Summary
Immune checkpoint inhibitors are emerging as a therapeutic approach for patients with advanced or metastatic gastrointestinal malignancies following the recent Food and Drug Administration and Asian approvals for colorectal, gastric, and hepatocellular carcinoma. As discussed in earlier articles, phase I–II trials demonstrate quite positive clinical activity, particularly in patients with immunogenic cancer subtypes. This outreach paper discusses some of the next innovative immunotherapy strategies under development. Here, tumor-associated macrophages, myeloid-derived suppressor cells, and regulatory T cells are increasingly coming into focus as new targets. Besides the well described use of checkpoint inhibitors, blockade of ‘Wnt’ or Csf1R signaling pathways as well as combinatorial treatment strategies offer promising examples for overcoming immune silencing within the resistant tumor microenvironment.
Keywords: Gastrointestinal cancer, Immunotherapy, Wnt blockade, Virotherapy
Introduction
Gastrointestinal tumors are aggressive cancers and known for their resistances to chemotherapeutic treatment approaches. Gastrointestinal tumors are very heterogeneous, and hence there is a strong clinical need to clearly define predictive biomarkers such as Epstein-Barr virus in virally-induced cancers [1] or the consensus molecular subtypes (CMS) [2, 3]. Additionally, it is of scientific significance to further increase our understanding of the different interactions between tumor cells, the tumor microenvironment, and the immune system to identify and develop more effective therapies, particularly in non-responsive tumors [4].
Checkpoint Blockade
As shown in previous articles [1–8], the immune system is a gifted therapeutic partner alongside chemotherapy, irradiation, or other innovative strategies. Blockade of the B7 immunoglobulin superfamily molecules programmed cell death protein 1 (PD-1) (nivolumab, pembrolizumab), cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (tremelimumab), and programmed death ligand 1 (PD-L1) (durvalumab, atezolizumab, avelumab) has shown promising results in many trials with patients with gastrointestinal malignancies. The PD-1-blocking antibodies pembrolizumab and nivolumab obtained treatment results in deficient mismatch repair (dMMR)/microsatellite instability high (MSI-H) colorectal cancer patients [2]. For this reason, the Food and Drug Administration approved both checkpoint inhibitors for dMMR or MSI-H refractory or metastatic colorectal and esophageal cancer patients in the United States (US). Also, for the treatment of hepatocellular carcinoma, nivolumab reached approval in the US as second-line treatment, while pembrolizumab induced a clinical benefit in dMMR/MSI-H biliary tract tumors and PD-L1-positive neuroendocrine neoplasms. An entirely different picture is shown for gastric cancer where pembrolizumab is approved for PD-L1-positive tumors only, but nivolumab in for unresectable advanced or recurrent gastric cancer that has progressed after chemotherapyInnovative Strategies
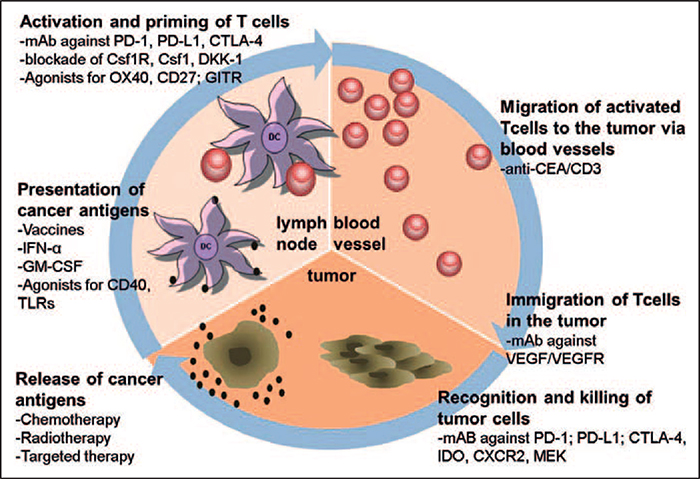
In view of tumors being able to overcome checkpoint blockade and relapse, improved treatment strategies and combinatorial therapies might enhance the efficacy of immunotherapeutic approaches [9]. Correspondingly, innovative approaches, to the largest part relating to the ‘cancer immunity cycle’, are depicted in figure 1 [10] and will be highlighted below. Discussed are novel strategies currently under development, including tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), and regulatory T cells (Tregs) as new immunotherapeutic targets. Additionally, the blockade of glucocorticoid-induced TNFR-related protein (GITR), indoleamine 2 3-dioxygenase (IDO), colony-stimulating factor 1 receptor (Csf1R), and Wnt signaling pathways as combination strategies offers capable approaches for overcoming immune silencing within the non-responsive or resistant tumor microenvironment.
Fig. 1.
Summary of therapeutic strategies in the context of the ‘cancer immunity cycle’. As described in the text, combining different treatment strategies leads to additional benefit and can prevent tumor resistance mechanisms (adapted from [6, 27]).
In comparison to circulating Tregs, GITR is highly expressed on tumor-infiltrating Tregs. Overexpression of GITR correlates with poor prognosis in colorectal, hepatocellular, and renal cancers. Therapeutic modulation of GITR is associated with Treg depletion and stronger antitumor immunity via increased levels of cytotoxic T cells [11].
IDO as a strategic target shows only low expression in normal healthy tissues, whereas strong upregulation is seen in different tumor entities. Overexpression of IDO in non-small cell lung cancer (NSCLC), cervical, colon, pancreatic, and liver cancers was associated with poor prognosis and a higher frequency of lymph node metastasis. As previously described for esophageal adenocarcinoma [5], the IDO gene is upregulated in pre- and post-treatment biopsies. IDO in primary tumors leads to a lack of tryptophan and subsequent decrease in T lymphocytes [12]. In ongoing phase I/II trials, the new IDO inhibitor epacadostat is combined with nivolumab (NCT02327078) or pembrolizumab (NCT03085914) in colorectal cancer patients.
Csf1R is normally expressed by monocytes, monocytic MDSC, and macrophages. Suppression of the Csf1R signaling pathway in pancreatic cancer was associated with decreased TAM and Tregs, reduced tumor progression, and increased T-cell infiltration. An adverse effect was the upregulated expression of checkpoint molecules such as CTLA-4 on T cells and PD-L1 on tumor cells, which suggests that a combination with checkpoint inhibitors may be warranted [13, 14]. As described for colorectal and pancreatic cancer [2, 4], Csf1R blockade induced synergistic effects when combined with other treatment strategies, such as CD40 agonism. One trial combined the Csf1R inhibitor cabiralizumab with nivolumab, with or without chemotherapy, in metastatic pancreatic cancer (NCT03336216 and NCT002526017). The combination was well tolerated. In comparison to single-agent treatment, combined nivolumab and cabiralizumab induced prolonged tumor control and increased overall survival in individual patients.
Constitutive activation of the canonical Wnt signaling pathway is an important event in gastrointestinal tumorigenesis. Upregulation of Wnt-associated proteins such as Wnt-1, −2, and −5a and loss of Wnt inhibitors such as Dickkopf-related protein 1 (DKK-1) and Axin have been shown in lung, esophageal, pancreatic, hepatocellular, bile duct, and gastric cancers [15, 16]. Serum levels of DKK-1 in gastric cancer patients seemed to correlate with TNM staging and poor prognosis [17–19]. As DKK-1 promotes an immunosuppressive tumor milieu by stimulation of MDSC, Gomceli et al. [19] proposed serum DKK-1 levels as a novel serological marker. Currently, there are 2 DKK-1-neutralizing antibodies (BHQ880 and DKN-01) which are well tolerated and clinically effective in advanced tumors such as multiple myeloma or NSCLC. Similar results are expected for the treatment of gastric cancers [20]. In a running clinical trial, patients with gastric cancer are receiving DKN-01 with paclitaxel or pembrolizumab (NCT02013154).
Virotherapy
Finally, oncolytic viruses, such as measles, herpes simplex (HSV), or parvovirus, may be additional novel treatment options to redirect and reboost the human immune system by release of additionaly new tumor antigens [10, 21, 22]. T-VEC, a double mutated HSV-1 virus, releasing GM-CSF, was well tolerated by patients with breast, head/neck, and gastrointestinal cancers [23]. Due to the upregulated expression of checkpoint molecules, such as PD-L1, after virotherapy, combinations with checkpoint inhibitors have shown synergistic effects and may enhance response rates [24, 25]. Combination studies with pembrolizumab or Atezolizumab are currently under investigation in hepatocellular carcinoma, breast cancer and gastrointestinal liver metastases (NCT02509507 and NCT03256344). A further running clinical trial is testing the effect of T-VEC with capecitabine and chemoradiation in rectal cancer patients (NCT03300544). The modified vaccinia poxvirus Pexa-Vec (JX-594) is also currently under development with nivolumab in advanced hepatocellular carcinoma (NCT03071094), and with durvalumab ± tremelimumab in colorectal cancer (NCT03206073), and with sorafenib in hepatocellular carcinoma (NCT02562755). Further analyses and clinical trials will clarify the treatment efficacy of these oncolytic viruses in gastrointestinal cancers [26].
Acknowledgments
Disclosure Statement
Markus Moehler acknowledges receiving the following: grants/research support: AIO, Amgen, BMS, EORTC, Merck, MSD, Taiho, Roche; honoraria or consultation fees: Amgen, AstraZeneca, BMS, Falk, Lilly, MCI, Merck, MSD, Nordic, Pfizer, Roche, Servier. Katrin Gopfert declares no conflict of interest. Heinz-Josef Lenz received advisory board fees and clinical trial support from BMS, Merck KG and Genentech/Roche.
References
- 1.Moehler M: Immunotherapy in gastrointestinal carcinoma – how to separate hope from hype. Oncol Res Treat 2018; 41 DOI: 10.1159/000489048. [DOI] [PubMed] [Google Scholar]
- 2.Stein A, Folprecht G: Immunotherapy of colon cancer. Oncol Res Treat 2018; 41 DOI: 10.1159/000488917. [DOI] [PubMed] [Google Scholar]
- 3.Smyth E, Thuss-Patience P: Immunotherapy of gastric cancer. Oncol Res Treat 2018; 41 DOI: 10.1159/000489099. [DOI] [PubMed] [Google Scholar]
- 4.Cheung PF, Lutz M, Siveke JT: Immunotherapy and combination strategies in pancreatic cancer: current status and emerging trends. Oncol Res Treat 2018; 41 DOI: 10.1159/000488917. [DOI] [PubMed] [Google Scholar]
- 5.Alsina M, Moehler M, Lorenzen S: Immunotherapy of esophageal cancer: current status, many trials and innovative strategies. Oncol Res Treat 2018; 41 DOI: 10.1159/000488120. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich B, Czauderna C, Marquardt JU: Immunotherapy of hepatocellular carcinoma. Oncol Res Treat 2018; 41 DOI: 10.1159/000488916. [DOI] [PubMed] [Google Scholar]
- 7.Tariq N, Vogel A, Namarav Mc, Valle JW: Biliary tract cancer: implicated immune-mediated pathways and their associated potential targets. Oncol Res Treat 2018; 41 DOI: 10.1159/000488997. [DOI] [PubMed] [Google Scholar]
- 8.Weber MM, Fottner C: Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat 2018; 41 DOI: 10.1159/000488996. [DOI] [PubMed] [Google Scholar]
- 9.Alsina M, Moehler M, Hierro C, Guardeno R, Tabernero J: Immunotherapy for gastric cancer: a focus on immune checkpoints. Target Oncol 2016; 11: 469–477. [DOI] [PubMed] [Google Scholar]
- 10.Moehler M, Delic M, Goepfert K, Aust D, Grabsch HI, Halama N, Heinrich B, Julie C, Lordick F, Lutz MP, Mauer M, Alsina Maqueda M, Schild H, Schimanski CC, Wagner AD, Roth A, Ducreux M: Immunotherapy in gastrointestinal cancer: recent results, current studies and future perspectives. Eur J Cancer 2016; 59: 160–170. [DOI] [PubMed] [Google Scholar]
- 11.Knee DA, Hewes B, Brogdon JL: Rationale for anti-GITR cancer immunotherapy. Eur J Cancer 2016; 67: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Liu H, Li F, Li H, Yu J, Ren X: The correlation between the subsets of tumor infiltrating memory T cells and the expression of indoleamine 2,3-dioxygenase in gastric cancer. Dig Dis Sci 2013; 58: 3494–3502. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG: CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014; 74: 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T: Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 2016; 5:e1151595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiurillo MA: Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med 2015; 5: 84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M, Nakayama H, Miyagi Y, Tsuchiya E, Kondo S, Nakamura Y, Daigo Y: Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res 2010; 70: 5326–5336. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang GF, Tan Y, Zeng JT, Zhang JW, Tang J, Zeng SP, Qin X: Expression of serum Dickkopf-1 in gastric cancer patients. Asian Pac J Trop Med 2015; 8: 870–872. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SJ, Zhuo SR, Yang XQ, Qin CX, Wang ZL: Serum Dickkopf-1 expression level positively correlates with a poor prognosis in breast cancer. Diagn Pathol 2014; 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomceli I, Bostanci EB, Ozer I, Kemik AS, Turhan N, Tez M, Kilic S, Demiriz B, Akoglu M: A novel screening biomarker in gastric cancer: serum Dickkopf-1. Hepatogastroenterology 2012; 59: 1661–1664. [DOI] [PubMed] [Google Scholar]
- 20.Kagey MHH, Xi: Rationale for targeting the Wnt signaling modulator Dickkopf-1 for oncology. Br J Pharmacology 2017; 174: 4637–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinrich B, Goepfert K, Delic M, Galle PR, Moehler M: Influence of the oncolytic parvovirus H-1, CTLA-4 antibody tremelimumab and cytostatic drugs on the human immune system in a human in vitro model of colorectal cancer cells. Onco Targets Ther 2013; 6: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrich B, Klein J, Delic M, Goepfert K, Engel V, Geberzahn L, Lusky M, Erbs P, Preville X, Moehler M: Immunogenicity of oncolytic vaccinia viruses JX-GFP and TG6002 in a human melanoma in vitro model: studying immunogenic cell death, dendritic cell maturation and interaction with cytotoxic T lymphocytes. Onco Targets Ther 2017; 10: 2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungerechts G, Engeland CE, Buchholz CJ, Eberle J, Fechner H, Geletneky K, Holm PS, Kreppel F, Kuhnel F, Lang KS, Leber MF, Marchini A, Moehler M, Muhlebach MD, Rommelaere J, Springfeld C, Lauer UM, Nettelbeck DM: Virotherapy research in Germany: from engineering to translation. Hum Gene Ther 2017; 28: 800–819. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL: Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun 2017; 8: 14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV: Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017; 170: 1109–1119.e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuhara H, Ino Y, Todo T: Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci 2016; 107: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DS, Mellman I: Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]