Highlights
-
•
The provision of high quality of TB care is crucial for the End TB Strategy.
-
•
COVID-19 pandemic will have grave consequences for TB patients.
-
•
Delay in the diagnosis of TB is a surrogate of the quality of TB care.
-
•
There is considerable delay in the diagnosis of TB in The Gambia.
-
•
Need for a better understanding of patient pathways to care.
Keywords: Tuberculosis, Diagnostic delay, Care-seeking, The Gambia
Abstract
Objectives
To investigate the pattern of tuberculosis (TB) care initiation and risk factors for TB diagnostic delay in The Gambia.
Methods
In this cross-sectional study, adult patients diagnosed with pulmonary TB (pTB) in public facilities in the Greater Banjul Area of The Gambia were consecutively recruited from October 2016 to March 2017. Diagnostic delay was defined as >21 days from the onset of at least one symptom suggestive of pTB to diagnosis. Logistic regression analyses were used to investigate risk factors for diagnostic delay.
Results
Overall, 216 pTB patients were included in the study; the median (Interquartile Range (IQR)) age was 30 (23–39) years and 167 (77%) were male patients. Of the 216 patients, 110 (50.9%) of them initiated care-seeking in the formal and informal private sector and 181/216 (83.8%) had TB diagnostic delay. The median (IQR) duration from the onset of symptoms to TB diagnosis was 34 (28–56) days. Age groups 18–29 years (aOR 3.2; 95% CI 1.2–8.8 [p = 0.02]) and 30–49 years (aOR 5.1; 95% CI 1.6–16.2 [p = 0.006]) and being employed (aOR 4.2; 95% CI 1.7–10.5 [p = 0.002]) were independent risk factors for TB diagnostic delay.
Conclusion
There is considerable TB diagnostic delay in The Gambia, and this is likely to be worsened by the COVID-19 pandemic.
Introduction
Globally, tuberculosis (TB) is the leading cause of death from a single infectious disease (Boggiano et al., 2017). An estimated 10 million new cases of TB and 1.5 million TB deaths were reported in 2018 (World Health Organization (WHO), 2019). The current public health strategy for the control of TB in high-burden settings is geared towards the early detection of infectious TB patients, mostly through passive, as opposed to active, case detection, followed by the prompt initiation of treatment (Sreeramareddy et al., 2014). In low- and middle-income countries (LMICs) with high TB burden, the focus has been on passive case detection of TB in national health systems (Fuge et al., 2018). Passive TB case detection is influenced by several factors, including the subject’s perception of the severity of the illness, awareness of the likely cause of their symptoms, stigma, access to health facilities well-equipped to screen for TB and on the level of TB symptoms recognition that will trigger an investigation by the care provider at the time of first presentation by the patients (Shewade et al., 2019). However, this approach has been less effective in LMICs where there is poor access to health care facilities with appropriate diagnostic tools, poor TB symptom awareness and inadequate community and social mobilisation to support effective passive case finding (Shewade et al., 2019). This has resulted in considerable TB diagnostic delay that negatively impacts the whole cascade of care, fuelling the increasing number of missed cases of TB each year, presently estimated globally at 3 million (World Health Organization (WHO), 2019).
There is growing interest in the quality of TB care in TB endemic settings (Satyanarayana et al., 2015). The provision of high quality of care for TB patients, with emphasis on rapid and accurate diagnosis and on patient-centred outcomes, is one of the cardinal objectives of the End TB Strategy of the WHO (Uplekar et al., 2015). Surya et al. reported that 75% of TB patients in Indonesia initiated TB care in the private sector lacking appropriate diagnostic facilities for TB (Surya et al., 2017). A combined patients pathway analysis (PPA) from 13 countries, which included six African countries with the highest TB burden on the continent, reported that about 60% of all TB patients initiated care from the private sector, and less than 50% of estimated TB patients had documented treatment success (Chin and Hanson, 2017). A cascade of TB patient care analysis in South Africa reported that only 53% of TB patients in South Africa were diagnosed and successfully treated (Naidoo et al., 2017). Taken together, the current public health approach for the control of TB in high-burden countries is characterised by TB diagnostic delay as well as delay in the initiation of effective treatment with poor rates of treatment success, all of which are considered surrogates for the quality of TB care (Cazabon et al., 2017).
The diagnosis of TB and initiation of treatment in The Gambia is based primarily on passive case detection (Lienhardt et al., 2001). Therefore, we aimed to investigate the pattern of TB care initiation and the risk factors associated with TB diagnostic delay, among newly diagnosed adult pulmonary TB (pTB) patients in The Gambia.
Methods
Settings, sites and study participants
In The Gambia, the incidence rate of TBis 174 cases/100,000 population (World Health Organization (WHO), 2019), while the prevalence of HIV infection among adults aged 15–49 years is 1.6% (Gambian Ministry of Health and Social Welfare, 2016). Also, 7% of newly diagnosed TB patients in The Gambia are HIV positive (Stop TB Partnership, 2018).
This exploratory cross-sectional study was conducted in the Greater Banjul Area (GBA) of The Gambia between October 2016 and March 2017, using the TB Case-Contact (TBCC) platform at the Medical Research Council Unit The Gambia at LSHTM (MRCG at LSHTM) (Hill and Ota, 2010). Ethical approval was obtained from the Gambia Government/MRC joint ethics committee. The GBA is a mixed urban, peri-urban and rural setting, with an approximate population of 700,000 (Egere et al., 2016), where an estimated 70% of all TB cases in The Gambia are notified (Gambian National Leprosy and Tuberculosis Control Programme (NLTP), 2012).
Our study population were consecutive adult subjects (age ≥18 years) who were newly diagnosed with sputum smear positive pTB disease at either of three health centres (Brikama, Fajikunda and Serrekunda Health Centres) or the MRC Gambia TB clinic in the GBA. Sputum smear microscopy is still the first and only tool for routine diagnosis of TB in most public health facilities providing comprehensive primary health care, including TB diagnosis, in The Gambia. GeneXpert test is additionally available at the MRC Gambia at entry point for potential research participants in the TBCC cohort. The TB clinics in these four public health facilities collectively account for approximately 80% of all TB diagnosis in the GBA annually (Gambian National Leprosy and Tuberculosis Control Programme (NLTP), 2012, Lienhardt et al., 2001).
Study participants were interviewed by trained nurse/field workers within one week of their TB diagnosis. A structured questionnaire was used to collect data on the sociodemographic characteristics, symptoms at presentation and health care seeking behaviours of the study participants (see supplementary material). All study participants were initiated on standard anti-TB treatment by direct observation according to the Gambian National TB Guidelines (Gambian National Leprosy and Tuberculosis Control Programme (NLTP), 2012). Health care providers are persons consulted by patients for their ailments with the aim of obtaining advice or prescription, as previously described (Lienhardt et al., 2001).
Data analysis and statistical methods
TB diagnostic delay was defined as more than 21 days from the onset of at least one symptom of TB before diagnosis, according to the WHO (World Health Organization (WHO), 1997). Participants’ characteristics were summarised using numbers (percentages) for categorical variables and median (interquartile range [IQR]) for quantitative variables. We investigated factors associated with TB diagnostic delay by univariate and multivariable logistic regression analysis. Age, sex and variables with p-value < 0.20 in the univariate analysis were included in the multivariable analysis. All analyses were performed by using the STATA version 16 (StataCorp, College Station, Texas, United States). p-values < 0.05 were considered statistically significant.
Results
All the 216 subjects diagnosed with sputum smear positive pTB in the four public facilities over the study period agreed to participate in the study. The median (IQR) age of the study participants was 30 (23–39) years, and all of them were HIV negative. One hundred and sixty-seven (77.3%) were male patients and 106 (49.1%) were unemployed (Table 1 ). Cough (214 [99.1%]) and malaise (214 [99.1%]) were the most frequent symptoms at presentation, followed by weight loss (211 [97.7%]), fever (196 [90.7%]), loss of appetite (194 [89.8%]), drenching night sweat (131 [60.6%]) and haemoptysis (30 [14.0%]).
Table 1.
Socio-demographic and clinical characteristics of study participants (N = 216).
| Variables | n (%) |
|---|---|
| Age (years) | |
| 18–29 | 102 (47.2) |
| 30–49 | 83 (38.4) |
| ≥50 | 31 (14.4) |
| Sex | |
| Male | 167 (77.3) |
| Female | 49 (22.7) |
| Marital status | |
| Married | 91 (421) |
| Unmarried | 125 (57.9) |
| Formal education | |
| Yes | 196 (90.7) |
| None | 20 (9.3) |
| Tribe | |
| Mandinka | 71 (32.9) |
| Wolof | 36 (16.7) |
| Fula | 45 (20.8) |
| Jola | 31 (14.3) |
| Others | 33 (15.3) |
| Employment status | |
| Employed | 110 (50.9) |
| Unemployed | 106 (49.1) |
| Symptoms | |
| Cough | 214 (99.1) |
| Fever | 196(90.7) |
| Weight loss | 211 (97.7) |
| Malaise | 214 (99.1) |
| Loss of appetite | 194 (89.8) |
| Drenching night sweat | 131(60.6) |
| Haemoptysis | 30(14.0) |
| Care Provider Pattern | |
| Public facility only | 57 (26.4) |
| Mixed public and private | 159 (73.6) |
TB care-seeking pattern
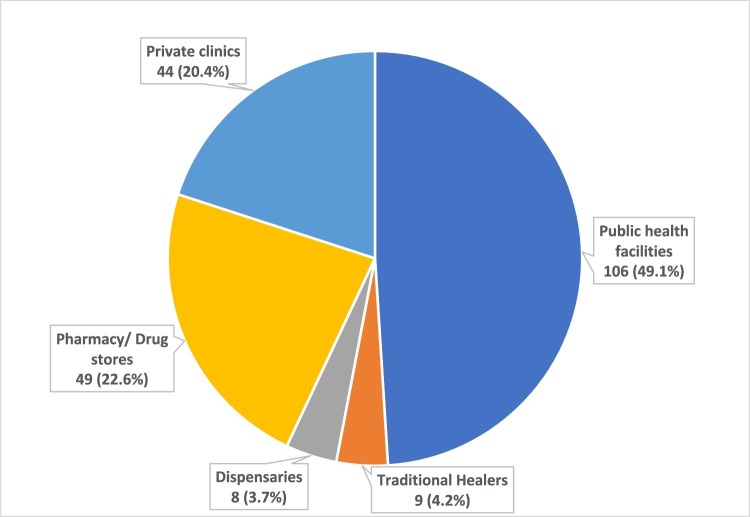
Of the 216 patients with pTB, 110 (50.9%) initiated TB care-seeking in the formal and informal private sector, comprising (traditional healers (n = 9), dispensaries (n = 8), pharmacy shops (n = 49) and private clinics (n = 44). (Figure 1 ). The median (IQR) number of visits to health facilities before TB diagnosis were 3 (2–4) visits. The median (IQR) duration from the onset of at least one symptom suggestive of TB to diagnosis was 34 (28 and 56) days, and 181 patients (83.8%) had more than 21 days from the onset of TB symptoms to diagnosis. One hundred and fifty-nine (73.6%) out of all the newly diagnosed participants with pTB in this analysis reported that they had sought care at both private and public health care facilities before diagnosis.
Figure 1.
Initiation of TB care: Distribution of the facilities at which newly diagnosed patients with pTB first sought care in the Greater Banjul Area of The Gambia (N = 216).
Factors associated with TB diagnosis delay
In the univariate analysis, we found that pTB patients aged 30–49 years (crude OR 5.2; 95% CI 1.8–15.2 [p = 0.003]) and those employed (crude OR 3.6; 95% CI 1.6–8.2 [p = 0.001]) had significantly higher odds of TB diagnostic delay relative to patients aged ≥50 years and those with no employment, respectively. Sex, marital status, tribe, having formal education and the care provider pattern were not associated with TB diagnosis delay (Table 2 ).
Table 2.
Determinants of TB diagnostic delay.
| Variables | Crude OR (95% CIa) | Crude p | Adjusted OR (95% CIa) | Adjusted p |
|---|---|---|---|---|
| Age (years) | ||||
| 18–29 | 2.2 (0.9–5.5) | 0.09 | 3.2 (1.2–8.8) | 0.02 |
| 30–49 | 5.2 (1.8–15.2) | 0.003 | 5.1 (1.6–16.2) | 0.006 |
| ≥50 | Reference | Reference | ||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.8 (0.4–1.9) | 0.64 | 1.3 (0.5–3.3) | 0.59 |
| Marital status | ||||
| Unmarried | Reference | |||
| Married | 1.3 (0.6–2.7) | 0.52 | ||
| Formal education | ||||
| No | Reference | Reference | ||
| Yes | 0.3 (0.1–1.9) | 0.19 | 0.1 (0.1–1.2) | 0.07 |
| Employed | ||||
| No | Reference | Reference | ||
| Yes | 3.6 (1.6–8.2) | 0.001 | 4.2 (1.7–10.5) | 0.002 |
| Tribe | ||||
| Mandinka | Reference | |||
| Wolof | 2.1 (0.7–7.0) | 0.21 | ||
| Fula | 2.1 (0.7–6.4) | 0.17 | ||
| Jola | 1.4 (0.5–4.2) | 0.56 | ||
| others | 1.2 (0.4–3.5) | 0.72 | ||
| Symptoms | ||||
| Cough | ||||
| No | Reference | |||
| Yes | 5.3 (0.3–86.7) | 0.24 | ||
| Weight loss | ||||
| No | Reference | Reference | ||
| Yes | 3.6 (0.6–22.4) | 0.17 | 5.1 (0.7–39.5) | 0.12 |
| Fever | ||||
| No | Reference | |||
| Yes | 0.9 (0.3–3.3) | 0.88 | ||
| Loss of appetite | ||||
| No | Reference | |||
| Yes | 1.6 (0.6–4.7) | 0.39 | ||
| Haemoptysis | ||||
| No | Reference | |||
| Yes | 1.0 (0.3–2.7) | 0.94 | ||
| Drenching night sweat | ||||
| No | Reference | |||
| Yes | 1.5 (0.7–3.1) | 0.27 | ||
| Care provider pattern | ||||
| Public facility only | Reference | |||
| Mixed public and private | 1.1 (0.5–2.6) | 0.75 | ||
95% CI = (95% confidence interval).
The multivariable logistic regression model, with the inclusion of age group categories, sex and variables in the univariate analysis with p-value < 0.20 as covariates, showed that age groups 18–29 years (aOR 3.2: 95% CI 1.2–8.8 [p = 0.02]) and 30–49 years (aOR 5.1: 95% CI 1.6–16.2 [p = 0.006]), and being employed (aOR 4.2: 95% CI 1.7–10.5 [p = 0.002]) were strong, significant and independent risk factors of TB diagnostic delay.
Discussion
Our study reports on the care-seeking pattern and diagnostic delay among newly diagnosed adult patients with pTB in The Gambia, West Africa, prior to the COVID-19 pandemic. We found that half of all newly diagnosed adult pTB patients in the GBA initiated care in the formal or informal private sector, and approximately 84% had delays to their TB diagnosis as defined by the WHO i.e. more than 21 days from the onset of symptoms suggestive of TB to diagnosis (World Health Organization (WHO), 1997). Also, the odds of TB diagnostic delay were, at least, three times higher in patients younger than 50 years of age and those who were employed than in older-aged patients or those who were unemployed. In The Gambia, sputum smear microscopy is still the first and only diagnostic test for TB in the routine public health system. The diagnosis of TB and provision of anti-TB drugs are only available in public health facilities (Gambian National Leprosy and Tuberculosis Control Programme (NLTP), 2012). Although 49% of our study participants initiated care-seeking in public facilities, the finding that more than 80% of all participants in this study had TB diagnostic delay and our finding of no significant difference in the odds of TB diagnostic delay between participants who sought care in public facilities only and those who had sought care in both public and private facilities before TB diagnosis, highly suggest that there could be delays in testing and missed opportunities for early TB diagnosis even in the public health facilities where TB diagnostic tests are available. The recognised limitations of sputum smear microscopy as the only TB diagnostic test available in routine public health system in The Gambia could have contributed to this delay. Thus, the findings further highlight the need for sputum smear microscopy replacement with WHO-endorsed rapid diagnostic tests (World Health Organization (WHO), 2020), and the potential for scaling up of molecular diagnostic tests for TB in The Gambia.
Furthermore, we found that TB patients made an average of three visits to health care providers while almost three-quarters sought care at both private and public health care facilities before diagnosis of TB was made. Our finding that TB patients made an average of three visits to health care facilities before diagnosis is similar to that of a systematic review of 23 studies from different parts of India in which patients made an average of three visits to health care providers before diagnosis (Sreeramareddy et al., 2014). This finding, however, seems to be an improvement over the finding in a previous study conducted in The Gambia that TB patients visited an average of four health care providers, and possibly even greater number of actual visits, before TB diagnosis (Lienhardt et al., 2001). However, another study from Italy, a high-income country, reported that TB patients had an average of two visits before diagnosis (Peri et al., 2018).
Approximately 51% of the adult TB patients in our study initiated care in the formal and informal private sector where studies in LMICs have shown that such providers often lack the knowledge of TB symptoms, and TB diagnostic facilities are frequently unavailable (Cazabon et al., 2020, Daniels et al., 2019, Das et al., 2015). This finding is comparable to the report of a combined PPA from 13 high TB burden countries in Africa and Southeast Asia, where about 60% of all TB patients initiated care in the private sector (Chin and Hanson, 2017).
The median duration from the onset of TB symptoms to diagnosis in our study was 34 days, which is comparable to the 30 days reported from a study in Ethiopia (Fuge et al., 2018). This duration in our study is, however, shorter than durations in the excess of 45 days reported from studies in Angola, Tunisia and Brazil (Ben Amar et al., 2016, Machado et al., 2011, Segagni Lusignani et al., 2013). In contrast to our study, these studies included patients with smear-negative TB, extra-pTB and culture-positive TB disease, which could explain the longer duration from the onset of TB symptoms to diagnosis.
Our study showed that age <50 years and being employed were strong and independent risk factors for TB diagnosis delay. These probably reflect the socio-economic realities of subjects battling ill-health in the low-income setting of The Gambia, where the need for economic survival trumps health care seeking. Older aged patients are probably retired and/or fully dependent on relatives for care. However, in contrast to our report, a systematic review of 45 studies from Asia reported that older age is strongly associated with delays in TB diagnosis (Cai et al., 2015). We did not find any association between gender, marital status, having formal education and TB diagnostic delay. Other studies had reported that female gender was associated with TB diagnostic delay (Ben Amar et al., 2016, Cai et al., 2015, Ndeikoundam Ngangro et al., 2012, Peri et al., 2018, Saldana et al., 2013).
It is very likely that the care-seeking behaviour and delays in TB diagnosis in The Gambia will be further impacted by the advent of the COVID-19 pandemic. Our recently published review article on the impact of the COVID-19 pandemic on TB patients and control programmes highlighted the likely grave consequences for existing and undiagnosed TB patients particularly in LMICs where TB is endemic and health services poorly equipped (Togun et al., 2020). This assertion is supported by another study that modelled the impact of the COVID-19 response on TB in high-burden countries that suggests that the two months of lockdown and of recovery will add an excess of 1.8 million TB cases over the next five years (Stop TB Partnership, 2020).
Our study has limitations: the small sample size might not have allowed sufficient statistical power to detect meaningful differences across strata. Also, the study was conducted in the largely urban Greater Banjul Area of The Gambia, and the findings might not be generalisable to the rural areas of The Gambia. However, the overwhelming majority of TB cases in The Gambia are notified in the densely populated GBA.
Conclusion
This exploratory study has highlighted the considerable delay in the diagnosis of TB in The Gambia, and provides a benchmark against which we can measure efforts to better understand and improve the overall quality of TB care in the country during the COVID-19 pandemic. There is an urgent need for larger prospective studies to better understand TB patients’ pathways to care, particularly the roles of age and socio-economic factors in care-seeking behaviour of presumed TB patients, and the best modalities for engaging the private sector in the national TB control programme. A standard cascade of TB care analysis is also needed to accurately estimate the proportions of total TB patients evaluated, diagnosed, initiated on treatment and successfully treated, and the losses at each step of the cascade.
Authors contribution
OAO and TT conceptualised and designed the study. OAO, MDG and AW coordinated the recruitment of study subjects. AOJ, MJ, GS and RJ recruited and interviewed study participants. SD coordinated the data management. OAO and TT carried out the data analysis. OAO and TT drafted the first version of the manuscript with input from JS and BK. All authors contributed to the revision and correction on multiple iterations of the manuscript.
Funding
This work was supported by the UK Medical Research Council and the U K Department for International Development (DFID, London, UK)under the MRC/DFID Concordant agreement. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interest
None declared.
Acknowledgements
The authors are grateful to the MRCG at LSHTM field and clinic teams (Nurses/field assistants, Nurse coordinators, field workers and supervisors, etc.) who facilitated the recruitment, screening and follow-up of the study participants; the National Tuberculosis and Leprosy programme managers and the study participants and their families who participated in the research project.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.09.029.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ben Amar J., Hassairi M., Ben Salah N., Charfi R., Tritar F., Fourati R. Pulmonary tuberculosis: diagnostic delay in Tunisia. Med Mal Infect. 2016;46(2):79–86. doi: 10.1016/j.medmal.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Boggiano C., Eichelberg K., Ramachandra L., Shea J., Ramakrishnan L., Behar S. Mycobacterium tuberculosis“The impact of immune evasion on protective immunity: implications for TB vaccine design”–meeting report. Vaccine. 2017;35(27):3433–3440. doi: 10.1016/j.vaccine.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Wang X., Ma A., Wang Q., Han X., Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazabon D., Alsdurf H., Satyanarayana S., Nathavitharana R., Subbaraman R., Daftary A. Quality of tuberculosis care in high burden countries: the urgent need to address gaps in the care cascade. Int J Infect Dis. 2017;56:111–116. doi: 10.1016/j.ijid.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazabon D., Pande T., Sen P., Daftary A., Arsenault C., Bhatnagar H. User experience and patient satisfaction with tuberculosis care in low- and middle-income countries: a systematic review. J Clin Tuberc Other Mycobact Dis. 2020;19:100154. doi: 10.1016/j.jctube.2020.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D.P., Hanson C.L. Finding the missing tuberculosis patients. J Infect Dis. 2017;216(suppl_7):S675–S678. doi: 10.1093/infdis/jix368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B., Kwan A., Pai M., Das J. Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South Africa. J Clin Tuberc Other Mycobact Dis. 2019;16:100109. doi: 10.1016/j.jctube.2019.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J., Kwan A., Daniels B., Satyanarayana S., Subbaraman R., Bergkvist S. Use of standardised patients to assess quality of tuberculosis care: a pilot, cross-sectional study. Lancet Infect Dis. 2015;15(11):1305–1313. doi: 10.1016/S1473-3099(15)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egere U., Sillah A., Togun T., Kandeh S., Cole F., Jallow A. Isoniazid preventive treatment among child contacts of adults with smear-positive tuberculosis in The Gambia. Public Health Action. 2016;6(4):226–231. doi: 10.5588/pha.16.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuge T.G., Bawore S.G., Solomon D.W., Hegana T.Y. Patient delay in seeking tuberculosis diagnosis and associated factors in Hadiya Zone, Southern Ethiopia. BMC Res Notes. 2018;11(1):115. doi: 10.1186/s13104-018-3215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambian Ministry of Health and Social Welfare . Republic of The Gambia; Banjul: 2016. National Health Policy: 2016–2020. Health is Wealth; pp. 1–52. [Google Scholar]
- Gambian National Leprosy and Tuberculosis Control Programme (NLTP) Department of State for Health, The Gambia; Banjul, The Gambia: 2012. National Guidelines for The Management of Tuberculosis. [Google Scholar]
- Hill P.C., Ota M.O. Tuberculosis case-contact research in endemic tropical settings: design, conduct, and relevance to other infectious diseases. Lancet Infect Dis. 2010;10(10):723–732. doi: 10.1016/S1473-3099(10)70164-X. [DOI] [PubMed] [Google Scholar]
- Lienhardt C., Rowley J., Manneh K., Lahai G., Needham D., Milligan P. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int J Tuberc Lung Dis. 2001;5(3):233–239. [PubMed] [Google Scholar]
- Machado A.C., Steffen R.E., Oxlade O., Menzies D., Kritski A., Trajman A. Factors associated with delayed diagnosis of pulmonary tuberculosis in the state of Rio de Janeiro, Brazil. J Bras Pneumol. 2011;37(4):512–520. doi: 10.1590/s1806-37132011000400014. [DOI] [PubMed] [Google Scholar]
- Naidoo P., Theron G., Rangaka M.X., Chihota V.N., Vaughan L., Brey Z.O. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl_7):S702–S713. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeikoundam Ngangro N., Chauvin P., Halley des Fontaines V. [Determinants of tuberculosis diagnosis delay in limited resources countries] Rev Epidemiol Sante Publique. 2012;60(1):47–57. doi: 10.1016/j.respe.2011.08.064. [DOI] [PubMed] [Google Scholar]
- Peri A.M., Bernasconi D.P., Galizzi N., Matteelli A., Codecasa L., Giorgio V. Determinants of patient and health care services delays for tuberculosis diagnosis in Italy: a cross-sectional observational study. BMC Infect Dis. 2018;18(1):690. doi: 10.1186/s12879-018-3609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana L., Abid M., McCarthy N., Hunter N., Inglis R., Anders K. Factors affecting delay in initiation of treatment of tuberculosis in the Thames Valley, UK. Public Health. 2013;127(2):171–177. doi: 10.1016/j.puhe.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Satyanarayana S., Subbaraman R., Shete P., Gore G., Das J., Cattamanchi A. Quality of tuberculosis care in India: a systematic review. Int J Tuberc Lung Dis. 2015;19(7):751–763. doi: 10.5588/ijtld.15.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segagni Lusignani L., Quaglio G., Atzori A., Nsuka J., Grainger R., Palma Mda C. Factors associated with patient and health care system delay in diagnosis for tuberculosis in the province of Luanda, Angola. BMC Infect Dis. 2013;13:168. doi: 10.1186/1471-2334-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewade H.D., Gupta V., Satyanarayana S., Pandey P., Bajpai U.N., Tripathy J.P. Patient characteristics, health seeking and delays among new sputum smear positive TB patients identified through active case finding when compared to passive case finding in India. PloS One. 2019;14(3) doi: 10.1371/journal.pone.0213345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramareddy C.T., Qin Z.Z., Satyanarayana S., Subbaraman R., Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–266. doi: 10.5588/ijtld.13.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stop TB Partnership . 2018. Tuberculosis Situation in The Gambia. Switzerland, Geneva. Available from: http://www.stoptb.org/resources/cd/GMB_Dashboard.html [cited 5 September 2020] [Google Scholar]
- Stop TB Partnership . 2020. The Potential Impact of the COVID-19 Response on Tuberculosis in High-Burden Countries: a Modelling Analysis. Geneva, Switzerland. Available from: http://www.stoptb.org/assets/documents/news/Modeling%20Report_1%20May%202020_FINAL.pdf [Cited 24 May 2020] 2020. [Google Scholar]
- Surya A., Setyaningsih B., Suryani Nasution H., Gita Parwati C., Yuzwar Y.E., Osberg M. Quality tuberculosis care in Indonesia: using patient pathway analysis to optimize public-private collaboration. J Infect Dis. 2017;216(suppl_7):S724–S732. doi: 10.1093/infdis/jix379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togun T., Kampmann B., Stoker N.G., Lipman M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann Clin Microbiol Antimicrob. 2020;19(1):21. doi: 10.1186/s12941-020-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uplekar M., Weil D., Lonnroth K., Jaramillo E., Lienhardt C., Dias H.M. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2nd ed. Vol. 1997. World Health Organization; Geneva: 1997. (Treatment of Tuberculosis: Guidelines for National Programmes). [Google Scholar]
- World Health Organization (WHO) 2019. Global Tuberculosis Report 2019. Geneva, Switzerland. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 [Cited 28 March 2020] 2019. [Google Scholar]
- World Health Organization (WHO) WHO; Geneva, Switzerland: 2020. Rapid Communication: Molecular Assays as Initial Tests for the Diagnosis of Tuberculosis and Rifampicin Resistance. Available from: https://apps.who.int/iris/rest/bitstreams/1264904/retrieve [cited 1 September 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.