a patient's ED stay to first, facilitate communication between the ED and the advanced heart failure team, and second, to facilitate timely and appropriate patient disposition. The VAD coordinator can and should be contacted even if the patient presents to a hospital different from where the VAD was implanted.
In patients without mechanical circulatory support, obtaining a blood pressure relies on the Korotkoff sounds to yield a systolic and diastolic blood pressure. As patients with LVADs have minimal (if any) left ventricular pulsatility, non‐invasive blood pressure recordings as well as pulse‐oximeter readings are unreliable. 19 A bedside measurement, called a “return to flow” is one way of assessing mean arterial pressure in patients with LVADs. This value may be obtained using a conventional sphygmomanometer and deflating the inflated cuff until a sound is heard. If this is unattainable and the clinical situation suggests hemodynamic compromise, placement of an arterial line is reasonable. Of note, mean arterial pressures > 90 mmHg have been reported to present a stroke risk in patients with LVADs. 20 In addition, guidelines suggest invasive blood pressure monitoring in patients with an LVAD who present with significant trauma. 21
As for all patients with heart failure, patient assessment should include a determination of volume status. Patients with LVADs may present to the ED hypovolemic (sepsis, over‐diuresis, or bleeding), hypervolemic (under‐diuresis), or euvolemic. Determination of fluid status in patients with LVADs should rely on patient history, physical exam, and point‐of‐care ultrasound (POCUS).
Point‐of‐care echocardiography can be challenging in patients with LVADs because of both foreign and distorted anatomy. Although LV contractility is a parameter that emergency physicians often assess, in patients with LVADs, LV contractility is nearly uniformly poor and provides little insight into patient presentation. POCUS may provide an assessment of inferior vena cava diameter and variability (whereby estimating right atrial pressure) as well as relative chamber sizes. Additional critical data points, such as local pericardial tamponade, may be difficult or impossible to obtain from transthoracic echocardiography.
Regarding patient disposition, if not already at an LVAD center, the patient should be transferred to an LVAD center. Patients with shock, regardless of etiology, should be sent to an intensive care unit facile with LVADs. If the patient has no evidence of hemodynamic compromise, a floor admission may be appropriate. Regardless of level of care of disposition, the advanced heart failure team should be consulted early.
6. ETIOLOGIES OF SHOCK IN PATIENTS WITH LVADS
Hemodynamic abnormalities in a patient with an LVAD requires a broad differential, from decreased intravascular volume (hemorrhage, dehydration), right heart failure, arrhythmia, obstruction (mechanical, thrombotic), and septic shock. 21 An algorithmic approach to patients with LVAD complaints can be seen in Figure 8.
FIGURE 8.
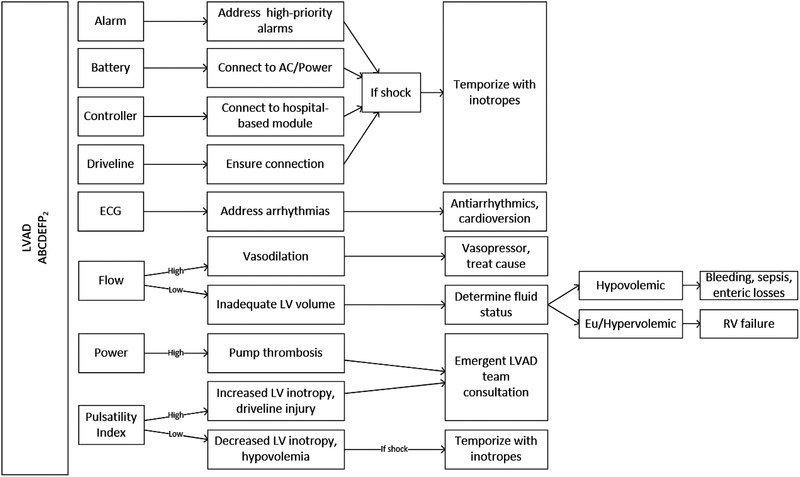
LVAD ABCs, an algorithm for patients with hemodynamic perturbations
States of hypoperfusion may be difficult to elucidate in patients with mechanical circulatory support. Clinical clues such as mental status, hypotension, excessive tachycardia, diaphoresis, and general appearance may provide clues to the emergency physician that the patient is experiencing visceral malperfusion. The LVAD itself, by alarming, may alert both the patient and the clinician that the patient is experiencing hemodynamic compromise. Both device manufacturers use tiered alarm priorities to signify their acuity. For example, high‐priority/critical alarms are displayed with a red indicator. In addition, both manufacturers have displays on their controllers where patients and clinicians can receive immediate instructions. Careful interpretation of the LVAD alarms may elucidate the etiology for a patient's hemodynamic decline.
Bleeding is the most common complication in patients with LVADs. 22 In addition to being on systemic anticoagulation, the etiologies behind bleeding diatheses in patients with LVADs are several, including an acquired Von Willebrand's deficiency. 22 , 23 Gastrointestinal bleeding (a significant number from arteriovenous malformations) and epistaxis are the most common bleeding complications after the immediate postoperative period. 21 , 23 Management of bleeding in a patient with an LVAD is particularly difficult as coagulopathy correction mandates consideration of both hemodynamic maintenance and potential thrombotic complications (namely, LVAD thrombosis and stroke). In the setting of a patient with an LVAD who is acutely bleeding, an elevated INR may signify either supratherapeutic levels of a vitamin K antagonist and/or congestive hepatopathy from a failing right ventricle.
Clinical situation permitting, hemoglobin thresholds for red blood cell transfusions should be discussed with the patient's heart failure team because if the patient has received an LVAD as a bridge to cardiac transplantation, excessive transfusions may result in alloimmunization, which may cause the patient to manufacture antibodies, later contributing to transplant rejection. 21 In addition to correcting anemia, correction of coagulopathy may be undertaken with prothrombin complex concentrate in order to avoid excess volume often encountered with fresh frozen plasma. Strategies to reverse a patient's anticoagulation should be discussed with the patient's advanced heart failure team in order to balance restoration of coagulation with appropriate anticoagulation so as to not place the patient at risk for device thrombosis.
A suction event occurs when the inflow cannula of the LVAD abuts the wall of the left ventricle. This event occurs because of an under‐filled LV cavity or because of an interventricular septum distending into the LV from either right ventricular distension or pericardial tamponade. When the inflow cannula adheres to the LV wall, pump inflow abruptly decreases. When a suction event is detected by an LVAD, typically by a decrease in power, LVADs have a preprogrammed protocol to decrease RPMs to allow the inflow cannula to distance itself from the wall or septum, followed by RPM recovery. 24 Typically, patients will be alerted to this event because of low flow and/or low PI alarms. Patients may complain of symptoms of low cardiac output during these events as the LVAD output is low. If a hospital‐based console is available, as seen in Figure 6, decremental speed changes may be undertaken on the console until hemodynamics improve. Otherwise, cautious administration of intravascular volume will reexpand the LV cavity allowing for restoration of LVAD flows. If right ventricular failure is the cause for the suction event, intravenous fluids may worsen the RV failure leading to more suction events.
Right heart failure is common after an LVAD insertion both in the immediate postoperative period and in the months thereafter. 21 , 22 , 25 Owing to the heterogeneity of how many studies define right ventricle (RV) failure, the incidence is quoted at anywhere between 3.9% and 53%. 2 Prior to LVAD insertion, the RV accommodated lower volumes as the left ventricle's stroke volume was low. After LVAD insertion, the left heart output abruptly increases and the RV must accommodate the higher venous return. This higher venous return may distend the RV and cause it to fail. An elevated central venous pressure (manifested, for example, as a plethoric inferior vena cava on POCUS) and hypotension should lead clinicians to suspect right heart failure. Right heart failure may be addressed in a variety of ways. In the emergent setting, inotropes such as epinephrine should be given to increase RV contractility. In the setting of severe hypotension, milrinone should be avoided. In addition, patients with RV failure will likely require diuretics to lower RV preload, pulmonary vasodilators to lower RV afterload, and rarely, insertion of a temporary right ventricular assist device (RVAD). In addition to the patient's LVAD as a cause for RV failure, clinicians must also examine for etiologies for elevated pulmonary vascular resistance, for example, hypoxemia.
A unique cause of RV failure is sustained ventricular arrhythmia. In the setting of, for example, ventricular fibrillation, the LV is continuously emptied by the LVAD. The RV, in the setting of ventricular fibrillation, does not contract but still has incoming blood volume as the LVAD continues to move blood from the arterial to the venous system. This increase in RV preload without corresponding RV contractility causes the RV to distend and fail.
Arrhythmias are common in patients with LVADs. The mechanisms behind arrhythmias in this patient populations are complex, however, they pertain to abnormal myocardium before LVAD implantation as well as ventricular remodeling therafter. 26 As it has been established that implantable cardioverter‐defibrillators (ICDs) improve survival in patients with both ischemic (and potentially non‐ischemic) cardiomyopathy, most patients who are considered for LVAD candidacy already have an ICD. 27 , 28 The frequency of ventricular arrhythmias decrease as the patient becomes further removed from their LVAD implantation. 26
In patients without mechanical support, malignant ventricular arrhythmias are hemodynamically poorly tolerated. Because LVADs provide continuous cardiac output, patients may be less clinically labile in what are ordinarily lethal rhythms. 29 Despite being in, for example, ventricular fibrillation, cardiac output may be maintained entirely by the LVAD and thus consciousness may not be impaired. Though less “emergent” than a typical cardiac arrest, it is important to correct malignant arrhythmias to prevent RV failure. Though typical treatment of ventricular arrhythmias with medications in patients with LVADs is seldom successful, standard medications such as amiodarone, lidocaine, and procainamide may be used. 26 , 30 Treatment of the etiology of the ventricular arrhythmia (such as electrolyte abnormalities or a suction event) may lead to its resolution. As in patients without mechanical support, in the setting of hemodynamic or mental status deterioration, it is appropriate to perform a synchronized cardioversion or defibrillation, especially if the patient's ICD is not firing.
One of the most feared complications of an LVAD is pump thrombosis. Thrombus can form anywhere along the LVAD machinery and may be one of two clot types: “red clot” or “white clot.” 23 “Red clots” are rich in red blood cells and are conceptually similar to a “typical” thrombus seen in a deep venous thrombosis. “White clots,” however, develop insidiously and consist of denatured fibrin that accumulates on the pump surface, potentially because of heat generated by the device. 23
Patients who are subtherapeutic on their anticoagulation as well as those who have low‐VAD‐flow states (either from low VAD speeds or obstruction) are at highest risk of thrombosis. 21 The HeartMate II's successor, the HeartMate 3, demonstrated a significantly decreased rate of pump thrombosis, potentially owing to the improved design of the pump. 31 Given the mechanism of LVADs, a certain degree of hemolysis is expected. As such, INTERMACS has published criteria for major and minor hemolysis available in Table 3. 32
TABLE 3.
Interagency Registry for Mechanically Assisted Circulatory Support hemolysis definitions 31
| Parameter | Minor hemolysis | Major hemolysis |
|---|---|---|
| Plasma‐free hemoglobin (mg/dL) | >20 | |
| Lactate dehydrogenase | >2.5× upper limit of normal range | |
| Pump function | Normal | Abnormal |
| Clinical signs | Absent |
Hemoglobinuria Anemia Hyperbilirubinemia |
The diagnosis of pump thrombosis should be considered in patients whose LVAD displays high power alarms. High power alarms (“power spikes”) suggest that higher wattage is required to maintain same RPMs. To support the diagnosis of LVAD thrombus, markers of hemolysis (lactate dehydrogenase, haptoglobin, plasma‐free hemoglobin) will likely be elevated. In more severe cases of LVAD thrombosis, cardiogenic shock becomes more evident (eg, narrowed pulse pressure) and inotropes are required to maintain acceptable hemodynamics. A lack of an auscultatory LVAD hum may suggest complete LVAD thrombosis. Computed tomography (CT) angiography of the LVAD is poorly sensitive although highly specific for LVAD thrombus. 33 The optimal therapy for LVAD thrombosis is debated; however, intravenous anticoagulation is commonly employed. Should these measures (with or without the administration of thrombolytics) fail, a pump‐exchange is the final solution for LVAD thrombosis.
LVAD infections are an independent predictor of mortality. 22 Given that LVADs require a connection between the pump and an external controller by way of the driveline that interfaces with skin, the driveline has historically been and continues to be the most common site for infection. 10 , 34 With an incidence estimated around 14%–40%, LVAD infection risk factors include a larger driveline surface area, poor hygiene, high body mass index, and pocket hematomas. 21 , 22 , 34 , 35 Most commonly, gram‐positive cocci are the culprit organisms. 22 , 34 Examination of the driveline site is important for diagnosis of a driveline infection as infections often present with a cellulitis around the site. ED workup of a suspected device‐related infection resembles the workup for infection as with any other indwelling vascular device. There is no universal algorithm dictating the initial management of a suspected LVAD infection; however, common practice is to provide broad‐spectrum antimicrobial coverage after the appropriate diagnostics are obtained.
In patients without mechanical support, the decision to start external chest compressions depends on the presence or absence of a palpable pulse. As patients with LVADs may not have a pulse, the diagnosis of cardiac arrest can be somewhat nebulous given that conventional diagnostic criteria for cardiopulmonary arrest do not apply. Historically, there has been significant trepidation on performing external chest compressions on patients with LVADs because of the concern of disrupting the graft and device anastomoses. Moreover, both continuous‐flow LVAD manufacturers advise caution when performing external chest compressions. 15 , 16
Despite no documented cases of an LVAD becoming dislodged after external chest compressions, many clinicians are hesitant to perform them. According to a statement from the American Heart Association in 2017, in a pulseless, unresponsive patient with an LVAD with a PETCO2 < 20 mmHg through an endotracheal tube, rescuers should start external chest compressions. 36 A summary of this protocol has been previously published. 36 As PETCO2 correlates with cardiac output and therefore systemic perfusion, it is unlikely that a cardiovascular demise is the etiology of a patient's unconsciousness if PETCO2 value is normal. Bedside cardiac ultrasound may also provide insight to a patient's native contractility.
Stroke occurs in anywhere from 13% to 30% of patients with LVADs. 22 The peak incidences of stroke are early in the postoperative course and 9–12 months after LVAD insertion. 21 Infectious processes increase pro‐coagulant pathways, and as such, increase stroke risk. 19 , 37 In the case of ischemic strokes, given the paucity of data in the LVAD patient population, neither thrombolytics nor clot retrieval are “routinely recommended.” 21 , 22 There are, however, cases of thrombolytics being used safely in patients with LVADs. 21 If a patient with an LVAD develops an intracerebral hemorrhage, reversal of their prescribed anticoagulation should be undertaken in consultation with the heart failure team.
7. CONCLUSION
Given the increase in the number of patients with indications for and implantations of LVADs, emergency clinicians must be cognizant of LVAD physiology and its consequences. Future directions in LVAD therapy will likely focus on minimally invasive implantation techniques, smaller device footprints, improved device durability, and augmented biventricular support. 38
Hockstein MA. Continuous‐flow left ventricular assist devices: Management in the emergency department JACEP Open. 2020;1:362–370. 10.1002/emp2.12178
Supervising Editor: Bernard P. Chang, MD, PhD.
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The author has stated that no such relationships exist.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Ali H‐JR, Kiernan MS, Choudhary G, et al. Right ventricular failure post‐implantation of left ventricular assist device: prevalence, pathophysiology, and predictors. ASAIO J. 2020;66(6):610‐619. [DOI] [PubMed] [Google Scholar]
- 3. Metra M, Teerlink JR. Heart failure. Lancet North Am Ed. 2017;390(10106):1981‐1995. [DOI] [PubMed] [Google Scholar]
- 4. Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2013;32(2):141‐156. [DOI] [PubMed] [Google Scholar]
- 5. Pal N, Stansfield J, Mukhopadhyay N, Nelson M. Marginal improvement in survival post‐heart transplantation in patients with prior left ventricular assist device: a temporal analysis of united network of organ sharing registry. J Cardiothorac Vasc Anesth. 2020;34(2):392‐400. [DOI] [PubMed] [Google Scholar]
- 6. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty‐second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34(10):1244‐1254. [DOI] [PubMed] [Google Scholar]
- 7. Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080‐1086. [DOI] [PubMed] [Google Scholar]
- 8. Kirklin JK, Rogers JG. Mechanical Circulatory Support: A Companion to Braunwald's Heart Disease. 2nd ed Elsevier Inc; 2019. [Google Scholar]
- 9. Rodriguez LE, Suarez EE, Loebe M, Bruckner BA. Ventricular assist devices (VAD) therapy: new technology, new hope. Methodist DeBakey Cardiovasc J. 2013;9(1):32‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345(20):1435‐1443. [DOI] [PubMed] [Google Scholar]
- 11. Mancini D, Colombo PC, Devices left ventricular assist. J Am Coll Cardiol. 2015;65(23):2542‐2555. 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 12. Levine A, Gass A. Third‐generation LVADs: has anything changed?. Cardiol Rev. 2019;27(6):293‐301. [DOI] [PubMed] [Google Scholar]
- 13. Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 14. Mehra MR, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device — Final Report. N Engl J Med. 2019;380(17):1618‐1627. [DOI] [PubMed] [Google Scholar]
- 15. Thoratec Corporation . HEARTMATE 3TM LEFT VENTRICULAR ASSIST SYSTEM: Instructions for Use. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160054C.pdf. Published online August 2017.
- 16. HeartWare HVAD System Instructions for Use. https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100047S090D.pdf
- 17. Kumar J, Elhassan A, Dimitrova G, Essandoh M. The lavare cycle: a novel pulsatile feature of the HVAD continuous‐flow left ventricular assist device. J Cardiothorac Vasc Anesth. 2019;33(4):1170‐1171. [DOI] [PubMed] [Google Scholar]
- 18. Soucy KG, Koenig SC, Giridharan GA, Sobieski MA, Slaughter MS. Defining pulsatility during continuous‐flow ventricular assist device support. J Heart Lung Transplant. 2013;32(6):581‐587. https://www.jhltonline.org/article/S1053-2498(13)01151-0/fulltext. [DOI] [PubMed] [Google Scholar]
- 19. Pratt AK, Shah NS, Boyce SW. Left ventricular assist device management in the ICU. Crit Care Med. 2014;42(1):158‐168. [DOI] [PubMed] [Google Scholar]
- 20. Willey JZ, Boehme AK, Castagna F, et al. Hypertension and stroke in patients with Left Ventricular Assist Devices (LVADs). Curr Hypertens Rep. 2016;18(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Givertz MM, DeFilippis EM, Colvin M, et al. HFSA/SAEM/ISHLT clinical expert consensus document on the emergency management of patients with ventricular assist devices. J Heart Lung Transplant. 2019;38(7):677‐698. [DOI] [PubMed] [Google Scholar]
- 22. Han JJ, Acker MA, Atluri P. Left ventricular assist devices. Circulation. 2018;138(24):2841‐2851. [DOI] [PubMed] [Google Scholar]
- 23. Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2017;36(11):1164‐1173. [DOI] [PubMed] [Google Scholar]
- 24. Moazami N, Fukamachi K, Kobayashi M, et al. Axial and centrifugal continuous‐flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant. 2013;32(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 25. Rich JD, Burkhoff D. HVAD flow waveform morphologies: theoretical foundation and implications for clinical practice. ASAIO J Am Soc Artif Intern Organs. 2017;63(5):526‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopinathannair R, Cornwell WK, Dukes JW, et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019;139(20):e967‐e989. [DOI] [PubMed] [Google Scholar]
- 27. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151‐2158. [DOI] [PubMed] [Google Scholar]
- 28. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877‐883. [DOI] [PubMed] [Google Scholar]
- 29. Fasseas P, Kutalek SP, Kantharia BK. Prolonged sustained ventricular fibrillation without loss of consciousness in patients supported by a left ventricular assist device. Cardiology. 2002;97(4):210‐213. [DOI] [PubMed] [Google Scholar]
- 30. Kadado AJ, Akar JG, Hummel JP. Arrhythmias after left ventricular assist device implantation: incidence and management. Trends Cardiovasc Med. 2018;28(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 31. Mehra MR, Goldstein DJ, Uriel N, et al. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378(15):1386‐1395. [DOI] [PubMed] [Google Scholar]
- 32.INTERMACS Executive Committee . INTERMACS Adverse Event Definitions: Adult and Pediatric patients. Published online May 15, 2013. Accessed February 1, 2020. Search Results Web results www.uab.edu›intermacs›images›protocol_4.0›protocol_4.0_MoP. https://biolincc.nhlbi.nih.gov/media/studies/intermacs/Manual_of_Procedures.pdf?link_time=2018-10-19_01:16:22.252330
- 33. Tran BC, Nijjar PS. Role of contrast CT for the diagnosis and the prognosis of suspected LVAD thrombosis. J Card Surg. 2017;32(2):162‐165. [DOI] [PubMed] [Google Scholar]
- 34. Blanco‐Guzman MO, Wang X, Vader JM, Olsen MA, Dubberke ER. Epidemiology of Left Ventricular Assist Device infections: findings from a large non‐registry cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 10.1093/cid/ciaa011. Published online January 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Horo JC, Abu Saleh OM, Stulak JM, Wilhelm MP, Baddour LM, Rizwan Sohail M. Left ventricular assist device infections: a systematic review. ASAIO J Am Soc Artif Intern Organs. 2018;64(3):287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peberdy MA, Gluck JA, Ornato JP, et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: a scientific statement from the American Heart Association. Circulation. 2017;135(24):e1115‐e1134. 10.1161/CIR.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 37. Nakajima I, Kato TS, Komamura K, et al. Pre‐ and post‐operative risk factors associated with cerebrovascular accidents in patients supported by left ventricular assist device. ‐Single center's experience in japan‐. Circ J Off J Jpn Circ Soc. 2011;75(5):1138‐1146. [DOI] [PubMed] [Google Scholar]
- 38. Kanwar MK, Bailey S, Murali S. Challenges and future directions in left ventricular assist device therapy. Crit Care Clin. 2018;34(3):479‐492. [DOI] [PubMed] [Google Scholar]


