Summary
Background
The anti-progesterone drug mifepristone and the prostaglandin misoprostol can be used to treat missed miscarriage. However, it is unclear whether a combination of mifepristone and misoprostol is more effective than administering misoprostol alone. We investigated whether treatment with mifepristone plus misoprostol would result in a higher rate of completion of missed miscarriage compared with misoprostol alone.
Methods
MifeMiso was a multicentre, double-blind, placebo-controlled, randomised trial in 28 UK hospitals. Women were eligible for enrolment if they were aged 16 years and older, diagnosed with a missed miscarriage by pelvic ultrasound scan in the first 14 weeks of pregnancy, chose to have medical management of miscarriage, and were willing and able to give informed consent. Participants were randomly assigned (1:1) to a single dose of oral mifepristone 200 mg or an oral placebo tablet, both followed by a single dose of vaginal, oral, or sublingual misoprostol 800 μg 2 days later. Randomisation was managed via a secure web-based randomisation program, with minimisation to balance study group assignments according to maternal age (<30 years vs ≥30 years), body-mass index (<35 kg/m2vs ≥35 kg/m2), previous parity (nulliparous women vs parous women), gestational age (<70 days vs ≥70 days), amount of bleeding (Pictorial Blood Assessment Chart score; ≤2 vs ≥3), and randomising centre. Participants, clinicians, pharmacists, trial nurses, and midwives were masked to study group assignment throughout the trial. The primary outcome was failure to spontaneously pass the gestational sac within 7 days after random assignment. Primary analyses were done according to intention-to-treat principles. The trial is registered with the ISRCTN registry, ISRCTN17405024.
Findings
Between Oct 3, 2017, and July 22, 2019, 2595 women were identified as being eligible for the MifeMiso trial. 711 women were randomly assigned to receive either mifepristone and misoprostol (357 women) or placebo and misoprostol (354 women). 696 (98%) of 711 women had available data for the primary outcome. 59 (17%) of 348 women in the mifepristone plus misoprostol group did not pass the gestational sac spontaneously within 7 days versus 82 (24%) of 348 women in the placebo plus misoprostol group (risk ratio [RR] 0·73, 95% CI 0·54–0·99; p=0·043). 62 (17%) of 355 women in the mifepristone plus misoprostol group required surgical intervention to complete the miscarriage versus 87 (25%) of 353 women in the placebo plus misoprostol group (0·71, 0·53–0·95; p=0·021). We found no difference in incidence of adverse events between the study groups.
Interpretation
Treatment with mifepristone plus misoprostol was more effective than misoprostol alone in the management of missed miscarriage. Women with missed miscarriage should be offered mifepristone pretreatment before misoprostol to increase the chance of successful miscarriage management, while reducing the need for miscarriage surgery.
Funding
UK National Institute for Health Research Health Technology Assessment Programme.
Introduction
Miscarriage is common, affecting one in five pregnancies.1 Miscarriage can cause physical harm, such as excessive bleeding and infection,2 and substantial psychological harm, including anxiety, depression, and post-traumatic stress disorder.3, 4 There are two main types of miscarriage that require medical intervention, missed miscarriage and incomplete miscarriage.5 A missed miscarriage, also known as a delayed or silent miscarriage, is diagnosed when a non-viable pregnancy is identified on ultrasound scan during the first 14 weeks of gestation. Often, women who have missed miscarriage are asymptomatic or have small amounts of vaginal bleeding or pain before the diagnosis is made.5, 6, 7 All pregnancy tissue is retained in the uterus in a missed miscarriage.5 By contrast, an incomplete miscarriage is diagnosed when pregnancy tissue has been partly expelled by the uterus.5
Research in context.
Evidence before this study
The National Institute for Health and Care Excellence identified the effectiveness of a mifepristone and misoprostol combination as a research priority, given the paucity of evidence on the optimal regimen for medical management of missed miscarriage, and the National Institute for Health Research subsequently issued a commission call to address this issue. We used MEDLINE to search for studies published from inception to Dec 15, 2015, in any language using the terms “missed miscarriage”, “silent miscarriage”, “delayed miscarriage”, “early pregnancy loss”, “miscarriage, medical”, “mifepristone”, and “misoprostol”. The abstracts of the studies identified by our search were reviewed by two independent reviewers to identify trials that compared mifepristone and misoprostol in combination with misoprostol alone in the medical management of missed miscarriage. We found two relevant trials, with a total of 242 participants. Meta-analysis indicated that the miscarriage completion rate for mifepristone and misoprostol in combination was similar to that of misoprostol alone in the medical management of missed miscarriage (risk ratio [RR] 0·97, 95% CI 0·82–1·13). However, given the imprecision that inevitably accompanies such small sample sizes, we could not draw firm inferences. Since our review, a Cochrane systematic review published in 2019 identified three relevant studies, including a total of 447 women. Meta-analysis of these three trials did not provide a clear consensus, resulting in the reviewers calling for further research.
Added value of this study
Our study was a large multicentre placebo-controlled trial that assessed the effectiveness of the combination of mifepristone and misoprostol versus misoprostol alone in the medical management of missed miscarriage. We recruited 711 women from 28 UK hospitals. We found that mifepristone and misoprostol in combination was more effective than misoprostol alone in achieving completion of missed miscarriage, with an associated reduction in the number of women requiring surgical intervention after failed medical management.
Implications of all the available evidence
Our trial found an increase in the 7-day miscarriage completion rate and a reduction in the need for surgery with the combination of mifepristone and misoprostol when compared with mifepristone alone for women with missed miscarriage. This finding is consistent with another open-label trial in 283 patients, which found a higher rate of miscarriage completion with combination treatment compared with misoprostol alone (RR 1·25, 95% CI 1·09–1·43). Therefore, combination treatment should be considered as first-line treatment for women who wish to have medical management of missed miscarriage. Formal health economic evaluation is underway.
Management of first trimester missed miscarriage can be done in one of the following three ways: expectant, medical, or surgical.6 The recommended first-line treatment for missed miscarriage in the UK is expectant management for a duration of 7 to 14 days, during which women wait for the uterus to expel the pregnancy tissue naturally.5 If expectant management is not successful or not acceptable to the woman, then medical management is preferred. Medical management expedites the expulsion of retained pregnancy tissue with the help of medical drugs.5 Medical management is the preferred option for many women, and is recommended in international clinical guidelines.5, 7, 8
Misoprostol, a prostaglandin analogue, is commonly used for the medical management of miscarriage to induce myometrial contractions to aid the expulsion of pregnancy tissue.8 However, misoprostol is not always effective, and 15–40% of women require an additional dose of misoprostol, thus prolonging the duration of treatment.9, 10, 11, 12, 13 Failure of medical management can result in more surgical procedures being done, which can be particularly undesirable to women who have chosen to have medical management.14, 15 To augment the effect of misoprostol, a steroidal anti-progesterone called mifepristone is sometimes used in combination. Mifepristone is a competitive progesterone receptor antagonist that primes the myometrium before prostaglandin exposure.8
The reported effectiveness of combination treatment with mifepristone and misoprostol for the medical management of missed miscarriage in previous clinical trials has ranged from 64% to 84%.16, 17, 18 However, given the lack of placebo-controlled studies, the usefulness of mifepristone in the management of missed miscarriage has remained unclear. Standard practice in the UK before 2012 was to offer mifepristone plus misoprostol for the medical management of missed miscarriage. However, in 2012, the National Institute for Health and Care Excellence (NICE) guideline (clinical guidance 154) recommended not offering mifepristone as a treatment for missed or incomplete miscarriage.19 This recommendation was based on the findings of a small randomised controlled trial of 115 women, which found no benefit for the use of mifepristone and misoprostol versus misoprostol alone.16 The recommendation to not use combination treatment remains in the current 2019 version of the guidance (NICE guideline 126).5 Therefore, the standard practice in the UK is to offer vaginal misoprostol alone at a dose of 800 μg for women diagnosed with incomplete or missed miscarriage.5
We investigated whether treatment with mifepristone in combination with misoprostol would result in a higher rate of completion of missed miscarriage compared with misoprostol alone.
Methods
Study design and participants
MifeMiso was a multicentre, parallel-group, double-blind, placebo-controlled randomised effectiveness trial in 28 hospitals across the UK. Women were eligible for enrolment if they were aged 16 years and older, diagnosed with a missed miscarriage by pelvic ultrasound scan in the first 14 weeks of pregnancy (by last menstrual period), chose to have medical management of miscarriage, and were willing and able to give informed consent. Diagnosis of missed miscarriage was made by trained ultrasonographers in early pregnancy units if a non-viable pregnancy with the presence of a gestational sac was identified on ultrasound scan. Findings had to be confirmed by a second trained ultrasonographer or, if a second ultrasonographer was unavailable, the ultrasound scan was repeated after a further 7 days, as per NICE guidance for the diagnosis and initial management of miscarriage.5
Women who opted for alternative methods of miscarriage management (expectant or surgical), had a diagnosis of incomplete miscarriage, life threatening bleeding, contraindications to mifepristone or misoprostol, or had participated in another trial of investigational medicinal products during their current pregnancy were excluded from the trial.
Research staff from recruiting sites approached women diagnosed with missed miscarriage to check eligibility for the trial. Verbal and written trial information was provided and written consent was obtained from the participant. A member of the local research team from the recruiting sites gathered and entered baseline data into a web-based database, which included the current amount of vaginal bleeding using a Pictorial Blood Assessment Chart (PBAC) score20 (0–4; 0=no bleeding, 4=heavy bleeding) and a pregnancy-related pain score that was obtained using a visual analogue scale (0–10).
This trial was approved by the UK Medicines and Healthcare Products Regulatory Authority, the UK National Research Ethics Service Committee (West Midlands—Edgbaston; reference 17/WM/0017), and the National Health Service Research and Development department at each participating hospital. The study protocol is available online.
Randomisation and masking
Participants were randomly assigned (1:1) to a single dose of oral mifepristone 200 mg, followed by a single dose of vaginal, oral, or sublingual misoprostol 800 μg 2 days later, or an oral placebo tablet followed by a single dose of vaginal, oral, or sublingual misoprostol 800 μg 2 days later. Identical mifepristone and matched placebo tablets were packaged and supplied by MODEPHARMA (London, UK). MODEPHARMA had no role in the design of the study, the collection, analysis, or interpretation of the data, or the writing of the report.
Randomisation was managed via a secure web-based randomisation program provided by MedSciNet (London, UK), with minimisation to balance study group assignments according to maternal age (<30 years vs ≥30 years), body-mass index (BMI; <35 kg/m2 vs ≥35 kg/m2), previous parity (nulliparous women vs parous women), gestational age (<70 days vs ≥70 days), amount of bleeding (PBAC score;20 ≤2 vs ≥3), and randomising centre. Participants, clinicians, pharmacists, trial nurses, and midwives were masked to study group assignment throughout the trial.
Procedures
Participants were randomly allocated to a single dose of oral mifepristone 200 mg or an oral placebo tablet. Administration of the oral tablets took place at early pregnancy units in the recruiting hospitals. Participants then left the early pregnancy unit with instructions to return to have their scheduled single dose of vaginal, oral, or sublingual misoprostol 800 μg 48 h later. The scheduled single dose of misoprostol 800 μg could be omitted if the participant had already passed the gestational sac after the mifepristone or placebo tablet. After 48 h of receiving the single dose of misoprostol 800 μg, if the participant had no or little bleeding, they were asked to contact the research team for consideration of a further dose of misoprostol.
Participants were advised to return for a pelvic ultrasound scan 7 days after random assignment. If participants had heavy vaginal bleeding or pain during the course of treatment, they were advised to contact their local recruiting site to undergo appropriate assessment and management. Emergency miscarriage surgery was done if clinically indicated (eg, heavy or life threatening vaginal bleeding or failure of medical management to expel the gestational sac with the participant choosing to undergo surgical management). If participants passed the gestational sac within 7 days after random assignment, they were asked to do a urinary pregnancy test 3 weeks after random assignment. If the urinary pregnancy test was negative, the participant was discharged from the trial. If participants failed to spontaneously pass the gestational sac within 7 days after random assignment, they were managed according to local hospital practice, which generally involved offering participants further doses of misoprostol 800 μg or surgical management of miscarriage after appropriate clinical assessment. Participants remained in the trial until they had a negative pregnancy test and were discharged from the early pregnancy unit.
Outcome data for participants were collated by research team members by reviewing the clinical case notes and entering data into a web-based database. Participants were invited to take part in in-depth interviews following the resolution of their miscarriage. The findings from this mixed-methods study will be reported separately.
Outcomes
The primary outcome was failure to spontaneously pass the gestational sac within 7 days after random assignment. This primary outcome was the result of a consensus of a national panel of 152 UK health-care practitioners, informed by patient and public views. The prespecified key secondary outcome was surgical intervention to complete the miscarriage up to discharge from hospital care. Other secondary outcomes were surgical intervention to complete the miscarriage up to and including 7 days after random assignment and from 7 days after random assignment to discharge from hospital care, need for further doses of misoprostol within 7 days after random assignment and up to discharge from hospital care, diagnosis of infection associated with miscarriage requiring outpatient antibiotic treatment, diagnosis of infection associated with miscarriage requiring inpatient antibiotic treatment, negative pregnancy test result 21 days (± 2 days) after random assignment, and duration of bleeding as reported by the participant (days). Safety outcomes included requirement for blood transfusion, side-effects, any serious complications, and death. A masked endpoint review committee was convened to assess whether the primary outcome had been met for participants who did not undergo an ultrasound scan on day 6 or 7 and had not already passed their gestational sac according to an earlier scan. The masked endpoint review committee adhered to a prespecified committee charter. In brief, participants who did not undergo ultrasound scan on day 6 or 7 were contacted by the central trial management team and asked for details regarding the course of events, including vaginal bleeding, abdominal pain, and passage of pregnancy tissues. After trial recruitment was complete, the masked endpoint review committee convened to discuss the clinical details of each of these participants. The decision of whether the primary outcome was met needed to be decided unanimously by the committee.
Statistical analysis
We calculated that 335 women would need to be included in each trial group to provide 90% power to detect a minimally important absolute difference of ten percentage points between the mifepristone plus misoprostol group and the placebo plus misoprostol group for the primary outcome of failure to pass the gestational sac within 7 days (25% vs 15%) at a two-sided α level of 0·05. This minimally important difference was chosen on the basis of a national survey of health-care practitioners in the UK. The control group estimate was based on a systematic review by our research team at the time of trial design. We planned to include 710 women in the trial to account for an expected 5% loss to follow-up.
All analyses were prespecified in a statistical analysis plan. All randomly assigned women were included in the analysis and were analysed in the group to which they were randomly allocated, regardless of treatment received (intention-to-treat analysis).
For all binary outcomes, we used a log-binomial regression model to calculate the adjusted risk ratios (RR) and 95% CIs. For continuous outcomes, we used a linear regression model to estimate adjusted mean differences and 95% CIs. All estimates of treatment effects between groups were adjusted for the minimisation variables, where maternal age, BMI, and gestational age were treated as continuous fixed effects, parity and bleeding score as categorical fixed effects, and randomising centre as a random effect. If covariate adjustment was not possible, alternative models were explored. Where required, p values from the associated models were produced and used to assess statistical significance.
We prespecified a hierarchical testing procedure to allow for multiple comparisons. The null hypothesis for the primary outcome was tested first and if statistically significant at the 5% level, the key secondary outcome would be tested. Otherwise, no further hypothesis testing would be done. For all safety outcomes, to assess any signal within specific organ systems, p values are presented unadjusted for multiple testing. Results from all other secondary outcomes are treated as exploratory rather than confirmatory. No adjustments for multiple testing were made for CIs.
We analysed the treatment effect for the primary outcome in prespecified subgroups defined according to maternal age (<30 years vs ≥30 years), BMI (<35 kg/m2 vs ≥35 kg/m2), previous parity (nulliparous women vs parous women), gestational age (<70 days vs ≥70 days), and amount of bleeding (PBAC score20 ≤2 vs ≥3). The subgroup defined by gestational age was prespecified as of special interest; the results of other subgroup analyses should be treated with caution and used for the purposes of hypothesis generation only.21 The effects of these subgroups were examined by adding the variables for the interaction of subgroup with trial group to the regression model. We used a χ2 test to determine whether the effects of mifepristone and misoprostol or placebo and misoprostol differed in the various subgroups. Sensitivity analysis consisted of a restricted analysis excluding women for whom the primary outcome was determined using information from the masked endpoint review committee.
Analyses of principal safety and effectiveness outcomes were done on behalf of the data and safety monitoring committee by the trial statistician (who remained masked to treatment assignments) on two occasions during the recruitment period (at 251 patients and 507 patients). Because these analyses were done with the Peto principle,22 which applies a stringent stopping boundary of p<0·001, no adjustment was necessary to the final p values to determine significance. All analyses were done using SAS version 9.4. The trial is registered with the ISRCTN registry, ISRCTN17405024.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
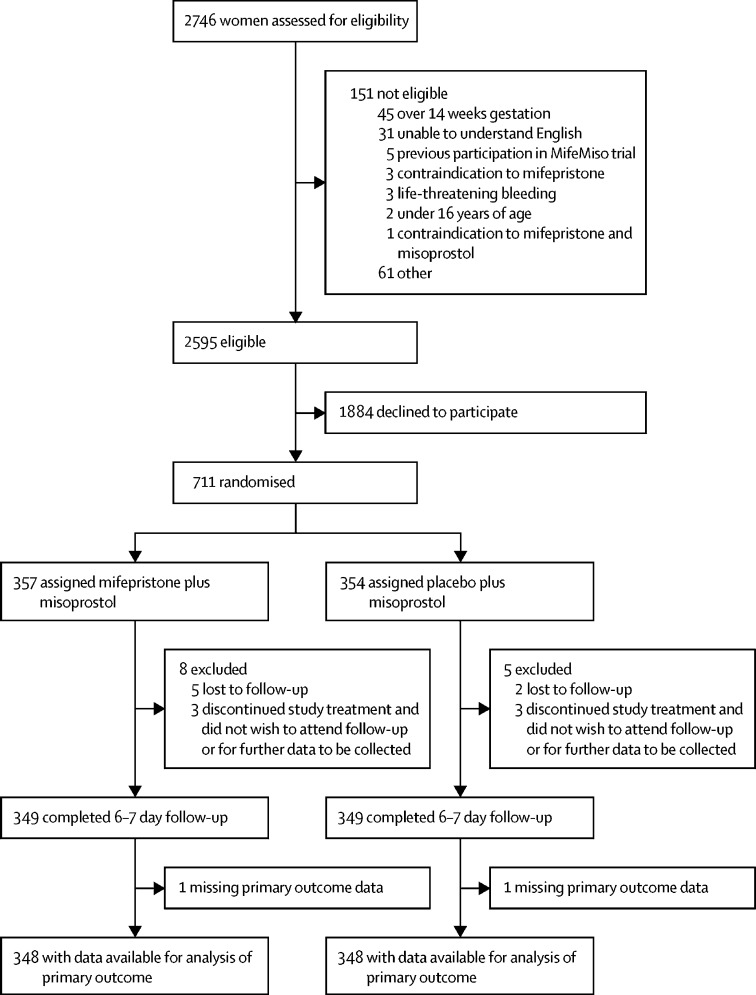
Between Oct 3, 2017, and July 22, 2019, 2595 women were identified as being eligible for the MifeMiso trial (figure 1). 711 women were randomly assigned to receive either mifepristone and misoprostol (357 women) or placebo and misoprostol (354 women). 696 (98%) of 711 women had available data for the primary outcome. Demographic and baseline characteristics were similar between the two trial groups (table 1; appendix p 3).
Figure 1.
Study flowchart
Table 1.
Baseline characteristics
| Mifepristone plus misoprostol (n=357) | Placebo plus misoprostol (n=354) | ||
|---|---|---|---|
| Maternal age, years* | |||
| <30 | 95 (27%) | 95 (27%) | |
| ≥30 | 262 (73%) | 259 (73%) | |
| Mean (SD) | 32·8 (5·6) | 32·7 (5·7) | |
| Body-mass index (kg/m2)* | |||
| <35 | 332 (93%) | 328 (93%) | |
| ≥35 | 25 (7%) | 26 (7%) | |
| Mean (SD) | 25·8 (5·6) | 26·5 (5·5) | |
| Previous parity* | |||
| Nulliparous | 167 (47%) | 168 (47%) | |
| Parous | 190 (53%) | 186 (53%) | |
| Gestational age, days* | |||
| <70 | 176 (49%) | 175 (49%) | |
| ≥70 | 181 (51%) | 179 (51%) | |
| Mean (SD) | 70·5 (13·1) | 70·7 (13·8) | |
| Amount of bleeding (Pictorial Blood Assessment Chart20 score)† | |||
| ≤2 | 351 (98%) | 348 (98%) | |
| ≥3 | 6 (2%) | 6 (2%) | |
| Ethnicity | |||
| White | 296 (83%) | 280 (79%) | |
| Black | 10 (3%) | 17 (5%) | |
| Asian | 38 (11%) | 42 (12%) | |
| Other | 12 (3%) | 15 (4%) | |
| Pregnancy-related pain score at random assignment‡ | 1·0 (1·8) | 1·2 (2·0) | |
| Number of gestational sacs | |||
| 1 | 351 (98%) | 348 (98%) | |
| 2 | 6 (2%) | 6 (2%) | |
Data are n (%) or mean (SD). Missing data are <1% unless otherwise presented. Further details of baseline characteristics are given in the appendix (p 3).
Minimisation variables.
0–4; 0=no bleeding, 4=heavy bleeding.
0–10; 0 indicates no pain, 10 indicates worst possible pain.
59 (17%) of 348 women in the mifepristone plus misoprostol group did not pass the gestational sac spontaneously within 7 days, versus 82 (24%) of 348 women in the placebo plus misoprostol group (RR 0·73, 95% CI 0·54–0·99; p=0·043; table 2).
Table 2.
Primary and secondary outcome results
| Mifepristone plus misoprostol (n=357) | Placebo plus misoprostol (n=354) | RR estimate*(95% CI) | p value | ||
|---|---|---|---|---|---|
| Primary outcome | |||||
| Failure to pass the gestational sac spontaneously within 7 days after random assignment | 59/348 (17%) | 82/348 (24%) | 0·73 (0·54 to 0·99) | 0·043 | |
| Key secondary outcome | |||||
| Surgical intervention to complete the miscarriage up to discharge | 62/355 (17%) | 87/353 (25%) | 0·71 (0·53 to 0·95) | 0·021 | |
| Other secondary outcomes | |||||
| Surgical intervention to complete the miscarriage up to and including day 7 after random assignment | 23/355 (6%) | 19/353 (5%) | 1·23 (0·68 to 2·21)† | .. | |
| Surgical intervention to complete the miscarriage from after day 7 and up to discharge | 39/355 (11%) | 68/353 (19%) | 0·56 (0·39 to 0·81) | .. | |
| Need for further doses of misoprostol within 7 days after random assignment | 34/356 (10%) | 48/354 (14%) | 0·71 (0·47 to 1·08) | .. | |
| Need for further doses of misoprostol up to discharge | 50/357 (14%) | 65/354 (18%) | 0·77 (0·55 to 1·09) | .. | |
| Infection requiring outpatient antibiotic treatment | 8/351 (2%) | 11/351 (3%) | 0·73 (0·29 to 1·82)‡ | .. | |
| Infection requiring inpatient antibiotic treatment | 5/351 (1%) | 4/351 (1%) | 1·25 (0·33 to 4·74)‡ | .. | |
| Negative pregnancy test result 21 days (±2 days) after random assignment | 237/308 (77%) | 230/302 (76%) | 1·03 (0·94 to 1·14)§ | .. | |
| Pregnancy test not provided | 33 | 28 | .. | .. | |
| Missing | 16 | 24 | .. | .. | |
| Duration of bleeding reported by woman, days | 16·0 (12·6; 326) | 16·3 (15·2; 330) | −0·3 (−2·5 to 1·8) | .. | |
| Time from random assignment to discharge, days | 27·0 (14·2; 340) | 27·3 (14·4; 337) | .. | .. | |
Data are n (%), n, or mean (SD; N), unless otherwise indicated. Missing data are <3% unless otherwise presented. RR=risk ratio.
Value <1 favours mifepristone plus misoprostol combination group; the RR was adjusted for the following minimisation variables: maternal age, body-mass index, previous parity, gestational age, and amount of bleeding; the clustering effect of randomising centres was accounted for by using robust standard errors at the centre level.
Centre removed from the model because of non-convergence.
Unadjusted model used because of non-convergence.
Poisson model used because of non-convergence.
62 (17%) of 355 women in the mifepristone plus misoprostol group of required surgical intervention to complete the miscarriage, versus 87 (25%) of 353 women in the placebo plus misoprostol group (RR 0·71, 95% CI 0·53–0·95; p=0·021; table 2). Among women who underwent surgery, the most common reason for surgery was pregnancy tissue remaining in the uterus, reported in 55 (89%) of 62 women in the mifepristone plus misoprostol group and 79 (91%) 87 women in the placebo plus misoprostol group.
50 (14%) of 357 women in the mifepristone plus misoprostol group required further doses of misoprostol up to discharge, versus 65 (18%) of 354 women in the placebo plus misoprostol group (RR 0·77, 95% CI 0·55–1·09). The mean time from randomisation to discharge was 27·0 days (SD 14·2) in the mifepristone plus misoprostol group versus 27·3 days (14·4) in the placebo plus misoprostol group.
A detailed description of the interventions is provided in the appendix (p 5). 337 (94%) of 357 women in the mifepristone plus misoprostol group and 341 (96%) of 354 women in the placebo plus misoprostol group adhered to the treatment regimen (appendix p 5). A sensitivity analysis excluding the findings of the masked endpoint review committee was consistent with the findings of the primary analysis (RR 0·75, 95% CI 0·55–1·02; p=0·062; appendix p 8).
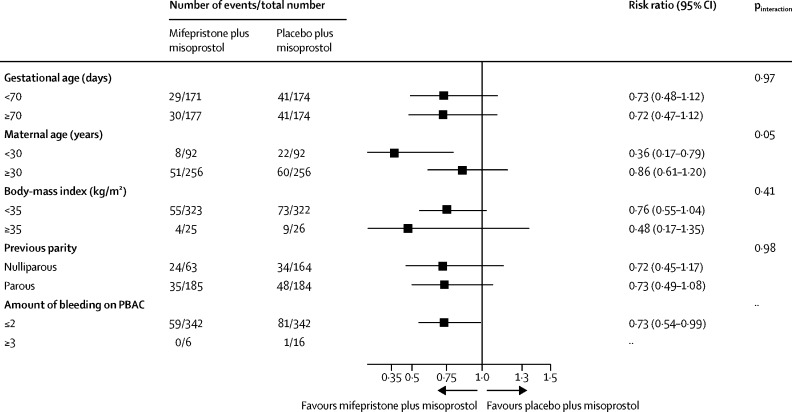
We found no evidence of a subgroup effect according to gestational age, which was prespecified as a subgroup of special interest (figure 2). We found no evidence of a between-group difference in the proportions of participants with serious adverse events, which were reported in five (1%) of 357 women in the mifepristone plus misoprostol group and two (1%) of 354 women in the placebo plus misoprostol group. The incidence of adverse side-effects and requirement for blood transfusion were also similar in both trial groups. There were no deaths in the trial population. All safety outcomes and adverse side-effects are reported in the appendix (p 9).
Figure 2.
Subgroup analyses
PBAC=Pictorial Blood Assessment Chart.
Discussion
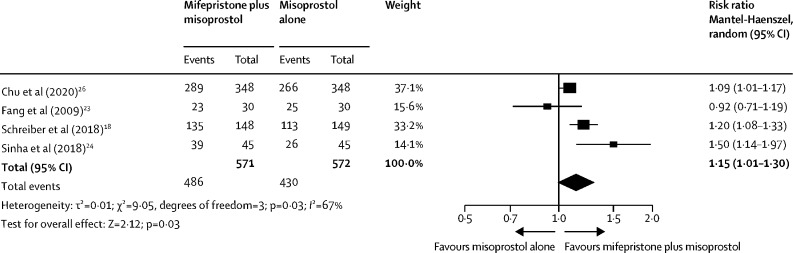
Our large multicentre, randomised, double-blind, placebo-controlled trial showed that the combination treatment of mifepristone plus misoprostol resulted in an increase in the number of missed miscarriages that were completed within 7 days compared with misoprostol alone. Furthermore, fewer incidences of surgical management to complete miscarriage were reported in the mifepristone plus misoprostol group compared with the misoprostol alone group. These findings are consistent with other previously published trials.18, 23, 24 A Cochrane review published in 201925 identified three previously published trials that evaluated the effectiveness of mifepristone and misoprostol versus misoprostol alone in the medical management of miscarriage. These trials included smaller numbers of participants (60 to 300 participants). Although these trials used the same dose of mifepristone as our trial (200 mg), the dose of misoprostol varied (400 to 800 μg). Additionally, these trials assessed the outcome of miscarriage management by varying methods, including clinical and ultrasound assessment, and at differing timepoints. We updated the Cochrane meta-analysis that investigated the effectiveness of mifepristone and misoprostol compared with misoprostol alone for the medical management of miscarriage to incorporate the findings from our trial (figure 3). We found a benefit for mifepristone plus misoprostol compared with misoprostol alone for the resolution of missed miscarriage (RR 1·15, 95% CI 1·01–1·30).
Figure 3.
Updated meta-analysis of mifepristone plus misoprostol compared with misoprostol alone for successful resolution of missed miscarriage
Our trial results show the importance of optimising the medical management of missed miscarriage using the combined mifepristone and misoprostol treatment regimen, which improves outcomes and safety by increasing the proportion of women who have miscarriage resolution and reducing the need for surgical management. Women who choose medical management of missed miscarriage often wish to have expedited treatment and resolution of their miscarriage, while avoiding the risks of surgery.26 The risks of miscarriage surgery are dependent on the clinical context, the setting in which the procedure is done, the surgeon, and available equipment. Complications of miscarriage surgery are rare, but can include bleeding, infection, and uterine perforation requiring more extensive surgery, which carries substantial morbidity.26 Our findings show that the combination treatment of mifepristone and misoprostol reduces the need for surgery after medical management, which could be of great importance to women who wish to undergo medical management of missed miscarriage.
The combination regimen is also likely to be cost-effective; we are in the process of a formal cost effectiveness analysis using resource use data collected within this trial. Similarly, qualitative interviews were done with a maximum variation sample of 42 women who participated in the trial to explore their satisfaction and experiences. A preliminary analysis of the mixed-methods work found a preference for active management of miscarriage to bring a timely resolution to physical symptoms, and women were more likely to express satisfaction with medical management. Detailed mixed-methods findings have been interpreted within the context of the clinical findings, and will be reported separately.
The strengths of this study include its multicentre approach, generalisability across a range of settings, and the placebo-controlled design with high adherence to treatment, enhancing internal validity. A pragmatic design was used in our study, which also adds to the generalisability of our findings. The route of administration of misoprostol reflected standard UK clinical practice and NICE guidance for the medical management of missed miscarriage.5 Most of our participants received vaginal misoprostol and the route of misoprostol administration was similar in both trial groups. The primary outcome of the MifeMiso trial was carefully selected through a consultation and survey of clinicians working with women diagnosed with miscarriage and the women themselves through patient involvement. We collated near-complete data for the primary outcome, which was aided by the use of a masked endpoint review committee. The committee convened using a strict charter and considered each individual participant's clinical data in turn. Clinical data were collected on a standardised case report form and the decision as to whether the participant had met the primary outcome could only be made unanimously. The sensitivity analysis excluding the findings of the masked endpoint review committee did not alter the findings of our trial and was consistent with the primary analysis.
However, some limitations of our study should also be considered. We studied the effect of study drugs in missed miscarriage, and therefore, the results are not generalisable to patients diagnosed with incomplete miscarriage where some pregnancy tissue has already been passed. The biological rationale for focusing exclusively on missed miscarriage was that the anti-progesterone effect of mifepristone was less likely to be relevant in incomplete miscarriage, in which the expulsion of pregnancy tissue has already begun.27
In conclusion, our trial showed that pretreatment with mifepristone followed by misoprostol resulted in a higher rate of resolution of missed miscarriage by 7 days compared with misoprostol treatment alone. Therefore, we recommend that women with missed miscarriage should be offered mifepristone pretreatment before misoprostol to increase the chance of successful miscarriage management, while reducing the need for miscarriage surgery. Clinical guidelines should be updated in light of this evidence.
Data sharing
After publication, trial data will be made available on reasonable request to the corresponding author. A proposal with a detailed description of study objectives and a statistical analysis plan will be needed for assessment of requests. Additional materials might also be required during the process of assessment. Deidentified participant data will be provided after approval by the chief investigator and trial management group.
Acknowledgments
Acknowledgments
This report presents independent research commissioned by the National Institute for Health Research (NIHR). A final report of data collected in this study will be published in the NIHR Journals Library. The views expressed in this Article are those of the author(s) and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health and Social Care. This project was funded by the NIHR Health Technology Assessment programme (15/160/02). The project was also supported by the Tommy's Charity, whose funding supports the UK National Miscarriage Research Network. We thank all the women who participated in this study; the following investigators for supervising recruitment and randomisation at the study centres: Amna Ahmed, Kalsang Bhatia, Cecilia Bottomley, Ying Cheong, Meena Choudhary, Shilpa Deb, Sangeetha Devarajan, Pratima Gupta, Judith Hamilton, Ismail Hassan, Frances Hodge, Andrew Horne, Feras Izzat, Yadava Jeve, Chitra Kumar, Jane Mears, Faizah Mukri, Natalie Nunes, Abigail Oliver, Joel Naftalin, Stewart Pringle, Kalpana Rao, Penny Robshaw, Jackie Ross, Anupama Shahid, Martyn Underwood, Nirmala Vaithilingham, and Linda Watkins; Janet Scollen for her outstanding contribution to recruitment and randomisation and all the other MifeMiso research nurses and midwives who assisted in collection of data; Mary Nulty and Hannah Noordali for support in administering the trial; Lee Middleton for his statistical support in the design of the trial; Rajendra Rai for chairing the trial steering committee; Maya Al-Memar and Ruth Bender-Atik for participating in the trial steering committee; Abha Maheshwari for chairing the data monitoring committee; Neelam Potdar and Mike Bradburn for participating in the data monitoring committee; and all those not otherwise mentioned above who have contributed to the MifeMiso trial.
Contributors
JJC, AJD, PH, TER, LLJ, RB-A, JB, KH, MC, AWH, SQ, and AC contributed to the study design and methodology. LEB was the trial manager and contributed to data collection. AA, MC, JN, CB, NN, AO, FI, KB, IH, YJ, JH, SD, JR, LW, MU, YC, CSK, SP, FH, PG, RS, AS, AWH, and SQ were responsible for the oversight of the study at their respective hospitals and contributed to the recruitment of participants. VC, YS, and PH were responsible for data analysis. IDG did the updated meta-analysis. All authors contributed to data interpretation. JJC, AJD, and AC wrote the first draft of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content and gave final approval.
Declaration of interests
AWH reports serving as a consultant for and receiving personal fees from AbbVie, Roche Diagnostics, Ferring, and Nordic Pharma and has received research support grants from the Medical Research Council, National Institute for Health Research, Chief Scientist's Office, Wellbeing of Women, Roche Diagnostics, AstraZeneca, and Ferring, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ. 1997;315:32–34. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantwell R, Clutton-Brock T, Cooper G. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118(suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy FA, Lipp A, Powles DL. Follow-up for improving psychological well being for women after a miscarriage. Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD008679.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farren J, Jalmbrant M, Falconieri N. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol. 2020;222:367. doi: 10.1016/j.ajog.2019.10.102. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence Ectopic pregnancy and miscarriage: diagnosis and initial management. April 17, 2019. https://www.nice.org.uk/guidance/ng126 [PubMed]
- 6.Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ. 2013;346 doi: 10.1136/bmj.f3676. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology ACOG Practice Bulletin No 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197–e207. doi: 10.1097/AOG.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; Geneva: 2018. Medical management of abortion. [PubMed] [Google Scholar]
- 9.Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks) Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD002253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen BA, Creinin MD. Contemporary management of early pregnancy failure. Clin Obstet Gynecol. 2007;50:67–88. doi: 10.1097/GRF.0b013e31802f1233. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761–769. doi: 10.1056/NEJMoa044064. [DOI] [PubMed] [Google Scholar]
- 12.Robledo C, Zhang J, Troendle J. Clinical indicators for success of misoprostol treatment after early pregnancy failure. Int J Gynaecol Obstet. 2007;99:46–51. doi: 10.1016/j.ijgo.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creinin MD, Huang X, Westhoff C, Barnhart K, Gilles JM, Zhang J. Factors related to successful misoprostol treatment for early pregnancy failure. Obstet Gynecol. 2006;107:901–907. doi: 10.1097/01.AOG.0000206737.68709.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major B, Cozzarelli C, Cooper ML. Psychological responses of women after first-trimester abortion. Arch Gen Psychiatry. 2000;57:777–784. doi: 10.1001/archpsyc.57.8.777. [DOI] [PubMed] [Google Scholar]
- 15.Pud D, Amit A. Anxiety as a predictor of pain magnitude following termination of first-trimester pregnancy. Pain Med. 2005;6:143–148. doi: 10.1111/j.1526-4637.2005.05030.x. [DOI] [PubMed] [Google Scholar]
- 16.Stockheim D, Machtinger R, Wiser A. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertil Steril. 2006;86:956–960. doi: 10.1016/j.fertnstert.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Grønlund A, Grønlund L, Clevin L, Andersen B, Palmgren N, Lidegaard Ø. Management of missed abortion: comparison of medical treatment with either mifepristone + misoprostol or misoprostol alone with surgical evacuation. A multi-center trial in Copenhagen county, Denmark. Acta Obstet Gynecol Scand. 2002;81:1060–1065. [PubMed] [Google Scholar]
- 18.Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161–2170. doi: 10.1056/NEJMoa1715726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence Ectopic pregnancy and miscarriage: diagnosis and initial management. Dec 12, 2012. www.nice.org.uk/CG154 [PubMed]
- 20.Bottomley C, Van Belle V, Pexsters A. A model and scoring system to predict outcome of intrauterine pregnancies of uncertain viability. Ultrasound Obstet Gynecol. 2011;37:588–595. doi: 10.1002/uog.9007. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 22.Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang A, Chen Q, Zheng W, Li Y, Chen R-Y. Termination of missed abortion in a combined procedure: a randomized controlled trial. Journal of Reproduction and Contraception. 2009;20:45–49. [Google Scholar]
- 24.Sinha P, Suneja A, Guleria K, Aggarwal R, Vaid NB. Comparison of mifepristone followed by misoprostol with misoprostol alone for treatment of early pregnancy failure: a randomized double-blind placebo-controlled trial. J Obstet Gynaecol India. 2018;68:39–44. doi: 10.1007/s13224-017-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmers M, Verschoor MA, Kim BV. Medical treatment for early fetal death (less than 24 weeks) Cochrane Database Syst Rev. 2019;6 doi: 10.1002/14651858.CD002253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu J, Hardy P, Beeson L, Coomarasamy A, Coomarasamy A. What is the best method for managing early miscarriage? BMJ. 2020;368 doi: 10.1136/bmj.l6438. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg J, van den Bent JM, Snijders MP, de Heus R, Coppus SF, Vandenbussche FP. Sequential use of mifepristone and misoprostol in treatment of early pregnancy failure appears more effective than misoprostol alone: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2014;183:16–19. doi: 10.1016/j.ejogrb.2014.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, trial data will be made available on reasonable request to the corresponding author. A proposal with a detailed description of study objectives and a statistical analysis plan will be needed for assessment of requests. Additional materials might also be required during the process of assessment. Deidentified participant data will be provided after approval by the chief investigator and trial management group.