Abstract
Tularemia is a rare zoonotic disease caused by Francisella tularensis. It can often present with varied clinical presentations, but meningitis is extremely rare. In this case study, we describe a patient who presented to our emergency department with a Tularemic infection coupled with acute atypical meningitis, after he was exposed to aerosolized rabbit hair from lawn mowing.
Prompt diagnosis of tularemic meningitis may be difficult without a known history of animal exposure. Despite what is taught in medical school, numerous studies have shown Kernig's sign, Brudzinski's sign, and nuchal rigidity do not have much diagnostic value in adults with meningitis. Yet, almost all patients with meningitis present with at least 2 of the 4 symptoms of fever, headache, altered mental status, and neck stiffness. For this reason, it is essential to stop using Kernig's sign and Brudzinski's sign as the only basis for diagnosing meningitis in every case.
With the rampant population increase of rabbits in states like Colorado, Missouri, and Illinois, and a growing number of tularemic patients from lawn mowing incidents popping up across the country, it is also vital to consider the diagnosis of tularemia in your differential diagnosis and send for a cerebrospinal fluid culture, based on a more detailed historytaking of your patient, specifically noting his/her outdoor activities during the initial assessment in the emergency department (ED). This would immensely speed up the process of diagnosing the patient and would ensure a timely start of antibiotics for a full recovery.
Keywords: aerosolized, arthropod, Francisella tularensis, infectious diseases, lawn mowing, tick, tularemia, tularemic meningitis, vector‐borne disease, zoonotic
1. INTRODUCTION
Tularemia is a rare zoonotic disease, whereas tularemic meningitis is even rarer still with only 17 cases previously reported in the United States. 1 Tularemia can present with oropharyngeal, oculoglandular, ulceroglandular, glandular, typhoidal, and pneumonic symptoms, but involvement of the central nervous system resulting in meningitis is extremely rare. We describe a rare case of tularemic meningitis presenting to the emergency department.
2. CASE DESCRIPTION
A 76‐year‐old man presented to the ED with a 1‐day history of fever, mild generalized myalgia, and resolving lethargy. The patient denied any neck pain or headache. The patient appeared well with no signs of neurologic impairment on physical examination. Initial evaluation in the ED was unremarkable, showing a slightly elevated creatinine level of 1.54 and normal WBC counts. The patient was discharged with a diagnosis of viral syndrome and advised to follow up with his primary care physician.
After discharge, the patient reported initial improvement with the ability to resume daily activities. However, 4 days later he started experiencing difficulty walking. He also reported losing his sense of balance.
Five days later, the patient returned to the ED, presenting with severe lethargy, confusion, and a maculopapular rash on his back (Figure 1). His vitals were: HR = 95 bpm; RR = 10 breaths/min; BP = 155/74 mm Hg; temp = 102.8°F; SpO2 = 90%.
FIGURE 1.
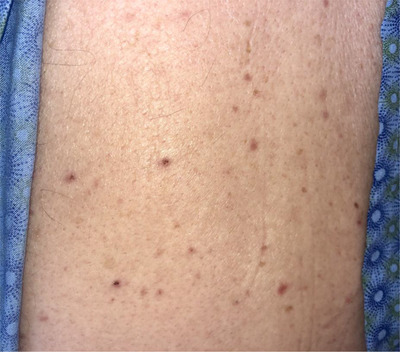
Maculopapular rash on the patient's back, noticed during his second visit to the ED
On examination, the patient was found to be febrile, tachypneic, and hypoxic. Our sepsis protocol was initiated, with labs showing a normal lactate and a slightly increased creatinine (1.6 mg/dL). Blood glucose was found to be 430 mg/dL with a sodium of 133 mmol/L. WBC count was 7200 μL. No ketoacidosis was present at presentation.
The patient received intravenous fluids, insulin for his hyperglycemia, and empiric broad spectrum antibiotics (vancomycin, ceftriaxone, and ampicillin). The emergency physicians performed a lumbar puncture. The patient was admitted to the hospital. Initial cerebrospinal fluid cell counts were suspicious for bacterial meningitis. Cerebrospinal fluid cultures revealed a gram‐negative coccobacillus. The microorganism was then identified as Francisella tularensis by the Colorado Department of Health.
On further questioning, the patient recalled driving a riding lawn mower over a dead rabbit carcass ≈1.5 weeks previously. The care team changed the antibiotics to gentamicin and doxycycline. The patient recovered and was discharged home stable, after 8 days of admission at the hospital. Patient was discharged with a prescription for gentamicin, which was to be continued for another 14 days to complete antibiotic regimen and to follow up with an ear, nose and throat specialist as soon as possible following discharge, to complete a baseline audiology exam.
3. DISCUSSION
Tularemia is a zoonotic disease caused by an intracellular, pleomorphic, non‐motile, non‐sporulating, aerobic, and a gram‐negative coccobacillus, Francisella tularensis.
The 2 main broad subtypes of F. tularensis that cause the most cases of tularemia are:
Type A: most virulent serotype for humans; it is most commonly found in rabbits and rodents in the United States and Canada; and
Type B: most commonly known to cause a mild ulceroglandular infection and is found in aquatic animals in Europe and Asia. 9
F. tularensis is a hardy organism capable of surviving for weeks at extremely low temperatures in moist soil, water, hay, and decaying animal carcasses. Small mammals like rabbits, squirrels, and mice are natural reservoirs for F. tularensis. Naturally acquired human infections of tularemia can occur through direct contact with infected rodents, bite of an infected arthropod vector (tick, deer flies, fleas), ingestion of contaminated food or water, or inhalation of aerosolized bacteria. 11
Tularemia can often present with varied clinical presentations, namely with oropharyngeal, oculoglandular, ulceroglandular, glandular, typhoidal, and pneumonic symptoms, but involvement of the central nervous system resulting in meningitis is extremely rare. 3 , 4 , 5 The onset of tularemic symptoms is usually sudden in nature, occurring 1–10 days after exposure to the disease process. The patient can present with headache, chills, nausea, vomiting, and an extremely high fever of between 103°F and 104°F. Subsequently, the patient might develop severe weakness, recurrent chills, and drenching sweats. In a patient infected with type A subtype, the patient's general condition tends to deteriorate with time, slowly developing rhabdomyolysis, and ultimately going into septic shock. Type B subtype is usually non‐lethal in humans, even when appropriate treatment is not given to the patient. 9
Early antibiotic therapy is highly recommended for persons exposed to or infected with tularemia. For decades, effective treatment for tularemia has consisted of an aminoglycoside, tetracycline, and fluoroquinolone, with doxycycline or a fluoroquinolone often used as prophylaxis after a high risk exposure. Because human‐to‐human transmission of tularemia is not known to occur, post‐exposure prophylaxis of close contacts to the patient is not required. 11 It should be noted that if gentamicin is used as a treatment option, the patient should be informed of its possible ototoxic side effects before the start of the treatment regimen, 12 and the patient should be advised to follow up as soon as possible with an ear, nose, and throat specialist to complete a baseline hearing test, post‐discharge.
Since 1931, only 17 cases of tularemic meningitis have been reported in the United States, of which only 10 survived (Figure 2). The worldwide incidence of tularemia is likely unknown and under‐recognized, but tularemia has not been infrequent in the United States. Between 2000 and 2017, 2831 cases of tularemia from 44 states have been reported to the Centers for Disease Control and Prevention (CDC). 2
FIGURE 2.
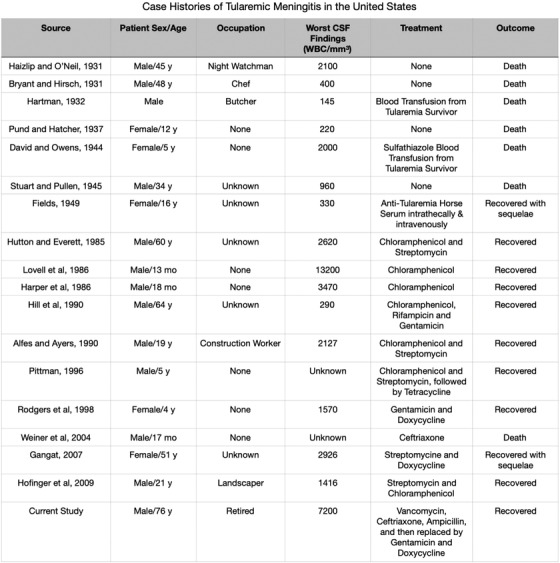
List of case histories of tularemic meningitis in the United States. CSF: cerebral spinal fluid
F. tularensis is classified as a Category A bioterrorism agent by the CDC. Tularemia was also one of the several biological weapons stockpiled by the US military in the late 1960s, all of which were destroyed by 1973. Tularemia is known to be endemic in several European countries, including the former Soviet Union, Turkey, Germany, Sweden, Kosovo, and Finland. F. tularensis subspecies, holarctica, a less virulent subspecies, is the most common cause of infection. There are suspicions that the rise of tularemia in Europe could have been because of the use of the causative agent of tularemia as a biological weapon between 1318 and 1320 BC. Most recently, there was a tularemia outbreak in Sweden in 2018. The primary vector of transmission has been identified as mosquitos, and 979 cases were reported as of October 6, 2019, recording the worst outbreak in Sweden since 1967. 8 In the United States, the most recent outbreak of tularemia occurred in 2000 in Martha's Vineyard, with 1 fatality. The last known study of a patient with tularemic meningitis in the United States was in 2009.
Our case is the first case report, and only the third reported instance in the world, of a patient being infected with tularemia directly attributed to lawn mowing. It is also only the 18th reported case of tularemic meningitis in the United States since 1931. Prompt diagnosis of tularemic meningitis may be difficult without a known history of animal exposure. Despite what is taught in medical school, numerous studies have shown Kernig's sign, Brudzinski's sign, and nuchal rigidity do not have much diagnostic value in adults with meningitis. 6 Yet, almost all patients with meningitis present with at least 2 of the 4 symptoms of fever, headache, altered mental status, and neck stiffness. 7 For this reason, it is essential to stop using Kernig's sign, Brudzinski's sign, and nuchal rigidity as the only basis for diagnosing meningitis in every case. Especially with the rampant population increase of rabbits in states like Colorado, Missouri, and Illinois, 13 and a growing number of tularemic patients from lawn mowing incidents popping up across the country, it is vital for emergency physicians in states with increased rabbit populations to consider the diagnosis of tularemia in your differential diagnosis and send for a cerebrospinal fluid culture, based on a more detailed history taking of the patient, specifically noting his/her outdoor activities during the initial assessment in the ED. This would immensely speed up the process of diagnosing the patient and would ensure a timely start of antibiotics for a speedy and full recovery.
4. CONCLUSIONS
We describe a case in which a patient presented to our ED with acute atypical meningitis after exposure to tularemia from lawn mowing, and the diagnosis was not made until his second ED visit, because of the absence of Kernig's sign, Brudzinski's sign, and nuchal rigidity. This case report highlights a rare case of tularemic meningitis in the United States and stresses the importance of detailed historytaking, specifically asking for animal exposure especially in states with increased populations of rabbits, during the patient's initial assessment in the ED itself.
ACKNOWLEDGMENT
We would like to thank Dr. Henry E. Wang, MD, MS, Editor in Chief for JACEP Open, for assistance with editing and his detailed suggestions that greatly improved this manuscript.
Venkatesan S, Johnston C, Mehrizi MZ. A rare case of tularemic meningitis in the United States from aerosolized Francisella tularensis . JACEP Open. 2020;1:238–241. 10.1002/emp2.12037
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Alejandro Martin‐Quiros, PhD
REFERENCES
- 1. Hofinger DM, Cardona L, Mertz GJ, Davis LE. Tularemic meningitis in the United States. Arch Neurol. 2009;66(4):523‐527. 10.1001/archneurol.2009.14. [DOI] [PubMed] [Google Scholar]
- 2. Statistics | Tularemia | CDC . Centers for Disease Control and Prevention. https://www.cdc.gov/tularemia/statistics/index.html. Accessed July 20, 2019.
- 3. Tularemia. NORD (National Organization for Rare Disorders) . https://rarediseases.org/rare-diseases/tularemia/. Published 2016. Accessed July 20, 2019.
- 4. Penn RL. Tularemia: clinical manifestations, diagnosis, treatment, and prevention Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc.; 2020. https://www.uptodate.com/contents/tularemia-clinical-manifestations-diagnosis-treatment-and-prevention. [Google Scholar]
- 5. Tularemia. Lyme Disease . https://www.columbia-lyme.org/tularemia. Published April 12, 2018. Accessed July 22, 2019.
- 6. Thomas KE, Hasbun R, Jekel J, Quagliarello VJ. The diagnostic accuracy of Kernig's sign, Brudzinski's sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis. 2002;35(1):46‐52. 10.1086/340979. [DOI] [PubMed] [Google Scholar]
- 7. Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31(3):167‐171. 10.1111/j.1526-4610.1991.hed3103167.x. [DOI] [PubMed] [Google Scholar]
- 8. Dryselius R, Hjertqvist M, Mäkitalo S, et al. Large outbreak of tularaemia, central Sweden, July to September 2019. Euro Surveill. 2019;24(42). 10.2807/1560-7917.es.2019.24.42.1900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bush LM, Perez MT. Tularemia ‐ Infectious Diseases. MSD Manual Professional Edition. https://www.msdmanuals.com/professional/infectious-diseases/gram-negative-bacilli/tularemia#v26500089. Published April 2018. Accessed January 15, 2020.
- 10. Tärnvik A, Berglund L. Tularaemia. Eur Respir J. 2003;21(2):361‐373. 10.1183/09031936.03.00088903 [DOI] [PubMed] [Google Scholar]
- 11.Francisella Tularensis (Tularemia). Johns Hopkins Center for Health Security. http://www.centerforhealthsecurity.org/our-work/publications/francisella-tularensis-fact-sheet. Published February 26, 2014. Accessed January 15, 2020.
- 12. Guerpillon B, Boibieux A, Guenne C, et al. Keep an ear out for Francisella tularensis: otomastoiditis cases after canyoneering. Front Med. 2016;3:9 10.3389/fmed.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Increase in human cases of Tularemia—Colorado, Nebraska, South Dakota, and Wyoming, January–September 2015. Ann Emerg Med. 2016;68(1):117‐118. 10.1016/j.annemergmed.2016.03.044 [DOI] [Google Scholar]


