Abstract
Background:
In the phase III RECOURSE trial, trifluridine/tipiracil (TAS-102) extended overall survival (OS) and progression-free survival (PFS) with an acceptable toxicity profile in patients with metastatic colorectal cancer refractory or intolerant to standard therapies. The present analysis investigated the efficacy and safety of trifluridine/tipiracil in RECOURSE subgroups.
Methods:
Primary and key secondary end-points were evaluated using a Cox proportional hazards model in prespecified subgroups, including geographical subregion (United States of America [USA], European Union [EU], Japan), age (<65 years, ≥65 years) and v-Ki-ras2 Kirsten rat sarcoma 2 viral oncogene homologue (KRAS) status (wild type, mutant). Safety and tolerability were reported with descriptive statistics.
Results:
Eight-hundred patients were enrolled: USA, n = 99; EU, n = 403; Japan, n = 266. Patients aged ≥65 years and those with mutant KRAS tumours comprised 44% and 51% of all patients in the subregions, respectively. Final OS analysis (including 89% of events, compared with 72% in the initial analysis) confirmed the survival benefit associated with trifluridine/tipiracil, with a hazard ratio (HR) of 0.69 (95% confidence interval [CI] 0.59–0.81; P = 0.0001). Median OS in the three regions was 6.5–7.8 months in the trifluridine/tipiracil arm and 4.3–6.7 months in the placebo arm (USA: HR 0.56; 95% CI 0.34–0.94; P = 0.0277; EU: HR 0.62; 95% CI 0.48–0.80; P = 0.0002; Japan: HR 0.75; 95% CI 0.57–1.00; P = 0.0470). Median PFS was 2.0–2.8 months for trifluridine/tipiracil and 1.7–1.8 months for placebo; HRs favoured trifluridine/tipiracil in all regions. Similar clinical benefits of trifluridine/tipiracil were observed in elderly patients and in those with mutant KRAS tumours. There were no marked differences among subregions in terms of safety and tolerability.
Conclusions:
Trifluridine/tipiracil was effective in all subgroups, regardless of age, geographical origin or KRAS status.
This trial is registered with ClinicalTrials.gov: NCT01607957.
Keywords: Fluoropyrimidine, Metastatic colorectal cancer, Randomised controlled trial, TAS-102, Tipiracil, Trifluridine
1. Introduction
Trifluridine/tipiracil (TAS-102, Lonsurf®; Taiho Oncology Inc., Princeton, NJ, USA) is an orally administered chemotherapy consisting of the antineoplastic thymidine-based nucleoside analogue trifluridine, and a thymidine phosphorylase inhibitor, tipiracil, at a molar ratio of 1:0.5 (weight ratio, 1:0.471). The primary cytotoxic mechanism of trifluridine is through incorporation into DNA, leading to DNA dysfunction and damage [1–3]. The addition of tipiracil improves the bioavailability of trifluridine by inhibiting its catabolism by thymidine phosphorylase [4].
Trifluridine/tipiracil has shown promise in a number of clinical trials, particularly in metastatic colorectal cancer [5–10]. In the phase III RECOURSE trial (NCT01607957), which was conducted in patients with metastatic colorectal cancer refractory to standard therapies, including fluoropyrimidines, irinotecan and oxaliplatin, treatment with trifluridine/tipiracil resulted in a significant improvement in median overall survival (OS) compared with placebo (7.1 versus 5.3 months; hazard ratio [HR] 0.68; P < 0.0001) and in median progression-free survival (PFS) (2.0 versus 1.7 months; HR 0.48; P < 0.0001) [5]. Trifluridine/tipiracil was well tolerated, with few serious adverse events (AEs) reported; neutropenia was the most frequently observed AE. Many patients in this trial [5], as well as all patients in the prior phase II trial [10], were Japanese. Therefore, it is of interest to compare the efficacy and safety of trifluridine/ tipiracil in Western populations with those reported from Japan. The current analyses were performed to further evaluate trifluridine/tipiracil compared with placebo among different patient groups, including geographical subregions, older patients aged ≥65 and ≥70 years, and v-Ki-ras2 Kirsten rat sarcoma 2 viral oncogene homologue (KRAS) status.
2. Patients and methods
2.1. Study design
The RECOURSE trial design has been previously described in detail (Supplementary Fig. 1) [5]. Briefly, RECOURSE was a global, phase III, multicentre, randomised, double-blind, placebo-controlled trial comparing trifluridine/tipiracil plus best supportive care on the one hand with placebo plus best supportive care on the other. Patients were stratified according to (1) KRAS status (wild type, mutant), (2) time since diagnosis of first metastasis (<18 months, ≥18 months) and (3) geographical region (Japan and Western [United States of America (USA), European Union (EU) including United Kingdom, and Australia]), and randomly assigned in a 2:1 ratio to receive trifluridine/tipiracil or placebo. Here, we assess results based on geographical subregions (Japan, USA and EU) and outcomes related to age and KRAS status. Patients were randomised at 21 different sites in the USA, 20 in Japan and 55 across the EU. Australia was omitted from this analysis because of the small number of enrolled patients (n = 32).
2.2. Patients
Patients with biopsy-proven/documented adenocarcinoma of the colon or rectum who had received two or more regimens of standard chemotherapies for metastatic disease were eligible for randomisation. For more details, please see the supplementary methods.
2.3. End-points
The primary end-point was OS, defined as the time from randomisation to death from any cause. Secondary end-points included PFS (time from randomisation to first radiographical confirmation of disease progression or death from any cause), overall response rate (proportion of patients with complete or partial response) and safety. Measurements are described in the supplementary methods.
2.4. Statistical analysis
The study protocol included a prespecified analysis of outcomes and safety according to geographical subregion, KRAS status and potential prognostic/predictive factors, including age <65 versus ≥65 years and <70 versus ≥70 years. OS and PFS were analysed in the intention-to-treat population in each geographical subregion with the use of a two-sided, stratified, logrank test, with the HR and two-sided 95% confidence intervals (CIs) based on a stratified Cox proportional hazards model and the associated Kaplan–Meier survival estimates. Median follow-up time for survival was calculated by means of the reverse Kaplan–Meier method. AEs and laboratory abnormalities were summarised for all patients who received at least one dose of study drug. The number and percentage of patients hospitalised, reason for hospitalisation and total duration of hospitalisation were summarised descriptively by treatment group for each region. Ad hoc analyses of outcomes across subregions according to age ≥65 years, age ≥70 years and KRAS status were also conducted using the same methodology.
The original results of RECOURSE were based on a cut-off date of 24th January 2014 [5]. A further analysis of OS outcomes was performed based on final survival data as of 8th October 2014 and is presented here. Additionally, a multivariate model of potential prognostic factors was developed to create a prognostic index for OS, and included KRAS status, time since diagnosis of first metastasis, geographical subregion, v-raf murine sarcoma viral oncogene homologue B1 (BRAF) status, age, race, gender, primary tumour site, Eastern Cooperative Oncology Group performance status (ECOG PS), number of prior regimens and number of metastatic sites [11].
3. Results
3.1. Baseline characteristics
Of the 800 patients (excluding the 32 from Australia) enrolled in the RECOURSE trial, 99, 403 and 266 were enrolled in the USA, EU and Japan, respectively. Baseline characteristics for patients randomised within these subregions are shown in Table 1. Elderly patients aged ≥65 and ≥70 years represented 44% and 23% of all patients enrolled within the three geographical subregions, respectively; patients with mutant KRAS tumours accounted for 51% of patients. There were some differences in the racial profile of patients from these subregions, with US and EU patients being predominantly Caucasian, and Japanese patients being Asian. More than 60% of patients in the EU and Japan were male individuals compared with approximately 50% in the USA. In Japan, the primary tumour site was evenly divided between colon and rectum, while in the EU, and particularly in the USA, the majority of primary tumour sites were in the colon. Japanese patients appeared to have less symptomatic disease, with 71% having an ECOG PS of 0 compared with 41% in the USA and 51% in the EU. In addition, 75.2% of Japanese patients had a normal baseline estimated glomerular filtration rate compared with 48% in the USA and 56% in the EU.
Table 1.
Baseline characteristics: geographical subgroups in RECOURSE.
| USA |
EU |
Japan |
||||
|---|---|---|---|---|---|---|
| FTD/TPI (n = 64) | Placebo (n = 35) | FTD/TPI (n = 271) | Placebo (n = 132) | FTD/TPI (n = 178) | Placebo (n = 88) | |
| Male, n (%) | 31 (48.4) | 18 (51.4) | 167 (61.6) | 82 (62.1) | 113 (63.5) | 58 (65.9) |
| Age, years, mean (SD) | 60.2 (11.86) | 58.5 (11.02) | 61.8 (9.98) | 62.1 (10.42) | 61.9 (10.09) | 62.1 (10.40) |
| Race, n (%) | ||||||
| Caucasian | 56 (87.5) | 27 (77.1) | 229 (84.5) | 119 (90.2) | 0 | 0 |
| Black/African-American | 3 (4.7) | 5 (14.3) | 1 (0.4) | 0 | 0 | 0 |
| Asian | 5 (7.8) | 3 (8.6) | 1 (0.4) | 1 (0.8) | 178 (100) | 88 (100) |
| Primary tumour site, n (%) | ||||||
| Colon | 47 (73.4) | 27 (77.1) | 176 (64.9) | 81 (61.4) | 100 (56.2) | 45 (51.1) |
| Rectum | 17 (26.6) | 8 (22.9) | 95 (35.1) | 51 (38.6) | 78 (43.8) | 43 (48.9) |
| ECOG PS, n (%) | ||||||
| 0 | 28 (43.8) | 13 (37.1) | 138 (50.9) | 68 (51.5) | 128 (71.9) | 60 (68.2) |
| 1 | 36 (56.3) | 22 (62.9) | 133 (49.1) | 64 (48.5) | 50 (28.1) | 28 (31.8) |
| KRAS statusa, n (%) | ||||||
| Wild type | 35 (54.7) | 17 (48.6) | 123 (45.4) | 68 (51.5) | 94 (52.8) | 40 (45.5) |
| Mutant | 29 (45.3) | 18 (51.4) | 148 (54.6) | 64 (48.5) | 84 (47.2) | 48 (54.5) |
| Time since diagnosis of first metastasisa, n (%) | ||||||
| <18 months | 14 (21.9) | 11 (31.4) | 61 (22.5) | 24 (18.2) | 33 (18.5) | 17 (19.3) |
| ≥18 months | 50 (78.1) | 24 (68.6) | 210 (77.5) | 108 (81.8) | 145 (81.5) | 71 (80.7) |
| Baseline renal functionb, n (%) | ||||||
| Normal (CrCL ≥90 ml/min/1.73 m2) | 43 (67.2) | 16 (45.7) | 164 (60.5) | 72 (54.5) | 86 (48.3) | 46 (52.3) |
| Mild impairment (CrCL 60–89 ml/min/1.73 m2) | 16 (25.0) | 17 (48.6) | 86 (31.7) | 41 (31.1) | 70 (39.3) | 33 (37.5) |
| Moderate impairment (CrCL 30–59 ml/min/1.73 m2) | 5 (7.8) | 2 (5.7) | 21 (7.7) | 16 (12.1) | 20 (11.2) | 9 (10.2) |
| Missing | 0 | 0 | 0 | 3 (2.3) | 2(1.1) | 0 |
| Baseline eGFRc, n (%) | ||||||
| Normal (CrCL ≥90 ml/min/1.73 m2) | 34 (53.1) | 14 (40.0) | 153 (56.5) | 72 (54.5) | 135 (75.8) | 65 (73.9) |
| Mild impairment (CrCL 60–89 ml/min/1.73 m2) | 24 (37.5) | 17 (48.6) | 92 (33.9) | 42 (31.8) | 30 (16.9) | 21 (23.9) |
| Moderate impairment (CrCL 30–59 ml/min/1.73 m2) | 5 (7.8) | 3 (8.6) | 18 (6.6) | 12 (9.1) | 9(5.1) | 1 (1.1) |
| Missing | 1 (1.6) | 1 (2.9) | 8 (3.0) | 6 (4.5) | 4 (2.2) | 1(1.1) |
Abbreviations: CrCL, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; eGFR, estimated glomerular filtration rate; FTD/TPI, trifluridine/tipiracil; SD, standard deviation.
As randomised.
CrCL based on Cockcroft-Gault using baseline creatinine.
eGFR (ml/min/1.73 m2) = 175 × (baseline creatinine)−1.154 × (age)−0.203 × (0.742 if female) × (1.212 if African-American).
3.2. Efficacy
Median OS in the three geographical subregions ranged from 6.5 months in the USA to 7.8 in Japan in the trifluridine/tipiracil arm and from 4.3 months in the USA to 6.7 in Japan in the placebo arm (Fig. 1A and B). Although the longest median OS was observed in Japan, the improvement was greater in the USA and the EU with HRs of 0.56 (95% CI 0.34–0.94; P = 0.0277) and 0.62 (95% CI 0.48–0.80; P = 0.0002), respectively, compared with 0.75 (95% CI 0.57–1.00; P = 0.047) in Japan. Similar trends were reported for PFS, with HRs favouring trifluridine/tipiracil in all three geographical subregions (Fig. 1C and D).
Fig. 1. Kaplan–Meier curves and forest plots for overall survival (OS) and progression-free survival (PFS).
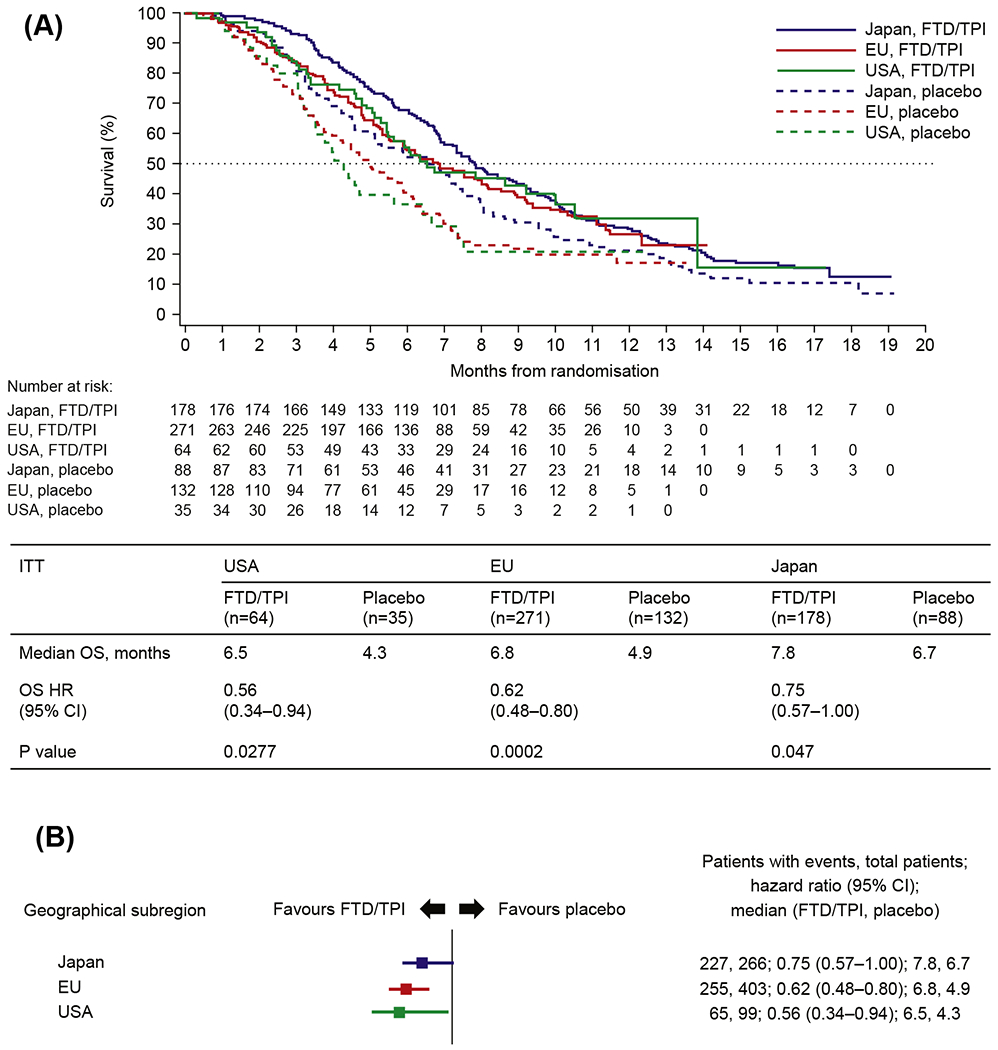

(A) Kaplan–Meier curve for OS by geographical subregion; (B) forest plot for OS by geographical subregion; (C) Kaplan–Meier curve for PFS by geographical subregion; (D) forest plot for PFS by geographical subregion. Abbreviations: CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; ITT, intention-to-treat.
At the time of the data cut-off for the final survival analysis (8th October 2014), 89% of the 800 patients randomised had died, accounting for 138 additional deaths to the 574 (72%) previously included in the original analysis [6]. Median OS in this updated analysis was 7.2 months for trifluridine/tipiracil versus 5.2 months for placebo, with an HR of 0.69 (95% CI 0.59–0.81; P < 0.0001) (Fig. 2). Although the median OS benefit was slightly longer than in the original analysis (2.0 versus 1.8 months), the outcome pattern remains consistent with the original analysis [5]. The final prognostic risk model identified primary tumour site (colon versus rectum), ECOG PS and number of metastatic sites as prognostic risk factors, in addition to the three stratification factors that were included by default. When patients were divided into quartiles by OS according to these prognostic risk factors, the median OS ranged from 4.6 to 10.5 months in the highest- and lowest-risk quartiles, respectively, in the trifluridine/tipiracil arm, and from 3.5 to 7.1 months, respectively, in the placebo arm (Supplementary Table 1). The HR favoured trifluridine/tipiracil over placebo in all quartiles, ranging from 0.67 in the lowest-risk quartile to 0.56 in the highest-risk quartile. Survival rates 1 year after randomisation in this final analysis were 27.1% (95% CI 23.3–30.9) and 16.6% (95% CI 12.4–21.4) in the trifluridine/tipiracil and placebo arms, respectively.
Fig. 2. Overall survival as of 8th October 2014 (intention-to-treat population).
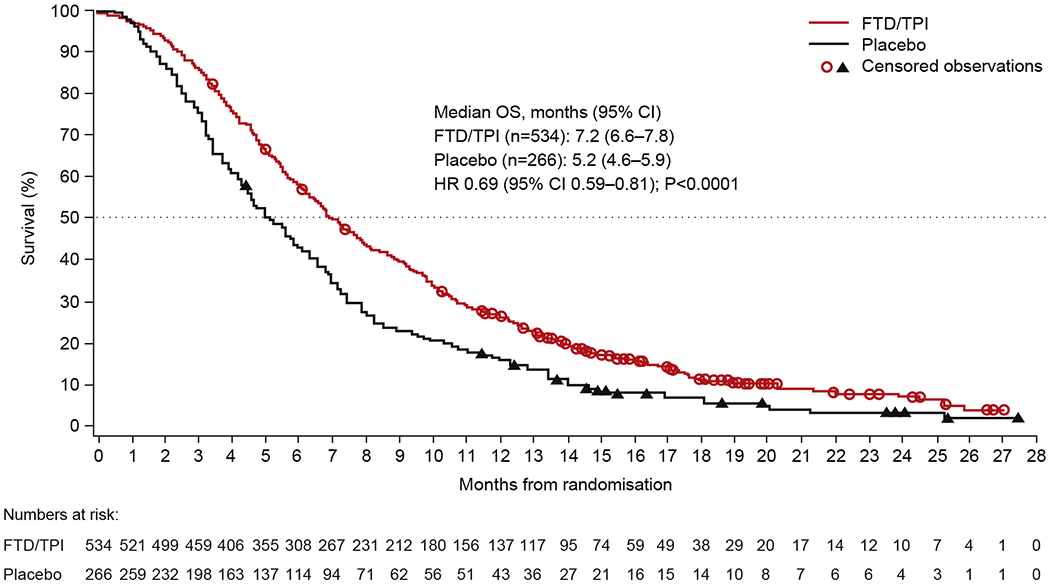
Abbreviations: CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; OS, overall survival.
Table 2 depicts HRs for OS and PFS for patients aged ≥65 years overall and in the USA, the EU and Japan. Median OS for patients aged ≥65 years was 7.0 months in the trifluridine/tipiracil arm, significantly longer than the 4.6 months reported in the placebo arm (HR 0.62; 95% CI 0.48–0.80; P = 0.0002). Similar to the population as a whole, the outcome results favoured trifluridine/tipiracil for OS and PFS in each region. Importantly, results for these elderly patients were similar to those for the overall population in each subregion, consistent with the prognostic model that had rejected age as a prognostic factor. Similar results were noted in an analysis of all patients aged ≥70 years across all three regions; median OS in this age group was 7.0 months in the trifluridine/tipiracil arm compared with 4.7 in the placebo arm (HR 0.65; 95% CI 0.45–0.94; P = 0.0231). HRs for OS and PFS by KRAS are shown in Table 3. OS was longer for patients with wild-type KRAS than for those with mutant KRAS in both the trifluridine/tipiracil and placebo arms overall and among all regions; HRs for OS favoured trifluridine/ tipiracil over placebo regardless of KRAS status, although this benefit for patients with mutant KRAS did not reach statistical significance (P = 0.0712). PFS was significantly longer for wild-type and mutant KRAS (both P < 0.0001) in the population as a whole; HRs again favoured trifluridine/tipiracil but did not reach statistical significance in all subregions.
Table 2.
OS and PFS in RECOURSE in elderly patients aged ≥65 years overall and by geographical subregion (ITT population).
| ITT | USA |
EU |
Japan |
Overall |
||||
|---|---|---|---|---|---|---|---|---|
| FTD/TPI (n = 27) | Placebo (n = 14) | FTD/TPI (n = 115) | Placebo (n = 61) | FTD/TPI (n = 86) | Placebo (n = 39) | FTD/TPI (n = 234) | Placebo (n = 118) | |
| Median OS, months | 7.8 | 4.0 | 6.3 | 4.4 | 7.8 | 6.8 | 7.0 | 4.6 |
| OS HR (95% CI) | 0.31 (0.13–0.79) | 0.59 (0.40–0.86) | 0.72 (0.47–1.09) | 0.62 (0.48–0.80) | ||||
| P value | 0.0099 | 0.0054 | 0.1143 | 0.0002 | ||||
| Median PFS, months | 3.9 | 1.6 | 2.1 | 1.8 | 2.0 | 1.8 | 2.1 | 1.8 |
| PFS HR (95% CI) | 0.24 (0.10–0.61) | 0.37 (0.26–0.53) | 0.52 (0.35–0.78) | 0.41 (0.32–0.52) | ||||
| P value | 0.0013 | <0.0001 | 0.0013 | <0.0001 | ||||
Abbreviations: CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PFS,progression-free survival.
Table 3.
OS and PFS in RECOURSE by KRAS status overall and in geographical subregions (ITT population).
| ITT | USA |
EU |
Japan |
Overall |
||||
|---|---|---|---|---|---|---|---|---|
| FTD/TPI | Placebo | FTD/TPI | Placebo | FTD/TPI | Placebo | FTD/TPI | Placebo | |
| Wild-type KRAS, n | 35 | 17 | 123 | 68 | 94 | 40 | 262 | 131 |
| Median OS, months | 6.7 | 4.3 | 8.8 | 5.5 | 9.0 | 6.8 | 8.0 | 5.7 |
| OS HR (95% CI) | 0.44 (0.22–0.87) | 0.56 (0.38–0.81) | 0.65 (0.44–0.96) | 0.58 (0.45–0.74) | ||||
| P value | 0.0152 | 0.0019 | 0.0309 | <0.0001 | ||||
| Median PFS, months | 2.8 | 1.7 | 2.0 | 1.7 | 2.1 | 1.8 | 2.1 | 1.7 |
| PFS HR (95% CI) | 0.51 (0.26–1.02) | 0.40 (0.28–0.56) | 0.58 (0.39–0.84) | 0.48 (0.38–0.60) | ||||
| P value | 0.0533 | <0.0001 | 0.0041 | 0.0001 | ||||
| Mutant KRAS, n | 29 | 18 | 148 | 64 | 84 | 48 | 272 | 135 |
| Median OS, months | 6.3 | 4.2 | 6.0 | 4.7 | 7.1 | 6.2 | 6.5 | 4.9 |
| OS HR (95% CI) | 0.77 (0.35–1.70) | 0.68 (0.48–0.97) | 0.87 (0.59–1.28) | 0.80 (0.63–1.02) | ||||
| P value | 0.5209 | 0.0345 | 0.4730 | 0.0712 | ||||
| Median PFS, months | 3.1 | 1.7 | 1.9 | 1.8 | 1.9 | 1.8 | 1.9 | 1.8 |
| PFS HR (95% CI) | 0.35 (0.17–0.70) | 0.43 (0.31–0.59) | 0.58 (0.40–0.84) | 0.49 (0.39–0.61) | ||||
| P value | 0.0021 | <0.0001 | 0.0037 | <0.0001 | ||||
Abbreviations: FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PFS, progression-free survival.
3.3. Safety and hospitalisation
There were no significant differences among the US, EU and Japanese subgroups and the overall population with respect to the incidence of AEs, grade ≥3 AEs, serious AEs or hospitalisations (Table 4). Almost all patients experienced an AE, with 98.5%, 96.9% and 99.4% of trifluridine/tipiracil patients and 91.6%, 100% and 92.0% of placebo patients having at least one AE of any grade in EU, US and Japanese subgroups, respectively. The incidence of AEs and haematological laboratory abnormalities of grade ≥3, which were reported in ≥5% of patients in any subregion, are shown in Table 4. The most commonly reported AEs were neutropenia in the USA and EU and anaemia in Japan, but there were no consistent differences between Asian and Western populations with respect to grade 3 or 4 clinical AEs or haematological abnormalities.
Table 4.
Adverse events and haematological laboratory abnormalities of grade ≥3 occurring in >5%, any group (as-treated population).
| n (%) | USA |
EU |
Japan |
|||
|---|---|---|---|---|---|---|
| FTD/TPI (n = 64) | Placebo (n = 35) | FTD/TPI (n = 270) | Placebo (n = 131) | FTD/TPI (n = 178) | Placebo (n = 88) | |
| Abdominal pain | 3 (4.7) | 4 (11.4) | 5 (1.9) | 4 (3.1) | 1 (0.6) | 1 (1.1) |
| Anaemia | 9 (14.1) | 2 (5.7) | 35 (13.0) | 2 (1.5) | 41 (23.0) | 3 (3.4) |
| Aspartate aminotransferase increased | 2 (3.1) | 2 (5.7) | 3 (1.1) | 1 (0.8) | 2 (1.1) | 4 (4.5) |
| Asthenia | 0 | 0 | 18 (6.7) | 8(6.1) | 0 | 0 |
| Blood alkaline phosphatase increased | 2(3.1) | 4 (11.4) | 9 (3.3) | 5 (3.8) | 7 (3.9) | 4 (4.5) |
| Blood bilirubin increased | 2(3.1) | 0 | 13 (4.8) | 7 (5.3) | 6 (3.4) | 2 (2.3) |
| Decreased appetite | 0 | 1 (2.9) | 9 (3.3) | 5 (3.8) | 10 (5.6) | 7 (8.0) |
| Dyspnoea | 1 (1.6) | 0 | 13 (4.8) | 7 (5.3) | 0 | 3 (3.4) |
| Fatigue | 3 (4.7) | 3 (8.6) | 12 (4.4) | 6 (4.6) | 5 (2.8) | 6 (6.8) |
| Febrile neutropenia | 4 (6.3) | 0 | 6 (2.2) | 0 | 10 (5.6) | 0 |
| Gamma-glutamyltransferase increased | 1 (1.6) | 0 | 9 (3.3) | 7 (5.3) | 7 (3.9) | 3 (3.4) |
| General physical health deterioration | 0 | 0 | 15 (5.6) | 9 (6.9) | 3 (1.7) | 3 (3.4) |
| Hepatic failure | 1 (1.6) | 2 (5.7) | 2 (0.7) | 2(1.5) | 0 | 4 (4.5) |
| Hyperbilirubinaemia | 2(3.1) | 2 (5.7) | 8 (3.0) | 2(1.5) | 1 (0.6) | 0 |
| Hypoalbuminaemia | 1 (1.6) | 2 (5.7) | 2 (0.7) | 0 | 1 (0.6) | 0 |
| Hypokalaemia | 6 (9.4) | 0 | 5(1.9) | 2 (1.5) | 1 (0.6) | 0 |
| Leukopenia | 4 (6.3) | 0 | 9 (3.3) | 0 | 0 | 0 |
| Neutropenia | 20 (31.3) | 0 | 79 (29.3) | 0 | 3 (1.7) | 0 |
| Peripheral oedema | 0 | 2 (5.7) | 0 | 0 | 1 (0.6) | 0 |
| Tumour pain | 0 | 0 | 1 (0.4) | 0 | 2(1.1) | 5 (5.7) |
| Vomiting | 3 (4.7) | 0 | 5(1.9) | 1 (0.8) | 3 (1.7) | 0 |
| Haematological laboratory abnormalities | ||||||
| Haemoglobin | 15 (23.8) | 2 (5.7) | 39 (14.6) | 3 (2.3) | 41 (23.0) | 3 (3.4) |
| Lymphocytes | 17 (27.0) | 3 (8.6) | 41 (15.6) | 11 (8.6) | 47 (26.4) | 11 (12.5) |
| Neutrophils | 28 (44.4) | 0 | 100 (37.3) | 0 | 67 (37.6) | 0 |
| Platelets | 5 (7.9) | 0 | 11 (4.1) | 0 | 11 (6.2) | 1(1.1) |
| Leukocytes | 18 (28.6) | 0 | 50 (18.7) | 0 | 43 (24.2) | 0 |
| Hospitalisations | ||||||
| Total hospitalisations | 17 (26.6) | 12 (34.3) | 84 (31.1) | 45 (34.4) | 52 (29.2) | 36 (40.9) |
| Median total days hospitalised | 7.0 | 10.5 | 7.5 | 9.0 | 13.0 | 19.5 |
| Median hospitalisation ratioa | 0.09 | 0.19 | 0.11 | 0.20 | 0.19 | 0.30 |
| Reason for hospitalisation | ||||||
| Serious adverse event | 16 (25.0) | 10 (28.6) | 79 (29.3) | 41 (31.3) | 45 (25.3) | 34 (38.6) |
| Febrile neutropenia | 4 (6.3) | 0 | 4(1.5) | 0 | 6 (3.4) | 0 |
| Elective preplanned surgery alone | 0 | 0 | 3(1.1) | 0 | 1 (0.6) | 0 |
| Hospice/palliative care alone | 0 | 1 (2.9) | 1 (0.4) | 3 (2.3) | 2(1.1) | 2 (2.3) |
| Palliative radiation alone | 0 | 1 (2.9) | 0 | 0 | 0 | 0 |
| Other | 1 (1.6) | 2 (5.7) | 8 (3.0) | 4 (3.1) | 5 (2.8) | 2 (2.3) |
Abbreviation: FTD/TPI, trifluridine/tipiracil.
Total days hospitalised divided by total days followed.
Table 4 also provides details of hospitalisation for any reason in the RECOURSE trial by subregion. Although there were no important differences in hospitalisation rates in association with trifluridine/tipiracil treatment, there was a slightly higher number of hospitalisations within the placebo cohort in Japan compared with the USA and EU. The hospitalisation rate was higher in the placebo arm than in the trifluridine/tipiracil arm in all regions. Duration of hospital stay was also longer in each region for the placebo arm than for the trifluridine/tipiracil arm. Median length of stay was longer in Japan than in the USA and the EU for both trifluridine/tipiracil and placebo arms. Serious AEs were the most common reason for hospitalisation.
4. Discussion
The efficacy and safety of trifluridine/tipiracil were similar across all three geographical subregions (USA, EU and Japan) and were consistent with the overall RECOURSE population. Improved OS and PFS were observed overall and within each geographical subgroup randomised to trifluridine/tipiracil versus placebo, with an acceptable safety profile. Notably, OS in both the trifluridine/tipiracil and placebo groups in Japan was somewhat longer than that observed in the same treatment groups in the other regions (7.8 versus 6.5–6.8 months for trifluridine/tipiracil; 6.8 versus 4.3–4.9 months for placebo). This result was consistent with what had been observed in an earlier phase II study conducted in a similar Japanese population in which the observed median OS values were 9.0 and 6.6 months in the trifluridine/tipiracil and placebo groups, respectively (HR 0.56; 95% CI 0.39–0.81; P = 0.011) [10].
Differences in baseline characteristics were observed between the Western and Japanese populations in the RECOURSE trial, which may help to explain regional differences in OS. As expected, the Japanese population was entirely Asian, whereas the US and EU populations were predominantly Caucasian. The similarity in efficacy among these regions encourages the generalised applicability of the results. Patients enrolled in Japan were more likely to have an ECOG PS of 0 and have a normal estimated glomerular filtration rate than patients enrolled in the USA or the EU, perhaps suggesting that Japanese patients had less advanced disease at study enrolment than their Western counterparts. This may explain the better prognosis at baseline and longer OS and PFS in both the trifluridine/tipiracil and placebo arms in Japan.
This updated survival analysis is based on reports of clinical events in 89% of randomised patients compared with the initial analysis that was based on reports of clinical events in 72% of such individuals [5]. The reported HR of 0.69 (95% CI 0.59–0.81; P < 0.0001) from this analysis, compared with 0.68 (95% CI 0.58–0.81; P < 0.001) in the original analysis, reveals that the OS benefit with trifluridine/tipiracil was maintained, with median OS increasing from 1.8 to 2.0 months. This survival benefit appears to be present in all patients regardless of their prognostic status at the time of trial entry. The prognostic risk model developed identified primary tumour site, ECOG PS and number of metastatic sites as meaningful prognostic risk factors, in addition to the default stratification factors, which included KRAS status, time since diagnosis of first metastasis and geographical subregion. A number of attempts have been made in recent years to develop prognostic risk models for metastatic colorectal cancer [12–15]. Although there are differences in methodology and patient populations among these analyses, ECOG PS, KRAS status and number of metastatic sites are factors common to many models. KRAS status was included in the model developed here by default as it was a stratification factor; however, our results demonstrated that patients with wild-type KRAS generally had a better overall outcome than those with mutant KRAS tumours. The importance of KRAS as a prognostic factor outside the context of targeted treatment is controversial [12], although some studies have demonstrated poorer outcomes in patients with KRAS mutations at codon 12 and in those with metastatic disease [16,17]. The model developed here is somewhat different from other models as it did not include treatment, allowing for the evaluation of trifluridine/tipiracil in different prognostic groups. Results showed all patients benefited equally from trifluridine/tipiracil, regardless of risk factor (including KRAS status), with no effect from the prognostic index. HRs for OS and PFS favoured trifluridine/tipiracil over placebo irrespective of KRAS status. However, these differences did not reach statistical significance in every subgroup across geographical regions, indicating that the decision to treat with trifluridine/tipiracil should not be influenced by prognostic factors if patients have a suitable physical condition with adequate organ function consistent with the entry criteria for this study. Results for patients aged ≥65 and ≥70 years were similar to those for the general population overall and in each subregion, indicating that trifluridine/tipiracil is a tolerable treatment option in older patients.
Trifluridine/tipiracil was generally well tolerated in the RECOURSE trial, with few differences among subregions. Any differences in the incidence of individual AEs between the Western and Japanese populations may be due to variations in the interpretation of terms used to define certain events, rather than any true differences in tolerability. For instance, the rate of neutropenia in Japan was reported as being substantially lower than elsewhere, although the rates of reduced neutrophil counts and febrile neutropenia were similar. Hospitalisation rates and reasons for hospitalisation were similar across subregions, and were consistently lower for trifluridine/tipiracil than for placebo. However, the median length of stay following hospitalisation for both treatment groups was longer in Japan, perhaps due to the lower cost of hospitalisation in this region.
Although the analysis of efficacy in geographical subregions was preplanned, the comparisons in this study are limited by the low number of patients in some subregions. Notably, the number of patients enrolled in the US subregion (n = 99) was substantially lower than that in the EU or Japanese subregions (n = 403 and n = 266, respectively). Therefore, despite being stratified by geographical area (Japan versus USA, EU and Australia combined), sample sizes may have been too small to detect differences between trifluridine/tipiracil and placebo in or between subregions. This issue may be compounded when examining ad hoc analyses of subpopulations, such as elderly patients, within these subregions.
5. Conclusion
OS and PFS benefits were observed in patients randomised to trifluridine/tipiracil compared with placebo in the USA, the EU and Japan, and were consistent with the results from the overall RECOURSE trial, with an acceptable safety profile. These benefits were observed in all defined prognostic subgroups, including elderly subpopulations. Overall, the results of this analysis provide confidence that trifluridine/tipiracil is safe and effective, regardless of age, KRAS status, racial/ethnic differences or other regional differences in geographically disparate patient populations.
Supplementary Material
Acknowledgements
The authors were responsible for the content and editorial decisions related to the development of this manuscript and received no honoraria or compensation associated with it. Editorial assistance in the preparation of this manuscript was provided by Phase Five Communications and Complete HealthVizion, supported by Taiho Oncology, Inc. We thank the following: all the patients, their families and the investigators; Manuel Aivado, Takako Nakajima and Ruben M. Ayzin Rosoky, the sponsor’s medical monitors, and independent data monitoring committee members John Marshall, Keisuke Aiba, Kees Punt and Peter Treasure; and Toshihiko Doi and Takayuki Yoshino for their contributions to the development of the protocol.
Funding
This work was supported by Taiho Oncology, Inc. No grant number is applicable.
Role of the funding source
This work was supported by Taiho Oncology, Inc. No grant number is applicable. Taiho were involved in the study design, in the collection, analysis and interpretation of the data, in the writing of the paper, and in the decision to submit for publication.
Conflict of interest statement
EVC reports grant support from Taiho for the conduct of the study. RJM reports a consulting/advisory role for Taiho. RW reports employment by, a leadership role for and travel, accommodation and expenses from Taiho. CG reports honoraria from Roche, Bayer, Merck and Meda and a consulting/advisory role for Bayer, Sanofi and Servier. MB reports honoraria from Roche, Merck and Sanofi and a consulting/advisory role for Roche, Amgen, Bayer, Merck, Sanofi, Lilly and Servier. FLM reports a consulting/advisory role for Lilly and travel, accommodation and expenses from Roche, Amgen and Lilly. FP reports travel, accommodation and expenses from Roche. FC reports participation in a speaker bureau for Roche, Merck Serono, Bayer, Lilly and Amgen and research funding from Bayer, Roche and Merck Serono. SS reports a consulting/advisory role for Amgen, Roche, Eli Lilly, Merck, Bayer and Sanofi. KY reports participation in a speaker bureau for Takeda, Lilly, Taiho, Chugai and Merck and research funding from Yakuruto-Honsha, Taiho, MSD, Ono, Chugai, Lilly and Merck. KM reports honoraria from Taiho, Chugai, Takeda and Eli Lilly. YT reports honoraria from Merck Serono, Eli Lilly Japan, Chugai, Taiho, Ono, Takeda, Daiichi Sankyo, Kyowakirin, Yakult Honsha, Nippon Kayaku and Medcon and travel, accommodation and expenses from Merck Serono. LM reports employment by Stathmi, Inc. and a consulting/advisory role for Taiho. HJL reports honoraria from, a consulting/advisory role for and research funding from Taiho. AO reports that an immediate family member is an employee of Celgene. All remaining authors have declared no conflicts of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejca.2017.10.009.
References
- [1].Tanaka N, Sakamoto K, Okabe H, Fujioka A, Yamamura K, Nakagawa F, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 2014;32:2319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sakamoto K, Yokogawa T, Ueno H, Oguchi K, Kazuno H, Ishida K, et al. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int J Oncol 2015;46:2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Emura T, Murakami Y, Nakagawa F, Fukushima M, Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med 2004;13:545–9. [PubMed] [Google Scholar]
- [4].Emura T, Suzuki N, Fujioka A, Ohshimo H, Fukushima M. Potentiation of the antitumor activity of α, α, α-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol 2005;27:449–55. [PubMed] [Google Scholar]
- [5].Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372:1909–19. 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- [6].Bendell JC, Rosen LS, Mayer RJ, Goldman JW, Infante JR, Benedetti F, et al. Phase 1 study of oral TAS-102 in patients with refractory metastatic colorectal cancer. Cancer Chemother Pharmacol 2015;76:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Doi T, Ohtsu A, Yoshino T, Boku N, Onozawa Y, Fukutomi A, et al. Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 2012;107:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Overman MJ, Kopetz S, Varadhachary G, Fukushima M, Kuwata K, Mita A, et al. Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest 2008;26:794–9. [DOI] [PubMed] [Google Scholar]
- [9].Overman MJ, Varadhachary G, Kopetz S, Thomas MB, Fukushima M, Kuwata K, et al. Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Invest New Drugs 2008;26:445–54. [DOI] [PubMed] [Google Scholar]
- [10].Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. [DOI] [PubMed] [Google Scholar]
- [11].Mayer RJ, Ohtsu A, Yoshino T, Falcone A, Garcia-Carbonero R, Tabernero J, et al. TAS-102 versus placebo plus best supportive care in patients with metastatic colorectal cancer refractory to standard therapies: final survival results of the Phase 3 RECOURSE trial. J Clin Oncol 2016;34(suppl 4S), abstract 634. [Google Scholar]
- [12].De Divitiis C, Nasti G, Montano M, Fisichella R, Iaffaioli RV, Berretta M. Prognostic and predictive response factors in colorectal cancer patients: between hope and reality. World J Gastroenterol 2014;20:15049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg 2011;35:684–92. [DOI] [PubMed] [Google Scholar]
- [14].Petrelli F, Coinu A, Cabiddu M, Borgonovo K, Lonati V, Ghilardi M, et al. Prognostic factors for survival with bevacizumab-based therapy in colorectal cancer patients: a systematic review and pooled analysis of 11,585 patients. Med Oncol 2015;32:456. [DOI] [PubMed] [Google Scholar]
- [15].Wilkinson KJ, Chua W, Ng W, Roohullah A. Management of asymptomatic primary tumours in stage IV colorectal cancer: review of outcomes. World J Gastrointest Oncol 2015;7:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Andreyev HJN, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer 2001;85:692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol 2010;17:572–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


